Abstract
Stem cell therapy offers potential in the regeneration of craniofacial bone defects; however, it has not been studied clinically. Tissue repair cells (TRCs) isolated from bone marrow represent a mixed stem and progenitor population enriched in CD90- and CD14-positive cells. In this phase I/II, randomized, controlled feasibility trial, we investigated TRC cell therapy to reconstruct localized craniofacial bone defects. Twenty-four patients requiring localized reconstruction of jawbone defects participated in this longitudinal trial. For regenerative therapy, patients were randomized to receive either guided bone regeneration (GBR) or TRC transplantation. At 6 or 12 weeks following treatment, clinical and radiographic assessments of bone repair were performed. Bone biopsies were harvested and underwent quantitative micro-computed tomographic (μCT) and bone histomorphometric analyses. Oral implants were installed, subsequently restored, and functionally loaded with tooth restorations. Reconstructed sites were assessed for 1 year following therapy. No study-related, serious adverse events were reported. Following therapy, clinical, radiographic, tomographic, and histological measures demonstrated that TRC therapy accelerated alveolar bone regeneration compared to GBR therapy. Additionally, TRC treatment significantly reduced the need for secondary bone grafting at the time of oral implant placement with a fivefold decrease in implant bony dehiscence exposure (residual bone defects) as compared to GBR-treated sites (p < 0.01). Transplantation of TRCs for treatment of alveolar bone defects appears safe and accelerates bone regeneration, enabling jawbone reconstruction with oral implants. The results from this trial support expanded studies of TRC therapy in the treatment of craniofacial deformities (ClinicalTrials.gov number CT00755911).
Keywords: Bone regeneration, Craniofacial tissue engineering, Stem cells, Cell therapy, Implants
INTRODUCTION
Treatment of oral and craniofacial diseases represent a substantial component of the global healthcare burden, with dental caries (tooth decay) and periodontal disease (gum disease) representing two of the most prevalent infections worldwide, oral cancer being the sixth most common cancer, and cleft lip and cleft palate representing two of the most common congenital defects (24,27). Though current treatment modalities provide functional and structural restorations of affected tissues, most approaches do not meet emerging concepts toward more biological treatment outcomes. New cell- and tissue-based strategies need development to overcome limitations of traditional treatments using allogeneic and synthetic substitutes for craniofacial reconstruction (29).
Bone marrow-derived mesenchymal stem cells are a commonly used cell type for utilization in cell-based regenerative approaches in craniofacial applications (8,9,15,17). Preclinical studies have demonstrated success in their use for bone regeneration (9,11,14,15), and more recently, several clinical reports have investigated the potential of autologous grafts containing bone marrow-derived cells in the repair of craniofacial defects (6,18–20,28). Though these preliminary reports hold promise, two limitations common to them are (1) the techniques used for cell isolation are crude and (2) the grafts used for transplantation are not well characterized.
To begin addressing these limitations, we report the first randomized, controlled clinical trial utilizing bone marrow-derived stem cells for the regeneration of craniofacial bone. This study compares a novel cell therapy for the treatment of localized jaw bone defects to a conventional guided bone regeneration procedure (GBR) (22). The cells employed in this study, tissue repair cells (TRCs or ixmyelocel-T, Aastrom Biosciences, Ann Arbor, MI, USA), are composed of a mixture of bone marrow-derived cells including expanded CD90+ mesenchymal stem cells, CD14+ monocytes/macrophages, as well as mononuclear cells from the original bone marrow aspirate (2,30). The central hypothesis underlying this feasibility trial was that a cell therapy approach using TRCs would be safe and efficacious in the regeneration of localized craniofacial bone defects.
MATERIALS AND METHODS
All chemicals are from Sigma-Aldrich (St. Louis, MO, USA) unless stated otherwise.
Patients
Following U.S. Food and Drug Administration and University of Michigan Institutional Review Board (IRB) approval, 24 patients requiring a tooth extraction were recruited to participate in this phase I/II trial (ClinicalTrials. gov number CT00755911). No power analysis was performed to determine the sample size, but instead, the number of participants for this trial was chosen for feasibility rather than statistical precision as a first-in-humans investigation. Healthy male and female participants, ages 20–70, were included if they were able and willing to read, understand, and sign an informed consent. Patients were excluded from participation if they had or exhibited one of the following conditions: blood dyscrasia, active infectious disease, liver or kidney dysfunction, endocrine disorders, cancer, current or history of bisphosphonate use, metabolic bone disorder, HIV+, pregnant, current smoker, < 2 mm of bone from apex of the tooth to be extracted to the floor of the maxillary sinus, < 4 mm of bone from apex of the tooth to be extracted to the crest of the alveolar bone.
Study Design
From March 2008 to October 2009, 24 osseous defects of 24 patients were treated and evaluated for bone regeneration. Half (12) of the subjects were assigned to receive the TRC therapy, and the other half received the control GBR therapy. Further, the participants were allocated within each treatment arm for either a 6- or 12-week bone core harvest and comprehensive safety and efficacy measures. Thus, six patients each were assigned to one of four possible patient groups (treatment arm crossed with time of outcome). Patients were randomized in blocks of four with a computer-generated randomization schedule, with one patient in each block assigned to one of the four patient groups. Due to the nature of the TRC group requiring autologous bone marrow aspiration, patient and surgeon masking was not possible. However, allocation of treatment was masked from examiners of primary outcome measures (radiographic, micro-computed tomography, histological and biochemical assessments). Additionally, patients assigned to the 12-week time point were not allowed to “cross over” to the 6-week time point.
