Abstract
AIM: To study the appearances of acute interstitial edematous pancreatitis (IEP) on non-enhanced MR imaging.
METHODS: A total of 53 patients with IEP diagnosed by clinical features and laboratory findings were underwent MR imaging. MR imaging sequences included fast spoiled gradient echo (FSPGR) fat saturation axial T1-weighted imaging, gradient echo T1-weighted (in phase), single shot fast spin echo (SSFSE) T2-weighted, respiratory triggered (R-T) T2-weighted with fat saturation, and MR cholangiopancreatography. Using the MR severity score index, pancreatitis was graded as mild (0-2 points), moderate (3-6 points) and severe (7-10 points).
RESULTS: Among the 53 patients, IEP was graded as mild in 37 patients and as moderate in 16 patients. Forty-seven of 53 (89%) patients had at least one abnormality on MR images. Pancreas was hypointense relative to liver on FSPGR T1-weighted images in 18.9% of patients, and hyperintense in 25% and 30% on SSFSE T2-weighted and R-T T2-weighted images, respectively. The prevalences of the findings of IEP on R-T T2-weighted images were, respectively, 85% for pancreatic fascial plane, 77% for left renal fascial plane, 55% for peripancreatic fat stranding, 42% for right renal fascial plane, 45% for perivascular fluid, 40% for thickened pancreatic lobular septum and 25% for peripancreatic fluid, which were markedly higher than those on in-phase or SSFSE T2-weighted images (P < 0.001).
CONCLUSION: IEP primarily manifests on non-enhanced MR images as thickened pancreatic fascial plane, left renal fascial plane, peripancreatic fat stranding, and peripancreatic fluid. R-T T2-weighted imaging is more sensitive than in-phase and SSFSE T2-weighted imaging for depicting IEP.
Keywords: Pancreas, Pancreatitis, Inflammation, Edema, Magnetic resonance
INTRODUCTION
Acute pancreatitis (AP) is a protean disease of wide clinical variation ranging from mild discomfort to severe multiorgan failure and death. Acute interstitial edematous pancreatitis (IEP), found in approximately 75% of patients presenting with AP, consists of edema and inflammation of the pancreas and is typically a self-limiting process with a mild clinical course[1,2]. Necrotizing pancreatitis (NP) is a far more severe form of pancreatitis, which is characterized by extensive fat necrosis, hemorrhage, and necrotic liquefaction of the pancreas. Dynamic CT is well established for differentiating IEP from NP[3]. However, CT is not necessary in depicting IEP or mild acute pancreatitis[2,4,5]. Patients with AP are often young and require multiple follow-up CT examinations; substitution of MRI for CT in some patients would reduce their collective radiation dose considerably. In addition, experimental study[6] and clinical retrospective analysis[7] of pancreatitis suggested that iodinated contrast medium might worsen or prolong attacks of acute pancreatitis.
Recent advances in abdominal MRI improve the spatial resolution of images and allow optimal imaging of the pancreas. MRI can have an important role in staging the severity of acute pancreatitis and may be superior to other imaging techniques for the characterization of peripancreatic collections[8-10]. Amano et al[11] reported 12 patients with mild AP who had CT and MRI examinations, and found non-enhanced MR imaging was superior to CT for depiction and confirmation of mild AP. However, there was a variety of intermediary forms from mild to severe pancreatitis which occurred in clinical practice[3]. We hypothesized that IEP may include mild AP and some intermediary forms based on clinical classification of AP, and that non-enhanced MR imaging could depict these abnormalities of IEP.
MATERIALS AND METHODS
Patients
All patients in this study were diagnosed as IEP based on clinical features and laboratory findings in our institute from January 2002 to March 2005. The recruitment criteria of patients were: (1) acute history; (2) pancreatitis at first onset; (3) elevated amylase or lipase, excluding other abnormalities with an elevated enzymes; (4) MRI examination; (5) maximum three-days interval between the MRI examination and the IEP onset; (6) minimal organ dysfunction or an uneventful recovery; and (7) no clinical or imaging appearance of pancreatic necrosis, pseudocyst, or abscess.
In this retrospective study, 53 patients (26 men and 27 women; average age: 47 ± 16 years, range: 10-73 years) with IEP were enrolled according to the aforementioned criteria. Medical records of the 53 patients were reviewed to investigate the recovery and the length of hospital stay. Review of images and medical records was conducted according to our Institutional Review Board’s guidelines, including approval for our department to conduct these reviews.
