Abstract
Molecular chaperone-based vaccines offer a number of advantages for cancer treatment. We have discussed the deployment of a vaccine prepared by gentle isolation of Hsp70 from tumor dendritic cell fusions (Hsp70 fusion vaccine). The vaccine was highly effective in triggering specific T cell immunity and in the treatment of tumor bearing mice and the preparation was shown to retain an increased amount of tumor antigens compared to other chaperone-based isolates. This approach has the further advantage that tumor sub-populations could be used to prepare the Hsp70 fusion vaccine. Cellular fusion vaccines were made to specifically target drug resistant cancer cells and tumor cell populations enriched in ovarian cancer stem cells (CSC). Such vaccines showed enhanced capacity to trigger T cell immunity to these resistant ovarian carcinoma populations. We have discussed the potential of using the cellular and Hsp70 fusion vaccine approaches in therapy of treatment resistant cancer cells and its deployment in combination with ionizing radiation or hyperthermia to enhance the effectiveness of both forms of therapy.
Introduction
Heat shock proteins (HSP) play a significant role in expressing the genome through the facilitation of protein folding (1, 2). Such ability to bind and fold client proteins has been depicted metaphorically as molecular chaperone activity (1). HSPs belong to five distinct families including HSPA (Hsp70), HSPB (small hsp), HSPC (Hsp90), HSPD (hsp60) and HSPH (large HSP) (3). The molecular chaperone abilities of these HSPs are utilized in the stress response, when cells are induced to express large quantities of each of the HSP families, leading to repair and reconstitution of the proteome (4). HSPs are also implicated in a number of pathologies, particularly cancer, in which they are expressed to high levels in many cancers and appear to mediate multiple facets of transformation and tumorigenesis (5–7). The relative effectiveness of the various HSPs as markers and indicators of prognosis have been discussed in detail in previous reviews. In general although HSPs are at high level in many cancers, they are not good indices of prognosis in many cases. In effect, heat shock factor 1 (HSF1), the transcriptional activator of HSP genes, is a better index at least in breast cancer and in fact correlates well with a bad prognosis. However, HSPs are envisioned as targets in cancer therapy and HSP-directed drugs are already in clinical trial directed against a number of cancers (8, 9). Currently Hsp90 directed drugs based on the natural products geldanomycin and are in trial as well as new synthetic Hsp90 drugs (9, 10). Drugs targeting other HSPs in cancer are also under development (11). Another approach to exploiting the HSPs in cancer therapy is in anticancer vaccine design (3, 12, 13). The principle idea behind this approach is that HSPs, as molecular chaperones should bind to target polypeptides in a selective but not very specific manner (reviewed (14)). HSPs would be expected to recognize hydrophobic sequences, as these are displayed on the exterior of denatured proteins but not specific amino acid sequences per se. HSPs might thus collect and chaperone tumor antigens and could be envisaged as Trojan horses that could deliver tumor antigens into the antigen processing pathways of APC and thus be used o stimulate cytotoxic lymphocytes directed against tumors (15, 16). Indeed it has been shown that a number of molecular chaperones including glucose regulated protein (GRP) 78, Hsp70, Hsp90, Hsp110, and GRP170 can bind to antigenic peptides and generate anti-tumor immunity (17–20). Significantly, it has been shown that large stress proteins such as Grp170 can complex with full-length tumor antigens in vivo indicating the potential of this approach (21).
