Abstract
High dose methotrexate (HDMTX), defined by doses of methotrexate (MTX) ≥ 1g/m2, is a widely used regimen known to cause renal toxicity. The reported incidence of renal toxicity in osteosarcoma patients is 1.8%, but the incidence in hematologic malignancies is not well characterized. In this retrospective study of 649 cycles of HDMTX in 194 patients, renal toxicity occurred in 9.1% of cycles in patients with lymphoma compared to 1.5% in patients with sarcoma. Older age, male sex, decreased baseline CrCl, and increased proton pump inhibitor use among the lymphoma population likely contributed to the observed difference. The incidence of renal toxicity was independent of the incidence of delayed MTX elimination, suggesting that kidney function is only one factor involved in MTX clearance. Renal toxicity prolonged the duration of hospitalization but severe renal insufficiency was uncommon. No significant impact on progression free or overall survival was observed.
Keywords: high-dose methotrexate, renal toxicity, lymphoma
Introduction
High-dose methotrexate (HDMTX) regimens, defined by MTX doses ≥ 1g/m2, and subsequent use of leucovorin to reduce toxicity, are used to treat a wide range of malignancies including lymphoma, breast carcinoma, sarcoma, and others [1-3]. Despite its broad application, the use of HDMTX is complicated by the risk of renal toxicity [4]. Widemann et al report that the incidence of MTX-mediated renal injury is 1.8% in patients with osteosarcoma, but the incidence in hematologic malignancies is not well characterized [5]. Dehydration and low urine pH are known to increase renal toxicity risk and therefore, intravenous hydration and urine alkalinization are standard adjuncts in HDMTX regimens [6]. Other factors associated with increased MTX-mediated nephrotoxicity risk include advanced age, male sex, MTX dose, low baseline CrCL, and the use of certain antibiotics [7-12]. Because MTX is excreted renally, the onset of nephrotoxicity delays MTX clearance and thereby increases the risk of other systemic toxicities [6].
Though renal toxicity is known to cause delayed MTX clearance, alterations in MTX elimination and a subsequent risk of systemic toxicities can also occur in the absence of renal toxicity. For example, proton pump inhibitors (PPIs) slow MTX clearance via inhibition of a specific drug transport protein in the kidney without otherwise affecting renal function [13-15]. Non-steroidals and probenecid have also been shown to delay MTX clearance via similar mechanisms [16]. The incidence of delayed MTX elimination in the absence of renal toxicity has not been reported [17].
In this retrospective analysis of 194 patients who received 649 cycles of HDMTX, we investigated the incidence, risk factors and outcomes, including length of stay (LOS), progression free survival (PFS), and overall survival (OS), associated with MTX-mediated renal toxicity and delayed elimination.
Materials and Methods
Patients
Eligible patients at Barnes Jewish Hospital (BJH) were identified by screening the BJH pharmacy database of medical oncology inpatients from January 1, 2005 through April 31, 2011. Patients at the Veterans Health Administration (VHA) were identified from a database of patients with diffuse large B-cell lymphoma (DLBCL) as described by Carson et al [18]. Inclusion criteria included 1) age ≥ 18 years and 2) HDMTX dose ≥ 1g/m2. Exclusion criteria included a lack of recorded MTX serum levels at 24, 48, and 72 hours after MTX. A total of 649 cycles of HDMTX in 194 patients were evaluated (504 cycles in 151 patients at BJH, 145 cycles in 43 patients at VHA). Thirty-five cycles were excluded due to incomplete MTX serum level records.
