Summary
Background
Given the immunogenicity of NYESO-1 peptides in prostate cancer, a phase I clinical trial was designed to evaluate HLA class-I and class-II restricted NYESO-1 peptides in metastatic castration-resistant prostate cancer (mCRPC).
Methods
Patients with progressive mCRPC, Zubrod Performance Status ≤2, PSA ≥10 ng/ml who had appropriate HLA class I (A2) and class II haplotypes (DR4, DP4) were eligible. Three groups with 3 patients each received the vaccine subcutaneously every 2 weeks for 6 doses. Group 1 received a peptide presented by an HLA class I haplotype (HLA-A2), Group 2 with a peptide presented by HLA class II haplotype (DR4, DP4), and Group 3 with peptides presented by both Class I and II haplotypes. Androgen-deprivation was continued. Owing to a myocardial infarction, the protocol was amended to omit the use of GMCSF.
Results
Fourteen patients were evaluable for toxicities and 9 received all 6 doses and were evaluable for efficacy. One death from myocardial infarction following GM-CSF occurred in a patient with generalized myalgias. After omitting GM-CSF, no grade >2 toxicities were observed. Among 9 patients evaluable for efficacy, the median PSA doubling time pre-therapy and during therapy were 3.1 and 4.92 months, respectively. NY-ESO-1 specific T-cell response observed by ELISPOT appeared more frequent in docetaxel-naïve patients (4 of 4) than docetaxel-pretreated patients (2 of 5).
Conclusion
In men with mCRPC, individualized HLA class-I and/or class-II restricted NY-ESO-1 peptides were tolerable, appeared to slow PSA doubling time and yielded antigen-specific T-cell responses more often in chemonaïve patients.
Keywords: NY-ESO-1, Castration-resistant prostate cancer, Peptide, Immunotherapy, HLA-restricted, Cancer vaccines
Introduction
Multiple new anti-tumor agents have become available to treat men with metastatic castration resistant prostate cancer (mCRPC) including cabazitaxel, abiraterone, enzalutamide and sipuleucel-T [1]. Immunotherapy with sipuleucel-T represents the first therapeutic vaccine demonstrated to improve outcomes in an advanced malignancy and provides a rationale to continue the investigation of immunotherapy for CRPC. Despite these advances, the median survival of mCRPC is ~20 months. Hence, the discovery of novel and tolerable agents remains a priority in this elderly population with generally multiple comorbidities.
Testis cancer antigens, including NY-ESO-1, are expressed in a variety of malignancies but not in normal tissues with the exception of testis [2, 3]. Additionally, NY-ESO-1 elicits humoral and cellular responses associated with anti-tumor activity [4–7]. NY-ESO-1 expression ranges from 5 % to 30 % of patients with mostly localized or locally advanced prostate cancer [8]. NY-ESO-1 was recently identified as an MHC class II restricted tumor antigen [9, 10]. Sixteen of 17 melanoma patients who developed a humoral response against NY-ESO-1 were HLA-DP4-positive [11].
Clinical and immune responses have been observed in melanoma patients treated with NY-ESO-1 peptides [12–16]. T cells can recognize NY-ESO-1 peptides presented by different HLA molecules, and have also been induced from peripheral blood mononuclear cells (PBMCs) of patients with prostate cancer [9–11, 17, 18]. Hence, we planned a phase I trial at the Baylor College of Medicine to primarily evaluate the feasibility and immunogenicity of tailored NY-ESO-1 class-I and/or class-II restricted peptides in men with mCRPC and corresponding HLA genotypes. We hypothesized that stimulation of T cell responses by MHC class I- and II-restricted peptides will be safe and will enhance anti-tumor immune response. Furthermore, we aimed to identify potential differentials in signals of immunogenicity by HLA genotype and preliminary signals of antitumor activity. We also planned to employ GM-CSF (granulocyte macrophage colony stimulating factor) as an adjuvant based on clinical trials demonstrating safety and activity as a single agent and as cellular GM-CSF secreting immunotherapy [19, 20].
Materials and methods
Patient eligibility
Patients with progressive mCRPC regardless of prior chemotherapy, Zubrod Performance Status ≤2, PSA ≥10 ng/ml and a castrate testosterone level (<50 ng/dL) were eligible. Progression was defined as symptomatic or measurable tumor progression, or PSA increase ≥50 % confirmed by repeating ≥2 weeks later. Adequate bone marrow, hepatic and renal function were required. The appropriate HLA class I (A2) and class II haplotypes (DR4, DP4) were required. Patients with central nervous system metastasis, immunosuppressive drugs intake and serious acute illness and those positive for hepatitis B surface antigen, Hepatitis C or HIV antibody were ineligible. Patients with a history of cardiac arrhythmia or ischemic heart disease were ineligible following an amendment owing to a myocardial infarction in one patient (see Feasibility and toxicities section below). The trial was approved by the BCM Institutional Review Board (IRB) and registered on clinicaltrials.gov (NCT00711334).