Enhancing HSP vaccines
Despite the early promise of HSP based vaccines, clinical trials involving the use of GRP96 and Hsp70 in an autologous context have proven only marginally effective (3, 22). Thus improvements in the vaccines would be desirable. The principle property required for the vaccines to be effective is ability to bind and retain antigenic peptides for delivery to APC when vaccines are injected into the host. This involves optimal choice of chaperone to be used. Indeed chaperones have a wide range of abilities to bind peptides with the HSPH family of proteins particularly effective in retaining antigens (23). In addition gentle and rapid isolation of HSP-peptide complexes (HSP-PC) improves vaccine effectiveness. Recently we have developed a method in which tumor-dendritic cell (DC) fusions are used as the source of HSP-PC which are isolated by gentle lysis and Hsp70-agarose affinity elution (24). It was shown originally that tumor-DC fusion could alone be a highly effective anticancer vaccine. The rationale behind this is that tumor antigens can be directly processed by the potent DC antigen processing machinery and then presented on the surface of the heterokaryons (24). We have used this cellular fusion vaccine approach to target cancer stem cells in a recent study (25) HSP-PC from tumor-DC fusion (HSP-fusion vaccine) have proved to be highly effective in provoking anti-tumor immunity and was markedly more potent compared to a similar vaccine from tumor alone (20, 26). We have illustrated the processes involved in generation of such a vaccine in Fig. 1. The HSP fusion vaccine was shown to retain an increased amount of the tumor antigen MUC1 (20). In addition, Hsp90 was co-isolated with the Hsp70 in the fusion vaccine and appeared to play a crucial role in immune effectiveness. Hsp70 and Hsp90 are known to associate in cells and mediate folding of client proteins (20). In addition, Hsp90 binds directly to peptides derived from the proteasome during antigen processing (27). Hsp90 may thus access antigenic peptides at source and may retain them within the Hsp70 fusion vaccine (27). Hsp90 inhibitory drugs were shown to prevent the effectiveness of the Hsp70 fusion vaccine when added to the fusion cells during vaccine preparation. Indeed it has been shown convincingly that Hsp90 bound to a model peptide from Ova is internalized by a receptor-mediated process in DC and lead to enhancement of cross presentation to cognate T cells (28).
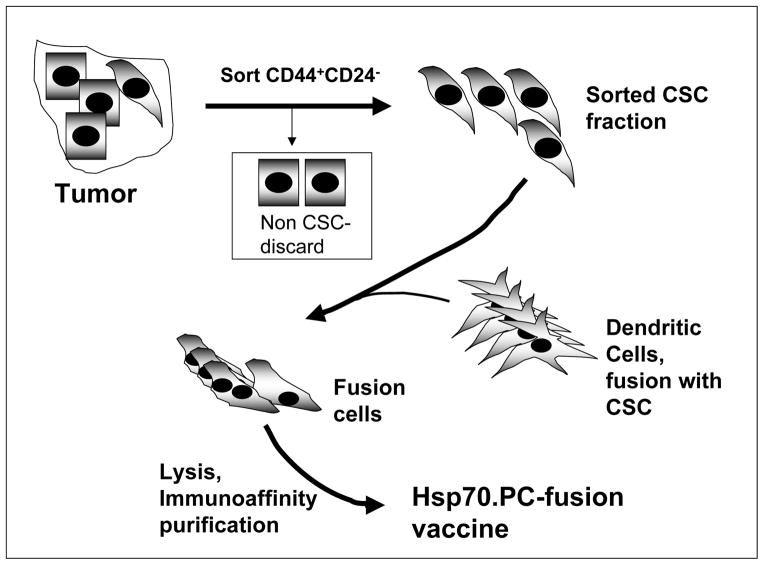
Figure 1. Preparation of HSP-fusion vaccine from CSC.
The tumor is depicted as a colony of cells containing CSC (spindle / mesenchymal shape) and more differentiated cells (cuboid shape). To prepare vaccine, cells are disaggregated and CSC are sorted by cell surface phenoptype (CD44+CD24−) using fluorescence–labeled monoclonal antibodies and cell sorting by FACS. CSC are then fused to autologous DC by the polyethylene glycol approach as described in Ref 19, leading to formation of fusion cells. Fusion cells can be used as vaccine in this state or lysed and the HSP fusion vaccine is prepared using Hsp70 antibody immunoaffinity chromatography as in Ref 19.
