Abstract
Langerhans cell histiocytosis (LCH) is a proliferation of Langerhans cells intermixed with inflammatory cells, in particular eosinophils, that may manifest as unisystem (unifocal or multifocal) or multisystem disease. Orbital involvement typically manifests as a solitary lesion that carries a favorable prognosis. Herein, we describe the clinical and histologic spectrum of LCH of the orbit on the basis of five cases. One patient exhibited multifocal unisystem disease, the other four patients presented with a localized process. The typical histologic features included numerous histiocytes with varying degrees of giant cell formation and scattered eosinophilic granulocytes. The presence of Langerhans cells was confirmed by CD1a and S100 immunostaining. Transmission electron microscopy exhibited characteristic intracytoplasmic Birbeck granules. The different ophthalmic manifestations of LCH and treatment strategies are reviewed in the context of previously reported cases. As LCH may solely involve the orbit, treatment is based on the degree of organ involvement. LCH has to be included in the differential diagnosis in tumors of the ocular adnexae, in particular in young children.
Keywords: Eosinophilic granuloma, Langerhans cell histiocytosis (LCH), Orbit
I. Introduction
Langerhans cell histiocytosis (LCH), previously called histiocytosis X, is a term describing a spectrum of proliferation of Langerhans cells that may manifest as unisystem (unifocal or multifocal) or multisystem disease. This entity was first described by Alfred Hand, Jr. in 1893. Varying manifestations of the disease have been described in the subsequent years as Hand-Schüller-Christian disease, Abt-Letterer-Siwe disease, and eosinophilic granuloma of bone that were all classified as “histiocytosis X” by Lichtenstein in 1953.1 Nezelof et al. identified the Langerhans cell as predominant pathologic cell in the disease leading to the term “Langerhans cell histiocytosis” in 1987. 2 Additionally, a division of histiocytic disorders into three classes was proposed: LCH (class I), non-LCH (class II), and malignant histiocytic disorders (class III). This classification was revised and nowadays dendritic cell-related disorders (class 1), macrophage-related disorders (class 2), and malignant disorders (class 3) are now recognized. LCH is by far the most common entity in class 1 histiocytic disorders. (http://www.histiocytesociety.org/site/c.mqISL2PIJrH/b.3809123/k.BD72/Home.htm)
A. Terminology
LCH represents a clonal proliferation of pathologic Langerhans cells.3–5 LCH is further defined by unisystem (unifocal or multifocal) or multisystem disease. A localized infiltrate involving only the orbit (unisystem, unifocal) or, as our case 3, involving the orbit and other bones including the skull and leg (unisystem, multifocal) was formerly referred to as eosinophilic granuloma or eosinophilic granuloma of bone. The triad of lytic defects in the skull, proptosis, and diabetes insipidus produced by multifocal bone lesions in somewhat older children had been termed Hand-Schüller-Christian disease. The term Abt-Letterer-Siwe disease was reserved for the acute and fulminant form of LCH that typically affects children less than two years of age and involves multiple organs systems with eczematous rash, hepatosplenomegaly, anemia, and lymphadenopathy resulting in a fatal outcome.1, 6 Liver, spleen and bone marrow are regarded as high-risk organ sites.7 Pulmonary LCH represent a polyclonal proliferation of Langerhans cells.
B. Epidemiology
Although LCH may be diagnosed at any age,8 LCH usually occurs in young children with a peak at the age of 1–4 years.9, 10 The incidence of LCH ranges from 2.6 – 8.9/106 children per year although several studies have shown an incidence of approximately 4–6/106 children per year in Europe.11–15 The incidence in children younger than 1 year of age is 9–15.3/106 per year.11–14 In children older than 10 years, the incidence is lower (about 0.7–2.0/106 per year). Unifocal and single-system disease dominates over multi-system disease in most studies.12, 14, 15 In particular, small children younger than 2–3 years of age suffer more often from multi-system LCH which harbors a more unfavorable outcome.11, 14, 16 Coexistence of LCH and hemophagocytic syndrome has been shown to be prognostically relevant as it usually occurs in younger children and is related to a less favorable outcome.17 LCH is more common in boys than girls.9, 11, 12, 14, 15, 18 LCH in boys is usually diagnosed at a higher age than in girls who present with more severe organ involvement.18 Caucasians seem to be more frequently affected than other races.14, 15, 18 However, our case series comprise African-American, Caucasian, and Hispanic patients. Some regard orbital involvement as uncommon,19, 20 while others reported relatively high incidence rates of orbital LCH (37.5% and 23%).21, 22 Orbital involvement is usually seen in patients with chronic lesions and uncommon in acute disseminated forms of LCH.22 In adults, the diagnosis of LCH is made at a mean age of 35 ± 14 years with a peak age ranging from 20 – 30 years.8
C. Pathogenesis
The pathogenesis of LCH is still unclear. 23 Although a clonal proliferation of Langerhans cells has been identified3–5, LCH is not unequivocally regarded as a true neoplasm and may represent an atypical immunoreaction.23 There is also evidence that the severity of this disease is directly influenced by an immature aberrant immune system.24 Another hypothesis assumes that the accumulation of Langerhans cells in LCH results from survival rather than uncontrolled proliferation, and is associated with the expansion of T-regs.25 There is also some evidence for an aberrant immune interaction between the clonal proliferation of dendritic cells and T cells leading to a “cytokine storm”.10 Uncontrolled cytokine production induced by several factors including viral infections may also contribute to transformation of precursor cells into pathologic Langerhans cells.26, 27 However, as the cell cycle is obviously not blocked in LCH lesions and chromosomal instability was found in LCH as well, a neoplastic process cannot be completely excluded.28
Although factors such as infections (such as EBV, CMV, HHV-6), genetic disorders, malignancies (medulloblastoma, retinoblastoma, medullary astrocytoma, glioma), seasonal and environmental influences have been implicated in the pathogenesis of LCH14, 15, 18, 19, 29, 30, there is no evidence for one factor to play a pivotal role.9, 11, 14, 31 However, each single factor may influence the immune system and thus predispose somewhat to LCH. For many patients - including our series - a history of a minor orbital trauma has been reported.18, 32 These findings may be biased since children are prone to have minor trauma at the head, however, it may be also a factor that is able to trigger an immune response and predisposed children may subsequently develop LCH.
II. Ocular Manifestations of LCH
Our series of five patients with typical clinical and histologic features of LCH is illustrated in Figs. 1–5. The clinical features of these patients are shown in Table 1. Isolated orbital infiltrates are the most common manifestation of LCH of the ocular adnexa.33, 34 They usually occur in children, but may also affect adults.35 One case of bilateral sequential orbital involvement has been reported.36 LCH was also described in the eyelid37, 38, conjunctiva39, caruncle18, 40, as an epibulbar nodule41, in the choroid, 42 the optic chiasm43, and the orbital apex and cavernous sinus manifesting as cavernous sinus syndrome.44
Figure 1.