MR Imaging
All examinations were conducted on a 1.5-T MR scanner with 38 mT/M gradients and 120 mT/M per second slope (Signa Excite; GE Medical Systems, Milwaukee, Wis), using phased-array torso-pelvis coil. The sequences included axial fast spoiled gradient echo (FSPGR) T1-weighted imaging with fat suppression, gradient-echo (GRE) T1-weighted in-phase and out-of-phase MR imaging, respiratory-triggered (R-T) axial fast recovery fast spin-echo (FRFSE) T2-weighted MR imaging with fat suppression, coronal and axial single shot fast spin-echo (SSFSE) T2-weighted MR imaging, and three-dimension (3D) FSPGR dynamic enhanced MR imaging.
FSPGR T1-weighted imaging with fat suppression was obtained in 1 or 2 breathholds, with the following parameters: TR ms/TE ms = 150-170/1.6; flip angle = 80°; matrix = 512 × 160-192; field of view = 26-32 cm; section thickness = 5 mm (gap ≤ 0.5 mm); number of signals acquired (NSA) = 1; and the sampling bandwidth = 20.8 kHz.
GRE in-phase and out-of-phase MR imaging were acquired in breath hold, with the following parameters: TR ms/TE ms = 150/4.4, 2.2; flip angle = 90°; matrix = 256× 192-224; field of view = 26-32 cm; section thickness = 5 mm (gap ≤ 0.5 mm); number of signals acquired (NSA) = 1; and the sampling bandwidth = 31 or 62 kHz.
R-T FRFSE T2-weighted sequence were obtained with the following parameters: repetition time [TR] ms/echo time [TE] ms = 10 000-12 000/90-100, TR determined by the frequency of respiration; section thickness = 5 mm; intersection gap = 0.5 mm; matrix = 256 × 192; number of signals acquired (NSA) = 3; and field of view = 34 cm × 34 cm. It took about 3-4 min to complete the acquisition.
Coronal and axial SSFSE T2-weighted images were obtained in breathhold, with the following parameters: TE= 90-100 ms; 2 s between slize acquisitions; section thickness = 5 mm; intersection gap = 0.5 mm; matrix = 384 × 224; one-half signal acquired; and field of view = 33 cm × 33 cm.
Radial oblique slab SSFSE images were obtained for MRCP with the following parameters: TE = 1300 ms; 6 s between image acquisitions; section thickness = 40 mm; matrix = 384 × 224; one-half signal acquired; and field of view = 30 cm × 30 cm.
Dynamic enhanced imaging was performed with axial fat saturated 3D FSPGR sequence. Gadolinium chelate was administered (0.2 mmol/L per kilogram of body weight) intravenously at approximately 3.5 mL/s by projector (Spectris MR Injection System, Medrad Inc., USA) injection, followed by a 20-mL saline solution flush. An additional delayed phase was acquired using 2D FSPGR fat suppression axial T1-weighted imaging.
Image interpretation
The original MR imaging data was loaded onto a workstation (GE, AW4.1, Sun Microsystems, Palo Alto, CA) to be reviewed. Two observers (with 4 and 6 years’ experience in interpreting abdominal MRI examinations), who were blinded to the laboratory data and clinical outcome, reviewed MR images. Any discrepancy between the two readers was settled by consensus.
The enlarged pancreas was defined as anterior-posterior diameter ≥ 3 cm on axial images[12]. Decreased signal intensity of pancreas on FSPGR T1-weighted imaging was defined as not higher than that of liver[13,14]. The signal intensity of normal pancreas was defined as slightly higher than that of liver on T2-weighted images[13]. Pancreatic lobular septum thickening (PLST) with increased signal intensity was defined as a thickness of the septum surrounding pancreatic lobules ≥ 2 mm and its signal intensity significantly higher than that of liver. The pancreatic fascial plane (PFP) was defined as linear signal intensity along the surface of the pancreas which was hypointense on in-phase images or hyperintense on T2-weighted images.
Peripancreatic fat stranding (PPFS) referred to the stranding with hypointensity on in-phase images or hyperintensity on T2 weighted images. Peripancreatic fluid (PPF) collections referred to poorly defined fluid collection in the anterior pararenal space around the gland and in the lesser peritoneal sac, with diameter larger than 2 cm. The renal fascial plane (RFP) was defined as renal fascia thickness over 3 mm[15], which was hypointense on in-phase and hyperintense on T2-weighted images. Perivascular fluid referred to fluid surrounding portal vein, superior mesentery vein (SMV) or artery (SMA), which was hyperintense on T2-weighted images.