The most common cancer types were lymphoma (72.8% of cycles, 151 patients), sarcoma (21% of cycles, 26 patients), and breast carcinoma (4.9% of cycles, 12 patients). Lymphoma histologies included primary central nervous system lymphoma (PCNSL) (337 cycles), DLBCL without CNS involvement (89 cycles), and Burkitt lymphoma (25 cycles). Other less common lymphoma histologies included mantle cell (3 cycles) and peripheral T-cell lymphomas (20 cycles). The majority of sarcoma patients were diagnosed with osteosarcoma (114 cycles) and angiosarcoma (12 cycles). Other sarcoma diagnoses included chondrosarcoma (5 cycles) and soft tissue sarcomas (6 cycles). There were 5 cycles with pre-B-cell acute lymphoblastic leukemia, one with small cell lung cancer, and one with pituitary carcinoma. All patients received similar supportive measures including hydration and urine alkalinization.
Data Collection
In patients who received HDMTX, additional information was retrieved from individual patient records. Data collected included patient age, sex, height, weight, race, diagnosis, baseline and peak serum creatinine, MTX dose, MTX serum level at 24/48/72 hours, administration of PPIs within 24 hours before or after MTX administration, and date of documented progression or death. For patients at BJH, an online social security number database was used to confirm date of patient death when none was recorded in the medical record.
Measurements and Definitions
Renal toxicity was defined by the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) v4.03 grading scale which is as follows: grade 1 = serum creatinine (SCr) > 1.1-1.5 × upper limit of normal (ULN); grade 2 = > 1.5-3.0x ULN; grade 3 = > 3.0-6.0x ULN; grade 4 = > 6.0x ULN [19]. Delayed MTX clearance was defined as serum MTX ≥ 15μmol/L at 24h, ≥1.5 μmol/L at 48h, or ≥ 0.15μmol/L at 72h [14]. Creatinine clearance was calculated using the CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) equation [20]. LOS was defined as the duration of time between MTX infusion and discharge. PFS was defined as the date of first HDMTX infusion to date of documented progression. OS was defined as date of first HDMTX infusion to date of death.
Statistics
Generalized Estimating Equation (GEE) was used to account for possible correlation between multiple MTX cycles in a single patient. Chi-square test, two sample T test, Fisher's exact test, Wilcoxon two sample test, and row mean score test were used for univariate analyses, where appropriate. A two-tailed α significance level of .05 was considered statistically significant. Cox proportional hazards models were used to assess the association of renal toxicity and delayed elimination with PFS and OS while controlling for variables identified during univariate analyses.
Results
Baseline Characteristics
Baseline patient characteristics of BJH and VHA patients are presented in Table I. The VHA population was older, predominantly male, and had a higher baseline serum creatinine (SCr) and lower baseline creatinine clearance (CrCl). Both groups received comparable MTX doses and number of cycles. There was a male sex predominance in the collective group (59.6% vs. 40.4%) secondary to both the demographics of the VHA patient population as well as higher rates of osteosarcoma and lymphoid malignancies in males [21,22].
Table I.
Baseline characteristics of the BJH and VAMC populations.
| Characteristics | BJH (%) | VHA (%) | p-value |
|---|---|---|---|
| Age* | 49.9 | 59.3 | <0.0001† |
| Sex | <0.0001^ | ||
| Male | 49.6 | 93.8 | |
| Female | 50.4 | 6.3 | |
| Baseline SCr | 0.76 | 0.99 | <0.0001† |
| Baseline CrCl (mL/min) | 0.0002§ | ||
| 30-59 | 7.3 | 18.8 | |
| 60-89 | 19.1 | 30.6 | |
| >90 | 71.8 | 50.7 | |
| MTX Dose (g/m2) | 0.9600§ | ||
| 1-2 | 5.4 | 15.3 | |
| >2-3 | 9.3 | 17.4 | |
| >3-4 | 64.1 | 26.4 | |
| >4-6 | 8.3 | 19.4 | |
| >6 | 12.9 | 21.5 | |
| Median # of cycles | 2 | 3 | 0.8104# |
| PPI use | 40.7 | 51.4 | 0.0220^ |
Key:
chi-square test,
two sample T test,
Wilcoxon two sample test,
row mean score test,
age values in mean years
Incidence of Mtx-Mediated Renal Toxicity and Delayed Mtx Elimination
Figure 1 depicts the incidence of grade 2-4 renal toxicity and delayed elimination for lymphoma and sarcoma patients. Grade 2-4 renal toxicity occurred in approximately 9% of cycles in lymphoma patients and just over 1% of cycles in sarcoma patients. This compares to the incidence of grade 2-4 renal toxicity in osteosarcoma patients as reported by Widemann et al of 1.8% [5]. Delayed elimination of MTX occurred in 32% of cycles in lymphoma patients and 15% of cycles in sarcoma patients. Of note, the incidence of toxicity and delayed elimination in lymphoma patients at BJH and VHA were comparable.