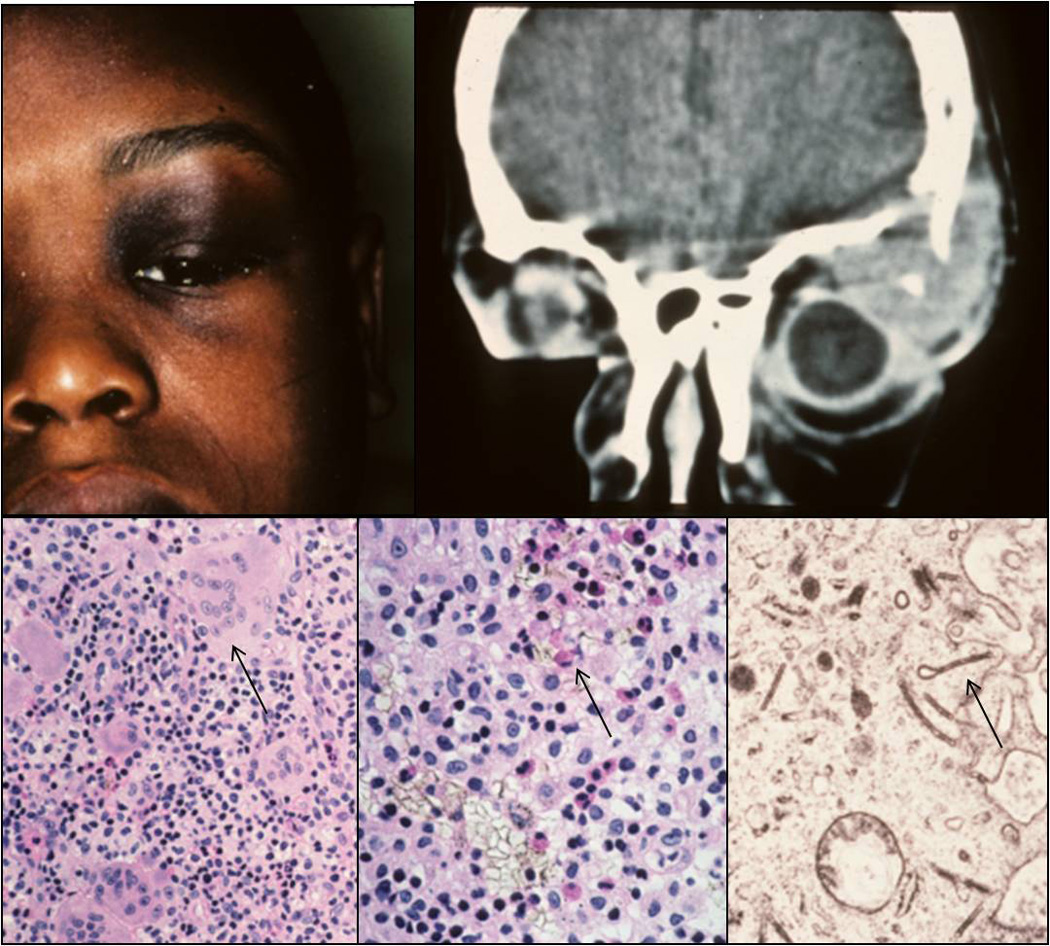
Clinical appearance of an 8-years-old patient with a superior temporal mass in the left orbit (A). Computed tomography (CT) demonstrating the orbital lesion with bone erosion (B). Histology of the lesion showing infiltrates composed of histiocytes, giant cells (C, arrow; H&E, 80x), eosinophils (D, arrow; H&E, 128x), and lymphocytes. Birbeck granules exhibiting the shape of tennis rackets were detected by transmission electron microscopy (E; TEM, 30000x).
Figure 5.
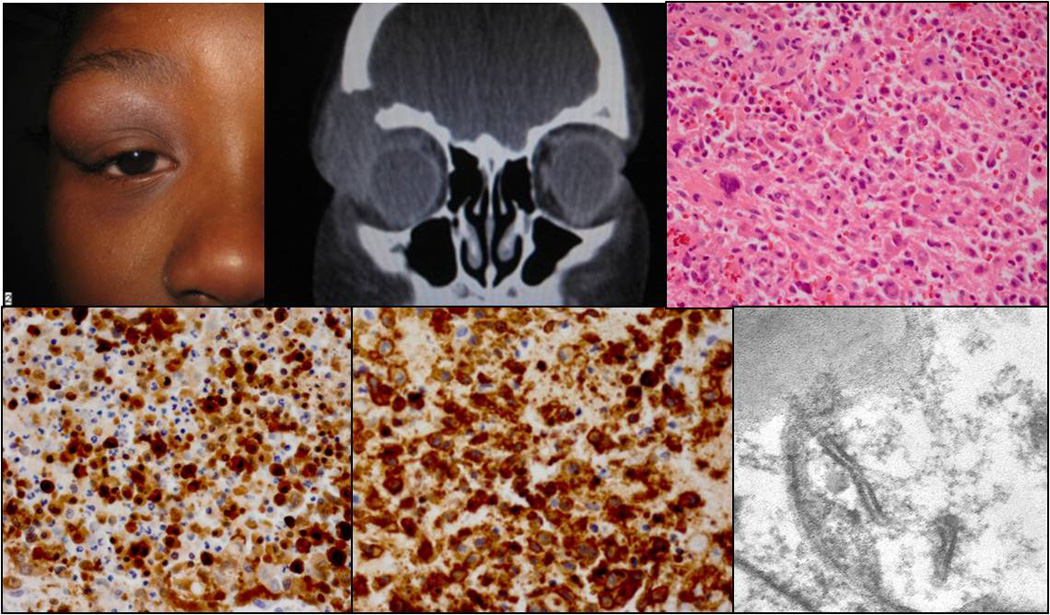
Clinical appearance of a nine-years-old patient with a mass in the right upper orbit (A). CT demonstrating the orbital lesion with bone erosion (B). Histology illustrating the Langerhans cell infiltrate with giant cells and eosinophils (C; H&E, 40x). The Langerhans cells were positive for S100 (D; 40x) and CD1a (E; 40x). Birbeck granules were detected by TEM (F; 14000x).
Table 1.
