Abstract
AIM: To compare the value of endoscopic retrograde cholangiography (ERC) and standard T2-weighted magnetic resonance cholangiography (MRC) in the evaluation process as adult-to-adult right lobe living donor liver transplantation (LDLTx) demands a successful outcome, and exact knowledge of the biliary tree is implicated to avoid biliary complications, postoperatively.
METHODS: After starting the LDLTx program, 18 liver transplant candidates were selected for LDLTx by a stepwise evaluation process. ERC and standard T2-weighted MRC were performed to evaluate the biliary system of the donor liver. The anatomical findings of ERC and MRC mapping were compared using the Ohkubo classification.
RESULTS: ERC allowed mapping of the whole biliary system in 15/15 (100%) cases, including 14/15 (93.3%) with biliary variants while routine MRC was only accurate in 2/13 (15.4%) cases. MRC was limited in depicting the biliary system proximal of the hepatic bifurcation. Postoperative biliary complications occurred in 2 donors and 8 recipients. Biliary complications were associated with Ohkubo type C, E or G in 6/8 recipients, and 2/3 recipients with biliary leak received a graft with multiple (≥2) bile ducts.
CONCLUSION: Pretransplant ERC is safe and superior over standard MRC for detection of biliary variations that occur with a high frequency. However, precise knowledge of biliary variants did not reduce the incidence of postoperative biliary complications.
Keywords: Living donor liver transplantation, Donors biliary tree, Endoscopic retrograde cholangiography, Magnetic resonance cholangiography
INTRODUCTION
Over the past decade, a critical shortage of cadaveric organs for adults in need of liver transplants has developed. The current mortality for patients awaiting liver transplantation (LTx) ranges from 20% to 30%. During this time, the waiting period for LTx and the mortality among patients on waiting lists have increased by a factor of more than 10 while the donor pool has expanded only marginally[1]. The use of adult-to-adult right lobe living donor liver transplantation (LDLTx) provides an alternative technique to reduce the waiting list mortality[2-4]. Hereby, a transplant candidate gains survival time and quality of life while a proven healthy individual undergoes liver resection for living donation of right or left lobe. The greatest risk in LDLTx is the death of the donor which is estimated to range between 0.1% to 0.5%[5-7]. Furthermore, a potentially uneventful outcome of the recipient is a psychological threat for the donor. Thus, healthy donors might undergo an extended invasive diagnostic during evaluation. Also, complications are relatively common in living liver donors[8]. Therefore, donor safety has the highest priority when LDLTx is performed[9], and selection criteria and management of living donors requires continuous refinement[10]. Finally, potential advantages in recipients must be faced against any potential risk of morbidity and mortality in the living donor.
Bile duct leaks and stenosis impact morbidity and mortality in the early and late phase after LTx[11]. After LDLTx, only few studies focused on anatomical variations of the biliary tree, which is the rule rather than the exception in liver surgery[12]. The reported incidences of absent hepatic duct are 26% for the right and 2% for the left side[12]. In LDLTx, anatomic variations of intrahepatic bile ducts can complicate both, the donor and the recipient operation[13]. Full hepatic lobectomy is required for adult-to-adult LDLTx, and the postoperative risk is greater after right lobe resection[14]. Preoperative delineation of the biliary system appears important to achieve a successful outcome[13].
Management of bile duct variations in LDLTx is technically demanding and lacking awareness of biliary variation increases the risk of postoperative complications[15,16]. The biliary anatomy is mainly evaluated by endoscopic retrograde cholangiography (ERC), magnetic resonance cholangiography (MRC) or intraoperative cholangiography. The last might cause technical complications or results in oversight of biliary anomalies with consecutive biliary complications[17]. Hitherto, about half of the LDLTx programs perform ERC for the evaluation of donor bile-duct anatomy, and about one third of the programs use MRC[8]. Therefore, we compared the value of ERC and standard MRC in living donors prior to LTx.
