Abstract
Introduction
Current efficacy data supporting the routine use of acellular dermal matrices (ADM) in postmastectomy tissue expander/implant reconstruction is limited. A multi-center, blinded, randomized controlled study was designed to evaluate the effectiveness of ADM in the setting of TE/I reconstruction. The primary objective of the study was to determine whether the use of ADM would decrease patient-reported, post-operative pain. The secondary objective was to determine whether the use of ADM accelerated the rate of post-operative expansion. Tertiary objectives included an evaluation of long-term aesthetic results, capsular contracture rates and patient satisfaction.
Methods
The randomized controlled trial was conducted at two centers in the US from 2008 to 2011. Immediately following mastectomy, all patients were randomized to one of two treatment arms: i) ADM-assisted, TE/I reconstruction and ii) placement of an expander in a completely submuscular position. All patients were blinded to their treatment arm.
Results
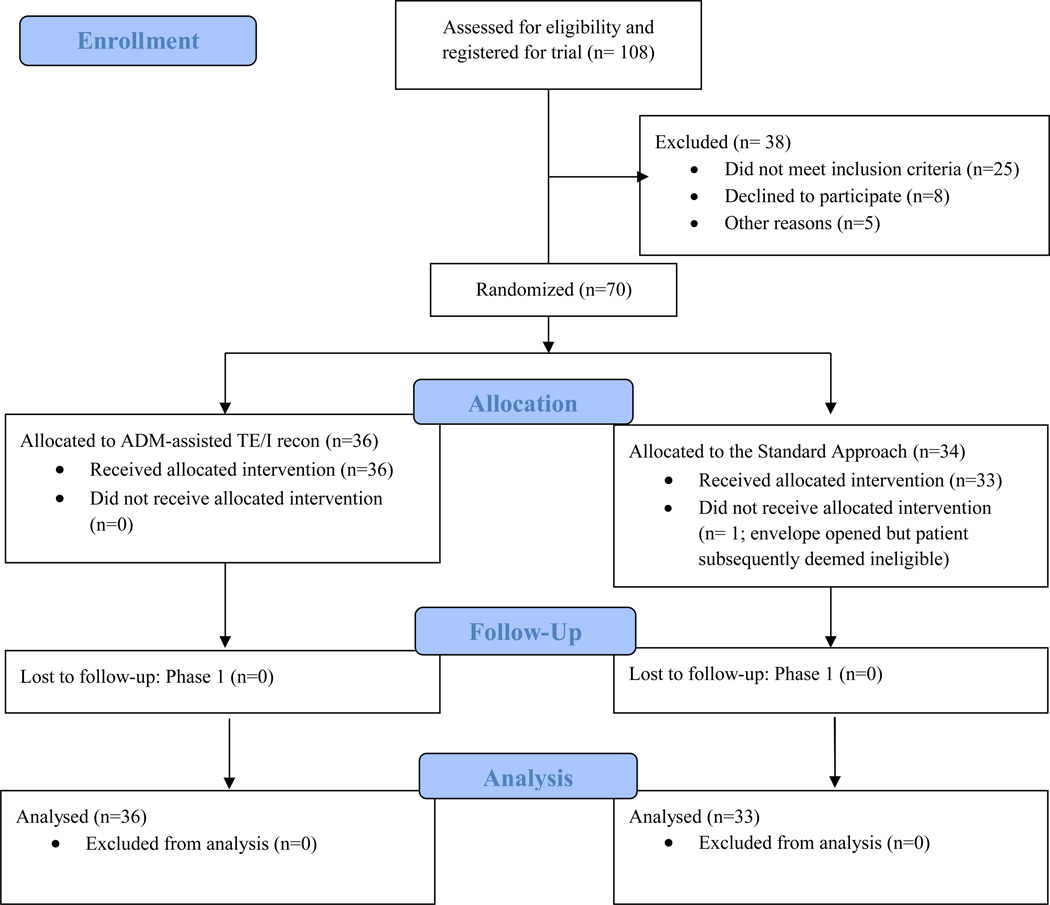
108 consented to participate, 38 of whom were excluded prior to randomization. Thus, in total, 70 patients were randomized. There were no differences between the two groups in pain in the immediate, post-operative period (p=0.19) and pain averaged during the expansion phase (p=0.65). There was similarly no difference in post-operative narcotic use between treatment arms (p=0.38). The rate of post-operative expansion did not differ between groups (p= 0.83). The evaluation of long-term outcomes is currently ongoing.
Conclusions
The results of this multicenter, blinded, randomized controlled trial suggest that the use of ADM in the setting of TE/I reconstruction neither reduces post-operative pain nor accelerates the rate of post-operative expansion. This data provides impartial evidence on the effectiveness of ADM in the early post-operative period. An examination of the efficacy of ADM in improving long-term outcomes following TE/I reconstruction is warranted.
Keywords: acellular dermal matrix, AlloDerm, breast reconstruction, breast implants, tissue expander/implant reconstruction, randomized controlled trial
Introduction
The routine use of acellular dermal matrices (ADM) in the setting of postmastectomy, tissue expander/implant (TE/I) reconstruction is advocated by some (1). Proponents suggest that the use of an implantable dermal matrix affords multiple advantages when compared to traditional techniques. Firstly, by using ADM in the creation of the infero-lateral expander pocket, elevation of the serratus fascia/musculature is avoided. It is hypothesized that the pain and sensory morbidity experienced due to the surgical disruption and subsequent expansion of the lateral intercostal nerves is therefore minimized (2–6). Secondly, because of the pliability of the acellular dermal matrix, it is suggested that the reconstructive process can be expedited by maximizing expansion volumes thereby minimizing the number of expansions required (5–9). Thirdly, it is theorized that by facilitating expansion in the lower pole of the breast, a breast with greater ptosis and more natural contours can be created (5,10–12).