Clinical and histologic features of our 5 patients with LCH.
| Case | Age | Sex | Race | History | Orbit | Site | Size [mm] |
Bone Erosion | Eye Exam | MF [per HPF] |
S100 | CD1a | Systemic disease | TEM | Follow-up | Therapy |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| # 1 | 8 yrs | ♂ | AA | trauma (orbital contusion with progressive swelling) | superotemporal (lacrimal gland area) | OS | 20x15 | + | VA 20/20; limitation of gaze superotemporally (OS) | 0 | + | N/A | no | BG | N/A | N/A |
| # 2 | 3 yrs | ♀ | C | progressive eyelid swelling after trauma (minor fall) | superior, preseptal lesion | OD | + | full extraocular muscle movement | 0 | N/A | N/A | no | N/A | 11 yrs w/o recurrence | etoposide (VP-16; 168 mg i.v. per week) over 12 weeks | |
| # 3 | 1 yrs | ♂ | C | orbital trauma (prior to complaints) | anterior | OS | N/A | + | ptosis | 1 | N/A | N/A | bony erosions leg, skull | N/A | 3 yrs w/o recurrence | etoposide (VP-16; 100mg i.v.) over 10 weeks + prednisone (5mg) |
| # 4 | 8 mo | ♂ | HA | right facial swelling, pretreatment with antibiotics | inferolateral | OD | 14×19×18 | + | VA 20/20; full extraocular muscle movement / no strabism | 0 | + | + | no | N/A | 2 yrs w/o recurrence | low dose methotrexate + prednisone (5 mg, bid) over 1 year |
| # 5 | 9 yrs | ♀ | AA | trauma (orbital contusion), pretreatment with topical antibiotics | superolateral | OD | 29×18×35 | + | VA 20/25; full extraocular muscle movement / no strabism | 0.1 =1 MF /10HPF | + | + | no | BG | under treatment | vinblastine (6mg/m2 i.v. per week) + 6-MP (15mg/m2 p.o. per week) + prednisone over 1 year |
AA: African-American; C: Caucasian; HA: Hispanic-American; HPF: high power field; MF: mitotic figures; mo: months; TEM: transmission electron microscopy; yrs: years; BG: Birbeck granules; wo:without; N/A: not available
LCH of the orbit usually presents as an isolated bone lesion with an associated soft tissue mass, although it can also be associated with multifocal or multisystemic disease.18, 33, 45, 46 It occurs predominantly in the superior or superolateral orbital roof and exhibits other features depending on the location of the Langerhans cell infiltrate such as a visible mass with ptosis and/or (erythematous) swelling if located in the anterior orbit. This may be misinterpreted as an infectious process. Proptosis is predominantly seen in lesions that involve the posterior orbit. This may be accompanied by diabetes insipidus and skull lesions forming the triad formerly called Hand-Schüller-Christian disease. Depending on the location and the size of the lesion as well as the infiltrated structures such as extraocular muscles ocular movement may be impaired resulting in diplopia and nerve palsies may occur. Visual acuity may be somewhat affected in young patients due to ptosis and the development of amblyopia.18 Fundus abnormalities such as optic disc edema, dilated venous channels, and macular edema, have been reported in eyes with orbital LCH (Table 2).47
Table 2.
Diagnostic clinical and microscopic features of LCH
Diagnostic imaging, including computed tomography (CT) and magnetic resonance tomography (MRT)18 shows well-defined bony lesions with a classic “punched-out” lytic appearance that are often accompanied by soft tissue involvement in the orbit.24, 48 Lesions without bone erosions have also been reported.44, 46 As orbital lesions may be part of a multisystem disease,21, 49 a complete medical history and systemic work-up is mandatory to rule out a multisystemic manifestation for all orbital LCH lesions.21 A scheme for systemic evaluation is shown in Table 3.7, 21, 27, 34
Table 3.
Tests for disease extension in LCH
|
None of the patients in our series with orbital LCH suffered from disease recurrence or progression although it has been reported in other case series.18, 21 Recurrence/progression in patients with localized orbital lesions occurs most likely within 12–18 months of initial treatment. However, recurrences after a 13 and 16 year disease free intervals have been described.50, 51 One report describes an orbital recurrence 10 years after involvement of the jaw.52 Thus, follow-up examinations are recommended at least within 2–3 years after the initial treatment.21
Several studies point towards an association between orbital involvement and development of diabetes insipidus/CNS disease.53 Since diabetes insipidus require aggressive treatment in patients with orbital LCH, this seems to contradict a favorable outcome in patients with localized orbital involvement.1, 54 It appears that patients with limited orbital involvement as initial manifestation of LCH have a good prognosis whereas those with extended orbital and periocular involvement may be more likely to develop diabetes insipidus. Similar conclusions regarding the location and size of orbital lesions and their association with neurodegeneration were made by Vosoghi and co-workers who investigated nine patients with orbital lesions that occurred predominantly in the context of multifocal bone or multisystemic disease.21 They concluded that the orbit reflects a spectrum of the disease and thus treatment should be based on variable factors such as location, size, and type of involvement (unifocal unisystem, multifocal unisystem, multisystem disease). In particular, a distinction between anterior and posterior LCH lesions may be helpful for risk group assignment.
III. Pathologic features of LCH
A. Histology
Histopathologically, the histiocytic infiltrate consists - despite its relatively benign-looking histologic features - predominantly of a clonal proliferation of pathologic Langerhans cells that resemble tissue macrophages rather than the typical dendritic shape of Langerhans cells in the skin.1 The Langerhans cell infiltrate is accompanied by a varying amount of giant cells and eosinophils which are not mandatory for the diagnosis of LCH. Lymphocytes, plasma cells, polymorphonuclear leukocytes, macrophages (histiocytes) and varying amount of necrosis can be also present (Tables 1, 2; Figs. 1–5). The lesions are very often vascular and erythrocytes are frequently observed due to hemorrhage.6 Mitotic figures (MF) are occasionally found. In our series, mitotic figures were present in two of five cases. One lesion (unifocal disease; Fig. 5) exhibited only 1 MF/10 high power fields (HPF), the other orbital lesion (multifocal, unisystem disease; Fig. 3) exhibited 1 MF/HPF. Larger studies have shown that there is no correlation between the histologic features and the disease severity.55 Older lesions may exhibit foamy (xanthomatous) histiocytes as sign of lipidization. Thus, in some cases LCH needs to be distinguished from Erdheim-Chester disease. Some bony lesions may undergo spontaneous healing resulting in a fibrotic scar.6
Figure 3.