Figure 1.
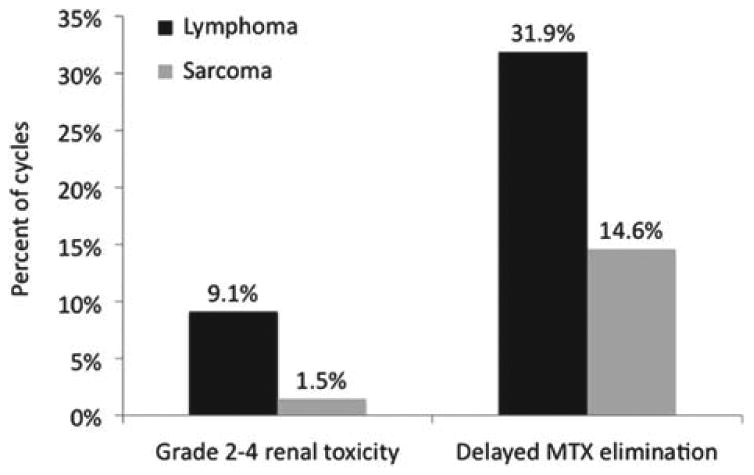
Comparison of the incidence of HDMTX-mediated renal toxicity and delayed MTX elimination in patients with lymphoma and sarcoma.
Relationship Between Grade 2-4 Renal Toxicity and Delayed Mtx Elimination
Figure 2 is a Venn diagram illustrating the overlap between the occurrence of grade 2-4 renal toxicity and the occurrence of delayed MTX elimination. A total of 9 out of 46 cycles (20%) associated with grade 2-4 renal toxicity were not associated with delayed MTX elimination. A total of 134 out of 171 cycles (78%) associated with delayed MTX elimination were not associated with grade 2-4 renal toxicity. In 37 out of 179 cycles (21%), both grade 2-4 renal toxicity and delayed MTX elimination occurred.
Figure 2.
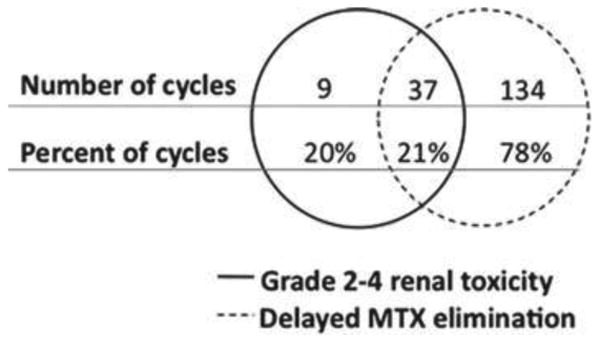
Venn diagram depicting the relationship between renal toxicity and delayed elimination. Th e diagram is composed of two circles, one indicating grade 2 – 4 renal toxicity and one indicating delayed MTX elimination. Th e space between represents agreement between the two metrics. Values in the left circle represent the number and percent of encounters that fi t grade 2 – 4 renal toxicity but did not fi t delayed MTX elimination criteria. Values in the right circle represent the number and percent of cycles that fi t criteria for delayed MTX elimination but did not fi t the criteria for grade 2 – 4 renal toxicity
Risk Factors for Hdmtx Renal Toxicity
Risk factors analyzed included age, sex, baseline CrCl, MTX dose (g/m2), and PPI use. Each risk factor was evaluated for both grade 2-4 renal toxicity and delayed MTX elimination. Table II presents results from all patients but results in the lymphoma subset were comparable. Correlations between risk factors were tested for but none were identified.