Tumor heterogeneity and significant cellular sub-populations
Tumor cell populations are highly heterogeneous. The tumor population is heterogeneous in terms of pathophysiology: perfusion and oxygenation vary in different parts of tumors and this heterogeneity affects resistance to radiation therapy in particular as hypoxic cells are radioresistant (29). In addition heterogeneity is encountered in terms of cell biology in that there is now considerable evidence to suggest that tumorigenesis is restricted to a sub-population that resembles tissue stem cells (cancer stem cells or CSC) and that these cells initiate the formation of tumors and may fuel metastasis (30, 31). CSC constitute a particular challenge for cancer therapy in being resistant to chemotherapy and radiation therapy (25, 32–34). A further source of tumor heterogeneity is provided by the penetration of normal cell such as macrophages, mesenchymal stem cells, tumor associated fibroblasts (TAF), regulatory T cells (Treg) and myeloid derived suppressor cells (MDSC) into tumors. One result of these normal cells appears to be the creation of an immunosuppressive tumor environment: immunosuppressive cytokines such as interleukin 10 and tumor growth factor B are secreted by MDSC and TAF and MDSC and Treg suppress the activity of DC and cytotoxic T cells (CTL) (35, 36).
Targeting of CSC and drug resistant cells by fusion vaccines
The fusion vaccine approach has the advantage that theoretically any tumor population could be used in the preparation of the vaccine as long as it can be isolated from the bulk population. The presence of surface markers on stem cells suggested the possibility of isolating such CSC using specific antibodies coupled with cell sorting. Initial experiments were carried out in ovarian carcinoma cells (37). Our initial experiments, to establish the principle of the approach have been carried out using tumor-DC fusion vaccines (cellular fusion vaccine). We aim to proceed to using Hsp70 fusion vaccines (molecular fusion vaccines) in subsequent experiments. Most patients with stage III/IV ovarian carcinoma (OVCA) develop resistance to standard therapies and this may be associated with increases in drug resistant CSC populations (38). CSC subpopulations have been determined in OVCA cell lines and express stem cell associated proteins such as Oct4, Notch-1, nesting, BM1-1, and surface markers CD44 and CD177 (35, 39). It was found that OVCA cells surviving carboplatin expressed cell surface CD44 and exhibited a CSC phenotype (25). Fusion vaccine prepared from CD44+ sorted OVCA led to the preferentially killing of CD44+ cells as well as carboplatin resistant OVCA by specific CTL populations. This vaccine was also highly effective in killing cells from the bulk population (25). The vaccine is thus selective for the minority of tumor initiating cells in the OVCA population and targets drug resistant cells, indicating the power of this approach (25). Targeting CSC is particularly important as these cells are not only capable of initiating primary tumors but are also the major cells involved in the seeding of metastases (40). We have shown that metastasis is an early event in mammary tumorigenesis in mice and largely fueled by CSC (40). Thus selective elimination of CSC by the fusion vaccine may be important in regression of both primary and secondary tumors. In addition to maintenance of CSC populations by renewal mechanisms, such cells may arise by reprogramming of differentiated cells or progenitors in a process that resembles the events in inducible pluripotent stem cell IPSC programming (41–43). For instance ionizing radiation can trigger stem cell reprogramming in tumor cells through a process involving the transcription factor STAT3, a key factor in IPSC programming (41, 42). This process may be of high significance in cancer treatment in that such therapy may preferentially kill non-CSC as well as triggering cells with a CSC phenotype with high tumor initiating and metastatic potential and increased treatment resistance. Inclusion of immunotherapy targeting CSC within conventional treatment protocols may thus be indicated.