To date, however, the evidence to support these efficacy claims is based solely on limited evidence, namely expert opinion, and retrospective series (1–12). While much has been written about the rate of complications in this setting, the clinical efficacy of ADM-assisted TE/I reconstruction has not been clearly demonstrated (13) Before a practice is established as routine care, it should be tested under controlled conditions to generate evidence of efficacy (14,15). The relatively high cost of AlloDerm and the current lack of high-level evidence supporting its efficacy underscore this need.
We therefore conducted a multi-center, blinded, randomized controlled trial to evaluate the efficacy of ADM in the setting of immediate, tissue expander/implant reconstruction.
Phase I
-
▪
The primary objective of this trial was to determine whether using ADM to create the inferior-medial portion of the expander pocket will decrease patient-reported pain in both the immediate post-operative period and during the tissue expansion phase.
-
▪
The secondary objective was to determine whether using ADM to create the inferior-medial portion of the expander pocket will have an effect the rate of tissue expansion.
Phase II
-
▪
The tertiary objective was to determine the effect of ADM on the long-term aesthetic outcome, rate of capsular contracture, patient satisfaction and quality of life following tissue expander/implant reconstruction.
Phase I has now been completed and the results presented here. The results of Phase II will be presented separately.
Research Design and Methods
Design
This was a multicenter, blinded, randomized controlled trial conducted in the United States from 2008 to 2011. Institutional Review Board approval was obtained at three sites prior to trial initiation.
Participants
All women aged 21 or over who elected to undergo immediate, postmastectomy tissue expander/implant reconstruction were invited to participate. Exclusion criteria included the performance of single-stage, implant reconstruction and/or combined autogenous tissue-expander/implant reconstruction, a history of prior irradiation to the ipsilateral breast or chest and/or a history of prior axillary lymph node dissection. Patients were similarly deemed ineligible intraoperatively by the attending surgeon if they had evidence of significant mastectomy flap ischemia prior to initiation of the reconstructive procedure, and/or if they underwent an axillary lymph node dissection at the time of mastectomy.
Treatment / Intervention
Immediately following mastectomy, prior to initiation of the reconstructive procedure, all patients were randomized to one of two treatment arms. Group A (ADM-assisted Reconstruction) was composed of patients in whom the inferior-lateral portion of tissue expander pocket was created using implantable ADM. The sole ADM used in this trial was a 4 × 16 cm2 thick sheet of AlloDerm (LifeCell Corp, Branchburg, NJ).
In this group, neither the serratus muscle/fascia nor the rectus abdominis fascia was elevated. Laterally, the AlloDerm was sutured to the serratus musculature in line with the anterior axillary fold. Group B (Standard Approach) was composed of patients in whom a standard, submuscular expander pocket was created without the use of ADM. Creation of the submuscular pocket in these patients involved elevation of the serratus muscle/fascia and the rectus abdominis fascia.
The appropriate expander was selected by the attending surgeon based on its base dimensions and volume capability. After wound closure, intraoperative expansion was performed to tissue tolerance. One or two drain(s) were placed deep to the mastectomy skin flaps depending on surgeon preference. All patients received peri-operative antibiotic therapy.
During hospitalization, all patients received a standardized pain control management. Immediately following surgery, intravenous analgesia was administered by PCA pump in the recovery unit. Patients were transferred to the floor after usual discharge criteria were met. While on the floor, transition to oral analgesia was made when the patient demonstrated the ability to tolerate an oral diet.
All patients underwent outpatient, post-operative expansion. Office expansion began as early as 10 days post-operatively and was continued on a weekly or bi-weekly basis, depending on patient preference. Expansions were performed by a plastic surgery physician assistant or plastic surgery nurse, each of whom was blinded to the individual patient’s treatment arm. With each expansion, endpoints for injection included: i) patient discomfort; and/or ii) tissue tolerance. Expansion was considered complete when the patient felt satisfied with the overall size of her breast mound. Overexpansion by approximately 10–20% was then typically performed.
If required, postoperative chemotherapy was generally given during the expansion period. Tissue expanders were then exchanged a minimum of one month after the completion of chemotherapy.
Outcome Measures
Patients were evaluated at 6 time points during Phase I: i) pre-operatively, ii) 24 hours following tissue expander insertion, iii) immediately following the first, second and third post-operative outpatient expansions, and iv) following completion of tissue expansion prior to the exchange procedure. Two patient-reported outcomes measures were used at each time point: i) the Visual Analog Scale (VAS) (16); and, ii) the BREAST-Q© Physical Well-Being: Chest and Upper Body Scale (17).
Pain intensity was measured on the Visual Analog Scale (VAS). The scale consists of a straight line of a specified length with verbal descriptors at each end. The descriptors are short phases that describe the variable being measured (i.e. “no pain at all”; “worst possible pain”). Patients are instructed to place a mark on the line to report the intensity of pain experienced. The distance from the low end of the scale to the patient’s mark is measured in centimeters.
The BREAST-Q© is a newly developed, patient-reported outcome measure that was specifically designed to measure quality of life and patient satisfaction among breast surgery patients. The instrument was developed and validated with adherence to guidelines set by the Scientific Advisory Committee of the Medical Outcomes Trust (2002) (18) and the US Federal Drug Administration (19). The ‘BREAST-Q© Reconstruction Module’s 11-item scale, ‘Physical Well-being: Chest and Upper Body’ was used here. This scale addresses issues such as neck, shoulder and rib pain, pain in the muscles of the chest, difficulty lifting or moving arms, difficulty sleeping because of discomfort in the breast area, and tightness, nagging and throbbing in the breast area. A Likert-type response format is used. The 11 items in the scale are summed and transformed on a 0 to 100 scale, with higher values representing a more favorable outcome. Psychometric evaluation of the scale has shown high levels of internal consistency and test-retest reliability (Cronbach’s alpha = 0.91; Intraclass correlation coefficient = 0.96)(17).