Table II.
Risk factors for HDMTX-mediated renal toxicity and delayed MTX elimination.
| Grade 2-4 renal toxicity | Delayed MTX elimination | |||||
|---|---|---|---|---|---|---|
| Yes (%) | No (%) | p-value | Yes (%) | No (%) | p-value | |
| Age* | 60.8 | 51.3 | <0.0001† | 57.6 | 50 | <0.0001† |
| Sex | 0.0069^ | <0.0001^ | ||||
| Male | 78.3 | 58 | 73.7 | 52.4 | ||
| Female | 21.7 | 42 | 26.3 | 47.6 | ||
| Baseline CrCl (mL/min) | 0.0001# | <0.0001§ | ||||
| 30-59 | 41.3 | 9 | 18.7 | 7 | ||
| 60-89 | 28.3 | 21.1 | 29.8 | 19.3 | ||
| >90 | 30.4 | 69.9 | 51.5 | 73.7 | ||
| MTX dose (g/m2) | 0.0671§ | 0.1077^ | ||||
| 1-2 | 13 | 7.1 | 2.3 | 5 | ||
| >2-3 | 6.5 | 11.5 | 12.3 | 5.7 | ||
| >3-4 | 69.6 | 54.7 | 45 | 54 | ||
| >4-6 | 2.2 | 11.5 | 1.2 | 8.8 | ||
| >6 | 8.7 | 15.3 | 39.2 | 26.5 | ||
| PPI use | 50 | 42.5 | 0.3237^ | 53.8 | 39.3 | 0.0011^ |
Key:
chi-square test,
two sample T test,
Wilcoxon two sample test,
row mean score test,
age values in mean years
Effects on Length of Stay
Mean hospitalization time for cycles where the patient developed grade 2-4 renal toxicity was 8.9 days compared to 5.6 days for those without renal toxicity (p=0.0005). Mean hospitalization time for cycles where the patient experienced delayed MTX elimination was 7.8 days compared to 4.5 days in those without (p=<0.0001).
Effects on Progression Free Survival (Pfs) and Overall Survival (Os) in Lymphoma Patients
Neither renal toxicity nor delayed elimination had a significant effect on OS (HR 0.677, 95% CI 0.404-1.134; HR 0.891, 95% CI 0.562-1.415). Renal toxicity increased median PFS from 10.7 months to 35.7 months (HR 0.508, 95% CI 0.305-0.845) while delayed elimination had no effect (HR 0.891, 95% CI 0.562-1.415).
Discussion
In this patient cohort treated with HDMTX, the incidence of renal toxicity was 9.1% of cycles in lymphoma patients and 1.5% of cycles in sarcoma patients. The incidence of delayed MTX elimination was higher than that of renal toxicity but exhibited a similar imbalance between lymphoma and sarcoma populations (31.9% vs. 14.6%).
These findings highlight two important points. First, the incidence of delayed elimination is frequently not associated with measurable renal toxicity. Only 21% of cycles associated with delayed elimination resulted in any renal toxicity. Although MTX is renally excreted, this suggests that the pharmacokinetics is more complex than previously thought. For example, biliary excretion may be important for some patients [23,24]. Another possible mechanism is genetic variation in proteins involved in MTX metabolism may act independently of renal failure. Genome-wide association studies have already identified specific genes, such as anion transporter gene SLCO1B1, that are associated with inter-individual variability in MTX transport and could therefore explain why so few patients with delayed excretion had renal failure [25].