Clinical Procedures
The bioreactor production of TRCs has been previously described (7). Briefly, following University of Michigan Institutional Review Board (IRB) approval, 12 healthy human participants giving informed consent underwent a bone marrow aspiration of the posterior ilium under conscious sedation and local anesthetic (ClinicalTrials.gov number CT00755911). Collected marrow was transferred to a sterile blood bag and then inoculated into a bioreactor, which is a proprietary computer-controlled, automated cell processing unit (Aastrom Biosciences). The cell cassette within this system provides a functionally closed, sterile environment in which cell production occurs. The fluid pathway in the cell cassette includes the cell growth chamber, a medium supply container, a mechanism for medium delivery, a waste medium collection container, and a container for the collection of harvested cells. The culture medium consists of Iscove’s modified Dulbecco’s medium (IMDM), 10% fetal bovine serum, 10% horse serum, 5 μM hydrocortisone. This system incorporates single-pass perfusion in which fresh medium flows slowly over cells without retention of waste metabolites or differentiating cytokines. After cultivation for 12 days at 37°C and 5% CO2, the TRC product was harvested by trypsinization, resuspended, and collected into a sterile bag where it was stored until the time of transplantation. According to previously described methods for characterization of TRCs (7,10), flow cytometry was performed immediately following enrichment of TRCs with fluorochromes conjugated to antibodies to CD90, CD34, CD19, vascular endothelial growth factor receptor 1 (VEGFR1), Tie2 (tyrosine kinase with immunoglobulin-like and epidermal growth factor-like domains 2), CD145, CD45, CD3, CD144, CD184, (Beckman Coulter, Indianapolis, IN, USA). The flow cytometry data confirmed the characterization of these cells as previously reported (7,10); hence, the data are not shown here.
For the 24 patients selected to participate in this study, tooth extraction of a nonrestorable tooth was performed and its removal resulted in an area of a localized osseous defect. In the TRC group (n = 12), 1 ml of the TRC suspension (approximately 1.5 × 107 cells/ml) was placed onto an absorbable gelatin sponge (Gelfoam®, Pfizer, New York, NY, USA) sized to a dimension of ~2 cm3. The sponge was then delivered into the extraction socket. In the GBR group (n = 12), only the sponge, soaked in 1 ml sterile saline, was transplanted into the socket. In both treatment groups, a bioabsorbable collagen barrier membrane (Biomend®, Zimmer Dental, Carlsbad, CA, USA) was placed over the sponge to contain the cell construct and the tissues were closed. Surgical reentry of the treated osseous defect was performed 6 or 12 weeks post-surgery, and a bone core of 2 × 7 mm was harvested with a trephine drill (Ace Surgical Supply Co., Brockton, MA, USA). The core was prepared for micro-computed tomography (μCT) and histomorphometry through which bone mineral density (BMD), bone volume fraction (BVF), and bone area/tissue area (BA/TA) were all measured.
Outcome Assessments
Radiographic bone height was assessed at the time of bone biopsies. Standardized digital radiographs were assessed using Emago® software (Exan Academic, Inc., Port Coquitlam, BC, Canada). In comparison to baseline images, the follow-up images (6 and 12 weeks) were corrected for changes in gamma exposure and angulation differences. Linear measurements were taken from the base of the extraction socket to the alveolar crest along the long axis of the socket in the baseline, 6-week, and 12-week images from both GBR and TRC treatment groups. “Linear radiographic bone height” measures were calculated as a percentage of the regenerated bone height over the height of the initial defect.
If sufficient bone volume and density (23) was present at the time of surgical reentry of treated sites, oral implants (Screwplant®, Implant Direct, Inc., Agoura Hills, CA, USA) of appropriate sizes were placed. If residual bone deficiencies remained at the time of implant placement causing implant exposure, an additional bone grafting procedure was performed. The degree to which implants were exposed was measured as the percent linear implant exposure (length of exposed area of implant divided by the total implant length) and the amount of secondary graft material was recorded at the time of surgery. This secondary grafting was performed with either an allogeneic bone graft material (Oragraft®, Salvin Dental Specialties, Inc., Charlotte, NC, USA) alone or in combination with a barrier membrane.
The ability of the oral implant fixtures to sustain biomechanical forces under functional loading with single tooth implant restorations (crowns) was assessed at 3 and 6 months postloading, with qualitative radiographic evaluation of changes in crestal bone height at these time points.
Micro-computed tomographic (μCT) analyses of bone core biopsies were performed as previously described (25). Briefly, the nondecalcified core was captured with the scanning direction parallel to the longitudinal axis of the core specimen. High-resolution scanning with an in-plane pixel size and slice thickness of 24 μm was performed. To cover the entire thickness of the bone core biopsy, the number of slices was set at 400. MicroView® software (GE Medical Systems, London, ON, Canada) was used to make three-dimensional (3D) reconstructions from the set of scans. To obtain 3D images, a threshold value of 1,100 was used. On 3D images of the specimen, the total volume (TV, mm3), bone volume (BV, mm3), and bone mineral density (BMD) of the bone core was measured directly, and the fractional bone volume (3D BV/TV, %) was calculated. After scanning, the core was decalcified and, following decalcification, was prepared histologically for standard hematoxylin and eosin (H&E) staining to perform bone histomorphometry, as previously described (14).