Twenty-four hour narcotic use and expander dynamic data were collected prospectively. Expander dynamic data included: expander size (measured by recommended fill volume), volume of fluid injected intraoperatively, volume of fluid injected administered at each office visit, number of percutaneous injections, total volume of fluid injected and total duration of the expansion phase.
Peri-operative complications and revisional procedures performed were prospectively recorded. Demographic data, past medical history and oncologic data (including breast cancer stage adjuvant therapies received) were extracted through review of the medial record.
Sample Size
The primary outcomes of the study were immediate post-operative pain and pain during the expansion phase, both measured by the VAS. Existing literature suggests that the mean pain score as measured using the VAS in patients undergoing standard tissue expander reconstruction is 6.8 (standard deviation 1.9) in the immediate postoperative period and 5.6 (standard deviation 2.8) during expansion (20). Furthermore, a clinically significant decrease in patient-reported pain symptomatology has been considered a two point reduction in patient-reported pain on the VAS (21) (i.e. reducing the mean pain score to 4.8 in the immediate postoperative period, and to 3.6 during the expansion period). Given that multiple expansions are performed on each patient, the average pain score during the expansion period for each patient will be calculated. Assuming 90% power and a type I error of 0.025 for each of the two comparisons, it was determined that 25 patients per arm were necessary to compare the difference in postoperative pain between groups and 49 patients per arm were necessary to compare differences in pain during the expansion phase using two-sided t-tests. Forty-nine patients per arm were therefore recruited for a total sample size of 98.
Randomization
Randomization was accomplished using randomly permuted blocks, and the randomization list was prepared by a biostatistician with no clinical involvement in the trial. Randomization was stratified on the basis of center and laterality of reconstruction (i.e. unilateral versus bilateral reconstruction). Although there is no published data to support the hypothesis that patients who undergo bilateral reconstruction have more post-operative pain than those who undergo unilateral reconstruction, it was hypothesized that bilaterality could be a confounder.
Allocation Concealment
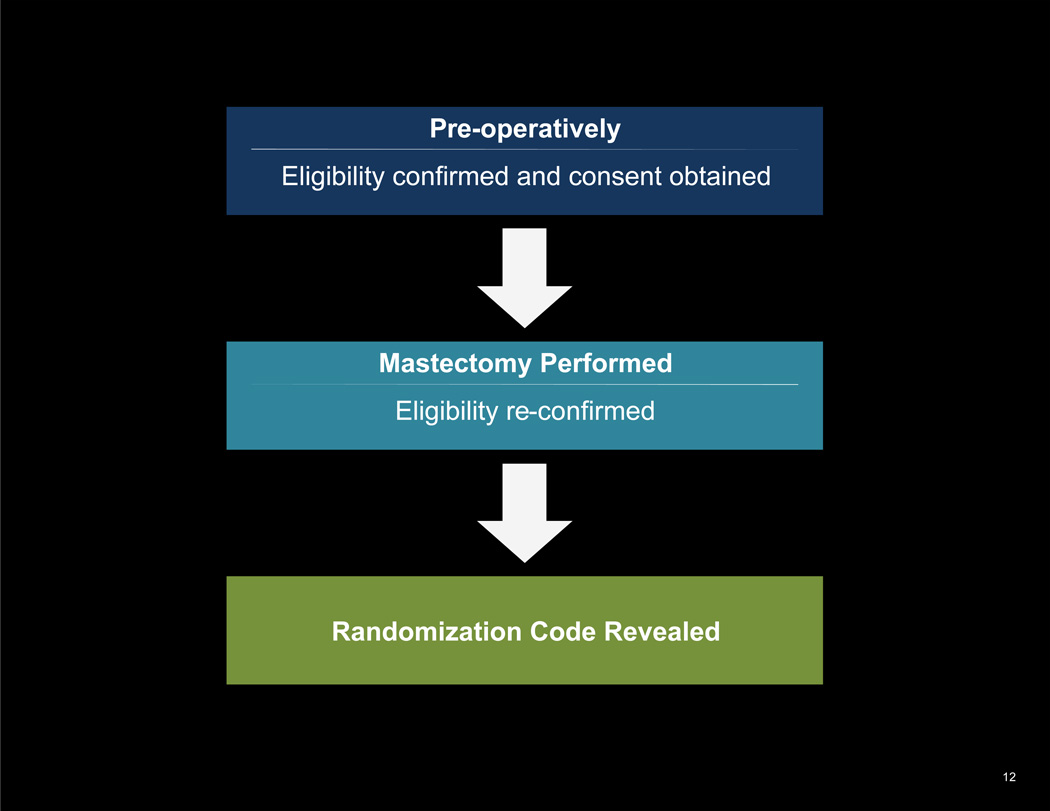
The allocation sequence was concealed by placing each randomization assignment in a sequentially numbered, opaque, sealed envelope. Consecutive envelopes were delivered to the operating room by the onsite research study assistant once the mastectomy was complete. To prevent subversion of the allocation sequence, envelopes were not opened until the attending physician confirmed the absence of significant mastectomy flap necrosis and the fact that a patient did not undergo a concurrent axillary lymph node dissection. (See Figure 1.)
Figure 1.
Two-stage eligibility confirmation and randomization
Blinding
All patients were blinded to their treatment arm. Once the treatment arm was revealed, the attending surgeon advised the surgical team (surgical assist(s), operating room nurses) if the ADM was to be prepared. While the surgical team was aware of the randomization code, the rest of the post-operative care team (including recovery room, floor nursing staff and clinic staff performing outpatient expansions), outcome assessors and data analysts were kept blinded to the intervention performed.