Combination of HSP fusion vaccines with conventional treatments
As mentioned previously, tumor microenvironments tend to be immunosuppressive due to infiltration of Treg, MDSC and TAM and exclusion of CTL from the tumor microcirculation (35). Such an environment could be reversed by induction of local inflammatory killing that might bias the cytokine milieu in an immunostimulatory direction (44). One highly promising candidate for such an effect would be treatment of the tumor with ionizing radiation, a modality that has been shown to be pro-inflammatory and immunogenic (45). Radiation of the tumor locally would kill primary tumor cells as well as reversing the immunosuppressive tumor milieu. Immunotherapy functions best with minimal residual disease and activated T cells are able to kill metastatic tumor cells. One potential problem with immunotherapy that is beginning to emerge is stem cell reprogramming by the radiation (42, 46, 47). CSC are markedly radioresistant a property that may be a consequence of reduced rates of proliferation that characterize stem cells and / or the expression of polycomb family gene such as Bmi1 that increase the rates of DNA repair (42, 46, 47). CSC are also highly metastatic suggesting the potential for radiation to trigger metastases (40). We have shown that Hsp70 fusion vaccines can be prepared that can lead to preferential killing of CSC and treatment resistant cells (J. Gong & S.K. Calderwood, in revision). Combined radiotherapy and Hsp70 stem cell / DC fusion vaccines could thus be mutually reinforcing in dealing with pathways of tumor treatment resistance and be synergistic in tumor cell killing. The optimal ordering of the component arms in such an approach would clearly be desirable in order to maximize the potential of this multi-faceted treatment protocol. One could also envisage the use of thermal therapy in combination with Hsp70 fusion vaccines. Although the ability of conventional hyperthermia at 42–45°C to enhance immunity is uncertain, higher ablative heating above 50°C is markedly immunostimulatory and could be used to boost the effects of the Hsp70 fusion vaccine (reviewed in detail (48)). Necrosis is known to be the dominant form of cell death in this temperature range (49). Necrotic cell killing is classically immunogenic (50). In addition, fever range hyperthermia (FRH) at 39–40 °C, below the conventional hyperthermia at 42–45°C range, also increases tumor immunity through multiple stimulatory effects on immune effector cells. Combined Hsp70 fusion vaccines with FRH may also be strongly indicated (51).
One further problem related to this approach that could arise is that patients with advanced cancer may be deficient in CTL activation due to long-term chemotherapy and may only mount a weak immune response during the radioimmunotherapy (52, 53). One treatment strategy in such a scenario could involve the ex vivo stimulation of patients’ CD8+ T lymphocytes with tumor antigens and re-introduction of the activated T cells into the patient by adoptive transfer. Hsp70 antigen complexes from stem cell /DC fusion or from treatment resistant tumor cells could be used to program such CTL to attack tumor initiating cells in vitro prior to introduction of the CTL into patients by adoptive transfer.
Finale
Although molecular chaperone vaccines offer many advantages for tumor immunotherapy, their performance in the clinic has not been overwhelming so far. We have attempted to develop a novel vaccine based on extracting Hsp70 chaperone complexes from tumor dendritic fusions, with some success. The approach has the advantage of high antigen retention and ability to prompt antigen specific tumor immunity in vivo. This method also has the merit of permitting the use of malignant subpopulations such as CSC and drug or radiation resistant cells in vaccine preparation. These populations can then be selectively targeted. We envisage the use of these Hsp70 fusion vaccines clinically in combination with conventional therapeutic approaches such as chemotherapy and radiation therapy.
Acknowledgments