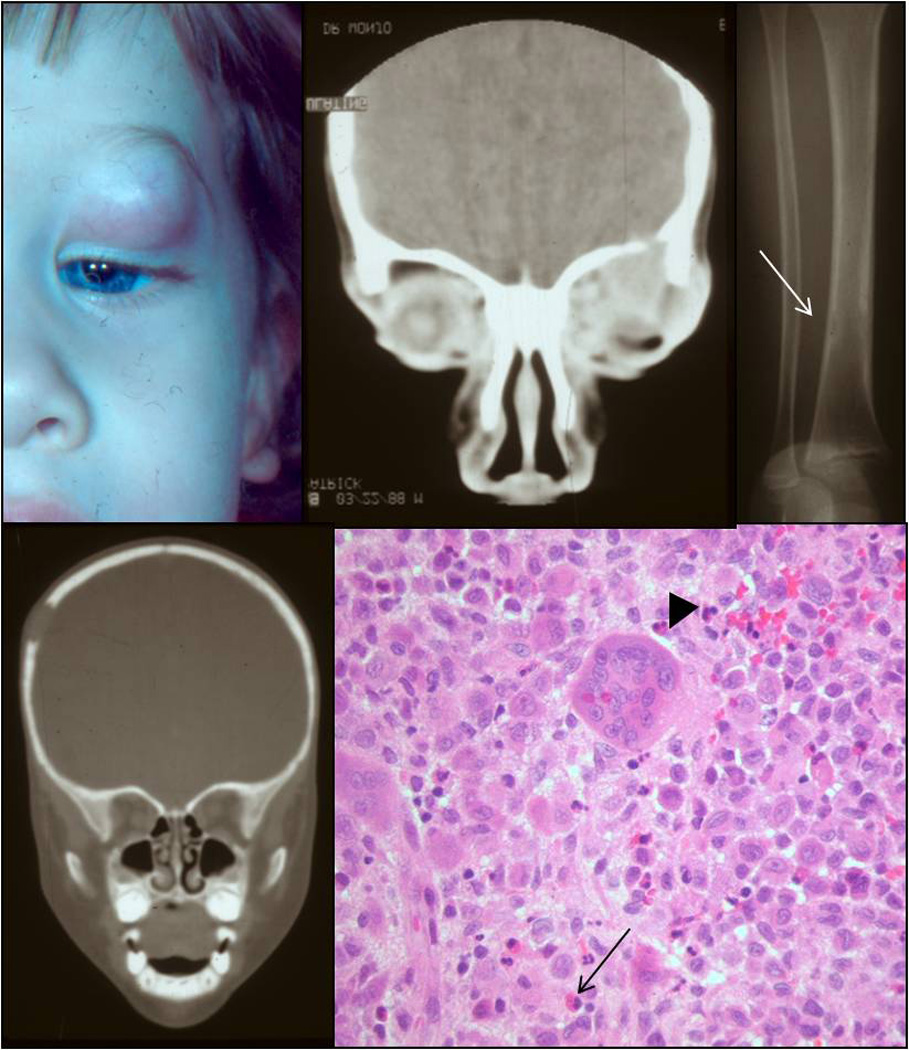
Clinical appearance of a one-years-old patient with a mass in the anterior orbit (A). CT revealing bony erosion in the orbital rim (B), the tibia (C), and the skull (D). Histology showing sheets of histiocytes intermixed with giant cells (arrowhead) and eosinophils (arrow) (E; H&E, 40x).
B. Immunohistochemistry
The combination of immunopositivity for the neuronal marker S100 and CD1a (Table 2, Fig. 5), which is specific for Langerhans cells (and thymocytes) and is not expressed by macrophages, helps to confirm the diagnosis. Other immunohistochemical markers that may be positive in LCH include adenosine triphosphatase, peanut lectin binding, alpha-mannosidase, CD207 (Langerin), and fascin.1, 19, 34, 56
C. Transmission electron microscopy
Small rod- or tennis racket-shaped bodies, called Birbeck granules, may be found in the cytoplasm of regular and pathologic Langerhans cells (Table 2, Fig. 1 and 5). Although only present in about 50–70% of the lesions, Birbeck granules are regarded as pathognomonic for LCH.57, 58 In lesions with Birbeck granules, they may be found in 2–29% of the pathologic Langerhans cells.59 Charcot-Leyden crystals are sometimes also found in histiocytes in LCH.60, 61
IV. Differential Diagnosis
The clinical differential diagnosis of ophthalmic LCH includes periorbital cellulitis, acute dacryocystitis, (ruptured) dermoid cyst, hematoma, inflammatory pseudotumor, pilomatrixoma, leukemia, sarcoma, metastastic neuroblastoma, rhabdomyosarcoma, and other tumors.62 Some of these lesions may be differentiated from LCH by the absence of lytic bony erosions that are typical for LCH.
The histologic differential diagnosis of LCH include giant cell reparative granuloma,63 hemorrhagic cyst, cholesterol granuloma (cholesteatoma),64 Erdheim-Chester disease,65 giant cell aneurysma of bone, giant cell tumor, and histiocytic sarcoma. These entities exhibit histologic features, are composed of CD1a- macrophages (not CD1a+ Langerhans cells) and can be excluded from LCH by immunohistochemical staining. CD68+ macrophages may be also present in LCH, in particular if complicated by the hemophagocytic syndrome.
V. Treatment
As the pathogenesis of LCH is still unknown, treatment is empirical.18 The choice of therapeutic regime is based on disease severity and degree of systemic involvement.66 Different treatment possibilities are shown in Table 4. In general, the diagnosis of LCH should be proven by a biopsy. Incisional and excisional biopsies are preferred over fine-needle aspiration biopsy because the latter might not provide enough material for a sufficient histologic diagnosis.27 In cases of sequential multifocal lesions, observation is one option.67
Table 4.
Treatment strategies of orbital LCH.
| Treatment | Type of LCH | References |
|---|---|---|
| observation | LCH, diagnosed elsewhere (sequential multifocal LCH) | 67 |
| incisional biopsy (w/o curettage) | unisystem, unifocal LCH | 68, 69 |
| biopsy and curettage/complete excision | unisystem, unifocal LCH | 18, 20, 45, 46, 72 |
| subtotal curettage and intralesional methylprednisolone (30–125 mg) | unisystem, unifocal LCH (recurrence - 1 case report) | 27, 45, 52 |
| radiation (400–800cGy ) | unisystem, unifocal LCH | 27, 45, 46 |
| chemotherapy (various combinations of prednisone, vinblastine, etoposide, methometrexate, 2-cda, 6-mercaptopurine) | unisystem, unifocal LCH with contact to the Dura Mater multifocal/multisystem LCH recurrent disease | 19, 66, 71, 72, 77 |
| surgical excision, radiation & chemotherapy | single report of “aggressive” LCH | 73 |
As single bone lesions have a far better outcome than multiple bone lesions and multiorgan disease,16 most physicians recommend biopsy and curettage for solitary orbital lesions, although remission after incisional biopsy alone has been reported.18, 68, 69 Localized lesions of the caruncle and eyelid have also been successfully treated with an excisional biopsy.37, 40
Careful curettage/excision in combination with intralesional steroids may be another effective treatment for primary unifocal lesions of the orbit27, 45, 70 and recurrent lesions.52 One study group observed the absence of recurrence in 20 patients with unifocal, unisystem bone lesions of the orbit treated with excision and curettage alone but recurrences occurred in 3 patients treated with excision and adjuvant chemotherapy (n=2) and excision and intralesional triamcinolone (n=1), respectively.18 Although different doses of steroids have been used, 125 mg methylprednisolone is recommended as it might have an inhibitory effect on osteolysis.27, 45 The favorable outcome of excision and curettage alone may be attributed to changes in the microenvironment leading to the disruption of the pathological cascade.27, 45 This observation highlights the paradox of an aggressive bone destruction in LCH and its response to minimal intervention. However, recurrences have been reported after this treatment and thus close follow-up is recommended.71
Radiation is predominantly used to treat recurrences.45, 46 Side-effects or long-term sequelae such as secondary neoplasms are still a concern with (low-dose) radiation therapy.