Alkaline Phosphatase (AP) Activity
To quantify the AP activity of TRCs, following 1 week of culture in “osteogenic” media [minimum essential medium (MEM) with 15% fetal bovine serum, 5 μg/ml gentamycin, 5 mM β-glycerol phosphate, 100 nM dexamethasone, and 50 μM ascorbic acid 2-phosphate], cells were lysed in passive lysis buffer (Promega Corporation) according to the manufacturer’s instructions and cell lysates were sonicated and centrifuged. The supernatant was recovered for the quantitative colormetric AP assay as previously described (19). The cell pellet was used for DNA isolation and the determination of the DNA concentration using the Quant-iT™ dsDNA BR Assay (Invitrogen, Life Technologies Corporation, Grand Island, NY, USA) as per the manufacturer’s instructions.
Mineralization Assay (Von Kossa)
To detect TRC mineralized matrix formation indicative of osteogenic differentiation, all 12 TRC populations were plated at a density of 30,000 cells per well in 12-well plates. At 100% confluence (which was reached following 3–5 days of culture in plates), cells were induced with “osteogenic media” (described above) for 21 days. TRCs were fixed in 4% paraformaldehyde for 30 min, immersed in fresh 5% silver nitrate and incubated in the dark for 30 min. After washing in water, TRCs were exposed to ultraviolet light for 30 min followed by a 4-min incubation in 1% sodium thiosulfate. Photographs were taken of the stained plates. These photographs were analyzed using the Mac software ImageJ (from the NIH, Bethesda, MD, USA), and the mineral density measurements were performed on the photographs. Mineral density calculations were performed by taking a measurement from the control Von Kossa plate (uninduced cells) and a measurement from the osteogenically induced plate. The density measurement from the control plate (indicative of background staining) was subtracted from the measurement of the induced plate, and this difference was reported as a relative value (“Von Kossa relative staining intensity”; % of control).
Statistical Analysis
A data safety monitoring board (DSMB) provided oversight throughout the course of the investigation and findings from this committee were submitted along with the yearly reports to the U.S. FDA. The outcomes of the investigation followed the study protocol for the primary outcome variables as well as for several post hoc analyses, including correlation of BMD/BVF with osteogenic differentiation/mineralization, percentage of implant exposure, and need for additional bone grafting. Safety analyses were accumulated at each postbaseline visit and included reporting of adverse events by body system, severity, and relationship to TRC therapy, as assessed by the investigator. Statistical analysis was performed with the statistical software package R (R Foundation for Statistical Computing, Vienna, Austria). Data are reported as means ± standard deviation, and 95% confidence intervals (CI) for the mean differences between groups (GBR and TRC) are reported in the tables; differences in means between the treatment groups were assessed with a two-sample t test. Correlation was based upon Pearson’s product–moment correlation coefficient (r), with significance based on Fisher’s Z transformation. Statistical significance was defined as p < 0.05.
RESULTS
Study Design and Patients
Throughout the study, there were no serious, study-related adverse events that were reported in examination of comprehensive safety assessments during the trial. It should be noted that one patient was lost to follow-up and did not return for the final two study visits (following restoration). The baseline demographic characteristics of all study participants are shown in Table 1. Figure 1A shows the trial timeline, and Figure 1B displays the consort diagram of the patient allocation to groups. It should be noted that, in order to obtain a total of 12 patients in the TRC groups (six for 6 weeks and six for 12 weeks), it required a total of 16 participants to be randomized to this group because aspirates could not be obtained from four patients due to high body mass index (BMI) values ( >25). This necessitated the requirement for an amended protocol which, from this point, excluded study participation of patients who presented at bone marrow aspiration with a BMI > 25.
Table 1.
Patient Demographics
| 6-Week Time Point
|
12-Week Time Point
|
|||
|---|---|---|---|---|
| GBR | TRC | GBR | TRC | |
| % Female | 66.7 | 50 | 50 | 50 |
| Ethnicity | 6 Caucasians | 1 Asian, 5 Caucasians | 1 African American, 4 Caucasians, 1 Hispanic | 1 African American, 5 Caucasians |
| Mean age (range, years) | 53 (38–62) | 48 (31–57) | 53 (46–63) | 44 (38–56) |
| Maxillary/mandibular teeth | 4/2 | 4/2 | 5/1 | 5/1 |
| Reason for extraction | Fractured tooth = 5 | Fractured tooth = 4 | Fractured tooth = 4 | Fractured tooth = 4 |
| Periodontal disease = 1 | Periodontal disease = 1 | Periodontal disease = 0 | Periodontal disease = 1 | |
| Tooth decay = 0 | Tooth decay = 1 | Tooth decay = 2 | Tooth decay = 1 | |
| Number of patients with bone defect present at time of extraction | 3 | 4 | 4 | 4 |
| Mean implant diameter (range, mm) | 4.5 (3.7–4.7) | 4.2 (3.7–4.7) | 4.2 (3.7–4.7) | 4.4 (3.7–4.7) |
| Mean implant length (range, mm) | 12.0 (10–13) | 12.3 (10–13) | 11.5 (10–13) | 12.3 (10–13) |
| Mean (keratinized) gingival thickness, mm (SD) | 1.8 (1.2) | 1.5 (0.5) | 1.8 (0.7) | 1.1 (0.2) |
| Mean (keratinized) gingival tissue width, mm (SD) | 4.8 (2.9) | 3.8 (0.7) | 5.2 (1.2) | 4.7 (1.8) |
GBR, guided bone regeneration; TRC, tissue repair cell.