MATERIALS AND METHODS
Subjects
Between March 1st, 2000 and October 5, 2005, 18 liver transplant candidates were selected for LDLTx by a stepwise evaluation process including identification of potential candidacy, assessment of clinical status and risk factors, assessment of liver function and anatomy, and final measures[18].
Mean age of 18 donors (11 female, 7 male) was 50.4 ± 9.7 years, and 47 ± 9.2 years in 18 recipients (12 male, 6 female). Seven donors had genetic and 11 emotional relation to their recipient. Indications for LTx were hepatitis B or hepatitis C related cirrhosis (n = 6), postalcoholic cirrhosis (n = 4), hepatic metastasis of neuroendocrine tumor (n = 2), primary biliary cirrhosis (n = 1), primary sclerosing cholangitis (n = 1), cryptogenic cirrhosis (n = 1), bile duct cysts (n = 1), hepatic metastasis of gastrointestinal stroma tumor (n = 1), and echinococcus multilocularis hydatits with postalcoholic cirrhosis (n = 1). Patients with cirrhosis were staged Child A (n = 3), B (n = 6), and C (n = 5) according to the Child-Pugh-Turcotte classification.
Evaluation of biliary anatomy
Biliary anatomy was evaluated by standard MRC and ERC. Standard MRC technique was provided by a radiologist in daily routine with Half-Fourier Spin-Echo in single shot technique and by Turbo-Spin-Echo with multiplanar reconstruction in T2-weighted images. Standard ERC was performed by one experienced hepato-gastroenterologist. Patients received midazolam, propofol, and butylscopolaminiumbromide immediately before ERC. Serum amylase and lipase were controlled before and after ERC. Additionally, biliary anatomy was assessed by graft preparation and intraoperative cholangiography.
The biliary anatomy of MRC and ERC films were classified retrospectively according to Ohkubo[12] by the specialists in radiology, hepato-gastroenterology, and transplantation surgery at our institution (Table 1).
Table 1.
Preoperative MRC, ERC, Ohkubo classification[12] and intraoperative findings in donors, biliary reconstruction of the graft and postoperative biliary complications of recipients
| Donor | Recipient | |||||||
|
No. |
MRC |
ERC |
Ohkubo
classifi-
cation |
Intraoperative
findings |
Biliary
complications |
Biliary
reconstruction |
Stents |
Biliary
complication |
| 1 | NA | NA | NA | 3 BD | None | HJS | 2 THS | Leak due to dislocated stent |
| 2 | Suspicion of early branches to SV and VI from RHD | SIV drains into RHD, branch of SV drains into LHD | Type K | 3 BD | None | 2 D/D | T-drain | None |
| 3 | SV drains into LHD | SV and VI drain separately into LHD | Type C | 2 BD | None | 2 D/D | 2 THS | None |
| 4 | Suspicion of doubled RHD | 2 separate RHD | Type E | 2 BD | Leak at cutting surface | 2 D/D | 2 THS | None |
| 5 | No variation | SVI drains separately into CHD | Type G | 2 BD | None | 2 D/D | 2 THS | Leak at cutting surface |
| 6 | Early division of CHD | Early division of CHD | Type A | 2 BD | None | 2 D/D | T-drain | None |
| 7 | NA | NA | NA | 1 BD | None | 1 D/D | T-drain | None |
| 8 | No variation | SVII drains into LHD, SIVa drains into RHD | Type E Type K | 1 BD | None | 1 D/D | T-drain | None |
| 9 | No variation | Early division of RHD, SV drains directly into CHD | Type C | 1 BD | BD stenosis | HJS | 1 THS | SVI necrosis, intrahepatic bilioma |
| 10 | SVI drains directly into CHD | SVI and VII drains directly into CHD | Type E | 2 BD | None | 2 D/D | T-drain | BD stenosis 2 years after LDLTX |
| 11 | Doubled RHD | 2 separate RHD | Type E | 2 BD | None | 2 D/D | T-drain | BD necrosis |
| 12 | NA | NA | NA | 1 BD | None | 1 D/D | 1 THS | Leak at cutting surface |
| 13 | Not evaluable because of artifacts | No variation | Type A | 1 BD | None | 1 D/D | 1 THS | None |
| 14 | Suspicion of early division of RHD | Trifurcation of RHD to SV, VI, and VIII | Type B | 3 BD | None | 3 D/D | 1 THS | None |
| 15 | Suspicion of early division of RHD | Trifurcation of RHD to SV, VI, and VIII | Type B | 3 BD | None | 3 D/D | None | None |
| 16 | No variation | 2 separate RHD | Type E | 2 BD | None | HJS | None | BD stenosis 3 mo after LDLTX |
| 17 | NA | Early division of RHD | Type C | 1 BD | None | HJS | 1 THS | BD stenosis 6 mo after LDLTX |
| 18 | NA | Early division of RHD | Type C | 1 BD | None | 1 D/D | T-drain | None |
NA: Not available; BD: Bile duct; HJS: Hepaticojejunostomy; THS: Transhepatic stent; S: Segment; RHD: Right hepatic duct; LHD: Left hepatic duct; D/D: Duct-to-duct; CHD: Common hepatic duct; LDLTX: Living donor liver transplantation.