The diameter of the pancreatic main duct was measured at the level of the head or body of the pancreas on MRCP images. Pancreatic necrosis was also noted on gadolinium chelate 3D FSPGR dynamic enhanced images.
The grade of the pancreatitis was scored with the MR severity score index (MRSI) (Table 1), which was derived from the CT severity score index developed by Balthazar and others[4,15-17]. The severity of pancreatitis was expressed as mild (score = 0-2 points), moderate (score = 3-6 points), and severe (score = 7-10 points).
Table 1.
MR severity score index
| Prognostic indications | Characteristic | Points |
| Pancreatic | Normal pancreas | 0 |
| Inflammation | Focal or diffuse enlargement of the pancreas | 1 |
| Intrinsic pancreatic abnormalities with inflammatory changes in pancreatic fat | 2 | |
| Single, poorly defined fluid collection or phlegmon | 3 | |
| Two or more poorly defined collection or presence of gas in or adjacent to the pancreas | 4 | |
| Pancreatic necrosis | No necrosis | 0 |
| 30% | 2 | |
| 30%-50% | 4 | |
| > 50% | 6 |
Statistical analysis
Results were expressed as mean ± SD for continuous data. Two-tailed student’s t test was used to calculate differences between two observers. The Chi-square test was used to evaluate the difference for MR findings among IEP patients on in-phase, SSFSE T2- and R-T T2- weighted imaging, as well as to compare the prevalence of MR findings for IEP on R-T T2- weighted images. Relationship of length of hospitalization variables to the severity of IEP was tested using t tests, and to the continuous variables with Pearson product-moment correlations. P < 0.05 was considered statistically significant.
RESULTS
Sample characteristics
In this study, 49% (26/53) of patients had cholecystopathy or cholelithiasis confirmed by surgery or laparoscopy. One patient had congenital choledochocele and cholecystitis, confirmed at laparotomy. Of them, 17% (9/53) patients had alcohol abuse, and 34% (18/53) were idiopathic.
MRI findings
The anterior-posterior diameter of the pancreas on FSPGR T1-weighted imaging was, respectively, 2.3 ± 0.4 cm and 2.4 ± 0.4 cm (P = 0.1) for observer 1 and observer 2. Only one patient had a diameter of the pancreas larger than 3 cm.
Of the 53 patients with IEP, 89% (47/53) had at least one abnormality on MR images, whereas 11% (6/53) showed normal pancreas. We found that 19% (10/53) of patients showed decreased pancreatic signal intensity on FSPGR T1-weighted images, while 81% (43/53) of patients showed normal pancreatic signal intensity. In addition, 25% (13/53) and 30% (16/53) of patients showed increased signal intensity on SSFSE T2-weighted and R-T T2-weighted images (Figure 1), respectively (χ2 = 0.5-1.8, P > 0.05). As shown in Figures 1 and 2, 21% (11/53) and 40% (21/53) of patients had PLST with increased signal intensity on SSFSE T2-weighted and R-T T2-weighted images, respectively (χ2 = 4.5, P < 0.05).
Figure 1.
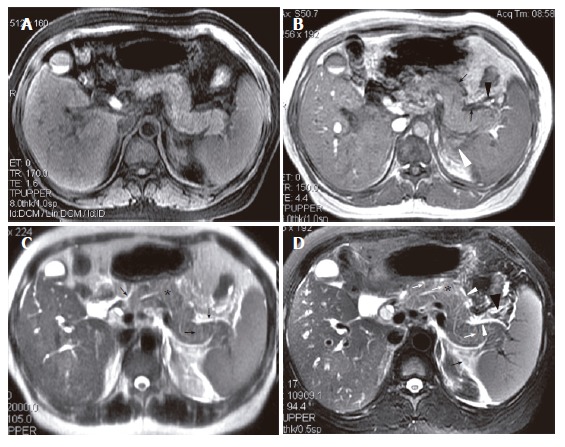
A 42-year-old woman with interstitial edematous pancreatitis. A: SPGR fat-suppressed T1-weighted (TR/TE = 170/1.6 ms) image shows pancreatic signal intensity comparable to that of liver; B: GRE in-phase (TR/TE = 150/4.4 ms) image shows pancreatic fascial plane (arrows) and peripancreatic fat stranding (arrow heads); C: SSFSE T2-weighted (TE = 90 ms) image shows increased pancreatic signal intensity (asterisk). Stranding in the pancreatic fascial plane (large arrow) and peripancreatic fat and thickened pancreatic lobular septum (small arrow) can also be seen; D: R-T T2-weighted (TR/TE = 11 800/93 ms) image shows above findings much better (Asterisk indicates pancreatic parenchyma, white arrows indicate thickened pancreatic lobular septum, white arrow head indicates pancreatic fascial plane, and black arrow and arrow head indicate fat stranding).