Synthesis of GMP grade NY-ESO-1 peptides
The most immunogenic peptides restricted by DR4, DP4 and HLA-A2 restricted NY-ESO-1 peptide were employed [11]. The peptide sequences are as follows: DR4-restricted NY-ESO-1 peptide: PGVLLKEFTVSG (ESO DR4-1P), DP4-restricted NY-ESO-1 peptide: YGRKKRRQRRRSLLMWITQAFLPV, and the A2-retricted peptide: SLLMWITQC.
Trial design and administration of therapy
A phase I trial was designed with a primary endpoint of safety and a secondary endpoint of immune response. Three groups with ≥3 patients in each group received the peptides every 2 weeks for 6 doses. If class I or class II binding peptides were safe individually in patients with class I and class II genotypes respectively, subsequent patients with both HLA class I and class II types were planned to be able to receive both types of peptides in combination. Group 1 received a peptide presented by an HLA class I haplotype (HLA-A2), Group 2 was treated with a peptide binding to an HLA class II haplotype (DR4, DP4), and Group 3 received peptides presented by both Class I and II haplotypes. Logistically, if the patient was HLA-A2 positive, he was assigned to treatment group 1, regardless of the DR or DP type, until treatment group 1 accrual was complete. If group 1 filled, the HLA-A2 positive patient was enrolled in group III if positive for DR4 or DP4. If HLA-A2 was negative, and DR4 or DP4 was expressed, the patient was assigned to group 2. If one class II marker was positive, the patient received the corresponding peptide. If more than one class II marker was positive, then the peptide chosen targeted DR4 preferably, and then DP4. The decision to prefer DR4 was made arbitrarily, but was intended to have a consistent schema rather than random selection of DR4 or DP4. If grade ≥3 toxicity up to 1 month following the last dose attributable to therapy was observed in either Group I or Group II, then enrollment was held in Group III. If only one subject in a cohort experienced grade ≥3 toxicity, then the cohort was expanded by 3 patients, and the cohort terminated if grade ≥3 toxicity occurred in an additional patient. If grade ≥3 toxicitywas observed in >1 patient in any group, then no further patients were enrolled into that group or Group III. If toxicity grade ≥3 occurred in 3 patients, the study was to be terminated.
Group I and Group II received 1 dose of 1 mg and Group III received doses of 1 mg for each of the 2 peptides. This dose was chosen based on the activity of this dose in prior clinical studies in other malignancies and there was no dose escalation [16]. The peptides were stored at −20 °C in 0.5 ml and mixed with Montanide ISA 51 prior to administration. The dose and formulation have been derived from previous clinical trials in other malignancies [16, 21–23]. The vaccine was delivered subcutaneously in the upper arm in a volume of 1 ml using standard methods of good clinical practice and the patient was observed for 1 h. Androgen-deprivation was continued. Granulocyte-macrophage colony stimulating factor (GM-CSF) was to be given for 5 days, beginning 2 days before peptide administration, but the protocol was amended to remove the administration of GM-CSF due to toxicity in an initial patient (see below).
Clinical and laboratory assessments during therapy
Clinical tumor evaluation assessment was performed at baseline and at least every 12 weeks. History and physical exam were performed at baseline and at weeks 5 and 13. Complete blood cell counts, comprehensive metabolic profile, LDH, PSA and urinalysis were performed at baseline and weeks 5 and 11. Blood was drawn prior to treatment, week 5 and 11 and at post-treatment evaluation in week 13 for immune monitoring studies. The tubes were transported to the laboratory where PBMCs were isolated by Ficoll-Paque gradient (Pharmacia AB, Uppsala, Sweden) and cryopreserved until ≥3 sequential specimens were obtained for a single experiment, thereby minimizing inter-assay variability.
Measurement of PBMCs/T-cell response to NY-ESO-1
Increased Th1 cytokine elaboration compared to baseline by the patient’s PBMCs against NY-ESO-1 peptides was measured in vitro by Enzyme-linked immunosorbent spot (ELISPOT) assays. PBMCs from patients were suspended in RPMI-1640 supplemented with 10 % heat-inactivated AB serum (Valley Biomedical, Winchester, USA), 2 mM lglutamine, 100 U/ml penicillin and 100 μg/ml streptomycin. Cells were cultured in 24-well plates, and individual NY-ESO-1 peptides were added to a final concentration of 20 μg/ml per well and incubated for 7 days at 37 °C, 5 % CO2 in humidified air. Recombinant hIL-2 (Proleukin; Chiron, Emeryville, CA, USA) 300 U/ml was added on day 1. Cells were harvested on day 7, washed twice in RPMI-1640 and used for ELISPOT assay. The IFN-γ ELISPOT assay was performed as previously described to quantify peptide-specific PBMCs/T cells after in vitro expansion [24].
Measurement of antibody response to NY-ESO-1
Antibodies to NY-ESO-1 were measured by Enzyme Linked Immunosorbent Assay (ELISA), which was performed as previously described with a slight modification [25]. A positive reaction is defined as an OD value of diluted serum that exceeds the mean OD value of sera from normal donors by 2 SD values.