Statistical Methods
Differences in pain using the VAS were compared using Analysis of Covariance in order to adjust for baseline pain scores. Differences between group scores using the Breast-Q Physical Well-being Scale were evaluated using t-tests.
Expansion variables (expander size, volume of fluid injected intraoperatively and at each post-operative visit, number of percutaneous injections, total volume of fluid injected, and duration of expansion) were summarized for both groups and compared using the Wilcoxon rank sum test. The number of percutaneous injections included both the intra-operative fill as well as the total number of postoperative fills. Duration of expansion was calculated from the date of surgery until the last expansion. The rate of expansion was defined as the total volume divided by the number of percutaneous injections.
In-hospital narcotic use over the first 24 hours was summarized and differences between groups compared using the Wilcoxon rank sum test. Doses of various narcotics administered were converted to oral codeine equivalent doses for the sake of comparison.
Patients who underwent the premature explantation of a temporary expander (n= 1), were evaluated using an intention-to-treat analysis for all outcomes. For the expansion analyses, only the data from this patient’s first expander was used.
Results
Recruitment
Eligible patients were recruited at two sites: i) Memorial Sloan-Kettering Cancer Center, New York, NY; and, ii) the University of North Carolina at Chapel Hill, NC from 2008 to 2011. Although institutional review board approval was obtained at a third site, the trial was closed at this center prematurely due to difficulties encountered in recruiting patients. In total, six plastic surgeons participated in the trial.
Reasons for Stopped Trial
The trial was subject to annual review by the Data Safety Monitoring Board at the lead institution, MSKCC. At its June 2011 meeting, the Data Safety Monitoring Board recommended that an interim analysis of the study be performed due to concerns about the length of time to reach target accrual. At the time of the interim analysis, 69 patients (70% of planned accrual) had been recruited and randomized.
An unplanned interim analysis was therefore conducted using sequential analysis methodology to evaluate the likelihood that the trial would yield a positive result (i.e. a result suggesting that ADM has a significant, positive effect on reducing pain and sensory morbidity in the setting of TE/I reconstruction) if the study completed its planned accrual. The probability of achieving a positive result based on the primary endpoints of immediate post-operative pain and pain during the expansion period was calculated to be at most 11% and < 1%, respectively. Based on these results, the decision was made to close the trial to accrual early. Importantly, all participants continue to complete their full 12 month follow-up at this time, despite the fact that the study is closed to accrual.
Baseline Data
There was no difference in patient age, breast cancer stage, mean mastectomy weight, or the proportion of patients receiving neoadjuvant or adjuvant chemotherapy between the two groups. Since patients were stratified by unilateral versus bilateral reconstruction, there was similarly no difference in these proportions between treatment arms. There was also no difference in baseline pre-operative VAS (p=0.33) and physical well-being (p=0.88) between groups. Baseline clinical/demographic data is summarized in Table 1.
Table 1.
Baseline Clinical/Demographic Variables
| Variable | Category | ADM-assisted Reconstruction (n=36) |
Standard Approach (n=33) |
p-value |
|---|---|---|---|---|
| Age | Median (range) | 49 (29–69) | 53 (32–72) | 0.15 |
| Breast Cancer Stage | 0 | 14 (39%) | 13 (39%) | 0.93 |
| IA | 14 (39%) | 11 (33%) | ||
| IIA | 7 (19%) | 8 (24%) | ||
| IIB | 1 (3%) | 1 (3%) | ||
| Chemotherapy | None | 25 (69%) | 21 (64%) | 0.92 |
| Neoadjuvant | 2 (6%) | 2 (6%) | ||
| Adjuvant | 9 (25%) | 10 (30%) | ||
| Laterality | Unilateral | 16 (44%) | 16 (48%) | 0.81 |
| Bilateral | 20 (56%) | 17 (52%) | ||
| Mastectomy weight (grams) | Median (IQR) | 548 (362–882) | 527 (395–874) | 1.0 |
| Mean (std) | 597 (308) | 643 (408) | ||
| Missing | 10 | 7 | ||
| Pre-op VAS | Median (IQR) | 0 (0, 7) | 0 (0, 1.8) | 0.33 |
| Mean (std) | 7 (14.9) | 1.4 (3.2) | ||
| Missing | 3 | 1 | ||
| Pre-op Physical Well-being | Median (IQR) | 85 (77, 100) | 85 (81, 100) | 0.70 |
| Mean (std) | 85.6 (13.5) | 86.9 (12.4) | ||
| Missing | 2 | 2 |
Numbers analyzed
No patient was lost to follow up in Phase I of the trial; thus, data from 69 patients were available for the intention-to-treat analysis. (See Figure 2.)
Figure 2.
