Abstract
Objective:
To investigate the effect of externally applied cyclical (vibratory) forces on the rate of tooth movement, the structural integrity of the periodontal ligament, and alveolar bone remodeling.
Methods:
Twenty-six female Sprague-Dawley rats (7 weeks old) were divided into four groups: CTRL (unloaded), VBO (molars receiving a vibratory stimulus only), TMO (molars receiving an orthodontic spring only), and TMO+VB (molars receiving an orthodontic spring and the additional vibratory stimulus). In TMO and TMO+VB groups, the rat first molars were moved mesially for 2 weeks using Nickel-Titanium coil spring delivering 25 g of force. In VBO and TMO+VB groups, cyclical forces at 0.4 N and 30 Hz were applied occlusally twice a week for 10 minutes. Microfocus X-ray computed tomography analysis and tooth movement measurements were performed on the dissected rat maxillae. Tartrate-resistant acid phosphatase staining and collagen fiber assessment were performed on histological sections.
Results:
Cyclical forces significantly inhibited the amount of tooth movement. Histological analysis showed marked disorganization of the collagen fibril structure of the periodontal ligament during tooth movement. Tooth movement caused a significant increase in osteoclast parameters on the compression side of alveolar bone and a significant decrease in bone volume fraction in the molar region compared to controls.
Conclusions:
Tooth movement was significantly inhibited by application of cyclical forces.
Keywords: Orthodontic tooth movement, Cyclical forces, Osteoclasts, Alveolar bone remodeling
INTRODUCTION
Orthodontic tooth movement (OTM) requires a bone remodeling sequence that consists of bone resorption in the direction of tooth movement and bone formation on the opposite side.1 The average orthodontic treatment can take several years, resulting in significant side effects. Efforts to shorten the time of orthodontic treatment and accelerate the alveolar bone response would be beneficial to the patient and the profession.
Animal studies have shown that the application of cyclical forces in conjunction with an orthodontic force could increase the rate of OTM.2,3 Nishimura et al.2 showed that cyclical forces at resonant frequency (∼60 Hz) externally applied to the maxillary first molars increased the rate of OTM. The limitation of this experimental design was the method of force application. The use of an expansion spring can lead to possible skeletal effects (sutural expansion) that can overestimate the actual amount of dental tooth movement. As a result, the mechanism of enhanced tooth movement, the magnitude of cyclical force used, and the effect of cyclical forces on the periodontium still remain unclear.
Recently, a randomized clinical trial explored the effect of cyclical forces on OTM in humans.4 However, this study was unable to show enhanced tooth movement in the alignment of the mandibular anterior teeth with a vibratory device.
During tooth movement, alveolar bone undergoes rapid remodeling that results in a significant osteopenia.5,6 In the past decade, a number of studies have investigated the effect of low-magnitude cyclical forces on weight-bearing bones. They have shown that vibratory signals promote bone formation, enhance bone density, and attenuate the negative effects associated with catabolic stimuli.7–11 An anabolic response has also been observed in craniofacial structures,12,13 including the alveolar bone.14
Osteoclasts are the key participants in modulating bone mass. In contrast to the Nishimura et al.2 findings, a study showed that low-magnitude acceleration (0.5–2 g) suppressed osteoclast activity in vitro.15 It was also found that low-magnitude, high-frequency vibration can inhibit the RANKL-induced (receptor activator of nuclear factor-kappa B ligand) osteoclast differentiation of RAW 264.7 cells.16
Therefore, the objective of this pilot study was to examine the effect of externally applied cyclical forces on the rate of sagittal OTM, the structural integrity of the periodontal ligament, and the alveolar bone remodeling during OTM.
MATERIALS AND METHODS
Study Design
All experiments were performed under an institutionally approved protocol for the use of animals in research and in accordance with the National Guiding Principles for Animal Research. In total, 26 female Sprague Dawley (7-week-old) rats were used in this pilot study.
The experimental design used four groups: CTRL group (four rats without orthodontic spring and no vibratory stimulus), VBO group (four rats received occlusal vibratory stimulus twice a week without orthodontic spring), TMO group (nine rats received the orthodontic spring only), and TMO+VB group (nine rats received the spring and additional vibratory stimulus twice a week).