Statistical considerations
Assuming that the population incidence of HLA-A2 is 50 % and 78 % of patients will be positive for HLA-DR4, -DP4, or -DR13 molecules, to enroll ≥3 patients for each of the 3 groups, 16–18 prequalified patients were planned to be screened for HLA-A2 (http://www.ashi-hla.org/publicationfiles/archives/prepr/mori_abd.htm) [26]. The primary goal of the Phase I trial was to evaluate safety. Evaluability for toxicity required completion of the first four treatments with vaccine unless serious toxicity resulted in treatment modification before then, in which case the patient was evaluable regardless of the number of weeks of treatment. For NY-ESO-1 peptides, the sample size of 3 patients in each group assured that there was 83.4 % probability of observing at least one patient with a toxic event if the true rate of toxicity for the whole population is 45 %, and 70.3 % probability if the true rate is 33.3 %. Summary statistics were performed and implausible and missing values were identified and double-checked with laboratory personnel for accuracy. Mean differences between immune parameters prior to the first vaccination and after treatments were assessed using paired t-tests when the normality assumption was true based on the Kolmogorov-Smirnov test. Otherwise, the Wilcoxon signed rank test was employed. The criterion for significance (α) was 0.05. All tests were 2-tailed and analysis was conducted using SPSS version 11. The baseline and on-trial PSA-doubling time (DT) was calculated by using an online calculator (http://nomograms.mskcc.org/Prostate/PsaDoublingTime.aspx) and by employing ≥3 PSA values over a period of 1–4 months immediately preceding and during the 12 weeks of trial therapy, respectively.
Results
Patient characteristics
Patients were enrolled between June 2006 and March 2011 in the Michael E. DeBakey Veterans Affairs Medical Center, an affiliate of Baylor College of Medicine, Houston, Texas(Table 1). Fourteen patients received any protocol related therapy and were considered evaluable for toxicities. Three of the 14 patients received GM-CSF with 0, 2 and 5 doses of vaccine, respectively. Two other patients received 1 and 2 doses of the vaccine, respectively, without GM-CSF. Thus, 9 patients received all 6 doses without GM-CSF and were considered evaluable for efficacy, i.e. clinical and immune responses. The median PSA was 56.7 ng/ml in evaluable patients. All patients had bone metastasis and pain was present in 6 of 9 evaluable patients. Of the 9 evaluable patients, 4 were chemonaïve and 5 had received docetaxel-based chemotherapy.
Table 1.
Patient demographics (N=14)
| Parameter | Overall N=14 | Evaluable N=9 |
|---|---|---|
| Age (years) | 70.5 (54–81) | 69 (54–81) |
| Ethnicity | ||
| Caucasian | 6 | 4 |
| African | 8 | 5 |
| ECOG performance status | ||
| 0–1 | 13 | 9 |
| NA | 1 | 0 |
| Metastasis site (PCWG-2 subtype) | ||
| Bone +/− soft tissue | 8 | 5 |
| Visceral+/− other sites | 4 | 2 |
| Soft tissue/lymph node | 2 | 2 |
| Median PSA (ng/ml) | 96.6 (17.7–4583.7) | 56.7 (17.7–4583.7) |
| Prior docetaxel (N) | 6 | 5 |
| Prior radiation to metastatic site (N) |
2 | 1 |
| Prior definitive local therapy | ||
| Radical prostatectomy | 5 | 3 |
| Radiotherapy | 6 | 4 |
| Baseline Hemoglobin (gm/dl) |
11.1 (9.1–13.1) | 12.4 (10.3–13.1) |
| Baseline pain (N) | 8 | 6 |
Feasibility and toxicities
The protocol initially specified that GM-CSF was to be given for 5 days, beginning 2 days before the peptide administration. A fatal myocardial infarction occurred in one patient after his first dose of GM-CSF was administered but before any peptide was given. The patient had diabetes, hypertension, and multiple bony metastases. He experienced significant pain probably from the growth factor, which was considered to be a possible precipitating factor that led to his myocardial infarction. Following this event, GM-CSF was omitted from the conditioning regimen and patients with prior cardiovascular diseases were excluded from the protocol. These decisions were made based on the comments from the data safety review board since the potential risks from GM-CSF were considered to outweigh the potential benefits. The exclusion of patients with cardiac disease was predicated on the possibility that arthralgias and myalgias from the vaccine itself may potentially pose a risk.
Of the remaining 2 men who received GM-CSF with vaccine, one patient was removed from trial after 2 doses due to grade 4 anemia, and another patient was removed after 5 doses due to grade 3 syncope unlikely related to therapy. Of the 2 men who received 1 or 2 doses of the vaccine without GM-CSF, one was removed after 2 doses due to grade 2 chest pain possibly related to therapy and another patient was removed after one dose due to disease progression. After this modification in the protocol, no dose-limiting or grade ≥2 toxicities were observed that were possibly related to therapy (Table 2). Potential therapy related grade 1 toxicities were local injection site erythema (n=5), fatigue (n=3), flu-like symptoms (n=1), myalgias (n=1), anorexia (n=2), nausea (n=2) and leukocytosis (n=2).