Flow Diagram
Outcomes
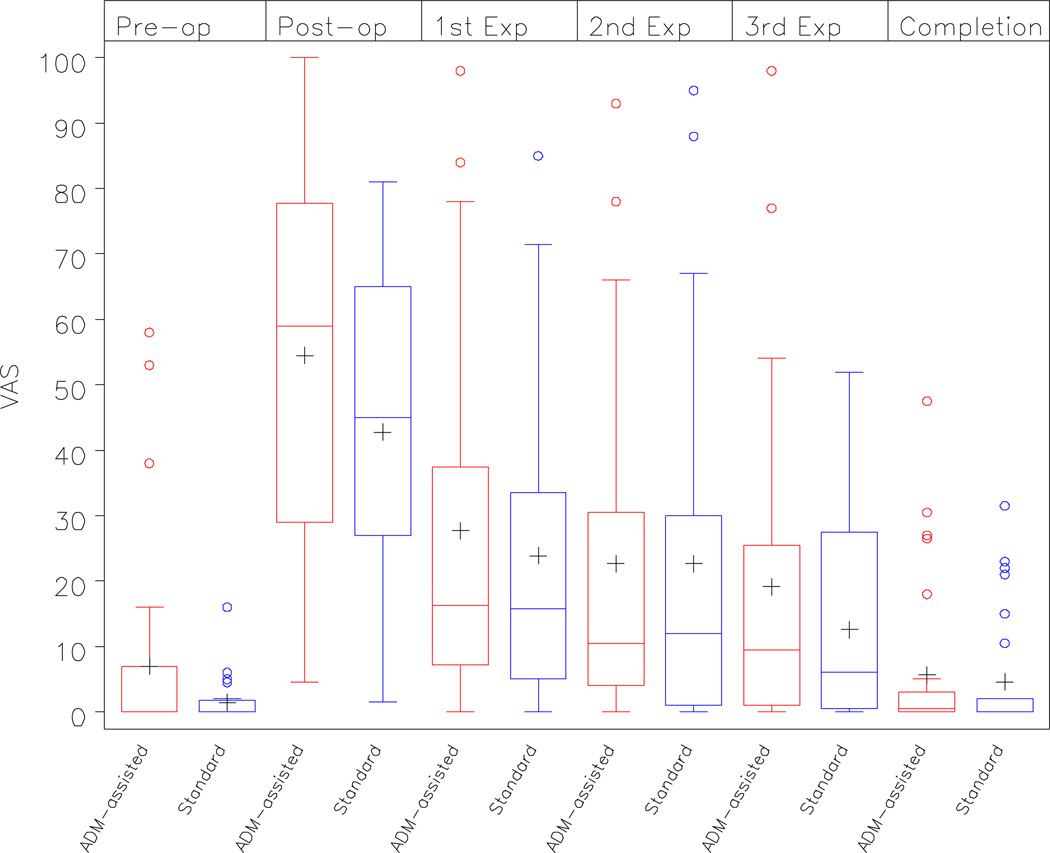
There were no differences in VAS scores between the two groups for the two primary outcomes: pain in the immediate, post-operative period (p=0.19) and pain averaged during the expansion phase (p=0.65). There was also no difference in pain prior to the exchange procedure (p=0.93). These results were consistent after removing patients from the analysis who had unusually high baseline pain values (i.e. > 30 on the pre-operative VAS). Summary statistics are presented in Table 2A. (See Figure 3).
Table 2.
| A. Patient-reported pain (VAS) over time | |||
|---|---|---|---|
| ADM-assisted Reconstruction |
Standard Approach | p-value | |
| Mean (std) | Mean (std) | ||
| Baseline | 7 (14.9) | 1.4 (3.2) | 0.33 |
| Immediate 24 hour Post-operative Period |
54.6 (27.6) | 42.8 (24.5) | 0.19* |
| Expansion Phase† | 17.0 (15.9) | 4.6 (8.9) | 0.65* |
| Completion of Expansion | 5.6 (11.6) | 4.6 (8.9) | 0.93* |
| B. Patient-reported Physical Well-being: Chest and Upper Body (BREAST-Q©) over time | |||
|---|---|---|---|
| ADM-assisted Reconstruction |
Standard Approach | p-value* | |
| Mean (std) | Mean (std) | ||
| Baseline | 85.6 (13.5) | 86.9 (12.4) | 0.70 |
| Immediate 24 hour Post-operative Period |
65.8 (12.7) | 68.2 (13.7) | 0.52 |
| Expansion Phase† | 68.6 (10.6) | 69.3 (7.9) | 0.77 |
| Completion of Expansion | 79.7 (15.1) | 80.5 (13.3) | 0.82 |
p-value calculated using ANCOVA to adjust for baseline VAS
VAS scores averaged during expansion phase
Scores averaged during expansion phase
Figure 3.
VAS scores in the ‘ADM-assisted’ and ‘Standard Approach’ cohorts over time
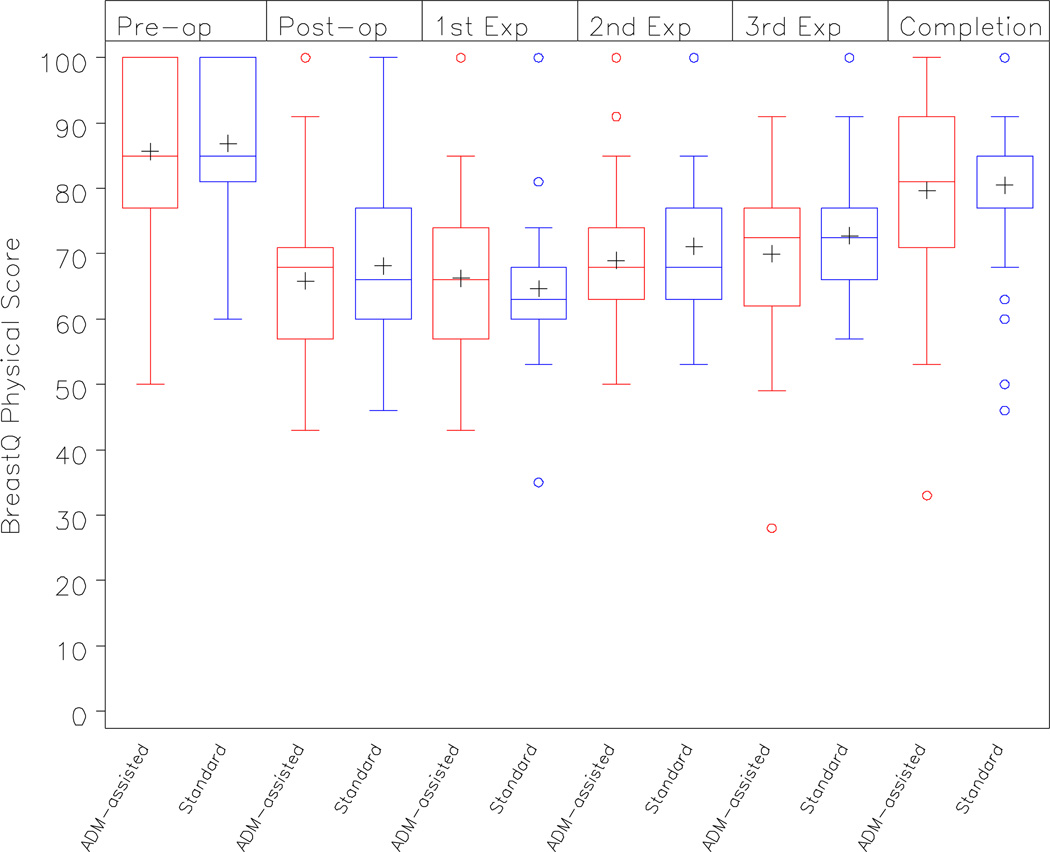
There were similarly no differences in physical well-being in the immediate post-operative period, during the expansion phase, or prior to the exchange period (p= 0.52, p=0.77, p=0.82, respectively). Summary statistics are presented in Table 2B. (See Figure 4).