RESULTS
Evaluation of donor biliary anatomy
ERC did not cause procedure-related complications. ERC (MRC) images were completely available in 15 (13) donors for retrospective analysis (Table 1). ERC allowed mapping of the whole biliary system in 15/15 cases (100%), while routine MRC was only accurate in 2/13 cases (15.4%). MRC was limited to depict the biliary anatomy only up to the first hepatic branches (Figure 1, Figure 2, Figure 3). Interestingly, 14/15 (93.3%) donors had biliary variants detected by ERC. Biliary anatomical variants classified according to Ohkubo et al[12] are depicted in Table 1. Donor livers were classified type A (n = 2), B (n = 2), C (n = 4), E (n = 5), G (n = 1), and K (n = 1). Right hepatic duct (RHD) was present in 2 (13.3%) and absent in 12 (80.0%) donor livers. Left hepatic duct (LHD) was absent in one donor liver.
Figure 1.
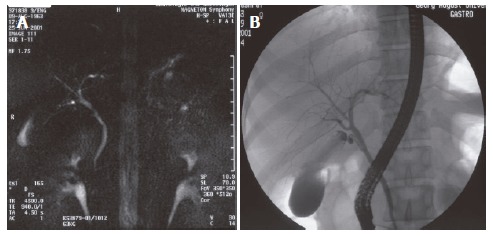
Preoperative MRC and ERC of donor No.11 with RHD. A: MRC, doubled; B: ERC, two separate.
Figure 2.
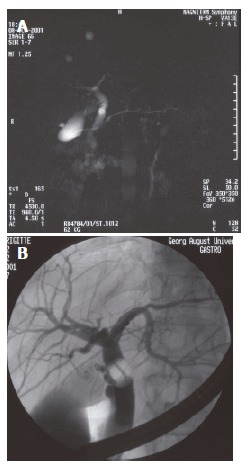
Preoperative MRC and ERC of donor No.14 with RHD. A: MRC, suspicion of early division; B: ERC, trifurcation of RHD to SV, VI, and VIII.
Figure 3.
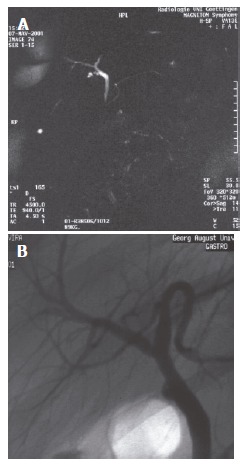
Preoperative MRC and ERC of donor No.8. A: MRC, no variation; B: ERC, SVII drains into LHD, SIVa drains into RHD.
The biliary anatomy determined by preoperative ERC was identical to the intraoperative findings (Table 1). Seven of 18 (38.9%) grafts had one, 7/18 (38.9%) two, and 4/18 (22.2%) three bile ducts. In total, 11/18 (61.1%) right lobe grafts presented with two or more bile ducts.