Figure 2.
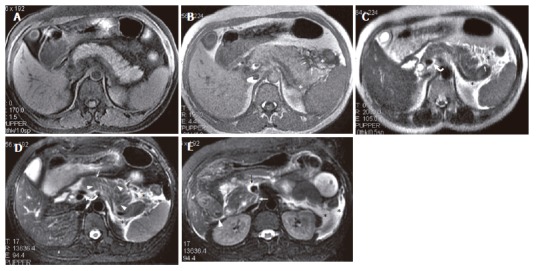
A 28-year-old man with IEP. A: SPGR fat-suppressed T1-weighted (TR/TE = 170/1.6 ms) image shows normal pancreatic signal intensity; B: pancreatic fascial (arrows) and peripancreatic fat (asterisk) stranding can be seen on GRE in-phase (TR/TE, 150/4.4 ms) image; C: SSFSE T2-weighted (TE = 90 ms) image; D: R-T T2-weighted (TR/TE = 12300/98 ms) image. Perivascular fluid (curve arrow) can be seen on latter two sequences. Thickened pancreatic lobular septum (arrow heads) can only be seen on R-T T2-weighted image (D); E: At the level of the head of the pancreas, R-T T2-weighted image (E) shows pancreatic fascial plane (white arrow), right renal fascia plane (white arrow head), fluid surrounding SMV (black arrow), and left anterior pararenal space and lateroconal plane fluid collections (asterisk).
The other MR findings of IEP are shown in Table 2, and illustrated in Figure 1, Figure 2, Figure 3, Figure 4. The prevalence of most findings of IEP on R-T T2-weighted imaging was higher than that on in-phase or SSFSE T2-weighted imaging (Table 2). The commonest finding of IEP on R-T T2-weighted imaging was PFP (85%), left RFP (77%) and PPFS (55%), whose prevalence was significantly higher than those for PPF, right RFP, perivascular fluid and free ascites, respectively (Table 2) (χ2 = 78.5, P < 0.001).
Table 2.
MR findings of IEP in 53 patients
| PFP n (%) | PPF n (%) | PPFS n (%) | LRFP n (%) | RRFP n (%) | PVF n (%) | FA n (%) | |
| In-phase | 18 (34) | 10 (19) | 15 (28) | 26 (49) | 8 (15) | 1 (2) | 7 (13) |
| SSFSE T2W | 33 (62.3) | 13 (25) | 24 (45) | 31 (58) | 16 (30) | 14 (26) | 10 (19) |
| R -T T2W | 45 (85) | 13 (25) | 29 (55) | 41 (77) | 22 (42) | 24 (45) | 10 (19) |
| χ2 (P values)1 | 28.9 (< 0.001) | 0.6 (0.7) | 7.8 (0.02) | 9.3 (0.01) | 9.1 (0.01) | 27.1 (< 0.001) | 0.8 (0.7) |
PFP: Pancreatic fascial plane; PPF: Peripancreatic fluid; PPFS: Peripancreatic fat stranding; LRFP: Left renal fascial plane; RRFP: Right renal fascial plane; PVF: Perivascular fluid; FA: Free ascites.
Comparison among three sequences.
Figure 3.
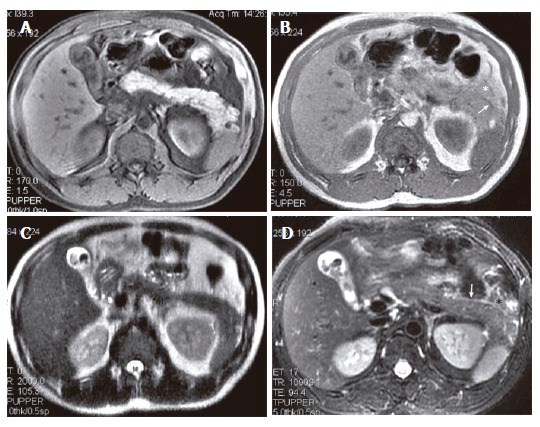
A 45-year-old woman with IEP and cholelithiasis. (A) SPGR fat-suppressed T1-weighted (TR/TE = 170/1.6 ms) image shows normal pancreatic signal intensity. GRE in-phase (TR/TE = 150/4.4 ms) (B) and SSFSE T2 weighted (TE = 90 ms); (C) images show the pancreatic fascia plane (arrows) and the peripancreatic fat stranding (asterisk), which appear more extensive on R-T T2-weighted (TR/TE = 12 300/98 ms) image (D).