Figure 4.
BREAST-Q Physical Well-being: Chest and Upper Body Scores in the ‘ADM-assisted’ and ‘Standard Approach’ cohorts over time
Immediate, 24-hour postoperative narcotic use is summarized in Table 3. There was no difference in narcotic use between treatment arms (p=0.38).
Table 3.
In-hospital narcotic use reported as oral codeine equivalents (mg)
| ADM-assisted Reconstruction | Standard Approach | p-value | |||||
|---|---|---|---|---|---|---|---|
| N | Mean (std) | Median (IQR) | N | Mean (std) | Median (IQR) | ||
| 0–6 hrs post-op | 33 | 228 (153) | 200 (100, 300) | 30 | 256 (197) | 200 (123, 340) | 0.77 |
| 6–24 hrs post-op | 33 | 619 (519) | 520 (200, 850) | 30 | 715 (533) | 620 (267, 920) | 0.38 |
| Total 24 hr period |
36 | 776 (602) | 600 (295, 1143) | 32 | 910 (634) | 890 (365, 1222) | 0.38 |
Expander dynamics are presented in Table 4. There was no difference in intra-operative fill volume between the two treatment arms (p=0.86). The mean number of percutaneous injections was 6.4 (std 1.6) in the ADM-assisted reconstruction and 7.3 (std 1.8) in the Standard approach. This difference was statistically significant (p=0.04).
Table 4.
Expander Dynamics
| ADM-assisted Reconstruction | Standard Approach | p-value | |||
|---|---|---|---|---|---|
| Mean (std) | Median (IQR) | Mean (std) | Median (IQR) | ||
| Expander size | 431 (94) | 400 (350–500) | 465 (123) | 450 (400–500) | 0.23 |
| Intra-op fill (cc) | 145 (82) | 120 (60–180) | 141 (100) | 120 (60–180) | 0.86 |
| No. percutaneous injections |
6.4 (1.6) | 7 (5–7.5) | 7.3 (1.8) | 8 (5–9) | 0.04 |
| Total volume of fluid injected (cc) |
524 (146) | 480 (420–660) | 587 (167) | 580 (440–660) | 0.11 |
| Duration of expansion phase (days) |
91 (59) | 73 (54–115) | 108 (65) | 90 (65–148) | 0.19 |
|
Expansion rate (cc/fill) |
85 (23) | 80 (70–98) | 84 (26) | 79 (64–103) | 0.83 |
Adverse Events
The proportion of patients experiencing any adverse event was similar between the two groups (p=1.00). See Table 5. Two patients developed a post-operative hematoma requiring a return to the operating theater. One of these patients had undergone ADM-assisted reconstruction; the other two had undergone Standard reconstruction. Four patients developed a post-operative seroma, only one of whom underwent ADM-assisted reconstruction. All post-operative seromas were managed by percutaneous drainage and/or observation. A single patient in the ADM-assisted reconstruction cohort developed a peri-prosthetic infection necessitating the premature removal of the device. Following resolution of the infection, the patient then returned to the OR for delayed tissue expander placement. Three additional patients were treated for presumed cellulitis of the breast mound with antibiotics. All responded without surgical intervention.
Table 5.
Adverse events
| ADM-assisted Reconstruction |
Standard Approach | ||
|---|---|---|---|
| No. (%) | No. (%) | ||
| Hematoma | 1 | 1 | |
| Seroma | 1 | 3 | |
| Infection | 3 | 1 | |
| Premature removal of device | 1 | 0 | p-value |
| TOTAL COMPLICATIONS | 6 (17%) | 5 (15%) | 1.00 |
Discussion
In this multicenter, blinded, randomized controlled trial, the use of ADM in the setting of tissue expander/implant reconstruction failed to reduce immediate post-operative pain as well as pain during the expansion phase. Prior studies have suggested, by contrast, that ADM-assisted reconstruction reduces pain symptomatology after two-stage implant reconstruction. Importantly, however, these studies enrolled small numbers of patients and were retrospective in nature (1–12).