Biliary complications
Postoperatively, 2/18 donors developed biliary complications (11.1%) including one biliary leak (Ohkubo type E, 2 bile ducts) and one bile duct stenosis (Ohkubo type C, one bile duct). Both complications occurred in livers with absent RHD. The biliary leak was located at the cutting surface and resolved without intervention, but the T-drain was kept for a longer period. Bile duct stenosis was successfully treated by endoscopic stenting. Eight of 18 (44.4%) recipients developed biliary complications (Table 1) including 3 biliary leakages, one bilioma, one bile duct necrosis, and 3 bile duct stenoses. Biliary leakages were associated with Ohkubo type G (n = 1) or not classified (n = 2). One leakage was related to stent dislocation. This graft had 3 bile ducts reconstructed by hepaticojejunostomy (HJS) with insertion of transhepatic stents. Two leakages were located at the cutting surface. A segment 6 necrosis occurred in one recipient who received an Ohkubo type C graft with one bile duct that was reconstructed by HJS and placement of a transhepatic stent. This patient also developed intrahepatic bilioma. Segmental necrosis might be related to transsection of the arterial supply. One recipient developed bile duct necrosis located at the T-tube insertion side. Bile duct stenosis occurred in 3 recipients. One of these patients had received an Ohkubo type E graft including two bile ducts that were reconstructed duct-to-duct with insertion of a T-drain. Bile duct stenosis developed two years after transplantation and was successfully treated by endoscopic stenting. A second recipient also received an Ohkubo type E graft with two bile ducts reconstructed by hepaticojejunostomy. Bile duct stenosis occurred 3 mo after transplantation and was successfully treated by placement of a Yamakawa prosthesis. A third patient with an Ohkubo type C graft and one bile duct developed bile duct stenosis 6 mo after transplantation that was also successfully treated by a Yamakawa prosthesis.
In summary, preoperative ERC detected biliary variants in 93.3% of donors. MRC confirmed ERC findings in only 15.4%, and was restricted in depicting the biliary anatomy above the hepatic bifurcation. Biliary complications occurred in 2 donors and 8 recipients. Ohkubo type C, E or G grafts were associated with biliary complications in 6/8 (75%) recipients. Two of 3 recipients with biliary leakage received a graft with multiple (≥ 2) bile ducts.
DISCUSSION
Determination of biliary anatomy in LDLTx can be performed preoperatively by ERC, MRC, contrast-enhanced CT-cholangiography, and intraoperative cholangiography. MRC is less invasive than ERC that can cause pancreatitis as a known complication. In a meta-analysis of 15 prospective trials using endoscopic retrograde cholangio-pancreaticography (ERCP), 14 risk factors of ERCP-associated pancreatitis have been identified involving nine related to the endoscopic technique (e.g. precut sphincterotomy) with a relative risk of 2.2 to 4.09[19]. In our series, we used ERC only diagnostically without intervention or pancreatic injection. ERC was well tolerated and none of the patients developed pancreatitis or other procedure related complications. In contrast to standard routine MRC, ERC allowed complete mapping of the biliary system. Preoperative ERC detected variations of biliary anatomy in 93.3% of donors that were confirmed by our intraoperative findings.