Table 2.
Toxicities in patients receiving any protocol therapy (N=14)
| Grade toxicity | Number of patients with Grade 1 toxicities |
Number of patients with Grade 2 toxicities |
Number of patients with Grade ≥3 toxicities |
|---|---|---|---|
| Injection site erythema | 5 | 0 | |
| Fatigue | 3 | 0 | |
| Flu-like symptoms | 1 | 0 | |
| Pain | 1* | ||
| Myalgias | 1 | 0 | |
| Anorexia | 2 | 0 | |
| Nausea | 2 | 0 | |
| Leukocytosis | 2 | 2 | |
| Neutrophilia | 1 | ||
| Eosinophilia | 1 | 1 | |
| Dyspnea | 1 | ||
| Tachycardia | 1 | ||
| Chest pain | 1 | ||
| Myocardial infarction | 1* |
9 patients received all 6 doses without GM-CSF, 3 patients received GM-CSF with 0,2 and 5 doses of vaccine, 2 patients received 1–2 doses of vaccine without GM-CSF;
1 death from myocardial infarction occurred following GM-CSF and was considered possibly from therapy (the patient did not receive the peptide); this patient also had generalized pain and myalgias after GM-CSF injection
Immunological response
PBMCs/T cell responses were observed against the corresponding NY-ESO-1 peptides as measured by ELISPOT assay (Fig. 1). Peptide-specific T cells were induced in 6 out of 9 evaluable patients at any time point up to 13 weeksafter baseline, but were not detectable in 3 patients even after receiving 6 peptide doses (Table 3). Increase in ELISPOT response with time was observed. Antigen-specific T cell responses were observed in patients who received peptides restricted by class I and/or class II molecules. Docetaxel-naïve patients appeared to exhibit T-cell responses more frequently (4 of 4) compared to docetaxel pretreated patients (2 of 5). Among the 6 patients with T-cell response, 3 exhibited slowing of PSA-doubling time (DT), while all 3 patients without a detectable T-cell response exhibited slowing of PSA-DT (Table 3). NY-ESO-1 antibody was only detectable in 1 of 9 patients (this patient had received a DR4 restricted NY-ESO-1 peptide immunization) (Fig. 2). However, the peptide immunization did not changethe NY-ESO-1 antibody titer in any patient during the course of vaccinations (Fig. 2).
Fig. 1.
Changes in the frequency of NY-ESO-1 peptide specific T cells in PBMCs from the prostate cancer patients. PBMCs were collected prior to and after vaccinations with peptides, and then were stimulated with the peptides in vitro for a week. Vaccination-induced T cell responses against the corresponding NY-ESO-1 peptides were measured by ELISPOT analysis and data show the mean number of peptide specific IFN-γ spot-forming cells (SFC) in response to a given peptide by 1×106 in vitro stimulated PBMCs. Numbers of non-specific IFN-γ spot-forming cells are subtracted
Table 3.
PSA doubling time pretherapy and on-therapy in evaluable patients who completed all 6 planned doses of peptide (N=9)
| Patient # ID # |
HLA restriction |
Prior docetaxel |
PSA-doubling time (mo) |
Slowing of PSA doubling Timea |
T-cell Response (ELISPOT) |
Antibody response |
|
|---|---|---|---|---|---|---|---|
| Baseline | On- therapy |
||||||
| 1 (10) | DR4 | No | +3.46 | +9.50 | + | + | − |
| 2 (12) | DP4 | No | +28.8 | +7.80 | − | + | − |
| 3 (13) | A2 | No | +4.44 | +4.09 | − | + | − |
| 4 (16) | A2 | Yes | +0.97 | +1.99 | + | + | − |
| 5 (18) | A2 | Yes | +2.51 | +1.38 | − | + | − |
| 6 (23) | A2, DP4 | No | +3.81 | +4.92 | + | + | − |
| 7 (24) | DR4 | Yes | +1.40 | +10.6 | + | − | − |
| 8 (25) | A2, DR4 | Yes | +1.35 | +2.27 | + | − | − |
| 9 (29) | A2, DP4 | Yes | +3.1 | −4.83a | + | − | − |
The baseline and on-trial PSA-doubling time (DT) was calculated on an online calculator (http://nomograms.mskcc.org/Prostate/PsaDoublingTime.aspx) and by using ≥3 PSA values over 1–4 months immediately preceding and during the 12 weeks of trial therapy, respectively. A negative PSA-DT indicates a negative overall slope
Fig. 2.