Application of Orthodontic Force
Animals were anesthetized with an intraperitoneal injection of ketamine (80 mg/kg) and xylazine (8 mg/kg). A 9-mm, nickel-titanium (NI-Ti), 0.010 × 0.030-inch closed-coil spring (Rocky Mountain Orthodontics, Denver, Co) delivering 25 g of force was used for the application of the orthodontic force. Prior to appliance delivery, a 0.008-inch stainless-steel (SS) ligature was threaded through the contact between the first and second left maxillary molars and attached to the Ni-Ti spring. A second 0.008-inch SS ligature was placed around the incisors, and the spring was then activated for ∼2 mm. Grooves, 0.5 mm from the gingiva, were prepared on the facial, lingual, and distal surfaces of the maxillary central incisors to prevent the ligatures from dislodging. After the ligatures were tied and cut, composite resin (Transbond XT Light Cure Adhesive Paste; 3M Unitek, Monrovia, Calif) was placed over the wire to prevent slipping, gingival irritation, and pulpal irritation (Figure 1A,B). The incisal grooves were renewed and the coil spring retied once per week to compensate for the continual incisal eruption. In order to achieve a constant force on the first molar, springs were reactivated as needed. The duration of the experiments was 14 days.
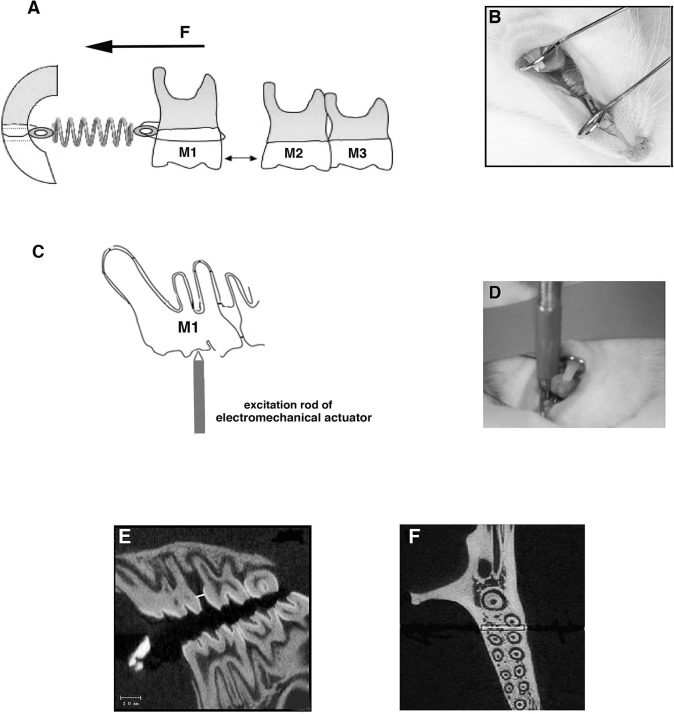
Figure 1.
The model used in this study. (A) Schematic of the OTM model showing the appliance design with the spring being activated from the left maxillary first molar to the incisor. Arrow shows the direction of orthodontic force. (B) Inserted appliance. (C) External application of cyclical force on the left maxillary molar. The controlled feedback loop/electromechanical actuator is used to apply cyclical force (30 Hz at compression force 0.1–0.4 N) vertically on the occlusal surface of the first molar. (D) Rod of electromechanical actuator being placed on the molar of the anesthetized rat. (E) Sagittal two-dimensional micro-CT section used for measuring intermolar (1M-2M) distance. (F) Axial micro-CT section of the left maxillae. The square shows the ROI; the white line represents the center of the analysis.
Application of Cyclical Forces
Upon adequate anesthesia, a custom mouth prop was placed between the maxillary and mandibular incisors to hold the rat's mouth open. An electromechanical actuator was used to apply unilateral cyclical forces of 0.4 N vertically to the occlusal surface of the left first maxillary molar (Model 3230; Bose/EnduraTec, Minnetonka, Minn; Figure 1C,D). The range of compression force applied (0.1–0.4 N) was controlled by a sensor on the top of the vibrational rod. Loading protocols for individual animals consisted of 10 minutes at a frequency of 30 Hz (cycles/second). The rats were subjected to the vibratory stimulation twice per week, with a total of five applications.
Microfocus X-Ray Computed Tomography Analysis and Tooth Movement Measurements
Upon completion of the experiments, at day 14, the rats were euthanized by CO2. Maxillae were dissected, hemisected, and placed in 10% neutral-buffered formalin at 4°C for 7 days. During that period, three-dimensional image arrays of the maxilla were collected using microfocus X-ray computed tomography (micro-CT). Tooth movement was defined and measured as the distance between the maxillary first and second molars at the most mesial point of the second molar crown and the most distal point of the first molar crown (1M-2M distance). The measurements were made within the near-sagittal plane of tooth movement, locating the image plane that revealed the most root structure (Figure 1E).
The region of interest (ROI) for the analysis of bone volume, total volume, and tissue density included a square that extended 200 µm from the mesial surface of the distobuccal and distolingual roots of the first maxillary left molar (Figure 1F).