The inaccuracy of standard routine MRC in our series might be explained by the imaging slice thickness and artifacts. Recently, new modified MRC techniques such as volumetric mangafodipir trisodium enhanced MRC, heavily T2-weighted thin section MRCP, and gadobenate dimeglumine enhanced MRC have been developed[20-24]. Sensitivity of heavily T2-weighted axial/coronal thin section MRCP for normal biliary anatomy was 89.5% and 71.4% for variants in LDLTx. The accuracy in depicting biliary anatomy was 84.6%[22]. In another series, the sensitivity, specificity, positive predictive, and negative predictive value of preoperative heavily T2-weighted radial slab MRC was 92%, 100%, 100%, and 94%[24]. Mangafodipir trisodium enhanced 3D MRC in combination with conventional T2-weighted MRC depicted the intrahepatic biliary anatomy correctly in 94% of living liver donors. Especially right duct variants were more accurately depicted with mangafodipir trisodium enhanced 3D MRC compared to conventional T2-weighted MRC[21]. Another new technique of mapping the biliary anatomy is multidetector computed topography cholangiography (MDCT-CA) with 1 mm collimation[16]. This method depicted anatomical biliary variations up to the fourth level of intrahepatic branches. Superiority of MDCT-CA over conventional MRC is explained by better spatial resolution using 1 mm instead of 4 mm slice thickness. Also, conventional MRC is susceptible for artifacts in T2-weighted images. Therefore, the use T1-weighted images is recommended in combination with Gd-EOB-DTPA or Gd-BOPTA, both biliary excreted contrast-agents[25,26]. Also, Gd-BOPTA-MRC can be combined with CE-MRA without injection of a second contrast agent[16,26]. Gd-BOPTA-enhanced MRC is superior over T2-weighted MRC sequences in mapping the biliary tree, but spatial resolution remains moderate[25,26]. In principal, both imaging modalities can be performed as an all-in-one-protocol for evaluation of liver parenchyma, arterial, venous, and biliary anatomy. However, MDCT-CA harbors potential risks like X-ray exposure and allergic reaction to contrast medium. Both risk factors can be avoided with Gd-BOPTA T1-weighted MRC[26]. However, the role of the above-mentioned procedures needs to be defined for evaluation of biliary anatomy in living liver donors in the near future. At present these new techniques have not been compared to preoperative ERC yet.
In our series, biliary complications occurred in 2/18 (11.1%) donors. In larger series, incidence of biliary complications ranged from 4% to 13%[27-31]. Incidence of biliary leaks at the cutting surface is approximately 5% in living donors[32-36] and was 5.6% (1/18 donors) in our series. Notable, donors with absent RHD (Ohkubo type C and G) grafts are at risk to develop biliary leakage or obstruction[12]. Both biliary complications in our donors occurred in livers with absent RHD (Ohkubo type C and E). In recipients, biliary complications are the Achilles’ heel of all segmental LTx[9]. Incidence of biliary complications in LDLTx recipients is 15%-40%[8,9,15]. In our series, 8/16 (44.4%) recipients had complications despite exact preoperative determination of biliary variants by ERC. Nevertheless, anatomic variations of the biliary tract are common but may not contraindicate donation[13,37]. The risks of biliary complications increase with the number of bile duct openings in the graft[38]. Rogiers et al[9] focused on two complex biliary variations: (1) Ramus posteriomedialis (RPM) drains into the common hepatic duct (CHD), ramus lateralis (RL) merges with segment (S) II, and drain together into the left hepatic duct (LHD), and (2) RPM drains into CHD; SI, SII, SIII, and SIV drain separately or together into RL. These two biliary variations cease splitting of the liver in selected cases. Recently, a case was published in which an anatomic biliary variation was seen as a contraindication for right lobe LDLTx[38].
In conclusion, biliary anatomy in living liver donors is highly variable, but does not exclude from donation. ERC is superior to the standard MRC technique for detailed preoperative mapping of intra- and extrahepatic bile ducts, but does not reduce the incidence of postoperative biliary complications.
