Abstract
Pancreatic cystic lesions comprise various entities with different histopathological characteristics and their differential diagnosis is often a challenge for clinicians. Autoimmune pancreatitis (AIP) is usually not considered in the differential diagnosis of cystic lesions, but often mimics the morphological aspects of pancreatic neoplasm. We report the case of a 64-year-old male patient with a cystic pancreatic head lesion (diameter 5 cm) and stenosis of the distal bile duct requiring repeated stenting. Because of the clinical presentation together with moderate elevation of serum CA19-9 and massive elevation of cyst fluid CA19-9 (122.695 U/L; normal range: < 37.0 U/L), the patient underwent explorative laparotomy and pylorus preserving partial pancreaticoduodenectomy. Histology revealed surprisingly AIP with an inflammatory pseudocyst. In conclusion, cyst fluid analysis of tumor markers and cyst fluid cytology lack high accuracy to clearly differentiate cystic pancreatic lesions. Although AIP is rarely associated with pseudocysts, the disease has to be considered in the differential diagnosis of cystic pancreatic lesions. Early examination of serum IgG, IgG4 and auto-antibodies might save these patients from unnecessary endoscopical and surgical procedures.
Keywords: Pseudocyst, Autoimmune pancreatitis, Pancreatic cancer, Tumor marker, CEA, CA19-9
INTRODUCTION
Autoimmune pancreatitis (AIP) is a benign disease that responds well to steroid treatment. Characteristics include radiological evidence of an irregular narrowing of the pancreatic main duct and a diffuse enlargement of the pancreas, together with increased levels of serum IgG and the IgG4 subclass as well as antinuclear (ANA), antilactoferrin(ALF), anticarbonic anhydrase II antibodies (ACA-II) and rheumatoid factor[1]. On histological examination, periductal lymphoplasmacytic infiltration, periductal fibrosis and venulitis are most frequently observed[2]. Although there is no international consensus on the diagnostic criteria for AIP, histology should be considered the gold standard in cases in which tissue diagnosis is possible. AIP predominantly affects male patients and is often associated with other autoimmune diseases such as Sjörgen’s syndrome, primary sclerosing cholangitis or diabetes mellitus which lead to the hypothesis that AIP might be part of a systemic autoimmune disorder with increased IgG4 and immune complexes (secondary AIP)[1]. Although AIP has become more and more accepted as a distinct disease entity during the last years[3], the presentation of the disease can be unusual and misleading as depicted in the reported case. The correct diagnosis of AIP is of great clinical relevance since this pancreatic disorder, in contrast to most other pancreatic pathologies, can be treated successfully by non-invasive therapies.
CASE REPORT
A 64-year-old male patient was referred to our department with a cystic lesion of the pancreatic head and a history of multiple biliary stent placements during a course of two years due to distal bile duct stenosis. There was no history of alcohol abuse. Three years earlier, the patient had undergone an explorative laparotomy, cholecystectomy and lymph node biopsy at a different hospital because of a pancreatic tumor of unknown etiology. However, intraoperative assessment and histology did not reveal neoplastic tumor growth. On follow-up, the patient developed recurrent attacks of abdominal pain with mild to moderate pancreatic enzyme elevation, and a distal bile duct stenosis requiring repeated stenting and dilation (Figure 1A). Fine needle aspiration cytology of the pancreas was consistent with chronic pancreatitis. On further follow-up, a cyst of the pancreatic head (diameter 5 cm) was diagnosed (Figures 1B and C). Cyst fluid analysis showed a CEA of 55 ng/L (normal range: < 3.4 ng/L) and a CA19-9 of 122.695 U/L (normal range: < 37.0 U/L), without evidence of malignant cells. The patient was referred to our department for surgical therapy with the suspicion of a cystic neoplasm. Pertinent laboratory data on admission to our department were (in brackets: normal range): lipase 108 U/L (< 51 U/L), γ-glutamyl transferase 158 U/L (< 60 U/L), bilirubin 0.4 mg/dL (within normal range), calcium 2.32 mmol/L (within normal range), WBC 6.91/nL (within normal range), CA19-9 43.4 U/mL (< 37.0 U/mL), CEA 3.2 ng/mL (< 2.5 ng/mL).