Chemotherapy is regarded as a treatment for multifocal/multisystem disease or recurrences.19, 66 The most common chemotherapeutic agents are vinblastine, prednisone, etoposide, and methotrexate in various combinations. Chemotherapy might be also considered for orbital lesions with dura involvement - as in our case 2.72 Some authors have treated aggressive LCH of the orbit and skull base with intracranial extension and cranial nerve involvement with surgical excision followed by radiation and chemotherapy.73
Bone marrow transplantation and immunoglobulin therapy are reserved for uncontrolled disease recurrences and CNS involvement, respectively.74, 75 The Histiocyte society provides up-to-date information regarding the latest treatment strategies and clinical trials. Since these trials are mostly designed for multi-system disease and are not conducted by ophthalmologists, the interested reader may should refer to the more comprehensive publications regarding this topic.7, 76
VI. Conclusion
Our cases exhibited the typical features of LCH such as a proliferation of pathologic Langerhans cells interspersed with giant cells and eosinophils. Immunohistochemical stains for S100 and CD1a and/or the presence of Birbeck granules by TEM supported the diagnoses. Biopsy of orbital lesions allows for a histopathological diagnosis and may be even curative in some cases. Based on clinical evidence from the literature, the treatment of solitary anterior orbital lesions with biopsy and curettage and - where required - intralesional methylprednisone alone appears justified and may prevent overtreatment with subsequent possible systemic side effects/complications from radiation or chemotherapy. An interdisciplinary approach is recommended to rule out multifocal or multi-systemic disease and develop an appropriate treatment strategy. Long-term surveillance is advisable to adequately diagnose and treat recurrences. Future knowledge about the detailed pathogenesis and etiology of LCH may allow for more specific and selective therapies.
VII. Literature Search
The literature selection of this review included a PubMed database search (http://www.ncbi.nlm.nih.gov/pubmed) from 1950 to 2012. The literature search was conducted using combinations of the following keywords: Langerhans cell histiocytosis, histiocytosis X, eosinophilic granuloma, orbit, eye. Articles written in English, German, and Chinese were considered. Many case reports were included due to the relatively rare occurrence of this disease but we limited them to reports contributing new information about characteristics, (differential) diagnosis, or treatment of the disease. Some reports were excluded because the diagnosis of LCH could not be unequivocally retraced.
Figure 2.
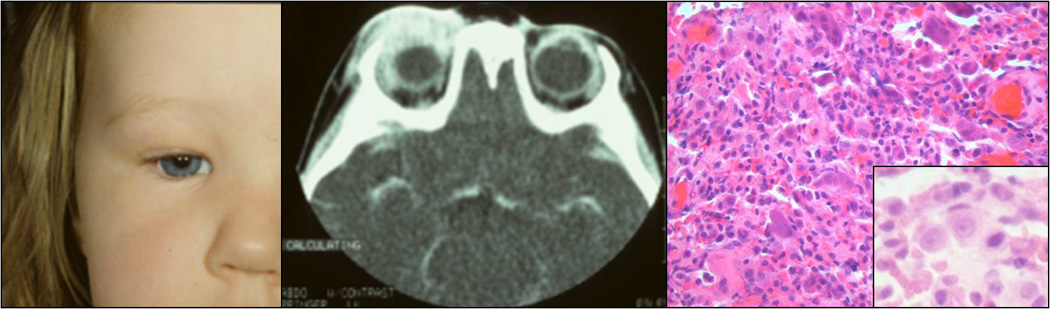
Clinical appearance of a three-years-old patient with an erythematous eyelid swelling (A). CT reavealing the orbital lesion with bone erosion (B). Histology showing sheets histiocytes (inset) intermixed with giant cells, lymphocytes, and a few eosinophils (C; H&E, 40x).
Figure 4.
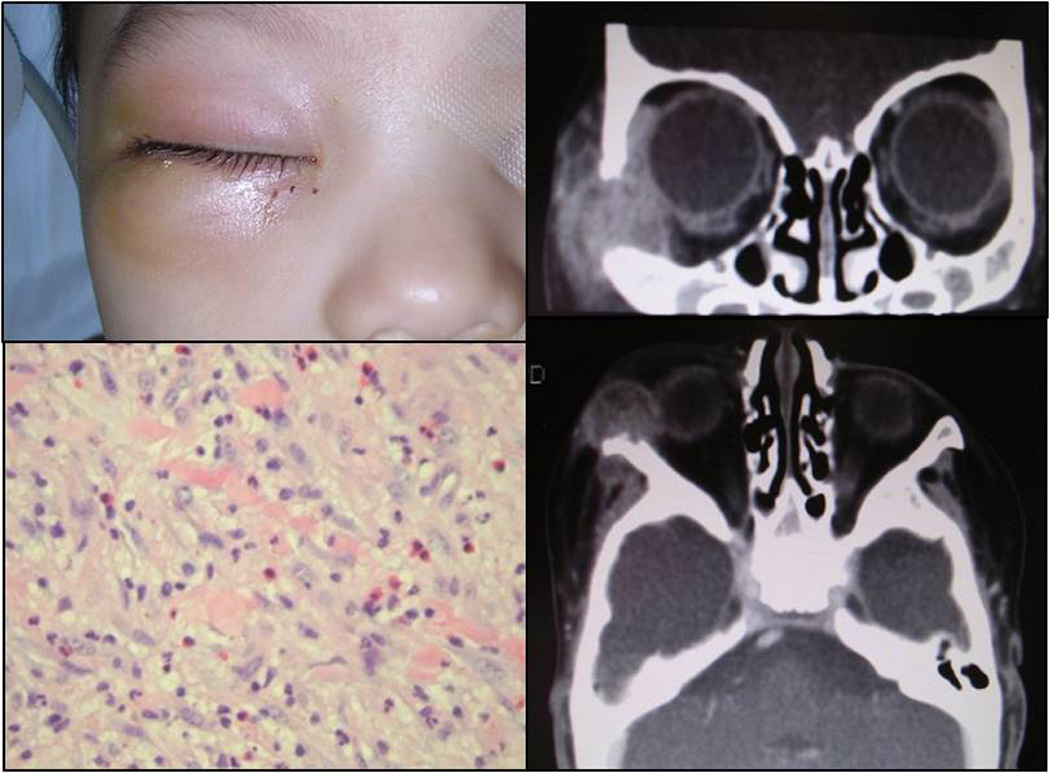
Clinical appearance of an eight-months-old patient with a right facial erythematous swelling (A). CT showing with a mass in his right temporal inferior orbit (B, C). Histologic picture illustrating the histiocytic infiltrate interspersed with occasional eosinophils (D; H&E, 40x).
Acknowledgments
VIII. Disclosure
This work was supported in part by an unrestricted departmental grant from Research to Prevent Blindness, Inc, NIH P30EY06360 (Hans E. Grossniklaus), and the German Research Foundation (DFG, Martina C. Herwig).
Footnotes
The authors report no proprietary or commercial interest in any product mentioned or concept discussed in this article.