Footnotes
S- Editor Pan BR L- Editor Ma JY E- Editor Liu WF
References
- 1.Everhart JE, Lombardero M, Detre KM, Zetterman RK, Wiesner RH, Lake JR, Hoofnagle JH. Increased waiting time for liver transplantation results in higher mortality. Transplantation. 1997;64:1300–1306. doi: 10.1097/00007890-199711150-00012. [DOI] [PubMed] [Google Scholar]
- 2.Marcos A, Fisher RA, Ham JM, Shiffman ML, Sanyal AJ, Luketic VA, Sterling RK, Posner MP. Right lobe living donor liver transplantation. Transplantation. 1999;68:798–803. doi: 10.1097/00007890-199909270-00012. [DOI] [PubMed] [Google Scholar]
- 3.Yamaoka Y, Washida M, Honda K, Tanaka K, Mori K, Shimahara Y, Okamoto S, Ueda M, Hayashi M, Tanaka A. Liver transplantation using a right lobe graft from a living related donor. Transplantation. 1994;57:1127–1130. [PubMed] [Google Scholar]
- 4.Wachs ME, Bak TE, Karrer FM, Everson GT, Shrestha R, Trouillot TE, Mandell MS, Steinberg TG, Kam I. Adult living donor liver transplantation using a right hepatic lobe. Transplantation. 1998;66:1313–1316. doi: 10.1097/00007890-199811270-00008. [DOI] [PubMed] [Google Scholar]
- 5.Lo CM. Complications and long-term outcome of living liver donors: a survey of 1,508 cases in five Asian centers. Transplantation. 2003;75:S12–S15. doi: 10.1097/01.TP.0000046534.45645.47. [DOI] [PubMed] [Google Scholar]
- 6.Schiano TD, Kim-Schluger L, Gondolesi G, Miller CM. Adult living donor liver transplantation: the hepatologist's perspective. Hepatology. 2001;33:3–9. doi: 10.1053/jhep.2001.21489. [DOI] [PubMed] [Google Scholar]
- 7.Trotter JF, Wachs M, Everson GT, Kam I. Adult-to-adult transplantation of the right hepatic lobe from a living donor. N Engl J Med. 2002;346:1074–1082. doi: 10.1056/NEJMra011629. [DOI] [PubMed] [Google Scholar]
- 8.Brown RS Jr, Russo MW, Lai M, Shiffman ML, Richardson MC, Everhart JE, Hoofnagle JH. A survey of liver transplantation from living adult donors in the United States. N Engl J Med. 2003;348:818–825. doi: 10.1056/NEJMsa021345. [DOI] [PubMed] [Google Scholar]
- 9.Broering DC, Sterneck M, Rogiers X. Living donor liver transplantation. J Hepatol. 2003;38 Suppl 1:S119–S135. doi: 10.1016/s0168-8278(03)00009-6. [DOI] [PubMed] [Google Scholar]
- 10.Sugawara Y, Makuuchi M. Advances in adult living donor liver transplantation: a review based on reports from the 10th anniversary of the adult-to-adult living donor liver transplantation meeting in Tokyo. Liver Transpl. 2004;10:715–720. doi: 10.1002/lt.20179. [DOI] [PubMed] [Google Scholar]
- 11.Testa G, Malagò M, Broelseh CE. Complications of biliary tract in liver transplantation. World J Surg. 2001;25:1296–1299. doi: 10.1007/s00268-001-0113-5. [DOI] [PubMed] [Google Scholar]
- 12.Ohkubo M, Nagino M, Kamiya J, Yuasa N, Oda K, Arai T, Nishio H, Nimura Y. Surgical anatomy of the bile ducts at the hepatic hilum as applied to living donor liver transplantation. Ann Surg. 2004;239:82–86. doi: 10.1097/01.sla.0000102934.93029.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheng YF, Huang TL, Chen CL, Chen YS, Lee TY. Variations of the intrahepatic bile ducts: application in living related liver transplantation and splitting liver transplantation. Clin Transplant. 1997;11:337–340. [PubMed] [Google Scholar]