Second, the incidence of both renal toxicity and delayed elimination are higher in the lymphoma population compared to patients with other malignancies. The baseline characteristics of patients with lymphoma compared to those with sarcoma or breast cancer, may explain these differences. The lymphoma cohort was older, had a higher percentage of males, had a lower baseline CrCl, and had a higher rate of PPI use than the solid tumor cohort (Table III). All of these factors were associated with increased risk of renal toxicity and/or delayed clearance.
Table III.
Baseline characteristics of the lymphoma and solid tumor populations.
| Characteristics | Lymphoma (%) | Solid Tumor (%) | p-value |
|---|---|---|---|
| Age* | 56.5 | 40 | <0.0001† |
| Gender | <0.0001^ | ||
| Male | 66.2 | 41.4 | |
| Female | 33.8 | 58.6 | |
| Baseline CrCl (mL/min) | 0.0087 § | ||
| <15 | 1.1 | 2.4 | |
| 15-29 | 0 | 0 | |
| 30-59 | 11.8 | 4.7 | |
| 60-89 | 24.3 | 14.8 | |
| >90 | 62.8 | 78.1 | |
| MTX Dose (g/m2) | <0.0001§ | ||
| 0-2 | 6.8 | 8.9 | |
| >2-3 | 14 | 3 | |
| >3-4 | 66.2 | 26.6 | |
| >4-6 | 6.1 | 24.3 | |
| >6 | 7 | 37.3 | |
| PPI use | 47.6 | 30.8 | 0.0002 ^ |
Key:
chi-square test,
two sample T test,
Wilcoxon two sample test,
row mean score test,
age values in mean years
Notably, of the four risk factors identified, PPI use was the only one that increased risk for delayed elimination but not renal toxicity, supporting the evidence that PPIs delay MTX clearance via transport protein inhibition rather than nephrotoxicity [26]. This finding suggests that the incidence of delayed elimination without renal toxicity could not only be related to biliary excretion or genetic variation in transport proteins as suggested above, but may also be secondary to drug-drug interactions.
The majority of incidents of renal toxicity in our study population were reversible without significant intervention. In the entire cohort, only one patient required emergent hemodialysis and compassionate-use carboxypeptidase. Though lasting renal injury was rare, other clinical consequences were investigated and shown to be associated with renal toxicity and delayed elimination. First, the occurrence of renal toxicity and/or delayed elimination was associated with increased duration of hospitalization. A longer length of stay increases costs and delays treatment, two arguments for the importance of preventing renal toxicity and clearance delays.
In contrast, the occurrence of renal toxicity was found to be associated with an increase in PFS. However, delayed elimination did not show this same correlation and neither delayed elimination nor renal toxicity were shown to affect OS. Ferreri et al have previously reported that lower baseline CrCl was associated with an increase in 3 year overall survival in a group of 45 patients with primary CNS lymphoma perhaps based on the premise that a lower CrCl could decrease MTX clearance, increase duration of exposure of cancer cells to the drug, and thereby lead to improved survival outcomes [8]. Though our data suggests a relationship between renal toxicity and PFS, the lack of correlation between delayed elimination and PFS as well as the lack of correlation between both metrics and OS does not support a significant survival benefit. Importantly, this survival data should be interpreted with caution as there was significant variability in the amount of treatment patients had received prior to HDMTX therapy.
In conclusion, the results of this study indicate that despite adequate hydration and urine alkalinization, HDMTX-mediated renal toxicity and delayed elimination continue to occur at a much higher rate in patients with lymphoma compared to solid tumors. This discrepancy in incidence is likely due, at least in part, to differences in the baseline characteristics of the lymphoma population including older age, predominance of male sex, lower baseline CrCl and higher rates of PPI use. Clinically, though occurrence of renal toxicity and clearance delays increase hospitalization duration and may delay subsequent treatments, such events do not appear to alter patient outcomes. Awareness of the higher incidence in lymphoma patients as well as an understanding of the risk factors that make this population more susceptible will hopefully allow clinicians to better anticipate and therefore avoid HD-MTX mediated renal toxicity and delayed elimination.