Figure 1.
Trial profile and consort diagram. (A) Trial timeline. (B) Consort diagram of patient distribution. μCT, micro-computed tomography; BVF, bone volume fraction; BMD, bone mineral density.
Clinical Outcomes: Bone Density and Residual Bone Defects
Figure 2A shows images of GBR and TRC treatment defects at the time of tooth extraction (baseline) and 6 weeks after therapy. At 6 weeks, there was greater radiographic bone height achieved in the TRC group than in the GBR group, as measured by the percentage of the radiographic bone fill within the extraction defect (Fig. 2B) (p = 0.01). At 12 weeks, the GBR group displayed 74.6 ± 3.3% bone fill while the TRC groups showed 80.1 ± 2.0% bone fill, p = 0.28 (Table 2).
Figure 2.
Tissue repair cells (TRCs) promote regeneration of alveolar bone defects. (A) Digital radiographic images of linear bone height density measures for guided bone regeneration (GBR) and TRC groups at the time of tooth extraction (baseline) and 6 weeks after treatment. (B) Standardized digital radiography (SDR) was used to assess linear changes in radiographic bone height from baseline to 6- and 12-week time points. In the baseline images (A), the (orange) lines show the full extent (height) of the original defect, created following extraction of the tooth. In the 6-week images, the (green) lines show the extent (height) to which there was radiographic bone fill. These heights were calculated and the linear length of the bone fill was determined by calculating the percentage of the original defect which was filled with radiographic evidence of bone. (C) Clinical photographs of the defect site created immediately following removal of the tooth, at reentry into the site 6 weeks following treatment, and 12 months following treatment after full restoration of the site with an oral implant supported crown. (D) In some patients, residual bony defects were noted at the time of reentry into the defect sites, and in other patients, remaining bone deficiencies were identified during implant placement. There was significantly greater implant exposure in those patients receiving GBR versus those patients receiving TRCs (p < 0.04). (E) Micro-computed tomographic and histomorphometric analyses. Micro-CT and histological evaluation (H&E staining) of bone formation in a representative specimen from a GBR-treated site and a TRC treated site 6 weeks following treatment (original magnification: 2×). Both sections show varying degrees of mature cortical bone with high vascularity, as indicated by the abundance of blood vessels. Bone volume fraction (BVF), bone mineral density (BMD), and histomorphometric measures were quantified and compared between TRC and GBR treated sites at 6 and 12 weeks.
Table 2.
Summary of Primary Outcome Measures
| 6-Week Time Point
|
12-Week Time Point
|
|||
|---|---|---|---|---|
| GBR | TRC | GBR | TRC | |
| Linear bone height (%) | ||||
| Mean | 55.3 | 78.9 | 74.6 | 80.1 |
| Mean diff GBR & TRC | 23.6 | 5.4 | ||
| 95% CI | 6.02, 41.09 | −12.11, 22.95 | ||
| p value | 0.01 | 0.28 | ||
| Bone volume fraction (BVF) | ||||
| Mean | 0.13 | 0.28 | 0.24 | 0.30 |
| Mean diff GBR & TRC | 0.15 | 0.05 | ||
| 95% CI | −0.03, 0.34 | −0.14, 0.24 | ||
| p value | 0.07 | 0.30 | ||
| Bone mineral density (BMD, mg/cc) | ||||
| Mean (mg/cc) | 85.5 | 195.0 | 146.6 | 186.8 |
| Mean diff GBR & TRC | 109.5 | 40.2 | ||
| 95% CI | −28.6, 247.5 | −97.8, 178.3 | ||
| p value | 0.08 | 0.29 | ||
| Bone area/tissue area (BA/TA) | ||||
| Mean | 0.196 | 0.335 | 0.351 | 0.352 |
| Mean diff GBR & TRC | 0.139 | 0.002 | ||
| 95% CI | −0.053, 0.332 | −0.191, 0.194 | ||
| p value | 0.09 | 0.49 | ||
Figure 2C shows photographic images of TRC and GBR treatment sites at baseline, 6 weeks after treatment, and fully restored 1 year after initial surgery. Clinically, in the GBR-treated sites, the regenerated tissues at 6 weeks appeared highly vascular and fibrous, and most specimens were notably soft during biopsy harvest. Overall, the regenerated tissues in the TRC sites exhibited a bone-like appearance clinically, were more dense, and demonstrated high vascularity during biopsy harvest.