Figure 1.
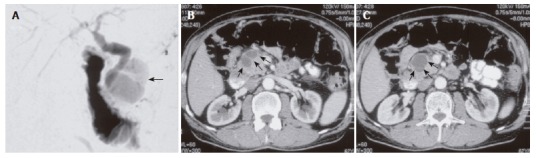
MRCP (A) depicting the dilated bile duct (13 mm) in close vicinity to the pancreatic cyst; CT scans (B, C) showing the cystic pancreatic lesion in the pancreatic head (arrows).
The patient underwent a pylorus preserving partial pancreaticoduodenectomy. The postoperative course was uneventful and the patient was discharged after seven days. Grossly, the pancreatic head measured 7 cm × 5 cm × 3 cm and showed a whitish induration of the tissue with a cyst of 4 cm in diameter (Figure 2A and B). Histological examination revealed surprisingly autoimmune pancreatitis with an inflammatory pseudocyst without evidence for malignancy (Figure 3A and F).
Figure 2.
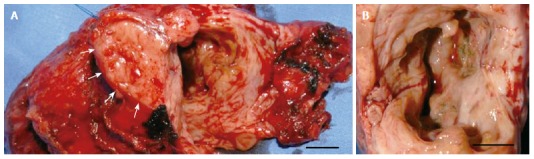
Intraoperative findings of the resected pancreatic specimen. A, B: Macroscopic appearance of the pancreaticoduodenectomy specimen (A) and the opened pseudocyst (B). Arrows indicate the pancreatic cut margin. Scale bar: 1 cm.
Figure 3.
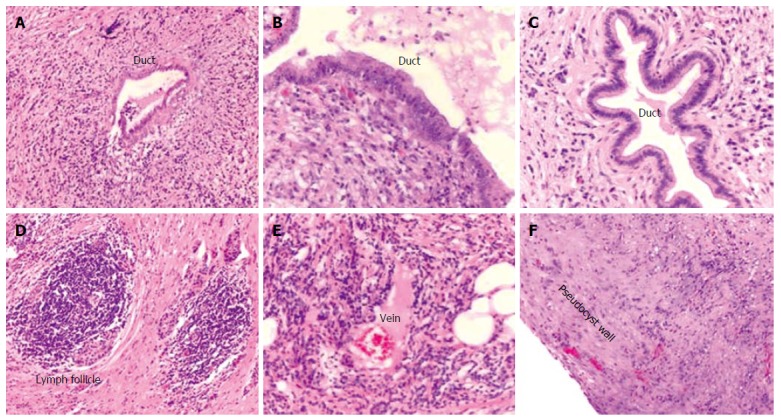
Histological findings of the resected pancreatic specimen. Histological examination displaying marked chronic periductal lymphoplasmacytic inflammation and fibrosis (A-C), intrapancreatic lymph follicle (D), and venulitis (E). F depicts the wall of the pseudocyst without evidence of epithelial lining.
DISCUSSION
Differentiating cystic pancreatic lesions remains a clinical challenge[4]. Adding serological analysis (e.g. CEA, CA19-9) of aspirated cyst fluid to conventional imaging modalities results in additional accuracy. Thus, of various markers including tumor markers and cytology, a CEA cutoff of 192 ng/mL (in the cyst fluid) demonstrated the highest accuracy in differentiating mucinous from non-mucinous cystic lesions[5]. However, the sensitivity and specificity rates remain unsatisfactory. Therefore, resection of cystic lesions remains the treatment of choice if malignant or pre-malignant cystic neoplasm cannot be ruled out[6].