While not fully understood, the development of pain and neurosensory symptoms following mastectomy with reconstruction is likely multifactorial. It has been hypothesized that the disruption of the lateral intercostal nerves during elevation of the serratus fascia/musculature contributes significantly to the development of postoperative pain in the setting of TE/I reconstruction. The results of this trial suggest, however, that there is no improvement in postoperative pain when elevation of the serratus fascia is avoided during ADM-assisted reconstruction. Instead, it may be that suture fixation of ADM to the lateral chest wall similarly results in nerve injury through suture ligation of sensory branches of the lateral intercostal nerves. Alternatively, the development of neurosensory symptoms following tissue expander/implant reconstruction with or without ADM may arise mostly from transection of these nerves during mastectomy and the subsequent proliferation of scar tissue surrounding these nerve endings, resulting in multiple, microscopic, traumatic neuromas. Further evaluation here is warranted.
Severe pain during submuscular expansion can result in inadequate expansion (24) or the premature surgical removal of an expander (25). Legeby et al recently investigated post-operative pain intensity in women after different types of breast cancer surgery. The subgroup of women who had immediate reconstruction using an expander prosthesis reported “unexpectedly high pain scores” and required more analgesics than patients undergoing mastectomy alone. Interestingly, the authors also concluded that one of the most important determinants of the development of chronic pain was the intensity of acute post-operative pain. (26).
The results of this blinded trial also suggest that ADM-assisted tissue expander/implant reconstruction confers no benefit in terms accelerating the rate of postoperative expansion. Notably, because outpatient visits are scheduled based on external factors such as patient and physician availability, systematic error could have been introduced in this analysis. For example, the rate of tissue expansion observed in this trial may have been limited by the ability to schedule office visits for expansions rather than the ability to proceed with expansion. That said, given that patients were randomized to treatment group, this phenomenon, if present, should have occurred to the same extent in each treatment arm, minimizing any bias introduced in favor of one reconstructive cohort.
The size of the ADM used in this study was limited to one sheet measuring 4 × 16 cm2. Notably, in two cases in this study the operating surgeon felt that the use of a larger sheet of ADM was indicated and thus deemed the patient ineligible for the trial, recognizing that is indeed possible that larger sheets of ADM may produce different clinical outcomes such as more rapid expansion. It follows that the results seen here may not be generalizable to cases where larger sheets of ADM are used.
Finally, complication data from Phase I of this trial suggests that there is no significant difference in peri-operative complications between treatment arms. Importantly, however, this specific analysis was not one of the objectives of this current trial and as such, this trial was not adequately powered to make such a determination. Ultimately, complication data obtained in this trial should be used in conjunction with other high-level evidence to support a meta-analysis focused on this research question.
The strengths of this current study clearly lie in its study design. By performing a randomized controlled trial, we have addressed potential concerns about selection bias, patient reporting biases, and confounding by factors that may be associated with the use of ADM. This results in the most impartial evidence of the efficacy of this surgical intervention. Similarly, by “blinding” patients and evaluators, preconceived views about an intervention cannot systematically bias the assessment of outcomes. Finally, by performing a multicenter trial, the generalizability of the trial results can be maximized (27).
Once data from both Phase I and II of this trial are collected, established tools should then be employed to perform cost-effectiveness analyses looking at the outcomes of ADM-assisted TE/I reconstruction in light of both the cost of its use and the potential for adverse outcomes. The overriding goal of this research program is to provide surgeons and patients with an unbiased understanding of the impact of ADM on postmastectomy TE/I reconstruction and, with this knowledge, to ultimately improve surgical care.
Acknowledgments
Funding
Grant support was received for the trial from the Plastic Surgery Foundation (PSF). The PSF had no role in study design, data collection and analysis, or preparation of the manuscript. Dr. Lee was supported by NIH/NCRR 1KL2RR025746-01.
Footnotes
Presented: American Society of Plastic Surgeons Annual Meeting, Denver, Colorado. Sept. 2011
Financial Disclosure
None of the authors has a financial interest in any of the products, devices, or drugs mentioned in this manuscript
References
- 1.Ngyuen J, et al. Use of human acellular dermal matrix in implant based breast reconstruction: Evaluating the evidence. J Plast Reconstr Aesthet Surg. 2011:02. doi: 10.1016/j.bjps.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 2.Spear SL, Pelletiere SC, Lockwood M. Immediate breast reconstruction with tissue expanders and AlloDerm. In: Spear SL, Willey SC, Robb GL, Hammond DC, Nahabedian MY, editors. Surgery of the Breast: Principles and Art. Philadelphia: Lippincott Williams & Wilkins; 2006. pp. 484–488. [Google Scholar]