References
- 1.Margo CE, Goldman DR. Langerhans cell histiocytosis. Surv Ophthalmol. 2008;53(4):332–358. doi: 10.1016/j.survophthal.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 2.Nezelof C, Basset F, Rousseau MF. Histiocytosis X histogenetic arguments for a Langerhans cell origin. Biomedicine. 1973;18(5):365–371. [PubMed] [Google Scholar]
- 3.Willman CL. Detection of clonal histiocytes in Langerhans cell histiocytosis: biology and clinical significance. Br J Cancer Suppl. 1994;23:S29–S33. [PMC free article] [PubMed] [Google Scholar]
- 4.Willman CL, Busque L, Griffith BB, et al. Langerhans'-cell histiocytosis (histiocytosis X)--a clonal proliferative disease. N Engl J Med. 1994;331(3):154–160. doi: 10.1056/NEJM199407213310303. [DOI] [PubMed] [Google Scholar]
- 5.Yu RC, Chu C, Buluwela L, Chu AC. Clonal proliferation of Langerhans cells in Langerhans cell histiocytosis. Lancet. 1994;343(8900):767–768. doi: 10.1016/s0140-6736(94)91842-2. [DOI] [PubMed] [Google Scholar]
- 6.Spencer: xxx. Edited by p
- 7.Allen CE, McClain KL. Langerhans cell histiocytosis: a review of past, current and future therapies. Drugs Today (Barc) 2007;43(9):627–643. doi: 10.1358/dot.2007.43.9.1088823. [DOI] [PubMed] [Google Scholar]
- 8.Arico M, Girschikofsky M, Genereau T, et al. Langerhans cell histiocytosis in adults. Report from the International Registry of the Histiocyte Society. Eur J Cancer. 2003;39(16):2341–2348. doi: 10.1016/s0959-8049(03)00672-5. [DOI] [PubMed] [Google Scholar]
- 9.Hamre M, Hedberg J, Buckley J, et al. Langerhans cell histiocytosis: an exploratory epidemiologic study of 177 cases. Med Pediatr Oncol. 1997;28(2):92–97. doi: 10.1002/(sici)1096-911x(199702)28:2<92::aid-mpo2>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 10.Laman JD, Leenen PJ, Annels NE, Hogendoorn PC, Egeler RM. Langerhans-cell histiocytosis 'insight into DC biology'. Trends Immunol. 2003;24(4):190–196. doi: 10.1016/s1471-4906(03)00063-2. [DOI] [PubMed] [Google Scholar]
- 11.Alston RD, Tatevossian RG, McNally RJ, Kelsey A, Birch JM, Eden TO. Incidence and survival of childhood Langerhans cell histiocytosis in Northwest England from 1954 to 1998. Pediatr Blood Cancer. 2007;48(5):555–560. doi: 10.1002/pbc.20884. [DOI] [PubMed] [Google Scholar]
- 12.Guyot-Goubin A, Donadieu J, Barkaoui M, Bellec S, Thomas C, Clavel J. Descriptive epidemiology of childhood Langerhans cell histiocytosis in France, 2000–2004. Pediatr Blood Cancer. 2008;51(1):71–75. doi: 10.1002/pbc.21498. [DOI] [PubMed] [Google Scholar]
- 13.Salotti J. Epidemiology of Langerhans cell histiocytosis: onwards and upwards! Pediatr Blood Cancer. 2008;51(1):3–4. doi: 10.1002/pbc.21552. [DOI] [PubMed] [Google Scholar]
- 14.Salotti JA, Nanduri V, Pearce MS, Parker L, Lynn R, Windebank KP. Incidence and clinical features of Langerhans cell histiocytosis in the UK and Ireland. Arch Dis Child. 2009;94(5):376–380. doi: 10.1136/adc.2008.144527. [DOI] [PubMed] [Google Scholar]
- 15.Stalemark H, Laurencikas E, Karis J, Gavhed D, Fadeel B, Henter JI. Incidence of Langerhans cell histiocytosis in children: a population-based study. Pediatr Blood Cancer. 2008;51(1):76–81. doi: 10.1002/pbc.21504. [DOI] [PubMed] [Google Scholar]
- 16.Jubran RF, Marachelian A, Dorey F, Malogolowkin M. Predictors of outcome in children with Langerhans cell histiocytosis. Pediatr Blood Cancer. 2005;45(1):37–42. doi: 10.1002/pbc.20364. [DOI] [PubMed] [Google Scholar]
- 17.Favara BE, Jaffe R, Egeler RM. Macrophage activation and hemophagocytic syndrome in langerhans cell histiocytosis: report of 30 cases. Pediatr Dev Pathol. 2002;5(2):130–140. doi: 10.1007/s10024001-0159-2. [DOI] [PubMed] [Google Scholar]
- 18.Maccheron LJ, McNab AA, Elder J, et al. Ocular adnexal Langerhans cell histiocytosis clinical features and management. Orbit. 2006;25(3):169–177. doi: 10.1080/01676830600669486. [DOI] [PubMed] [Google Scholar]
- 19.Kramer TR, Noecker RJ, Miller JM, Clark LC. Langerhans cell histiocytosis with orbital involvement. Am J Ophthalmol. 1997;124(6):814–824. doi: 10.1016/s0002-9394(14)71699-x. [DOI] [PubMed] [Google Scholar]
- 20.Mokal NJ, Shetty KP, Arora R, Thatte MR. Langerhans cell histiocytosis: orbital involvement as an unusual location. Plast Reconstr Surg. 2001;107(3):813–817. doi: 10.1097/00006534-200103000-00024. [DOI] [PubMed] [Google Scholar]
- 21.Vosoghi H, Rodriguez-Galindo C, Wilson MW. Orbital involvement in langerhans cell histiocytosis. Ophthal Plast Reconstr Surg. 2009;25(6):430–433. doi: 10.1097/IOP.0b013e3181b80cad. [DOI] [PubMed] [Google Scholar]
- 22.Moore AT, Pritchard J, Taylor DS. Histiocytosis X: an ophthalmological review. Br J Ophthalmol. 1985;69(1):7–14. doi: 10.1136/bjo.69.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Favara BE. Langerhans cell histiocytosis: an identity crisis. Med Pediatr Oncol. 2001;37(6):545. doi: 10.1002/mpo.1250. [DOI] [PubMed] [Google Scholar]
- 24.Azouz EM, Saigal G, Rodriguez MM, Podda A. Langerhans' cell histiocytosis: pathology, imaging and treatment of skeletal involvement. Pediatr Radiol. 2005;35(2):103–115. doi: 10.1007/s00247-004-1262-0. [DOI] [PubMed] [Google Scholar]
- 25.Senechal B, Elain G, Jeziorski E, et al. Expansion of regulatory T cells in patients with Langerhans cell histiocytosis. PLoS Med. 2007;4(8):e253. doi: 10.1371/journal.pmed.0040253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zelger B. Langerhans cell histiocytosis: a reactive or neoplastic disorder? Med Pediatr Oncol. 2001;37(6):543–544. doi: 10.1002/mpo.1249. [DOI] [PubMed] [Google Scholar]