At the time of reentry into the grafted sites, clinical assessments were performed to determine if there would be a need for additional bone grafting to stably place an oral implant. The decision was made to perform additional grafting if one of two possible scenarios occurred: (1) if residual bone defects persisted following initial treatment or (2) if bone deficiencies (i.e., dehiscences or fenestrations) were identified during oral implant placement (Fig. 2D). In all four groups, oral implants were able to be stably placed at their respective scheduled time points. However, due to a greater number of residual bone defects present in the groups initially treated with GBR, there was a greater need for the GBR groups (6 and 12 weeks) to receive secondary bone grafting procedures at the time of implant placement, relative to the need within the TRC-treated groups (Table 3). Additionally, in the GBR groups at both 6 and 12 weeks, there was about sixfold greater percent implant exposure which necessitated more extensive secondary grafting, relative to this need in the TRC treated groups (p < 0.04) (Fig. 2D and Table 3). Although different implant lengths and diameters were used at the time of implant installation, in general the sizes used were quite similar (Table 1). For each of the treatment groups following the regenerative procedures, all implants achieved clinical evidence of integration into the restored bone and were able to sustain biomechanical loading when restored with an implant restoration 6 months following placement. Implant stability was followed for 1 year, and all implants remained functionally integrated at study completion.
Table 3.
Residual Bone Defects Following Surgery
| 6-Week Time Point
|
12-Week Time Point
|
|||
|---|---|---|---|---|
| GBR | TRC | GBR | TRC | |
| Mean % linear implant exposure | 29.2 | 5.1 | 25.3 | 3.8 |
| 95% CI | (−1.2, 60) | (−8.5, 18.7) | (−0.9, 51.5) | (−6.1, 14) |
| p value | 0.04 | 0.04 | 0.03 | 0.03 |
| Cases Requiring secondary bone grafting | 5 | 2 | 3 | 2 |
| Mean amount additional graft used (cc) | 0.23 | 0.09 | 0.08 | 0.05 |
| 95% CI | (0.02, 0.44) | (−0.01, 0.2) | (−0.02, 0.2) | (−0.05, 0.16) |
| p value | 0.08 | 0.08 | 0.31 | 0.31 |
Biopsy Analyses: μCT and Histomorphometry
Bone core biopsies from the regenerative sites were analyzed 6 and 12 weeks following treatment using three-dimensional μCT and histomorphometry (Fig. 2E and Table 2). Bone volume fraction (BVF) and bone mineral density (BMD) were the primary μCT outcome measures, and analysis at 6 weeks showed that the BVF for the GBR group was 13 ± 6%, compared to 28 ± 8% for the TRC-treated group (p = 0.08). Similarly, in comparisons of BMD measures between GBR- and TRC-treated groups, bone repair in the TRC group had a greater than twofold higher BMD (195.0 ± 63.3 mg/cc) than regenerated bone in the GBR group (85 ± 46.3 mg/ cc; p = 0.1).
Following micro-CT analysis, bone core biopsies of regenerated bone were further evaluated histomorpho-metrically. When visualized under light microscopy, the majority of the samples were comprised of a highly cellular, dense connective tissue exhibiting the presence of an abundance of blood vessels, yet there were differences at 6 weeks between the specimens harvested from TRC- and GBR-treated sites with the presence of more bone in the TRC sites (Fig. 2E). Upon 6- and 12-week analyses, no statistically significant differences were found in measures of % bone area/tissue area (BA/TA). At 6 weeks, regenerated tissue from the GBR group had a BA/TA of 19.6 ± 4.2%, while tissue from the TRC-treated group had a BA/TA of 28.8 ± 9.1% (p = 0.10 between groups) (Table 2). At the 12-week time point, the percentage of regenerated bone tissue appeared to be similar between the GBR- and TRC-treated groups, with BA/TA measures for these groups being 35.1 ± 3.2% and 35.2 ± 8.9%, respectively (Table 2).
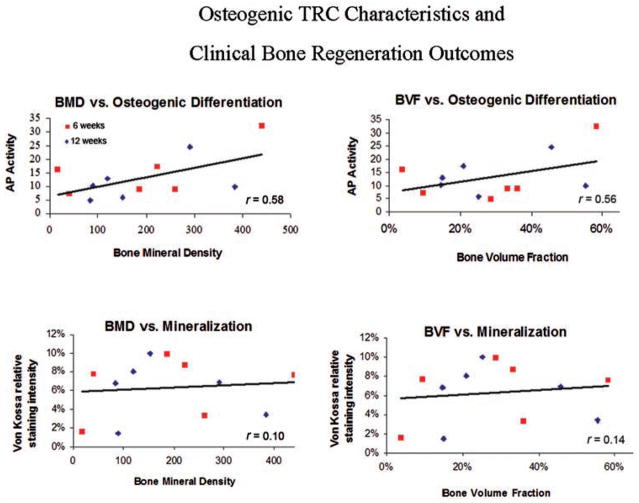
As an ad hoc analysis, in vitro osteogenic potential (AP activity) and mineralization (Von Kossa) ability of TRCs were correlated with the clinical outcome measures of BMD and BVF for each respective TRC population. These data demonstrated that there was a positive correlation between AP and BVF (r = 0.56, p = 0.058) and a statistically significant positive correlation between AP and BMD (r = 0.58; p = 0.049). Positive correlations with in vitro mineralization ability and BMD and BVF measures were not statistically significant (Fig. 3).
Figure 3.
In vitro TRC characteristics and clinical regeneration outcomes. The in vitro osteogenic potential [alkaline phosphatase (AP)-fold induction, top] and extracellular matrix mineralization (von Kossa staining, bottom) of each TRC population was correlated with its clinical capacity to form bone in vivo [as measured by BMD (mg/cc) and BVF]. Statistically significant differences (p < 0.05) were noted between BMD and osteogenic differentiation, while nonsignificant differences were found between mineralization and BMD/BVF.