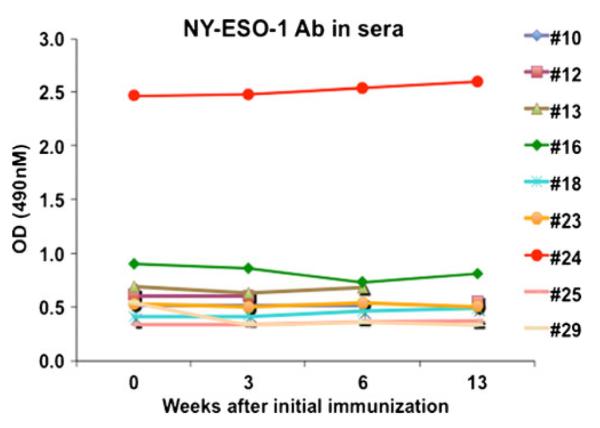
Presence of NY-ESO-1 antibody in the prostate cancer patients. Sera were collected prior to and after vaccinations with peptides, and were diluted in 2 % BSA/PBS at 1:100. ELISA assay was used for detection of the presence of NY-ESO-1 antibody in the diluted sera. Each symbol represents a patient at baseline (week 0), week 3, week 6 or 13 after initial peptide immunization. Positive reaction is defined as an OD value of diluted serum that exceeds the mean OD value of sera from normal donors by 2 SD values
Increase in PSA doubling time
The median PSA doubling time pre-therapy and during therapy were 3.1 and 4.92 months, respectively (Table 3). The PSA-DT was prolonged compared to baseline in 6 patients including a negative PSA slope (i.e. PSA decline) in one patient. This patient demonstrated a PSA decline of 26.9 % (4583.73→3351.41). Another patient demonstrated a minor PSA decline of 3.27 % (Fig. 3). Three of the 6 patients with slowing of PSA doubling also demonstrated T cell response to NY-ESO-1 peptides. Slowing of PSA-DT was observed in patients who received peptides restricted by class I and/or class II molecules. In accordance with Prostate Cancer Working Group (PCWG)-2 guidelines, all of the maximum levels of PSA changes during the 12 weeks of therapy are shown in the waterfall plot (Fig. 3) [27].
Fig. 3.
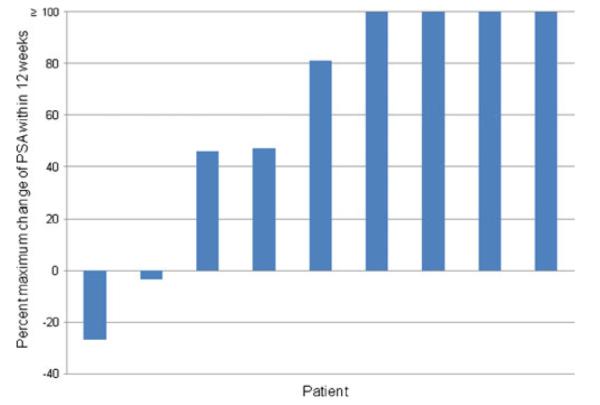
Waterfall plot of maximum percent change in PSA from baseline within 12 weeks
Discussion
This phase I clinical trial demonstrated feasibility and potential biologic anti-tumor activity for NY-ESO-1 peptides tailored for activity in patients expressing HLA class I (A2) and/or class II (DR4, DP4) molecules in men with mCRPC. Among 9 evaluable patients who received all 6 doses of the peptides, antigen-specific T cell responses, i.e. release of IFN-γ by PBMCs in response to NY-ESO-1 exposure in vitro, were observed in 6 patients. Moreover 6 of the 9 evaluable patients exhibited slowing of PSA-DT compared to baseline, including PSA decline of 26.9 % in one patient. Antigen-specific T cell response was observed in patients receiving peptides restricted by class I and/or class II molecules. In conjunction with this signal of biologic activity, the toxicity profile was excellent after the amendment to omit GM-CSF administration. Hence, HLA-restricted NY-ESO-1 naked peptides may warrant further evaluation as single agent or in combination regimens in patients with mCRPC.
Intriguingly, docetaxel-naïve patients appeared to exhibit ELISPOT immune responses more frequently (4 of 4 patients) compared to docetaxel-pretreated patients (2 of 5 patients). No clear association of slowing of PSA-DT and immune response was observed, probably due to a small number of patients. Additionally, although baseline PSADT appears to be prognostic, extension of PSA-DT after therapeutic intervention has not been validated to translate to improved clinical outcomes [28]. Slowing of PSA-DT after therapy may be indicative of a biologic effect of therapy as reported in other trials [19]. Moreover, the relatively rapid baseline PSA-DT (3.1 months) may render the slowing of PSA-DT easier to identify and unlikely to be due to chance alone. Guidelines to facilitate the standardization of methodology to measure PSA-DT should be adopted rapidly to assist in its validation as an intermediate endpoint [29].