- 3.Topol BM, Dalton EF, Ponn T, Campbell CJ. Immediate single-stage breast reconstruction using implants and human acellular dermal tissue matrix with adjustment of the lower pole of the breast to reduce unwanted lift. Ann Plast Surg. 2008;61:494–499. doi: 10.1097/SAP.0b013e31816d82d9. [DOI] [PubMed] [Google Scholar]
- 4.Salzberg CA. Nonexpansive immediate breast reconstruction using human acellular tissue matrix graft (AlloDerm) Ann Plast Surg. 2006;57:1e5. doi: 10.1097/01.sap.0000214873.13102.9f. [DOI] [PubMed] [Google Scholar]
- 5.Bindingnavele V, Gaon M, Ota KS, Kulber DA, Lee DJ. Use of acellular cadaveric dermis and tissue expansion in postmastectomy breast reconstruction. J Plast Reconstr Aesthet Surg. 2007;60:1214e8. doi: 10.1016/j.bjps.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 6.Namnoum JD. Expander/implant reconstruction with AlloDerm: Recent experience. Plast Reconstr Surg. 2009;124:387–394. doi: 10.1097/PRS.0b013e3181aee95b. [DOI] [PubMed] [Google Scholar]
- 7.Zienowicz RJ, Karacaoglu E. Implant-based breast reconstructionwith allograft. Plast Reconstr Surg. 2007;120:373e81. doi: 10.1097/01.prs.0000267340.31742.1. [DOI] [PubMed] [Google Scholar]
- 8.Nahabedian MY. AlloDerm performance in the setting of prosthetic breast surgery, infection, and irradiation. Plast Reconstr Surg. 2009;124:1743–1753. doi: 10.1097/PRS.0b013e3181bf8087. [DOI] [PubMed] [Google Scholar]
- 9.Sbitany H, Sandeen SN, Amalfi AN, Davenport MS, Sbitany H, Sandeen S, Amalfi AN, Davenport MS, Langstein HN. Acellular dermis-assisted prosthetic breast reconstruction versus complete submuscular coverage: A head-to-head comparison of outcomes. Plast Reconstr Surg. 2009;124:1735–1740. doi: 10.1097/PRS.0b013e3181bf803d. [DOI] [PubMed] [Google Scholar]
- 10.Langstein HN. Acellular dermis-assisted prosthetic breast reconstruction versus complete submuscular coverage: a head to-head comparison of outcomes. Plast Reconstr Surg. 2009;124:1735e40. doi: 10.1097/PRS.0b013e3181bf803d. [DOI] [PubMed] [Google Scholar]
- 11.Spear SL, Parikh PM, Reisin E, Menon NG. Acellular dermis-assisted breast reconstruction. Aesthetic Plast Surg. 2008;32:418–425. doi: 10.1007/s00266-008-9128-8. [DOI] [PubMed] [Google Scholar]
- 12.Gamboa-Bobadilla GM. Implant breast reconstruction using acellular dermal matrix. Ann Plast Surg. 2006;56:22e5. doi: 10.1097/01.sap.0000185460.31188.c1. [DOI] [PubMed] [Google Scholar]
- 13.Kim J, et al. A meta-analysis of human acellular dermis and submuscular tissue expander breast reconstruction". Plast Reconstr Surg. 2012;129(1):28. doi: 10.1097/PRS.0b013e3182361fd6. [DOI] [PubMed] [Google Scholar]
- 14.Memtsoudis SG, Besculides MC. Perioperative comparative effectiveness research. Best Pract Res Clin Anaesthesiol. 2011 Dec;25(4):535–547. doi: 10.1016/j.bpa.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 15.McCarthy CM, Collins ED, Pusic AL. Where do we find the best evidence? Plast Reconstr Surg. 2008 Dec;122(6):1942–1947. doi: 10.1097/PRS.0b013e31818d2098. [DOI] [PubMed] [Google Scholar]
- 16.Flaherty SA. Pain measurement tools for clinical practice and research. American Association of Nurse Anesthetists. 1996 Apr;64(2):133–140. [PubMed] [Google Scholar]
- 17.Pusic AL, Klassen AF, Scott AM, Klok JA, Cordeiro PG, Cano SJ. Development of a new patient-reported outcome measure for breast surgery: the BREAST-Q. Plast Reconstr Surg. 2009 Aug;124(2):345–353. doi: 10.1097/PRS.0b013e3181aee807. [DOI] [PubMed] [Google Scholar]
- 18.Scientific Advisory Committee of the Medical Outcomes Trust. Assessing health status and quality of life instruments: Attributes and review criteria. Qual Life Res. 2002;11:193–205. doi: 10.1023/a:1015291021312. [DOI] [PubMed] [Google Scholar]
- 19.U.S. Food and Drug Administration. Patient reported outcome measures: Use in medical product development to support labeling claims. 2006 doi: 10.1186/1477-7525-4-79. Available at: www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm071975.pdf. [DOI] [PMC free article] [PubMed]
- 20.Leyeeqye R, et al. Botulinum Toxin Infiltration for Pain Control After Mastectomy and Expander Reconstruction. Ann Surg. 2004;240(4):608–614. doi: 10.1097/01.sla.0000141156.56314.1f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Farrar JT, Berlin JA, Strom BL. Clinically important changes in acute pain outcome measures: a validation study. J Pain Symptom Management. 2003 May;25(5):406–411. doi: 10.1016/s0885-3924(03)00162-3. [DOI] [PubMed] [Google Scholar]
- 22.Cravioto H, Battista A. Clinical and ultrastructural study of painful neuroma. Neurosurgery. 1981:181–190. doi: 10.1227/00006123-198102000-00007. [DOI] [PubMed] [Google Scholar]
- 23.Vernadakis AJ, Koch H, Mackinnon SE. Management of neuromas. Clin Plast Surg. 2003 Apr;30(2):247–68. doi: 10.1016/s0094-1298(02)00104-9. vii. Review. [DOI] [PubMed] [Google Scholar]
- 24.Fodor J, et al. [Radiotherapy and delayed breast reconstruction with implant: examination of compatibility] Magy Onkol. 2002;46(4):323–326. Epub 2003 Feb 1. Hungarian. [PubMed] [Google Scholar]
- 25.Disa JJ, et al. The premature removal of tissue expanders in breast reconstruction. Plast Reconstr Surg. 1999 Nov;104(6):1662–1665. doi: 10.1097/00006534-199911000-00008. [DOI] [PubMed] [Google Scholar]
- 26.Legeby M, et al. Immediate reconstruction in breast cancer surgery requires intensive post-operative pain treatment but the effects of axillary dissection may be more predictive of chronic pain. The Breast. 2002;11(2):156–162. doi: 10.1054/brst.2001.0386. [DOI] [PubMed] [Google Scholar]
- 27.McCarthy CM. Randomized controlled trials. Plast Reconstr Surg. 2011 Apr;127(4):1707–1712. doi: 10.1097/PRS.0b013e31820da3eb. [DOI] [PubMed] [Google Scholar]