Figure 4.
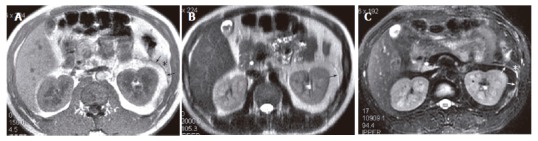
The same patient as in Figure 3. At the level of the head of the pancreas, left renal fascial plane (arrow) and anterior pararenal space fat stranding (asterisk) present on GRE in-phase (A), SSFSE T2-weighted (B) and R-T T2-weighted (C) images, but they are most prominent on R-T T2-weighted image.
All of the 29 (54.71%) patients with PPFS and 13 (24.52%) patients with PPF had PFP. All these 13 patients had lesser peritoneal sac fluid collections, including 5 with collections in the left anterior pararenal space fluid collections, 1 with a right anterior pararenal space fluid collection, and 2 with both right and left anterior pararenal space fluid collections.
Of 53 patients, 43 (81%) showed RFP, including 22 (42%) with both left and right RFP, 1 (2%) with only right RFP and 20 (38%) with only left RFP. The number of patients with left RFP was significantly higher than those with right RFP (42 vs 23 cases; χ2 = 14.4, P < 0.001).
On MRCP images, the main pancreatic duct was smooth, and its diameter was 2.2 ± 0.7 mm and 2.1 ± 0.6 mm (P = 0.7) for observer 1 and observer 2, respectively. No pancreatic necrosis was seen on gadolinium chelate 3D FSPGR dynamic MR imaging or other sequences.
Length of hospitalization and MRSI
The average length of hospitalization of the 53 patients was 14 ± 7 d (range: 4-33 d). The MRSI ranged from 0 points to 4 points with the average of 2.1 ± 1.2 points. The IEP was graded as mild in 37 (70%) patients, as moderate in 16 (30%) patients and as severe in 0 patients. The severity of pancreatitis was not related to the length of hospitalization (r = 0.1, P > 0.05).
DISCUSSION
In our study, we found 89% of patients with IEP had at least one abnormality on non-enhanced MR imaging, depicted primarily on T2-weighted images. These abnormalities mainly included pancreatic fascial plane (85%), left renal fascial plane (77%), peripancreatic fat stranding (55%), peripancreatic fluid (55%), perivascular fluid (45%), right renal fascial plane (42%), and pancreatic lobular septum thickening (40%). R-T T2-weighted imaging was much more sensitive than in-phase and SSFSE T2-weighted imaging in the depiction of the aforementioned abnormalities.
The pathology of IEP included pancreatic edema and mild cellular infiltration, sometimes with a few small scattered foci of necrosis and saponification in the peripancreatic fatty tissue[3]. The histological changes noted in experimental pancreatitis are progressive interstitial edema, and mononuclear-cell infiltration, without hemorrhage or necrosis, with regeneration of the pancreas in 6 d[18]. In our study, 19% of patients showed decreased pancreatic signal intensity on FSPGR T1-weighted images, while 25%-30% of patients showed increased pancreatic signal intensity on SSFSE T2-weighted and R-T T2-weighted images. The abnormal signal intensity of pancreatic parenchyma may partly reflect the inflammation of the pancreas in IEP. However, our results indicate that the signal intensity of the pancreatic parenchyma on both T1-weighted and T2-weighted imaging is not sensitive for depicting IEP. In the 12 patients with mild acute pancreatitis reported by Amano et al[11], 75% (9/12) of patients showed a prolonged T1 and T2 lesions of the pancreas. However, the patients selected in our study differ from those in theirs. IEP is not the same as mild acute pancreatitis, including a variety of intermediary forms from mild to severe pancreatitis which occur in clinical practice[3].
The pancreas does not have a well-developed fibrous capsule, but is surrounded by thin loose connective tissue, which is called the “fusion fascia of Treita” in the head and the “fusion fascia of Toldt” in the body and tail of the pancreas[19]. In our study, we found the commonest sign was PFP on T2-weighted images (85%). In the 29 patients with PPFS and 13 patients with PPF, all had PFP. We speculate that the pancreatic fascia may be first involved in acute pancreatitis. When the inflammation penetrated through the Toldt fusion fascia of the pancreas, peripancreas fat infiltration or fluid accumulation may develop. The peripancreatic vessels run in the fusion fascia[19]. It is possible that peripancreatic perivascular fluid in IEP resulted from inflammation of intra-fusion fascia of the pancreas.