Histological Analysis and Osteoclast Quantification
Following fixation, tissues were decalcified in 14% ethylenediaminetetraacetic acid at 4°C for 4 weeks, dehydrated in a graded series of ethanol, embedded in paraffin, and cut into serial 5-µm sagittal sections.
Staining for tartrate-resistant acid phosphatase (TRAP) activity was performed by using an acid phosphatase leukocyte kit (Sigma Chemical, St Louis, Mo) according to the manufacturer's instructions. Osteoclasts were considered as TRAP-positive multinucleated cells (>2 nuclei) and were counted on the alveolar bone surface on the compression side of the disto-buccal root of the maxillary first molars. The section that revealed the most root and pulp structure was chosen for the analysis. The area for histomorphometry and osteoclast quantification was defined as a square parallel to the sagittal axis of the disto-buccal root. One side of this square was half of the average width of the distobuccal root (∼200 µm). The adjoining side extended from bifurcation to root apex. Osteoclast surface was determined as a surface of active osteoclast divided by total bone surface. Histomorphometric analyses were carried out using Osteomeasure Software (OsteoMetrics Inc, Decatur, Ga).
Collagen Fibers Assessment
Deparaffinized, rehydrated histological sections were imaged on the multiphoton microscope (Ultima IV; Prairie Technologies, Middleton, Wis). Samples were scanned at 1-µm increments through the full thickness of the section at an excitation wavelength of 900 nm to image collagen via second-harmonic generation (SHG). The SHG signal was collected using the 435- to 485-nm bandpass filter. Z-projections through the 5-µm thickness were created using Fiji image analysis software.
Statistical Analysis
Statistical analyses were carried out using GraphPad Prism (GraphPad Software Inc, La Jolla, Calif). Statistical significance of differences among means was determined by one-way analysis of variance with a Bonferroni post-hoc test. Significance was accorded when P < .05.
RESULTS
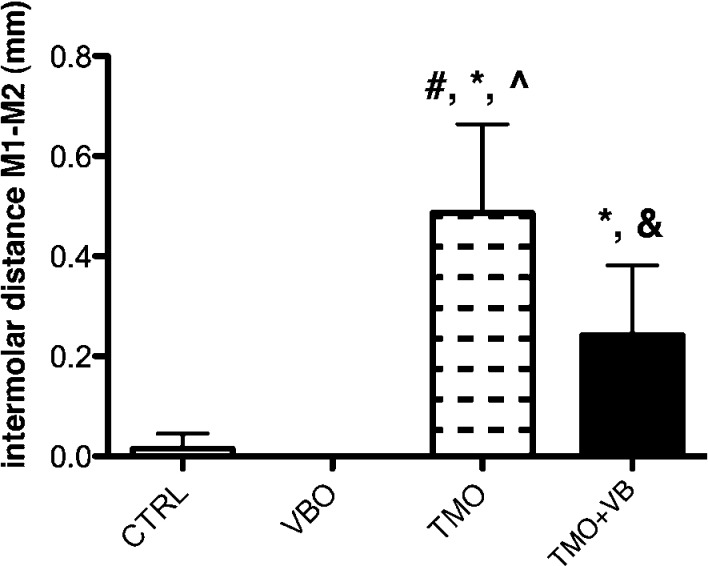
Tooth movement measurements were assessed on four CTRL rats, four VBO rats, nine TMO rats, and nine TMO+VB rats. The separation distance between the first and second maxillary molars in the CTRL and VBO group approximated 0 mm in each animal. Statistical significance was found when the TMO group (mean = 0.486 ± 0.178 mm) was compared to the CTRL, VBO, and TMO+VB groups and when the TMO+VB group (mean = 0.242 ± 0.139 mm) was compared to the VBO and TMO groups (one-way ANOVA, P < .0001). Significantly smaller intermolar distances in the TMO+VB group suggest that 30-Hz cyclical forces inhibited tooth movement (Figure 2).
Figure 2.
Intermolar distances at day 14. Each value represents the mean ± SD (n = 4–9). # significance compared to the CTRL group; * significance compared to the VBO group; ^ significance compared to the TMO+VB; & significance compared to the TMO group (one-way ANOVA, P < .0001). Note significantly less tooth movement in the TMO+VB group compared to the TMO group.