DISCUSSION
Regenerative medicine aims to use tissue engineering to restore damaged and lost tissue (16). In this report, we describe the first randomized, controlled, human trial employing stem cell therapy for the regeneration of craniofacial bone. TRCs were grafted into osseous defects of the jaw and biopsies harvested for analyses at 6 and 12 weeks. Reconstruction of these sites was completed with oral implant therapy and treatment sites were followed for 12 months postoperatively. Clinical and laboratory analyses of treatment sites demonstrated that the cell therapy accelerated the regenerative response as determined clinically, radiographically, and histologically. Further, there was a significantly reduced need for secondary bone grafting procedures in the group that originally received the cell therapy.
The extraction socket created following tooth removal serves as a reasonable model of bone repair being that the resulting defect is reproducible and has a limited capacity to heal completely without intervention. Pelegrine and colleagues used this model recently to investigate the potential of a bone marrow graft in preserving alveolar bone following tooth removal (26). Upon evaluation of the grafted sites at 6 months, the graft provided better results in maintaining the alveolar bone relative to when no graft was used. Though the results suggested that bone marrow constituents provided benefit, the cellular component of the graft was not characterized, and thus, homogeneity of the graft from patient to patient could not be ascertained. Other promising preliminary reports of successful craniofacial regenerative procedures using bone marrow-derived grafts are similarly confounded by the lack of characterization of the grafted biological cell type or construct (8,20,31). Meijer and colleagues spoke to this limitation in a study evaluating the use of bone marrow grafts for craniofacial reconstructions (21). These treatments resulted in clinical bone formation in only three of the six patients treated and because the constituents of the grafts were not characterized, the authors indicated that the cause for the 50% failure rate could not be definitively determined.
Another general critique of cell therapy approaches has been the lack of reproducible cell isolation and expansion protocols that can predictably yield consistent cell populations. Two defining elements of our study relative to other case reports employing bone marrow grafts for craniofacial reconstructions are (1) characterization of the cell population prior to grafting and (2) early 6-week regenerative healing time evaluated. Through the cell processing protocol used to generate TRCs, within a given range, cell surface marker characterization has demonstrated a relative degree of homogeneity in the TRCs produced between patients (1,10,13), namely enrichment of CD90+ cells. Preclinical studies of TRCs have demonstrated that high concentrations of CD90+ cells within TRC populations are positively correlated with ectopic bone formation (7). Even further, in correlating the in vitro osteogenic capacity of TRCs (AP) with the clinical regenerative BMD and BVF measures, we observed positive correlations (Fig. 3). These data provide important preliminary evidence underscoring the potential for targeted, individualized stem cell therapy approaches based upon initial cell characterization. Though the initial evidence appears promising, it is realized that in vitro analyses and clinical correlation studies need to be performed on larger subsets of TRC populations to more fully elucidate these correlations.
Another defining element of our study is the early 6-week time point in which we chose to reenter the regenerated sites. Most clinical protocols that employ grafting procedures for reconstruction of alveolar bone allow healing periods minimally of 3–6 months (5). In the natural healing environment of an extraction socket wound, the rate limiting step in the healing process is the recruitment and proliferation of progenitor cells from the local microenvironment (4,32). The rationale for our choosing the 6-week time point was to determine if wound repair could be accelerated by delivering cells to the defect at the time of tooth extraction. It was thus rather striking to see that TRC regenerated bone at 6 weeks similar to that observed at 12 weeks in both TRC and GBR groups. Furthermore, data shown in Table 3 and Figure 2 clearly demonstrate the ability of TRCs to augment bone regeneration of the healing socket by greatly reducing the need and extent of secondary grafting often performed at the time of oral implant installation. Additional studies would need to be performed to determine the mechanism of this accelerated regenerative response, yet two possible mechanisms for these observations exist: (1) the regenerated tissue is derived from the transplanted cells (12) and (2) the transplanted cells act in a trophic fashion and provide signals to the host cells thereby “jump-starting” the regenerative process (3).
The alveolar ridge defect model following tooth extraction is best viewed as a proof-of-concept approach using autologous cell therapy for the regeneration of craniofacial bone. Though our study was originally designed as a phase I/II first-in-humans trial to investigate safety, we included a control group (GBR) to which we could draw preliminary conclusions regarding efficacy. As such, the study is considered to be small, and though the early findings are promising, they are also considered with caution in the context of this proof-of-concept investigation. A next step would be to evaluate this approach in a larger patient population, which could lead to a strong foundation for utilization of this approach for treatment of larger, more debilitating craniofacial conditions.
Acknowledgments
This study was funded by a Career Award for Medical Scientists from the Burroughs Wellcome Fund (D.K.), the National Institutes of Health NIH/NCRR UL1RR024986 (W.V.G.), and National Institutes of Health NIH/NIDCR DE13397 (W.V.G.). The authors would like to thank Dr. Andrew Eisenberg for performing the bone marrow aspirations. The authors would also like to thank Judy Douville, Lea Franco, Anna Galloro, Emily Hall, Christina Huffman, and Emily Thorpe for administrative and clinical support. D.K. and W.V.G. were responsible for the concept and design of the study and obtained funding. D.K., G.P., C.H.P., and S.A.T. were responsible for acquisition of data. T.B. and D.K. did the statistical analysis. D.K. and W.V.G. supervised the study. R.L.B. provided administrative, material and technical support. D.K., W.V.G., G.P., and T.B. participated in the interpretation of data and critical revision of the report for intellectual content. All authors provided final approval of the submitted version. Ronnda L. Bartel is an employee of Aastrom Biosciences.