- 14.Marcos A, Fisher RA, Ham JM, Olzinski AT, Shiffman ML, Sanyal AJ, Luketic VA, Sterling RK, Olbrisch ME, Posner MP. Selection and outcome of living donors for adult to adult right lobe transplantation. Transplantation. 2000;69:2410–2415. doi: 10.1097/00007890-200006150-00034. [DOI] [PubMed] [Google Scholar]
- 15.Chen CL, Fan ST, Lee SG, Makuuchi M, Tanaka K. Living-donor liver transplantation: 12 years of experience in Asia. Transplantation. 2003;75:S6–11. doi: 10.1097/01.TP.0000046533.93621.C7. [DOI] [PubMed] [Google Scholar]
- 16.Schroeder T, Malagó M, Debatin JF, Testa G, Nadalin S, Broelsch CE, Ruehm SG. Multidetector computed tomographic cholangiography in the evaluation of potential living liver donors. Transplantation. 2002;73:1972–1973. doi: 10.1097/00007890-200206270-00026. [DOI] [PubMed] [Google Scholar]
- 17.Nery JR, Fragulidis GP, Scagnelli T, Weppler D, Webb MG, Khan MF, Tzakis AG. Donor biliary variations: an overlooked problem. Clin Transplant. 1997;11:582–587. [PubMed] [Google Scholar]
- 18.Wietzke-Braun P, Braun F, Lorf T, Ringe B, Ramadori G. Evaluation zur Lebendspende Lebertransplantation. Z Gastroenterol. 2003;XLI:117. [Google Scholar]
- 19.Masci E, Mariani A, Curioni S, Testoni PA. Risk factors for pancreatitis following endoscopic retrograde cholangiopancreatography: a meta-analysis. Endoscopy. 2003;35:830–834. doi: 10.1055/s-2003-42614. [DOI] [PubMed] [Google Scholar]
- 20.Ayuso JR, Ayuso C, Bombuy E, De Juan C, Llovet JM, De Caralt TM, Sánchez M, Pagés M, Bruix J, García-Valdecasas JC. Preoperative evaluation of biliary anatomy in adult live liver donors with volumetric mangafodipir trisodium enhanced magnetic resonance cholangiography. Liver Transpl. 2004;10:1391–1397. doi: 10.1002/lt.20281. [DOI] [PubMed] [Google Scholar]
- 21.Lee VS, Krinsky GA, Nazzaro CA, Chang JS, Babb JS, Lin JC, Morgan GR, Teperman LW. Defining intrahepatic biliary anatomy in living liver transplant donor candidates at mangafodipir trisodium-enhanced MR cholangiography versus conventional T2-weighted MR cholangiography. Radiology. 2004;233:659–666. doi: 10.1148/radiol.2333031977. [DOI] [PubMed] [Google Scholar]
- 22.Limanond P, Raman SS, Ghobrial RM, Busuttil RW, Lu DS. The utility of MRCP in preoperative mapping of biliary anatomy in adult-to-adult living related liver transplant donors. J Magn Reson Imaging. 2004;19:209–215. doi: 10.1002/jmri.10446. [DOI] [PubMed] [Google Scholar]
- 23.Lim JS, Kim MJ, Kim JH, Kim SI, Choi JS, Park MS, Oh YT, Yoo HS, Lee JT, Kim KW. Preoperative MRI of potential living-donor-related liver transplantation using a single dose of gadobenate dimeglumine. AJR Am J Roentgenol. 2005;185:424–431. doi: 10.2214/ajr.185.2.01850424. [DOI] [PubMed] [Google Scholar]
- 24.Kim RD, Sakamoto S, Haider MA, Molinari M, Gallinger S, McGilvray ID, Greig PD, Grant DR, Cattral MS. Role of magnetic resonance cholangiography in assessing biliary anatomy in right lobe living donors. Transplantation. 2005;79:1417–1421. doi: 10.1097/01.tp.0000159793.02863.d2. [DOI] [PubMed] [Google Scholar]
- 25.Bollow M, Taupitz M, Hamm B, Staks T, Wolf KJ, Weinmann HJ. Gadolinium-ethoxybenzyl-DTPA as a hepatobiliary contrast agent for use in MR cholangiography: results of an in vivo phase-I clinical evaluation. Eur Radiol. 1997;7:126–132. doi: 10.1007/s003300050125. [DOI] [PubMed] [Google Scholar]