In AP, the earliest changes appear to be in the acinar cells[20]. An increase in pancreatic ductal pressure has been described as the initial mechanism of acinar cell injury. The subsequent cellular events are not well understood, but the result appears to be activation of pancreatic digestive enzymes within the acinar cell[21]. Ultrastructural studies by Kloppel et al[22] indicate that an early event, irrespective of etiology, is the release of activated enzymes by acini at the periphery of lobules into the interstitium, resulting in perilobular fat necrosis. Experimentally, tracer material introduced by retrograde injection into the main pancreatic duct (MPD) is localized to the periacinar space[23] and human AP may show a similar pattern, irrespective of etiology[24]. Early microcirculatory changes in patients with AP include an increase in vascular permeability and the accumulation of extravagated fluid in the perilobular space. A decrease in flow velocity was noted 2 h after the onset of severe pancreatitis[25]. We found that 21%-40% of patients had PLST with increased signal intensity on T2-weighted images. We speculate that this resulted primarily from the accumulation of extravagated fluid in the perilobular space and perilobular fat necrosis.
CT has been helpful in revealing inflammatory thickening of the retroperitoneal fascial membranes and edema or lipolysis of the retroperitoneal fat resulting from acute pancreatitis. Nicholson et al[15] reported that 46% of patients with pancreatitis demonstrated renal fascial thickening and 27% of patients had increased fat density. Chintapalli et al[16] reported that renal fascial thickening was seen in 62% of patients with pancreatitis, involving left and right renal fascia in 59% (42/71) and 30% (21/71), respectively. The patients in the above two reports had various forms of pancreatitis. In our study, 81% of patients with IEP showed RFP, including 77% with left RFP and 42% with right RFP. The prevalence of left RFP was significantly higher than that of right RFP (P < 0.05), although the prevalence of RFP in MRI was higher than that reported in CT[15,16]. It is possible that MR imaging is more sensitive than CT for detecting renal fascia inflammatory thickening. The predominance of left RFP is consistent with earlier studies by CT, attributed to the proximity of the body and tail of the pancreas[16].
Mortele et al[26] reported that the pancreas appeared abnormal on MRI and MRCP in 57% (31/54) patients with asymptomatic mild serum hyperamylasemia and hyperlipasemia, while 43% of patients had normal pancreas. In our study, 11% (6/53) of patients showed normal pancreas on MR imaging. Each of these 6 patients had 3 times or more elevations of serum amylase and lipase at hospital admission, and serum amylase and lipase levels decreased within a few days. It is possible that the pancreatitis in these 6 patients was too mild to be seen with current non-enhanced MR imaging.
In our study, we did not find any correlation between the length of hospitalization and MRSI (r = 0.1, P > 0.05), which was in agreement with the findings by Lecesne et al[8]. Most of the patients with IEP were graded as mild (70%), and none were graded as severe in our study. Also, 49% of patients had cholecystopathy or cholelithiasis. We agree with Lecesne et al[8] that the length of hospitalization may not accurately represent morbidity, since social factors or selective surgery may prolong the length of stay, which was not included in the design of our study.
We also did not analyze the correlation of the level of serum amylase and lipase to the MR findings or to the length of hospitalization. Reports in the early literature indicated that the severity of acute pancreatitis was independent of the elevation in serum amylase/lipase level (≤ 3 or > 3 times of higher limit of normal range) on admission. Patients with only a slight increase can also have or develop severe acute pancreatitis[27]. In diabetic ketoacidosis, non-specific elevations of amylase and lipase occurred in 16%-25% of cases. Diagnosis of acute pancreatitis based solely on elevated amylase or lipase, even > 3 times normal, is not justifiable[28]. Mortele et al[26] reported that 43% of patients with asymptomatic serum hyperamylasemia and hyperlipasemia had normal pancreas on MRCP. The above reports indicate that the level of serum amylase and lipase was not relative to the findings on MR imaging and the severity of acute pancreatitis.
In our study only 3 patients had repeated MR imaging, so we were not able to document serial changes in the development of pancreatic inflammation. However, the short-term clinic status of all patients improved. Munoz-Bongrand et al[29] reported that serial computed tomography is rarely necessary in patients with acute pancreatitis, and repeated MR examinations may similarly be unnecessary in patients with IEP.