Footnotes
Potential Conflicts of Interests: The authors have no potential conflicts of interests.
References
- 1.Joerger M, Huitema ADR, Illerhaus G, Ferreri AJM. Rational administration schedule for high-dose methotrexate in patients with primary central nervous system lymphoma. Leukemia & Lymphoma. 2012;53:1867–1875. doi: 10.3109/10428194.2012.676177. [DOI] [PubMed] [Google Scholar]
- 2.Munzone E, Curigliano G, Burstein HJ, Winer EP, Goldhirsch A. CMF revisited in the 21st century. Annals of Oncology. 2012;23:305–311. doi: 10.1093/annonc/mdr309. [DOI] [PubMed] [Google Scholar]
- 3.D'Adamo DR. Appraising the current role of chemotherapy for the treatment of sarcoma. Seminars in Oncology. 2011;38:S19–S29. doi: 10.1053/j.seminoncol.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 4.Frei E, 3rd, Blum RH, Pitman SW, et al. High dose methotrexate with leucovorin rescue: Rationale and spectrum of antitumor activity. American Journal of Medicine. 1980;68:370–376. doi: 10.1016/0002-9343(80)90105-9. [DOI] [PubMed] [Google Scholar]
- 5.Widemann BC, Balis FM, Kempf-Bielack B, et al. High-dose methotrexate-induced nephrotoxicity in patients with osteosarcoma. Cancer. 2004;100:2222–2232. doi: 10.1002/cncr.20255. [DOI] [PubMed] [Google Scholar]
- 6.Abelson HT, Fosburg MT, Beardsley GP, et al. Methotrexate-induced renal impairment: clinical studies and rescue from systemic toxicity with high-dose leucovorin and thymidine. Journal of Clinical Oncology. 1983;1:208–216. doi: 10.1200/JCO.1983.1.3.208. [DOI] [PubMed] [Google Scholar]
- 7.Relling MV, Fairclough D, Ayers D, et al. Patient characteristics associated with high-risk methotrexate concentrations and toxicity. Journal of Clinical Oncology. 1994;12:1667–1672. doi: 10.1200/JCO.1994.12.8.1667. [DOI] [PubMed] [Google Scholar]
- 8.Ferreri AJM, Guerra E, Regazzi M, et al. Area under the curve of methotrexate and creatinine clearance are outcome-determining factors in primary CNS lymphomas. British Journal of Cancer. 2004;90:353–358. doi: 10.1038/sj.bjc.6601472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Green MR, Chamberlain MC. Renal dysfunction during and after high-dose methotrexate. Cancer Chemotherapy and Pharmacology. 2009;63:599–604. doi: 10.1007/s00280-008-0772-0. [DOI] [PubMed] [Google Scholar]
- 10.de Miguel D, García-Suárez J, Martín Y, Gil-Fernández JJ, Burgaleta C. Severe acute renal failure following high-dose methotrexate therapy in adults with haematological malignancies: a significant number result from unrecognized co-administration of several drugs. Nephrology, Dialysis, Transplantation. 2008;23:3762–3766. doi: 10.1093/ndt/gfn503. [DOI] [PubMed] [Google Scholar]
- 11.Blum R, Seymour JF, Toner G. Significant impairment of high-dose methotrexate clearance following vancomycin administration in the absence of overt renal impairment. Annals of Oncology. 2002;13:327–330. doi: 10.1093/annonc/mdf021. [DOI] [PubMed] [Google Scholar]
- 12.Dalle JH, Auvrignon A, Vassal G, Leverger G. Interaction between methotrexate and ciprofloxacin. Journal of Pediatric Hematology/Oncology. 2002;24:321–322. doi: 10.1097/00043426-200205000-00021. [DOI] [PubMed] [Google Scholar]