References
- 1.Boiret N, Rapatel C, Boisgard S, Charrier S, Tchirkov A, Bresson C, Camilleri L, Berger J, Guillouard L, Guerin JJ. CD34+ CDw90 (Thy-1)+ subset colocated with mesenchymal progenitors in human normal bone marrow hematon units is enriched in colony-forming unit mega-karyocytes and long-term culture-initiating cells. Exp Hematol. 2003;31(12):1275–1283. doi: 10.1016/j.exphem.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 2.Caldwell J, Palsson BO, Locey B, Emerson SG. Culture perfusion schedules influence the metabolic activity and granulocyte-macrophage colony-stimulating factor production rates of human bone marrow stromal cells. J Cell Physiol. 1991;147(2):344–353. doi: 10.1002/jcp.1041470221. [DOI] [PubMed] [Google Scholar]
- 3.Caplan AI. Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J Cell Physiol. 2007;213(2):341–347. doi: 10.1002/jcp.21200. [DOI] [PubMed] [Google Scholar]
- 4.Cardaropoli G, Araujo M, Hayacibara R, Sukekava F, Lindhe J. Healing of extraction sockets and surgically produced—augmented and non-augmented—defects in the alveolar ridge. An experimental study in the dog. J Clin Periodontol. 2005;32(5):435–440. doi: 10.1111/j.1600-051X.2005.00692.x. [DOI] [PubMed] [Google Scholar]
- 5.Coulthard P, Esposito M, Jokstad A, Worthington HV. Interventions for replacing missing teeth: Bone augmentation techniques for dental implant treatment. Cochrane Database Syst Rev. 2003;2003(3):CD003607. doi: 10.1002/14651858.CD003607. [DOI] [PubMed] [Google Scholar]
- 6.Delaere P, Vranckx J, Verleden G, De Leyn P, Van Raemdonck D. Tracheal allotransplantation after withdrawal of immunosuppressive therapy. N Engl J Med. 2010;362(2):138–145. doi: 10.1056/NEJMoa0810653. [DOI] [PubMed] [Google Scholar]
- 7.Dennis JE, Esterly K, Awadallah A, Parrish CR, Poynter GM, Goltry KL. Clinical-scale expansion of a mixed population of bone-marrow-derived stem and progenitor cells for potential use in bone-tissue regeneration. Stem Cells. 2007;25(10):2575–2582. doi: 10.1634/stemcells.2007-0204. [DOI] [PubMed] [Google Scholar]
- 8.Filho Cerruti H, Kerkis I, Kerkis A, Tatsui NH, da Costa Neves A, Bueno DF, da Silva MC. Allogenous bone grafts improved by bone marrow stem cells and platelet growth factors: Clinical case reports. Artif Organs. 2007;31(4):268–273. doi: 10.1111/j.1525-1594.2007.00374.x. [DOI] [PubMed] [Google Scholar]
- 9.Gao J, Dennis JE, Solchaga LA, Awadallah AS, Goldberg VM, Caplan AI. Tissue-engineered fabrication of an osteochondral composite graft using rat bone marrow-derived mesenchymal stem cells. Tissue Eng. 2001;7(4):363–371. doi: 10.1089/10763270152436427. [DOI] [PubMed] [Google Scholar]
- 10.Gastens MH, Goltry K, Prohaska W, Tschope D, Stratmann B, Lammers D, Kirana S, Gotting C, Kleesiek K. Good manufacturing practice-compliant expansion of marrow-derived stem and progenitor cells for cell therapy. Cell Transplant. 2007;16(7):685–696. doi: 10.3727/000000007783465172. [DOI] [PubMed] [Google Scholar]
- 11.Holtorf HL, Jansen JA, Mikos AG. Ectopic bone formation in rat marrow stromal cell/titanium fiber mesh scaffold constructs: Effect of initial cell phenotype. Biomaterials. 2005;26(31):6208–6216. doi: 10.1016/j.biomaterials.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 12.Jensen SS, Terheyden H. Bone augmentation procedures in localized defects in the alveolar ridge: Clinical results with different bone grafts and bone-substitute materials. Int J Oral Maxillofac Implants. 2009;24(Suppl):218–236. [PubMed] [Google Scholar]
- 13.Kaigler D, Avila G, Wisner-Lynch L, Nevins ML, Nevins M, Rasperini G, Lynch SE, Giannobile WV. Platelet-derived growth factor applications in periodontal and peri-implant bone regeneration. Expert Opin Biol Ther. 2011;11(3):375–385. doi: 10.1517/14712598.2011.554814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaigler D, Krebsbach PH, Wang Z, West ER, Horger K, Mooney DJ. Transplanted endothelial cells enhance orthotopic bone regeneration. J Dent Res. 2006;85(7):633–637. doi: 10.1177/154405910608500710. [DOI] [PubMed] [Google Scholar]