In conclusion, interstitial edematous pancreatitis primarily manifests on unenhanced MR images as the pancreatic fascial plane, left renal fascial plane and peripancreatic fat stranding, as well as peripancreatic fluid. Non-enhanced MR imaging, especially R-T T2-weighted imaging with fat suppression, is effective for depicting interstitial edematous pancreatitis.
Footnotes
Supported by Key project of Science and Technology Research, Ministry of Education, China, No. 206126
S- Editor Liu Y L- Editor Kumar M E- Editor Bai SH
References
- 1.Bradley EL 3rd, Allen K. A prospective longitudinal study of observation versus surgical intervention in the management of necrotizing pancreatitis. Am J Surg. 1991;161:19–24; discussion 24-25. doi: 10.1016/0002-9610(91)90355-h. [DOI] [PubMed] [Google Scholar]
- 2.Bradley EL 3rd. A clinically based classification system for acute pancreatitis. Summary of the International Symposium on Acute Pancreatitis, Atlanta, Ga, September 11 through 13, 1992. Arch Surg. 1993;128:586–590. doi: 10.1001/archsurg.1993.01420170122019. [DOI] [PubMed] [Google Scholar]
- 3.Balthazar EJ. Pancreatitis. In: Gore RM, Levive MS, editors. Textbook of Gastrointestinal Radiology. Philadelphia, PA: WB Saunders Company; 2000. pp. 1767–1795. [Google Scholar]
- 4.Balthazar EJ, Robinson DL, Megibow AJ, Ranson JH. Acute pancreatitis: value of CT in establishing prognosis. Radiology. 1990;174:331–336. doi: 10.1148/radiology.174.2.2296641. [DOI] [PubMed] [Google Scholar]
- 5.Morgan DE, Baron TH. Practical imaging in acute pancreatitis. Semin Gastrointest Dis. 1998;9:41–50. [PubMed] [Google Scholar]
- 6.Schmidt J, Hotz HG, Foitzik T, Ryschich E, Buhr HJ, Warshaw AL, Herfarth C, Klar E. Intravenous contrast medium aggravates the impairment of pancreatic microcirculation in necrotizing pancreatitis in the rat. Ann Surg. 1995;221:257–264. doi: 10.1097/00000658-199503000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McMenamin DA, Gates LK Jr. A retrospective analysis of the effect of contrast-enhanced CT on the outcome of acute pancreatitis. Am J Gastroenterol. 1996;91:1384–1387. [PubMed] [Google Scholar]
- 8.Lecesne R, Taourel P, Bret PM, Atri M, Reinhold C. Acute pancreatitis: interobserver agreement and correlation of CT and MR cholangiopancreatography with outcome. Radiology. 1999;211:727–735. doi: 10.1148/radiology.211.3.r99jn08727. [DOI] [PubMed] [Google Scholar]
- 9.Morgan DE, Baron TH, Smith JK, Robbin ML, Kenney PJ. Pancreatic fluid collections prior to intervention: evaluation with MR imaging compared with CT and US. Radiology. 1997;203:773–778. doi: 10.1148/radiology.203.3.9169703. [DOI] [PubMed] [Google Scholar]
- 10.Ward J, Chalmers AG, Guthrie AJ, Larvin M, Robinson PJ. T2-weighted and dynamic enhanced MRI in acute pancreatitis: comparison with contrast enhanced CT. Clin Radiol. 1997;52:109–114. doi: 10.1016/s0009-9260(97)80102-x. [DOI] [PubMed] [Google Scholar]
- 11.Amano Y, Oishi T, Takahashi M, Kumazaki T. Nonenhanced magnetic resonance imaging of mild acute pancreatitis. Abdom Imaging. 2001;26:59–63. doi: 10.1007/s002610000104. [DOI] [PubMed] [Google Scholar]
- 12.Kreel L, Haertel M, Katz D. Computed tomography of the normal pancreas. J Comput Assist Tomogr. 1977;1:290–299. doi: 10.1097/00004728-197707000-00002. [DOI] [PubMed] [Google Scholar]
- 13.Outwater EK, Mitchell DG. MR imaging techniques for evaluation of the pancreas. Top Magn Reson Imaging. 1996;8:248–264. [PubMed] [Google Scholar]
- 14.Gallix BP, Bret PM, Atri M, Lecesne R, Reinhold C. Comparison of qualitative and quantitative measurements on unenhanced T1-weighted fat saturation MR images in predicting pancreatic pathology. J Magn Reson Imaging. 2005;21:583–589. doi: 10.1002/jmri.20310. [DOI] [PubMed] [Google Scholar]