- 27.Woo KI, Harris GJ. Eosinophilic granuloma of the orbit: understanding the paradox of aggressive destruction responsive to minimal intervention. Ophthal Plast Reconstr Surg. 2003;19(6):429–439. doi: 10.1097/01.IOP.0000092800.86282.27. [DOI] [PubMed] [Google Scholar]
- 28.Abla O, Egeler RM, Weitzman S. Langerhans cell histiocytosis: Current concepts and treatments. Cancer Treat Rev. 2010;36(4):354–359. doi: 10.1016/j.ctrv.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 29.Sakata N, Toguchi N, Kimura M, Nakayama M, Kawa K, Takemura T. Development of Langerhans cell histiocytosis associated with chronic active Epstein-Barr virus infection. Pediatr Blood Cancer. 2008;50(4):924–927. doi: 10.1002/pbc.21249. [DOI] [PubMed] [Google Scholar]
- 30.Venkatramani R, Rosenberg S, Indramohan G, Jeng M, Jubran R. An exploratory epidemiological study of Langerhans cell histiocytosis. Pediatr Blood Cancer. 2012 doi: 10.1002/pbc.24136. [DOI] [PubMed] [Google Scholar]
- 31.Jeziorski E, Senechal B, Molina TJ, et al. Herpes-virus infection in patients with Langerhans cell histiocytosis: a case-controlled sero-epidemiological study, and in situ analysis. PLoS One. 2008;3(9):e3262. doi: 10.1371/journal.pone.0003262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beller AJ, Kornblueth W. Eosinophilic granuloma of the orbit. Br J Ophthalmol. 1951;35(4):220–225. doi: 10.1136/bjo.35.4.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jakobiec FA, Trokel SL, Aron-Rosa D, Iwamoto T, Doyon D. Localized eosinophilic granuloma (Langerhans' cell histiocytosis) of the orbital frontal bone. Arch Ophthalmol. 1980;98(10):1814–1820. doi: 10.1001/archopht.1980.01020040666015. [DOI] [PubMed] [Google Scholar]
- 34.Levy J, Monos T, Kapelushnik J, Maor E, Nash M, Lifshitz T. Ophthalmic manifestations in Langerhans cell histiocytosis. Isr Med Assoc J. 2004;6(9):553–555. [PubMed] [Google Scholar]
- 35.Zausinger S, Muller A, Bise K, Klauss V. Eosinophilic granuloma of the orbit in an adult woman. Acta Neurochir (Wien) 2000;142(2):215–217. doi: 10.1007/s007010050027. [DOI] [PubMed] [Google Scholar]
- 36.Demirci H, Shields CL, Shields JA, Eagle RC., Jr Bilateral sequential orbital involvement by eosinophilic granuloma. Arch Ophthalmol. 2002;120(7):978–979. [PubMed] [Google Scholar]
- 37.Weissgold DJ, Wulc AE, Frayer WC, Young M. Eosinophilic granuloma of the eyelid. Ophthal Plast Reconstr Surg. 1994;10(3):160–162. doi: 10.1097/00002341-199409000-00003. [DOI] [PubMed] [Google Scholar]
- 38.Ramzan M, Yadav SP, Bhalla S, Jamwal P, Grover AK, Sachdeva A. Eyelid nodule: a rare presentation of langerhans cell histiocytosis. J Pediatr Hematol Oncol. 2012;34(4):e158–e160. doi: 10.1097/MPH.0b013e3182332281. [DOI] [PubMed] [Google Scholar]
- 39.Knobel H, von Domarus D. [Eosinophilic granuloma of the conjunctiva] Ophthalmologica. 1978;177(1):47–52. doi: 10.1159/000308736. [DOI] [PubMed] [Google Scholar]
- 40.Stanowsky A, Krey HF, Wagner T. [Histiocytosis X (eosinophilic granuloma) of the caruncle] Klin Monbl Augenheilkd. 1991;199(5):359–361. doi: 10.1055/s-2008-1046096. [DOI] [PubMed] [Google Scholar]
- 41.Melamud A, Efrat M, Sova Y, Hod Y, Geyer O. Epibulbar nodule as a symptom of Langerhans cell histiocytosis. Arch Ophthalmol. 2002;120(10):1400–1401. doi: 10.1001/archopht.120.10.1400. [DOI] [PubMed] [Google Scholar]
- 42.EOPS.
- 43.Hervey-Jumper SL, Ghori A, Ziewacz JE, McKeever PE, Chandler WF. Langerhans cell histiocytosis of the optic chiasm: case report. Neurosurgery. 68(2):E556–E561. doi: 10.1227/NEU.0b013e31820206c7. [DOI] [PubMed] [Google Scholar]
- 44.Gross FJ, Waxman JS, Rosenblatt MA, Tabibzadeh SS, Solodnik P. Eosinophilic granuloma of the cavernous sinus and orbital apex in an HIV-positive patient. Ophthalmology. 1989;96(4):462–467. doi: 10.1016/s0161-6420(89)32855-7. [DOI] [PubMed] [Google Scholar]
- 45.Harris GJ, Woo KI. Eosinophilic granuloma of the orbit: a paradox of aggressive destruction responsive to minimal intervention. Trans Am Ophthalmol Soc. 2003;101:93–103. discussion 103–105. [PMC free article] [PubMed] [Google Scholar]
- 46.Gunduz K, Palamar M, Parmak N, Kuzu I. Eosinophilic granuloma of the orbit: report of two cases. J AAPOS. 2007;11(5):506–508. doi: 10.1016/j.jaapos.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 47.Zhou XD, Song GX, He YJ. [Clinical analysis of orbital histocytosis X] Zhonghua Yan Ke Za Zhi. 2003;39(11):673–677. [PubMed] [Google Scholar]
- 48.Shetty SB, Mehta C. Langerhans cell histiocytosis of the orbit. Indian J Ophthalmol. 2001;49(4):267–268. [PubMed] [Google Scholar]
- 49.Straatsma BR. Eosinophilic granuloma of bone. Trans Am Acad Ophthalmol Otolaryngol. 1958;62(6):771–776. [PubMed] [Google Scholar]
- 50.Escardo-Paton JA, Neal J, Lane CM. Late recurrence of Langerhans cell histiocytosis in the orbit. Br J Ophthalmol. 2004;88(6):838–839. doi: 10.1136/bjo.2003.031336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kilpatrick SE, Wenger DE, Gilchrist GS, Shives TC, Wollan PC, Unni KK. Langerhans' cell histiocytosis (histiocytosis X) of bone. A clinicopathologic analysis of 263 pediatric and adult cases. Cancer. 1995;76(12):2471–2484. doi: 10.1002/1097-0142(19951215)76:12<2471::aid-cncr2820761211>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 52.Wladis EJ, Tomaszewski JE, Gausas RE. Langerhans cell histiocytosis of the orbit 10 years after involvement at other sites. Ophthal Plast Reconstr Surg. 2008;24(2):142–143. doi: 10.1097/IOP.0b013e3181640839. [DOI] [PubMed] [Google Scholar]
- 53.Imashuku S, Kinugawa N, Matsuzaki A, et al. Langerhans cell histiocytosis with multifocal bone lesions: comparative clinical features between single and multi-systems. Int J Hematol. 2009;90(4):506–512. doi: 10.1007/s12185-009-0420-4. [DOI] [PubMed] [Google Scholar]