This work was supported by NIH research grants RO-1CA047407, R01CA119045 and RO-1CA094397
References
- 1.Ellis RJ. Protein misassembly: macromolecular crowding and molecular chaperones. Adv Exp Med Biol. 2007;594:1–13. doi: 10.1007/978-0-387-39975-1_1. [DOI] [PubMed] [Google Scholar]
- 2.Lindquist S, Craig EA. The heat shock proteins. Ann Rev Genet. 1988;22:631–7. doi: 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- 3.Murshid A, Gong J, Stevenson MA, Calderwood SK. Heat shock proteins and cancer vaccines: developments in the past decade and chaperoning in the decade to come. Expert Rev Vaccines. 2011 Nov;10(11):1553–68. doi: 10.1586/erv.11.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Craig EA. The stress response: changes in eukaryotic gene expression in response to environmental stress. Science. 1985 Nov 15;230(4727):800–1. doi: 10.1126/science.230.4727.800-a. [DOI] [PubMed] [Google Scholar]
- 5.Ciocca DR, Arrigo AP, Calderwood SK. Heat shock proteins and heat shock factor 1 in carcinogenesis and tumor development: an update. Arch Toxicol. 2012 Aug 11; doi: 10.1007/s00204-012-0918-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ciocca DR, Calderwood SK. Heat shock proteins in cancer: diagnostic, prognostic, predictive, and treatment implications. Cell Stress Chaperones. 2005 Summer;10(2):86–103. doi: 10.1379/CSC-99r.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Calderwood SK. Heat shock proteins in breast cancer progression--a suitable case for treatment? Int J Hyperthermia. 2010;26(7):681–5. doi: 10.3109/02656736.2010.490254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neckers L. Hsp90 inhibitors as novel cancer chemotherapeutic agents. Trends Mol Med. 2002;8(4):S55–61. doi: 10.1016/s1471-4914(02)02316-x. [DOI] [PubMed] [Google Scholar]
- 9.Trepel J, Mollapour M, Giaccone G, Neckers L. Targeting the dynamic HSP90 complex in cancer. Nat Rev Cancer. 2010 Aug;10(8):537–49. doi: 10.1038/nrc2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Neckers L, Workman P. Hsp90 molecular chaperone inhibitors: are we there yet? Clin Cancer Res. 2012 Jan 1;18(1):64–76. doi: 10.1158/1078-0432.CCR-11-1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Powers MV, Jones K, Barillari C, Westwood I, van Montfort RL, Workman P. Targeting HSP70: the second potentially druggable heat shock protein and molecular chaperone? Cell Cycle. 2011 Apr 15;9(8):1542–50. doi: 10.4161/cc.9.8.11204. [DOI] [PubMed] [Google Scholar]
- 12.Udono H, Srivastava PK. Heat shock protein 70-associated peptides elicit specific cancer immunity. J Exp Med. 1993 Oct 1;178(4):1391–6. doi: 10.1084/jem.178.4.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Calderwood SK, Ciocca DR. Heat shock proteins: stress proteins with Janus-like properties in cancer. Int J Hyperthermia. 2008 Feb;24(1):31–9. doi: 10.1080/02656730701858305. [DOI] [PubMed] [Google Scholar]
- 14.Calderwood SK, Theriault JR, Gong J. Message in a bottle: role of the 70-kDa heat shock protein family in anti-tumor immunity. Eur J Immunol. 2005 Sep;35(9):2518–27. doi: 10.1002/eji.200535002. [DOI] [PubMed] [Google Scholar]
- 15.Srivastava P. Interaction of heat shock proteins with peptides and antigen presenting cells: chaperoning of the innate and adaptive immune responses. Annu Rev Immunol. 2002;20:395–425. doi: 10.1146/annurev.immunol.20.100301.064801. [DOI] [PubMed] [Google Scholar]
- 16.Murshid A, Gong J, Calderwood SK. Purification, preparation, and use of chaperone-Peptide complexes for tumor immunotherapy. Methods Mol Biol. 2013;960:209–17. doi: 10.1007/978-1-62703-218-6_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Singh-Jasuja H, Toes RE, Spee P, Munz C, Hilf N, Schoenberger SP, et al. Cross-presentation of glycoprotein 96-associated antigens on major histocompatibility complex class I molecules requires receptor-mediated endocytosis. J Exp Med. 2000 Jun 5;191(11):1965–74. doi: 10.1084/jem.191.11.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang XY, Kazim L, Repasky EA, Subjeck JR. Immunization with tumor-derived ER chaperone grp170 elicits tumor-specific CD8+ T-cell responses and reduces pulmonary metastatic disease. Int J Cancer. 2003 Jun 10;105(2):226–31. doi: 10.1002/ijc.11058. [DOI] [PubMed] [Google Scholar]