- 15.Nicholson RL. Abnormalities of the perinephric fascia and fat in pancreatitis. Radiology. 1981;139:125–127. doi: 10.1148/radiology.139.1.7193897. [DOI] [PubMed] [Google Scholar]
- 16.Chintapalli K, Lawson TL, Foley WD, Berland LL. Renal fascial thickening in pancreatitis. J Comput Assist Tomogr. 1982;6:983–986. doi: 10.1097/00004728-198210000-00021. [DOI] [PubMed] [Google Scholar]
- 17.Arvanitakis M, Delhaye M, De Maertelaere V, Bali M, Winant C, Coppens E, Jeanmart J, Zalcman M, Van Gansbeke D, Devière J, et al. Computed tomography and magnetic resonance imaging in the assessment of acute pancreatitis. Gastroenterology. 2004;126:715–723. doi: 10.1053/j.gastro.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 18.Willemer S, Elsässer HP, Adler G. Hormone-induced pancreatitis. Eur Surg Res. 1992;24 Suppl 1:29–39. doi: 10.1159/000129237. [DOI] [PubMed] [Google Scholar]
- 19.Kimura W. Surgical anatomy of the pancreas for limited resection. J Hepatobiliary Pancreat Surg. 2000;7:473–479. doi: 10.1007/s005340070017. [DOI] [PubMed] [Google Scholar]
- 20.Lerch MM, Saluja AK, Dawra R, Ramaraò P, Saluja M, Steer ML. Acute necrotizing pancreatitis in the opossum: earliest morphological changes involve acinar cells. Gastroenterology. 1992;103:205–213. doi: 10.1016/0016-5085(92)91114-j. [DOI] [PubMed] [Google Scholar]
- 21.Steer ML. How and where does acute pancreatitis begin. Arch Surg. 1992;127:1350–1353. doi: 10.1001/archsurg.1992.01420110098019. [DOI] [PubMed] [Google Scholar]
- 22.Klöppel G, Dreyer T, Willemer S, Kern HF, Adler G. Human acute pancreatitis: its pathogenesis in the light of immunocytochemical and ultrastructural findings in acinar cells. Virchows Arch A Pathol Anat Histopathol. 1986;409:791–803. doi: 10.1007/BF00710764. [DOI] [PubMed] [Google Scholar]
- 23.Bockman DE, Schiller WR, Anderson MC. Route of retrograde flow in the exocrine pancreas during ductal hypertension. Arch Surg. 1971;103:321–329. doi: 10.1001/archsurg.1971.01350080237038. [DOI] [PubMed] [Google Scholar]
- 24.Bockman DE, Büchler M, Beger HG. Ultrastructure of human acute pancreatitis. Int J Pancreatol. 1986;1:141–153. doi: 10.1007/BF02788446. [DOI] [PubMed] [Google Scholar]
- 25.Sunamura M, Yamauchi J, Shibuya K, Chen HM, Ding L, Takeda K, Kobari M, Matsuno S. Pancreatic microcirculation in acute pancreatitis. J Hepatobiliary Pancreat Surg. 1998;5:62–68. doi: 10.1007/pl00009952. [DOI] [PubMed] [Google Scholar]
- 26.Mortelé KJ, Wiesner W, Zou KH, Ros PR, Silverman SG. Asymptomatic nonspecific serum hyperamylasemia and hyperlipasemia: spectrum of MRCP findings and clinical implications. Abdom Imaging. 2004;29:109–114. doi: 10.1007/s00261-003-0072-4. [DOI] [PubMed] [Google Scholar]
- 27.Lankisch PG, Burchard-Reckert S, Lehnick D. Underestimation of acute pancreatitis: patients with only a small increase in amylase/lipase levels can also have or develop severe acute pancreatitis. Gut. 1999;44:542–544. doi: 10.1136/gut.44.4.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yadav D, Nair S, Norkus EP, Pitchumoni CS. Nonspecific hyperamylasemia and hyperlipasemia in diabetic ketoacidosis: incidence and correlation with biochemical abnormalities. Am J Gastroenterol. 2000;95:3123–3128. doi: 10.1111/j.1572-0241.2000.03279.x. [DOI] [PubMed] [Google Scholar]
- 29.Munoz-Bongrand N, Panis Y, Soyer P, Riché F, Laisné MJ, Boudiaf M, Valleur P. Serial computed tomography is rarely necessary in patients with acute pancreatitis: a prospective study in 102 patients. J Am Coll Surg. 2001;193:146–152. doi: 10.1016/s1072-7515(01)00969-3. [DOI] [PubMed] [Google Scholar]