Our trial did not formally evaluate clinical anti-tumor activity, and had predefined objectives of demonstrating feasibility and immune responses. Indeed, early evidence of clinical anti-tumor activity is unlikely with immunotherapeutic agents, which generally demonstrate delayed benefits. Moreover, our trial did not stipulate specific post-trial therapy, which is likely to confound the interpretation of delayed benefits in the absence of a randomized trial design. Immune related response criteria have been proposed to address different patterns of response, which may even include initial progression before a delayed response or stability [30]. The trial was underpowered to detect lower rates of toxicities. However, phase I trials are typically underpowered to detect low rates of severe toxicities and we used standard Phase I conventions. Moreover, resource constraints did not allow expansion of cohorts.
Nevertheless, a solid rationale exists for earlier application of immunotherapy to chemonaïve patients. Furthermore, in this group of 9 evaluable patients, no specific HLA restricted peptide appeared to be clearly associated with more frequent slowing of PSA-DT or immune responses. While antibody responses against NY-ESO-1 were not observed, the major anti-tumor immune response is considered to be T-cell mediated. It is noteworthy that patients enrolled in our phase I trial had relatively advanced disease. Therefore, the administration of peptides to asymptomatic or minimally symptomatic and chemonaïve disease only may have elicited more potent immune and clinical responses. Moreover, given the excellent tolerability, higher doses of peptides may warrant evaluation. Unfortunately, our trial enrolled a small number of patients and lacked the resources to investigate higher doses of peptides in a larger number of cohorts. An alternative strategy of eliciting optimal immunity may entail overcoming immune suppression in the tumor microenvironment. We are currently evaluating whether inhibiting regulatory T (Treg) cell-mediated immune suppression can enhance therapeutic antitumor immunity.
We omitted GM-CSF after observing toxicities, which may have also attenuated the immunogenicity of the vaccine. The presumed mechanism of fatal myocardial infarction in a single patient is unclear. However, this unfortunate event was possibly partly from marrow expansion from GM-CSF in a patient with multiple bony metastases causing substantial pain, which led to stress and a subsequent myocardial infarction in the setting of several risk factors for coronary artery disease. Previous trials evaluating GM-CSF in trials enrolling prostate cancer patients have reported manageable mild toxicities, including fatigue, fever, injection site reactions, arthralgias, and nausea [19]. It is also recommended that GM-CSF should be used with caution in patients with preexisting cardiac disease due to a small risk of transient supraventricular arrhythmias (Leukine package insert, Bayer Healthcare Pharmaceuticals, Inc.).
Given that peptide-based immunotherapy requires a specific human leukocyte antigen (HLA) allele to enable recognition by host T lymphocytes, a strategy of administering a cocktail of peptides has been evaluated in order to account for all HLA types. A randomized phase II trial (n=57) comparing estramustine phosphate alone or with a personalized peptide derived from PSA, PAP, PSMA, multidrug-resistance protein and other epithelial tumor antigens in 57 HLA-A2–positive or HLA-A24–positive mCRPC patients demonstrated an extension of PFS [31].
While multiple immunotherapeutic modalities are emerging, naked peptide vaccines are advantageous since they appear highly tolerable, and are off the shelf products that may be prepared readily and cheaply, and can be individualized based on host HLA antigen profile. In conjunction with their clinical development, biomarkers predictive for efficacy and relevant markers for optimal immune response and intermediate clinical endpoints are urgently needed.
Acknowledgments
This work was in part supported by grants from the National Institutes of Health (Grant number NIH Specialized Programs of Research Excellence CA58204) as well as by grants from National Cancer Institute, NIH (R01CA090327, R01CA101795, R01CA116408 and R01CA121191) to R.F.W. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Ethical standards The research complies with the current laws of the United States.
Conflict of interest The authors declare that they have no conflict of interest.
Relevant conflicts of interest None.
Presented in part as a poster at the American Society of Clinical Oncology annual conference in June 2012, Chicago, IL.
Rong-Fu Wang and Teresa G. Hayes share the corresponding authorship.
Contributor Information
Guru Sonpavde, Department of Medicine, Section of Medical Oncology, Baylor College of Medicine, Houston, TX, USA; Michael E. DeBakey Veterans Affairs Medical Center, VA 111H, 2002 Holcombe Blvd, Houston, TX 77030, USA.
Mingjun Wang, Center for Inflammation and Epigenetics, The Methodist Hospital Research Institute and Weill Cornell Medical College of Cornell University, 6670 Bertner Street, Houston, TX 77030, USA.
Leif E. Peterson, Center for Biostatistics, The Methodist Hospital Research Institute and Weill Cornell Medical College of Cornell University, Houston, TX, USA
Helen Y. Wang, Center for Inflammation and Epigenetics, The Methodist Hospital Research Institute and Weill Cornell Medical College of Cornell University, 6670 Bertner Street, Houston, TX 77030, USA
Teresa Joe, Michael E. DeBakey Veterans Affairs Medical Center, VA 111H, 2002 Holcombe Blvd, Houston, TX 77030, USA.
Martha P. Mims, Department of Medicine, Section of Medical Oncology, Baylor College of Medicine, Houston, TX, USA
Dov Kadmon, Scott Department of Urology, Baylor College of Medicine, Houston, TX, USA.