- 19.Wang XY, Kazim L, Repasky EA, Subjeck JR. Characterization of heat shock protein 110 and glucose-regulated protein 170 as cancer vaccines and the effect of fever-range hyperthermia on vaccine activity. J Immunol. 2001 Jan 1;166(1):490–7. doi: 10.4049/jimmunol.166.1.490. [DOI] [PubMed] [Google Scholar]
- 20.Enomoto Y, Bharti A, Khaleque AA, Song B, Liu C, Apostolopoulos V, et al. Enhanced immunogenicity of heat shock protein 70 peptide complexes from dendritic cell-tumor fusion cells. J Immunol. 2006 Nov 1;177(9):5946–55. doi: 10.4049/jimmunol.177.9.5946. [DOI] [PubMed] [Google Scholar]
- 21.Arnouk H, Zynda ER, Wang XY, Hylander BL, Manjili MH, Repasky EA, et al. Tumour secreted grp170 chaperones full-length protein substrates and induces an adaptive anti-tumour immune response in vivo. Int J Hyperthermia. 2010;26(4):366–75. doi: 10.3109/02656730903485910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Srivastava PK. Therapeutic cancer vaccines. Curr Opin Immunol. 2006 Apr;18(2):201–5. doi: 10.1016/j.coi.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 23.Wang XY, Sun X, Chen X, Facciponte J, Repasky EA, Kane J, et al. Superior antitumor response induced by large stress protein chaperoned protein antigen compared with peptide antigen. J Immunol. 2011 Jun 1;184(11):6309–19. doi: 10.4049/jimmunol.0903891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weng D, Calderwood SK, Gong J. Preparation of a heat shock protein-based vaccine from dendritic cells. Methods in Molecular Biology. 2011 doi: 10.1007/978-1-61779-295-3_19. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weng D, Song B, Durfee J, Sugiyama V, Wu Z, Koido S, et al. Induction of cytotoxic T lymphocytes against ovarian cancer-initiating cells. Int J Cancer. 2010 Dec 10;129:1990–2001. doi: 10.1002/ijc.25851. [DOI] [PubMed] [Google Scholar]
- 26.Gong J, Zhang Y, Durfee J, Weng D, Liu C, Koido S, et al. A heat shock protein 70-based vaccine with enhanced immunogenicity for clinical use. J Immunol. 2010 Jan 1;184(1):488–96. doi: 10.4049/jimmunol.0902255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kunisawa J, Shastri N. Hsp90alpha chaperones large C-terminally extended proteolytic intermediates in the MHC class I antigen processing pathway. Immunity. 2006 May;24(5):523–34. doi: 10.1016/j.immuni.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 28.Murshid A, Gong J, Calderwood SK. Heat shock protein 90 mediates efficient antigen cross presentation through the scavenger receptor expressed by endothelial cells-I. J Immunol. 2010 Sep 1;185(5):2903–17. doi: 10.4049/jimmunol.0903635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hall EJ. Radiobiology for the Radiologist. Philadelphia: Lippincott-Raven; 1993. [Google Scholar]
- 30.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001 Nov 1;414(6859):105–11. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 31.Visvader JE. Keeping abreast of the mammary epithelial hierarchy and breast tumorigenesis. Genes Dev. 2009 Nov 15;23(22):2563–77. doi: 10.1101/gad.1849509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Phillips TM, McBride WH, Pajonk F. The response of CD24(−/low)/CD44+ breast cancer-initiating cells to radiation. J Natl Cancer Inst. 2006 Dec 20;98(24):1777–85. doi: 10.1093/jnci/djj495. [DOI] [PubMed] [Google Scholar]
- 33.Schatton T, Schutte U, Frank NY, Zhan Q, Hoerning A, Robles SC, et al. Modulation of T-cell activation by malignant melanoma initiating cells. Cancer Res. 2010 Jan 15;70(2):697–708. doi: 10.1158/0008-5472.CAN-09-1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schatton T, Frank MH. Antitumor immunity and cancer stem cells. Ann N Y Acad Sci. 2009 Sep;1176:154–69. doi: 10.1111/j.1749-6632.2009.04568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Calderwood SK, Stevenson MA, Murshid A. Heat shock proteins, autoimmunity, and cancer treatment. Autoimmune Dis. 2012;2012:486069. doi: 10.1155/2012/486069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mantovani A. Cancer: Inflaming metastasis. Nature. 2009 Jan 1;457(7225):36–7. doi: 10.1038/457036b. [DOI] [PubMed] [Google Scholar]