- 13.Joerger M, Huitema ADR, van den Bongard HJGD, et al. Determinants of the elimination of methotrexate and 7-hydroxy-methotrexate following high-dose infusional therapy to cancer patients. British Journal of Clinical Pharmacology. 2006;62:71–80. doi: 10.1111/j.1365-2125.2005.02513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Santucci R, Levêque D, Lescoute A, Kemmel V, Herbrecht R. Delayed elimination of methotrexate associated with co-administration of proton pump inhibitors. Anticancer Research. 2010;30:3807–3810. [PubMed] [Google Scholar]
- 15.Suzuki K, Doki K, Homma M, et al. Co-administration of proton pump inhibitors delays elimination of plasma methotrexate in high-dose methotrexate therapy. British Journal of Clinical Pharmacology. 2009;67:44–49. doi: 10.1111/j.1365-2125.2008.03303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takeda M, Khamdang S, Narikawa S, et al. Characterization of methotrexate transport and its drug interactions with human organic anion transporters. Journal of Pharmacology & Experimental Therapeutics. 2002;302:666–671. doi: 10.1124/jpet.102.034330. [DOI] [PubMed] [Google Scholar]
- 17.Widemann B, Adamson P. Understanding and managing methotrexate nephrotoxicity. The Oncologist. 2006;11:694–703. doi: 10.1634/theoncologist.11-6-694. [DOI] [PubMed] [Google Scholar]
- 18.Carson KR, Bartlett NL, McDonald JR, et al. Increased body mass index is associated with improved survival in united states veterans with diffuse large b-cell lymphoma. Journal of Clinical Oncology. 2012;30:3217–3222. doi: 10.1200/JCO.2011.39.2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.National Cancer Institute. Common Terminology Criteria for Adverse Events v4.03. NCI, NIH, DHHS. 2010 Jun 14; Internet. NIH publication #09-7473. [cited 2013 Aug 1]. Available from: http://evs.nci.nih.gov/ftp1/CTCAE/About.html.
- 20.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Annals of Internal Medicine. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mirabello L, Troisi RJ, Savage SA. Osteosarcoma incidence and survival rates from 1973 to 2004: data from the surveillance, epidemiology, and end results program. Cancer. 2009;115:1531–1543. doi: 10.1002/cncr.24121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morton LM, Wang SS, Devesa SS, Hartge P, Weisenburger DD, Linet MS. Lymphoma incidence patterns by who subtype in the United States, 1992-2001. Blood. 2006;107:265–276. doi: 10.1182/blood-2005-06-2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ito K, Oleschuk CJ, Westlake C, et al. Mutation of trp1254 in the multispecific organic anion transporter, multidrug resistance protein 2 (MRP2) (ABCC2), alters substrate specificity and results in loss of methotrexate transport activity. Journal of Biological Chemistry. 2001;276:38108–38114. doi: 10.1074/jbc.M105160200. [DOI] [PubMed] [Google Scholar]
- 24.Joannon P, Oviedo I, Campbell M, Tordecilla J. High-dose methotrexate therapy of childhood acute lymphoblastic leukemia: lack of relation between serum methotrexate concentration and creatinine clearance. Pediatric Blood & Cancer. 2004;43:17–22. doi: 10.1002/pbc.20032. [DOI] [PubMed] [Google Scholar]
- 25.Ramsey LB, Panetta JC, Smith C, et al. Genome-wide study of methotrexate clearance replicates SLCO1B1. Blood. 2013;121:898–904. doi: 10.1182/blood-2012-08-452839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Breedveld P, Zelcer N, Pluim D, et al. Mechanism of the pharmacokinetic interaction between methotrexate and benzimidazoles: potential role for breast cancer resistance protein in clinical drug-drug interactions. Cancer Research. 2004;64:5804–5811. doi: 10.1158/0008-5472.CAN-03-4062. [DOI] [PubMed] [Google Scholar]