Michael M. Ittmann, Michael E. DeBakey Veterans Affairs Medical Center, VA 111H, 2002 Holcombe Blvd, Houston, TX 77030, USA; Department of Pathology, Baylor College of Medicine, Houston, TX, USA
Thomas M. Wheeler, Department of Pathology, Baylor College of Medicine, Houston, TX, USA
Adrian P. Gee, Center for Cell and Gene Therapy, Baylor College of Medicine, Houston, TX, USA
Rong-Fu Wang, Center for Inflammation and Epigenetics, The Methodist Hospital Research Institute and Weill Cornell Medical College of Cornell University, 6670 Bertner Street, Houston, TX 77030, USA.
Teresa G. Hayes, Department of Medicine, Section of Medical Oncology, Baylor College of Medicine, Houston, TX, USA; Michael E. DeBakey Veterans Affairs Medical Center, VA 111H, 2002 Holcombe Blvd, Houston, TX 77030, USA
References
- 1.Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T immuno-therapy for castration-resistant prostate cancer. N Engl J Med. 363:411–22. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 2.Jungbluth AA, Chen YT, Stockert E, et al. Immunohistochemical analysis of NY-ESO-1 antigen expression in normal and malignant human tissues. Int J Cancer J Int Cancer. 2001;92:856–860. doi: 10.1002/ijc.1282. [DOI] [PubMed] [Google Scholar]
- 3.Odunsi K, Jungbluth AA, Stockert E, et al. NY-ESO-1 and LAGE-1 cancer-testis antigens are potential targets for immuno-therapy in epithelial ovarian cancer. Cancer Res. 2003;63:6076–6083. [PubMed] [Google Scholar]
- 4.Chen YT, Scanlan MJ, Sahin U, et al. A testicular antigen aberrantly expressed in human cancers detected by autologous antibody screening. Proc Natl Acad Sci U S A. 1997;94:1914–1918. doi: 10.1073/pnas.94.5.1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jager E, Nagata Y, Gnjatic S, et al. Monitoring CD8 T cell responses to NY-ESO-1: correlation of humoral and cellular immune responses. Proc Natl Acad Sci U S A. 2000;97:4760–4765. doi: 10.1073/pnas.97.9.4760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang RF, Johnston SL, Zeng G, Topalian SL, Schwartzentruber DJ, Rosenberg SA. A breast and melanoma-shared tumor antigen: T cell responses to antigenic peptides translated from different open reading frames. J Immunol. 1998;161:3598–3606. [PubMed] [Google Scholar]
- 7.Jager E, Karbach J, Gnjatic S, et al. Recombinant vaccinia/ fowlpox NY-ESO-1 vaccines induce both humoral and cellular NY-ESO-1-specific immune responses in cancer patients. Proc Natl Acad Sci U S A. 2006;103:14453–14458. doi: 10.1073/pnas.0606512103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fossa A, Berner A, Fossa SD, Hernes E, Gaudernack G, Smeland EB. NY-ESO-1 protein expression and humoral immune responses in prostate cancer. Prostate. 2004;59:440–447. doi: 10.1002/pros.20025. [DOI] [PubMed] [Google Scholar]
- 9.Zeng G, Touloukian CE, Wang X, Restifo NP, Rosenberg SA, Wang RF. Identification of CD4+ T cell epitopes from NY-ESO-1 presented by HLA-DR molecules. J Immunol. 2000;165:1153–1159. doi: 10.4049/jimmunol.165.2.1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zeng G, Wang X, Robbins PF, Rosenberg SA, Wang RF. CD4(+) T cell recognition of MHC class II-restricted epitopes from NY-ESO-1 presented by a prevalent HLA DP4 allele: association with NY-ESO-1 antibody production. Proc Natl Acad Sci U S A. 2001;98:3964–3969. doi: 10.1073/pnas.061507398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zeng G, Li Y, El-Gamil M, et al. Generation of NY-ESO-1-specific CD4+ and CD8+ T cells by a single peptide with dual MHC class I and class II specificities: a new strategy for vaccine design. Cancer Res. 2002;62:3630–3635. [PMC free article] [PubMed] [Google Scholar]
- 12.Rosenberg SA, Yang JC, Schwartzentruber DJ, et al. Immunologic and therapeutic evaluation of a synthetic peptide vaccine for the treatment of patients with metastatic melanoma. Nat Med. 1998;4:321–327. doi: 10.1038/nm0398-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jager E, Gnjatic S, Nagata Y, et al. Induction of primary NYESO-1 immunity: CD8+ T lymphocyte and antibody responses in peptide-vaccinated patients with NY-ESO-1+ cancers. Proc Natl Acad Sci U S A. 2000;97:12198–12203. doi: 10.1073/pnas.220413497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marchand M, van Baren N, Weynants P, et al. Tumor regressions observed in patients with metastatic melanoma treated with an antigenic peptide encoded by gene MAGE-3 and presented by HLA-A1. Int J Cancer J Int Cancer. 