AIP, on the other hand, is rarely associated with pseudocysts[7,8], but involvement of the pancreatic head can mimic pancreatic neoplasm[3]. AIP responds well to steroid treatment and is often associated with elevated serum IgG/IgG4 and the presence of different auto-antibodies (e.g. ANA, and others)[3,9]. In addition, pseudocysts in patients with AIP might represent a highly active inflammatory process, and these lesions have been shown to be steroid responsive if associated with AIP[7]. In the present case, histology demonstrated that the inflammatory process involved the distal common bile duct, which is most likely the reason for the patients’ history of recurrent bile duct stenosis.
The pseudocyst formation was detected almost 2 years after onset of abdominal symptoms. Muraki et al observed pancreatic cysts 1-3 years after onset of abdominal symptoms or diagnosis of AIP[7], indicating that pseudocyst development in AIP occurs after prolonged disease activity.
In our case, the consideration of AIP before or after the first surgical exploration together with a trial of steroid therapy would likely have saved the patient a number of endoscopical and ultimately surgical therapies. This case highlights the clinical problem of differentiating cystic pancreatic lesions, and the importance of considering AIP as a differential diagnosis early in the course of the disease. Nonetheless, given the high cure rate of pancreatic cystic tumors with surgical resection, laparotomy should not be delayed in case of suspected malignancy.
Footnotes
S- Editor Liu Y L- Editor Zhu LH E- Editor Ma WH
References
- 1.Okazaki K, Chiba T. Autoimmune related pancreatitis. Gut. 2002;51:1–4. doi: 10.1136/gut.51.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zamboni G, Lüttges J, Capelli P, Frulloni L, Cavallini G, Pederzoli P, Leins A, Longnecker D, Klöppel G. Histopathological features of diagnostic and clinical relevance in autoimmune pancreatitis: a study on 53 resection specimens and 9 biopsy specimens. Virchows Arch. 2004;445:552–563. doi: 10.1007/s00428-004-1140-z. [DOI] [PubMed] [Google Scholar]
- 3.Kim KP, Kim MH, Song MH, Lee SS, Seo DW, Lee SK. Autoimmune chronic pancreatitis. Am J Gastroenterol. 2004;99:1605–1616. doi: 10.1111/j.1572-0241.2004.30336.x. [DOI] [PubMed] [Google Scholar]
- 4.Brugge WR, Lauwers GY, Sahani D, Fernandez-del Castillo C, Warshaw AL. Cystic neoplasms of the pancreas. N Engl J Med. 2004;351:1218–1226. doi: 10.1056/NEJMra031623. [DOI] [PubMed] [Google Scholar]
- 5.Brugge WR, Lewandrowski K, Lee-Lewandrowski E, Centeno BA, Szydlo T, Regan S, del Castillo CF, Warshaw AL. Diagnosis of pancreatic cystic neoplasms: a report of the cooperative pancreatic cyst study. Gastroenterology. 2004;126:1330–1336. doi: 10.1053/j.gastro.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 6.Kleeff J, Friess H, Büchler MW. What is the most accurate test to differentiate pancreatic cystic neoplasms. Nat Clin Pract Gastroenterol Hepatol. 2004;1:18–19. doi: 10.1038/ncpgasthep0001. [DOI] [PubMed] [Google Scholar]
- 7.Muraki T, Hamano H, Ochi Y, Arakura N, Takayama M, Komatsu K, Komiyama Y, Kawa S, Uehara T, Kiyosawa K. Corticosteroid-responsive pancreatic cyst found in autoimmune pancreatitis. J Gastroenterol. 2005;40:761–766. doi: 10.1007/s00535-005-1622-z. [DOI] [PubMed] [Google Scholar]
- 8.Nishimura T, Masaoka T, Suzuki H, Aiura K, Nagata H, Ishii H. Autoimmune pancreatitis with pseudocysts. J Gastroenterol. 2004;39:1005–1010. doi: 10.1007/s00535-004-1436-4. [DOI] [PubMed] [Google Scholar]
- 9.Lara LP, Chari ST. Autoimmune pancreatitis. Curr Gastroenterol Rep. 2005;7:101–106. doi: 10.1007/s11894-005-0047-4. [DOI] [PubMed] [Google Scholar]