- 26.Goyen M, Barkhausen J, Debatin JF, Kühl H, Bosk S, Testa G, Malago M, Ruehm SG. Right-lobe living related liver transplantation: evaluation of a comprehensive magnetic resonance imaging protocol for assessing potential donors. Liver Transpl. 2002;8:241–250. doi: 10.1053/jlts.2002.30403. [DOI] [PubMed] [Google Scholar]
- 27.Brown RJ, Richardson M, Lai M, Everhart J, Russo MW, Hoofnagle JH. Adult living donor liver transplantation (LDLT) in the US: results of a survey from the NIH LDLT Meeting. Hepatology. 2001;34:235A. [Google Scholar]
- 28.Fujita S, Kim ID, Uryuhara K, Asonuma K, Egawa H, Kiuchi T, Hayashi M, Uemeto S, Inomata Y, Tanaka K. Hepatic grafts from live donors: donor morbidity for 470 cases of live donation. Transpl Int. 2000;13:333–339. doi: 10.1007/s001470050710. [DOI] [PubMed] [Google Scholar]
- 29.Ito T, Kiuchi T, Egawa H, Kaihara S, Oike F, Ogura Y, Fujimoto Y, Ogawa K, Tanaka K. Surgery-related morbidity in living donors of right-lobe liver graft: lessons from the first 200 cases. Transplantation. 2003;76:158–163. doi: 10.1097/01.TP.0000072372.42396.47. [DOI] [PubMed] [Google Scholar]
- 30.Miller CM, Gondolesi GE, Florman S, Matsumoto C, Muñoz L, Yoshizumi T, Artis T, Fishbein TM, Sheiner PA, Kim-Schluger L, et al. One hundred nine living donor liver transplants in adults and children: a single-center experience. Ann Surg. 2001;234:301–311; discussion 311-312. doi: 10.1097/00000658-200109000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yamaoka Y, Morimoto T, Inamoto T, Tanaka A, Honda K, Ikai I, Tanaka K, Ichimiya M, Ueda M, Shimahara Y. Safety of the donor in living-related liver transplantation--an analysis of 100 parental donors. Transplantation. 1995;59:224–226. [PubMed] [Google Scholar]
- 32.Bak T, Wachs M, Trotter J, Everson G, Trouillot T, Kugelmas M, Steinberg T, Kam I. Adult-to-adult living donor liver transplantation using right-lobe grafts: results and lessons learned from a single-center experience. Liver Transpl. 2001;7:680–686. doi: 10.1053/jlts.2001.26509. [DOI] [PubMed] [Google Scholar]
- 33.Broelsch CE, Malagó M, Testa G, Valentin Gamazo C. Living donor liver transplantation in adults: outcome in Europe. Liver Transpl. 2000;6:S64–S65. doi: 10.1053/jlts.2000.19015. [DOI] [PubMed] [Google Scholar]
- 34.Marcos A. Right lobe living donor liver transplantation: a review. Liver Transpl. 2000;6:3–20. doi: 10.1002/lt.500060117. [DOI] [PubMed] [Google Scholar]
- 35.Testa G, Malagó M, Valentín-Gamazo C, Lindell G, Broelsch CE. Biliary anastomosis in living related liver transplantation using the right liver lobe: techniques and complications. Liver Transpl. 2000;6:710–714. doi: 10.1053/jlts.2000.18706. [DOI] [PubMed] [Google Scholar]
- 36.Todo S, Furukawa H, Jin MB, Shimamura T. Living donor liver transplantation in adults: outcome in Japan. Liver Transpl. 2000;6:S66–S72. doi: 10.1053/jlts.2000.19009. [DOI] [PubMed] [Google Scholar]
- 37.Varotti G, Gondolesi GE, Goldman J, Wayne M, Florman SS, Schwartz ME, Miller CM, Sukru E. Anatomic variations in right liver living donors. J Am Coll Surg. 2004;198:577–582. doi: 10.1016/j.jamcollsurg.2003.11.014. [DOI] [PubMed] [Google Scholar]
- 38.Liu CL, Lo CM, Chan SC, Tso WK, Fan ST. The right may not be always right: biliary anatomy contraindicates right lobe live donor liver transplantation. Liver Transpl. 2004;10:811–812. doi: 10.1002/lt.20193. [DOI] [PubMed] [Google Scholar]