- 54.Harris GJ, Woo KI. Is unifocal Langerhans-cell histiocytosis of the orbit a"CNS-Risk" lesion? Pediatr Blood Cancer. 2004;43(3):298–299. doi: 10.1002/pbc.20055. author reply 300–291. [DOI] [PubMed] [Google Scholar]
- 55.Risdall RJ, Dehner LP, Duray P, Kobrinsky N, Robison L, Nesbit ME., Jr Histiocytosis X (Langerhans' cell histiocytosis). Prognostic role of histopathology. Arch Pathol Lab Med. 1983;107(2):59–63. [PubMed] [Google Scholar]
- 56.Pinkus GS, Lones MA, Matsumura F, Yamashiro S, Said JW, Pinkus JL. Langerhans cell histiocytosis immunohistochemical expression of fascin, a dendritic cell marker. Am J Clin Pathol. 2002;118(3):335–343. doi: 10.1309/N2TW-ENRB-1N1C-DWL0. [DOI] [PubMed] [Google Scholar]
- 57.Ye F, Huang SW, Dong HJ, Histiocytosis X. S-100 protein, peanut agglutinin, and transmission electron microscopy study. Am J Clin Pathol. 1990;94(5):627–631. doi: 10.1093/ajcp/94.5.627. [DOI] [PubMed] [Google Scholar]
- 58.Mierau GW, Favara BE. S-100 protein immunohistochemistry and electron microscopy in the diagnosis of Langerhans cell proliferative disorders: a comparative assessment. Ultrastruct Pathol. 1986;10(4):303–309. doi: 10.3109/01913128609064194. [DOI] [PubMed] [Google Scholar]
- 59.Schmitz L, Favara BE. Nosology and pathology of Langerhans cell histiocytosis. Hematol Oncol Clin North Am. 1998;12(2):221–246. doi: 10.1016/s0889-8588(05)70507-4. [DOI] [PubMed] [Google Scholar]
- 60.Amemiya T, Yoshida H. Electron microscopic study of the orbital lesion of Hand-Schuller-Christian disease. J Pediatr Ophthalmol. 1977;14(4):242–247. [PubMed] [Google Scholar]
- 61.Kumar N, Sayed S, Vinayak S. Diagnosis of Langerhans cell histiocytosis on fine needle aspiration cytology: a case report and review of the cytology literature. Patholog Res Int. 2011;2011:439518. doi: 10.4061/2011/439518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cheng P, Wei RL, Yue Y, Cai JP. [Clinical analysis of 11 cases of orbital Langerhans cell histiocytosis] Zhonghua Yan Ke Za Zhi. 2006;42(10):892–895. [PubMed] [Google Scholar]
- 63.Smolarz-Wojnowska A, Klein CM. [Tumour-like lesions of the orbit - clinical symptoms, difficulties in differentiation and options for therapy] Klin Monbl Augenheilkd. 2009;226(5):414–420. doi: 10.1055/s-0028-1109197. [DOI] [PubMed] [Google Scholar]
- 64.Loeffler KU, Kommerell G. Cholesterol granuloma of the orbit--pathogenesis and surgical management. Int Ophthalmol. 1997;21(2):93–98. doi: 10.1023/a:1005838915787. [DOI] [PubMed] [Google Scholar]
- 65.Platz R, Romeike BF, Pandey DK, Kalff R, Reichart R. Erdheim-Chester Disease - A Rare Differential Diagnosis of Eosinophilic Granuloma. A Case Report. Cen Eur Neurosurg. doi: 10.1055/s-0032-1309069. [DOI] [PubMed] [Google Scholar]
- 66.Das JK, Soibam R, Tiwary BK, Magdalene D, Paul SB, Bhuyan C. Orbital manifestations of Langerhans Cell Histiocytosis: A report of three cases. Oman J Ophthalmol. 2009;2(3):137–140. doi: 10.4103/0974-620X.57315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Berger B. Eosinophilic granuloma of orbit. Am J Ophthalmol. 1955;40(2):257–258. [PubMed] [Google Scholar]
- 68.Glover AT, Grove AS., Jr Eosinophilic granuloma of the orbit with spontaneous healing. Ophthalmology. 1987;94(8):1008–1012. doi: 10.1016/s0161-6420(87)33352-4. [DOI] [PubMed] [Google Scholar]
- 69.Smith JH, Fulton L, O'Brien JM. Spontaneous regression of orbital Langerhans cell granulomatosis in a three-year-old girl. Am J Ophthalmol. 1999;128(1):119–121. doi: 10.1016/s0002-9394(99)00055-0. [DOI] [PubMed] [Google Scholar]
- 70.Wirtschafter JD, Nesbit M, Anderson P, McClain K. Intralesional methylprednisolone for Langerhans' cell histiocytosis of the orbit and cranium. J Pediatr Ophthalmol Strabismus. 1987;24(4):194–197. doi: 10.3928/0191-3913-19870701-11. [DOI] [PubMed] [Google Scholar]
- 71.Song A, Johnson TE, Dubovy SR, Toledano S. Treatment of recurrent eosinophilic granuloma with systemic therapy. Ophthal Plast Reconstr Surg. 2003;19(2):140–144. doi: 10.1097/01.IOP.0000055980.85878.26. [DOI] [PubMed] [Google Scholar]
- 72.Holbach LM, Colombo F, Heckmann JG, Strauss C, Dobig C. [Langerhans-cell histiocytosis of the orbit, diagnosis, treatment and outcome in three patients -- children and adults] Klin Monbl Augenheilkd. 2000;217(6):370–373. doi: 10.1055/s-2000-9578. [DOI] [PubMed] [Google Scholar]
- 73.Binning MJ, Brockmeyer DL. Novel multidisciplinary approach for treatment of langerhans cell histiocytosis of the skull base. Skull Base. 2008;18(1):53–58. doi: 10.1055/s-2007-993048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gavhed D, Laurencikas E, Akefeldt SO, Henter JI. Fifteen years of treatment with intravenous immunoglobulin in central nervous system Langerhans cell histiocytosis. Acta Paediatr. 100(7):e36–e39. doi: 10.1111/j.1651-2227.2010.02125.x. [DOI] [PubMed] [Google Scholar]
- 75.Conter V, Reciputo A, Arrigo C, Bozzato N, Sala A, Arico M. Bone marrow transplantation for refractory Langerhans' cell histiocytosis. Haematologica. 1996;81(5):468–471. [PubMed] [Google Scholar]
- 76.Minkov M. Multisystem Langerhans cell histiocytosis in children: current treatment and future directions. Paediatr Drugs. 13(2):75–86. doi: 10.2165/11538540-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 77.Kasper EM, Aguirre-Padilla DH, Alter RY, Anderson M. Histiocytosis X: Characteristics, behavior, and treatments as illustrated in a case series. Surg Neurol Int. 2:57. doi: 10.4103/2152-7806.80122. [DOI] [PMC free article] [PubMed] [Google Scholar]