1999;80:219–230. doi: 10.1002/(sici)1097-0215(19990118)80:2<219::aid-ijc10>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 15.Dutoit V, Taub RN, Papadopoulos KP, et al. Multiepitope CD8(+) T cell response to a NY-ESO-1 peptide vaccine results in imprecise tumor targeting. J Clin Invest. 2002;110:1813–1822. doi: 10.1172/JCI200216428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khong HT, Yang JC, Topalian SL, et al. Immunization of HLA-A*0201 and/or HLA-DPbeta1*04 patients with metastatic melanoma using epitopes from the NY-ESO-1 antigen. J Immunother. 2004;27:472–477. doi: 10.1097/00002371-200411000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jager E, Chen YT, Drijfhout JW, et al. Simultaneous humoral and cellular immune response against cancer-testis antigen NYESO-1: definition of human histocompatibility leukocyte antigen (HLA)-A2-binding peptide epitopes. J Exp Med. 1998;187:265–270. doi: 10.1084/jem.187.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jager E, Jager D, Karbach J, et al. Identification of NY-ESO-1 epitopes presented by human histocompatibility antigen (HLA)-DRB4*0101-0103 and recognized by CD4(+) T lymphocytes of patients with NY-ESO-1-expressing melanoma. J Exp Med. 2000;191:625–630. doi: 10.1084/jem.191.4.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rini BI, Weinberg V, Bok R, Small EJ. Prostate-specific antigen kinetics as a measure of the biologic effect of granulocyte-macrophage colony-stimulating factor in patients with serologic progression of prostate cancer. J Clin Oncol. 2003;21:99–105. doi: 10.1200/JCO.2003.04.163. [DOI] [PubMed] [Google Scholar]
- 20.Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 21.Rosenberg SA, Yang JC, Schwartzentruber DJ, et al. Impact of cytokine administration on the generation of antitumor reactivity in patients with metastatic melanoma receiving a peptide vaccine. J Immunol. 1999;163:1690–1695. [PMC free article] [PubMed] [Google Scholar]
- 22.Disis ML, Grabstein KH, Sleath PR, Cheever MA. Generation of immunity to the HER-2/neu oncogenic protein in patients with breast and ovarian cancer using a peptide-based vaccine. Clin Cancer Res: Off J Am Assoc Cancer Res. 1999;5:1289–1297. [PubMed] [Google Scholar]
- 23.Muderspach L, Wilczynski S, Roman L, et al. A phase I trial of a human papillomavirus (HPV) peptide vaccine for women with high-grade cervical and vulvar intraepithelial neoplasia who are HPV 16 positive. Clin Cancer Res: Off J Am Assoc Cancer Res. 2000;6:3406–3416. [PubMed] [Google Scholar]
- 24.Wang M, Lamberth K, Harndahl M, et al. CTL epitopes for influenza A including the H5N1 bird flu; genome-, pathogen-, and HLA-wide screening. Vaccine. 2007;25:2823–2831. doi: 10.1016/j.vaccine.2006.12.038. [DOI] [PubMed] [Google Scholar]
- 25.Maio M, Coral S, Sigalotti L, et al. Analysis of cancer/testis antigens in sporadic medullary thyroid carcinoma: expression and humoral response to NY-ESO-1. J Clin Endocrinol Metab. 2003;88:748–754. doi: 10.1210/jc.2002-020830. [DOI] [PubMed] [Google Scholar]
- 26.Gonzalez-Galarza FF, Christmas S, Middleton D, Jones AR. Allele frequency net: a database and online repository for immune gene frequencies in worldwide populations. Nucleic Acids Res. 39:D913–9. doi: 10.1093/nar/gkq1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scher HI, Halabi S, Tannock I, et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol. 2008;26:1148–1159. doi: 10.1200/JCO.2007.12.4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Armstrong AJ, Garrett-Mayer ES, Yang YC, de Wit R, Tannock IF, Eisenberger M. A contemporary prognostic nomogram for men with hormone-refractory metastatic prostate cancer: a TAX327 study analysis. Clin Cancer Res. 2007;13:6396–6403. doi: 10.1158/1078-0432.CCR-07-1036. [DOI] [PubMed] [Google Scholar]
- 29.Arlen PM, Bianco F, Dahut WL, et al. Prostate Specific Antigen Working Group guidelines on prostate specific antigen doubling time. J Urol. 2008;179:2181–2185. doi: 10.1016/j.juro.2008.01.099. discussion 5–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hoos A, Eggermont AM, Janetzki S, et al. Improved endpoints for cancer immunotherapy trials. J Natl Cancer Inst. 2010;102:1388–1397. doi: 10.1093/jnci/djq310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Noguchi M, Kakuma T, Uemura H, et al. A randomized phase II trial of personalized peptide vaccine plus low dose estramustine phosphate (EMP) versus standard dose EMP in patients with castration resistant prostate cancer. Cancer Immunol Immunother. 59:1001–9. doi: 10.1007/s00262-010-0822-4. [DOI] [PMC free article] [PubMed] [Google Scholar]