- 37.Charafe-Jauffret E, Ginestier C, Birnbaum D. Breast cancer stem cells: tools and models to rely on. BMC Cancer. 2009;9:202. doi: 10.1186/1471-2407-9-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alvero AB, Chen R, Fu HH, Montagna M, Schwartz PE, Rutherford T, et al. Molecular phenotyping of human ovarian cancer stem cells unravels the mechanisms for repair and chemoresistance. Cell Cycle. 2009 Jan 1;8(1):158–66. doi: 10.4161/cc.8.1.7533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang S, Balch C, Chan MW, Lai HC, Matei D, Schilder JM, et al. Identification and characterization of ovarian cancer-initiating cells from primary human tumors. Cancer Res. 2008 Jun 1;68(11):4311–20. doi: 10.1158/0008-5472.CAN-08-0364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weng D, Penzner JH, Song B, Koido S, Calderwood SK, Gong J. Metastasis is an early event in mouse mammary carcinomas and is associated with cells bearing stem cell markers. Breast Cancer Res. 2012 Jan 25;14(1):R18. doi: 10.1186/bcr3102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ho JN, Kang GY, Lee SS, Kim J, Bae IH, Hwang SG, et al. Bcl-XL and STAT3 mediate malignant actions of gamma-irradiation in lung cancer cells. Cancer Sci. 2010 Jun;101(6):1417–23. doi: 10.1111/j.1349-7006.2010.01552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lagadec C, Vlashi E, Della Donna L, Dekmezian C, Pajonk F. Radiation-induced reprogramming of breast cancer cells. Stem Cells. 2012 May;30(5):833–44. doi: 10.1002/stem.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Welstead GG, Brambrink T, Jaenisch R. Generating iPS cells from MEFS through forced expression of Sox-2, Oct-4, c-Myc, and Klf4. J Vis Exp. 2008;(14) doi: 10.3791/734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kottke T, Sanchez-Perez L, Diaz RM, Thompson J, Chong H, Harrington K, et al. Induction of hsp70-mediated Th17 autoimmunity can be exploited as immunotherapy for metastatic prostate cancer. Cancer Res. 2007 Dec 15;67(24):11970–9. doi: 10.1158/0008-5472.CAN-07-2259. [DOI] [PubMed] [Google Scholar]
- 45.McBride WH, Chiang CS, Olson JL, Wang CC, Hong JH, Pajonk F, et al. A sense of danger from radiation. Radiat Res. 2004 Jul;162(1):1–19. doi: 10.1667/rr3196. [DOI] [PubMed] [Google Scholar]
- 46.Vissers JH, van Lohuizen M, Citterio E. The emerging role of Polycomb repressors in the response to DNA damage. J Cell Sci. 2012 Sep 1;125(Pt 17):3939–48. doi: 10.1242/jcs.107375. [DOI] [PubMed] [Google Scholar]
- 47.Vlashi E, McBride WH, Pajonk F. Radiation responses of cancer stem cells. J Cell Biochem. 2009 Oct 1;108(2):339–42. doi: 10.1002/jcb.22275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Calderwood SK. Hyperthermia the tumor environment and anti-tumor immunity: Hyperthermia the tumor environment and anti-tumor immunity. The Tumor Microenvironment. 2012 in press. [Google Scholar]
- 49.Mambula SS, Calderwood SK. Heat induced release of Hsp70 from prostate carcinoma cells involves both active secretion and passive release from necrotic cells. Int J Hyperthermia. 2006 Nov;22(7):575–85. doi: 10.1080/02656730600976042. [DOI] [PubMed] [Google Scholar]
- 50.Srivastava P. Hypothesis: controlled necrosis as a tool for immunotherapy of human cancer. Cancer Immun. 2003;3:4. [PubMed] [Google Scholar]
- 51.Segal BH, Wang XY, Dennis CG, Youn R, Repasky EA, Manjili MH, et al. Heat shock proteins as vaccine adjuvants in infections and cancer. Drug Discov Today. 2006 Jun;11(11–12):534–40. doi: 10.1016/j.drudis.2006.04.016. [DOI] [PubMed] [Google Scholar]
- 52.Rosenberg SA, Yang JC, Restifo NP. Cancer Immunotherapy: moving beyond current vaccines. Naure Medicine. 2004;10:909–15. doi: 10.1038/nm1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rosenberg SA. Shedding light on tumor immunotherapy of cancer. NEJM. 2004;350:1461–3. doi: 10.1056/NEJMcibr045001. [DOI] [PMC free article] [PubMed] [Google Scholar]