- 15.Krebsbach PH, Kuznetsov SA, Satomura K, Emmons RV, Rowe DW, Robey PG. Bone formation in vivo: Comparison of osteogenesis by transplanted mouse and human marrow stromal fibroblasts. Transplantation. 1997;63(8):1059–1069. doi: 10.1097/00007890-199704270-00003. [DOI] [PubMed] [Google Scholar]
- 16.Langer R, Vacanti JP. Tissue engineering. Science. 1993;260(5110):920–926. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 17.Lee CH, Shah B, Moioli EK, Mao JJ. CTGF directs fibroblast differentiation from human mesenchymal stem/ stromal cells and defines connective tissue healing in a rodent injury model. J Clin Invest. 2010;120(9):3340–3349. doi: 10.1172/JCI43230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Macchiarini P, Jungebluth P, Go T, Asnaghi MA, Rees LE, Cogan TA, Dodson A, Martorell J, Bellini S, Parnigotto PP, Dickinson SC, Hollander AP, Mantero S, Conconi MT, Birchall MA. Clinical transplantation of a tissue-engineered airway. Lancet. 2008;372(9655):2023–2030. doi: 10.1016/S0140-6736(08)61598-6. [DOI] [PubMed] [Google Scholar]
- 19.Manolagas SC, Burton DW, Deftos LJ. 1,25-Di-hydroxyvitamin D3 stimulates the alkaline phosphatase activity of osteoblast-like cells. J Biol Chem. 1981;256(14):7115–7117. [PubMed] [Google Scholar]
- 20.Marcacci M, Kon E, Moukhachev V, Lavroukov A, Kutepov S, Quarto R, Mastrogiacomo M, Cancedda R. Stem cells associated with macroporous bioceramics for long bone repair: 6- to 7-year outcome of a pilot clinical study. Tissue Eng. 2007;13(5):947–955. doi: 10.1089/ten.2006.0271. [DOI] [PubMed] [Google Scholar]
- 21.Meijer GJ, de Bruijn JD, Koole R, van Blitterswijk CA. Cell based bone tissue engineering in jaw defects. Biomaterials. 2008;29(21):3053–3061. doi: 10.1016/j.biomaterials.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 22.Melcher AH. On the repair potential of periodontal tissues. J Periodontol. 1976;47(5):256–260. doi: 10.1902/jop.1976.47.5.256. [DOI] [PubMed] [Google Scholar]
- 23.Misch CE. Bone classification, training keys to implant success. Dent Today. 1989;8(4):39–44. [PubMed] [Google Scholar]
- 24.Mossey PA, Little J, Munger RG, Dixon MJ, Shaw WC. Cleft lip and palate. Lancet. 2009;374(9703):1773–1785. doi: 10.1016/S0140-6736(09)60695-4. [DOI] [PubMed] [Google Scholar]
- 25.Park CH, Abramson ZR, Taba M, Jr, Jin Q, Chang J, Kreider JM, Goldstein SA, Giannobile WV. Three-dimensional micro-computed tomographic imaging of alveolar bone in experimental bone loss or repair. J Periodontol. 2007;78(2):273–281. doi: 10.1902/jop.2007.060252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pelegrine AA, da Costa CE, Correa ME, Marques JF., Jr Clinical and histomorphometric evaluation of extraction sockets treated with an autologous bone marrow graft. Clin Oral Implants Res. 2010;21(5):535–542. doi: 10.1111/j.1600-0501.2009.01891.x. [DOI] [PubMed] [Google Scholar]
- 27.Pihlstrom BL, Michalowicz BS, Johnson NW. Periodontal diseases. Lancet. 2005;366(9499):1809–1820. doi: 10.1016/S0140-6736(05)67728-8. [DOI] [PubMed] [Google Scholar]
- 28.Quarto R, Mastrogiacomo M, Cancedda R, Kutepov SM, Mukhachev V, Lavroukov A, Kon E, Marcacci M. Repair of large bone defects with the use of autologous bone marrow stromal cells. N Engl J Med. 2001;344(5):385–386. doi: 10.1056/NEJM200102013440516. [DOI] [PubMed] [Google Scholar]
- 29.Rayment EA, Williams DJ. Concise review: Mind the gap: Challenges in characterizing and quantifying cell- and tissue-based therapies for clinical translation. Stem Cells. 2010;28(5):996–1004. doi: 10.1002/stem.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schwartz RM, Palsson BO, Emerson SG. Rapid medium perfusion rate significantly increases the productivity and longevity of human bone marrow cultures. Proc Natl Acad Sci USA. 1991;88(15):6760–6764. doi: 10.1073/pnas.88.15.6760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Soltan M, Smiler D, Soltan C, Prasad HS, Rohrer MD. Bone grafting by means of a tunnel dissection: Predictable results using stem cells and matrix. Implant Dent. 2010;19(4):280–287. doi: 10.1097/ID.0b013e3181e40166. [DOI] [PubMed] [Google Scholar]
- 32.Trombelli L, Farina R, Marzola A, Bozzi L, Liljenberg B, Lindhe J. Modeling and remodeling of human extraction sockets. J Clin Periodontol. 2008;35(7):630–639. doi: 10.1111/j.1600-051X.2008.01246.x. [DOI] [PubMed] [Google Scholar]