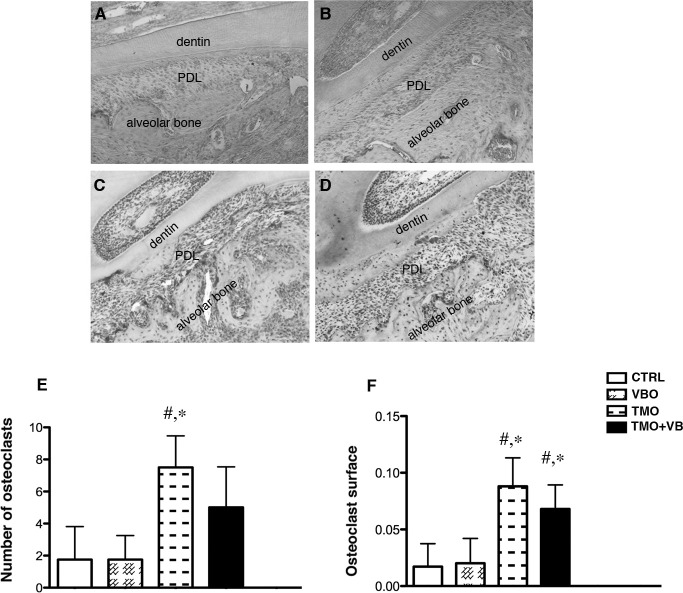
In the CTRL and VBO groups there were clearly fewer osteoclasts on the alveolar bone, and almost no osteoclasts were detected within the periodontal ligament (Figure 3). Statistical significance in the osteoclast number was found when the TMO group (mean = 7.50 ± 1.98) was compared to the CTRL (1.75 ± 2.06) and VBO (1.75 ± 1.50) groups (one-way ANOVA, P = .0012). No significance was detected when the TMO+VB group was compared to the CTRL and VBO groups (Figure 3E). This suggests that the formation of osteoclasts seen during OTM is altered by the application of cyclical forces. A similar trend was found with the osteoclast surface (one-way ANOVA, P < .0002; Figure 3F).
Figure 3.
Histological examination of osteoclast number and surface at day 14 in CTRL (A), VBO (B), TMO (C), and TMO+VB (D) groups. Many TRAP-positive cells were observed on the alveolar bone and within the periodontal ligament in the TMO and TMO+VB groups. Note no osteoclasts were seen within the periodontal ligament in the CTRL and VBO groups. (E) Quantification of active osteoclasts in alveolar bone; # significance compared to the CTRL group; * significance compared to the VBO group (one-way ANOVA, P = .0012). (F) Osteoclast surface parameters (one-way ANOVA, P < .0002). Each value represents the mean ± SD (n = 4–6).
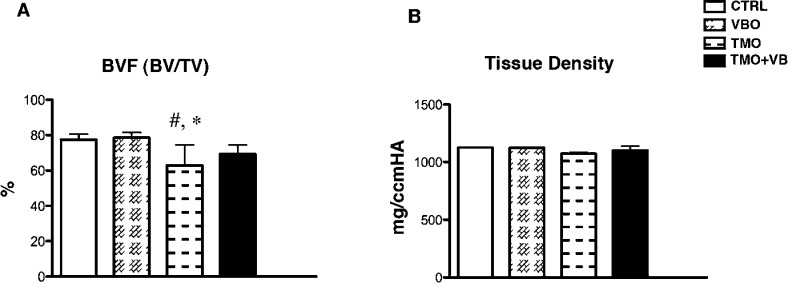
Micro-CT analysis showed a significant decrease in bone volume fraction (BVF) when the TMO group was compared to the CTRL and VBO groups. No significance was detected when the TMO+VB group was compared to the CTRL and VBO groups (one-way ANOVA, P = .0127; Figure 4A). A similar trend was found for tissue density (one-way ANOVA, P = .0252; Figure 4B).
Figure 4.
Micro-CT analysis of alveolar bone. A) BVF-bone volume divided by total volume (one-way ANOVA, P = .0127). # significance compared to the CTRL group; * significance compared to the VBO group. (B) Tissue density (one-way ANOVA, P = .0252). Each value represents the mean ± SD (n = 4–6).
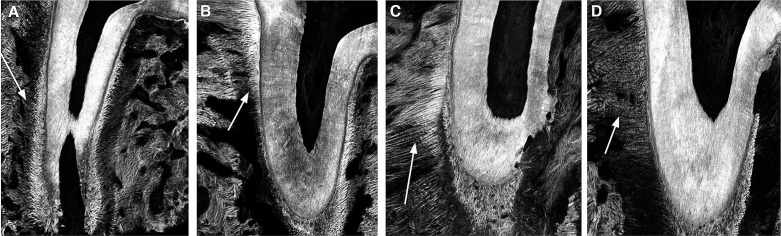
The structure of the collagen fibers was examined using two-photon microscopy on 5-µm sections of the distobuccal root of the first maxillary molar. Collagen fibers were evaluated on the tension area of the periodontal ligament (PDL). In the VBO group, collagen fibers showed slightly thicker morphology than those in the CTRL group (Figures 5A,B). Similar findings were observed in the TMO group, in which the fibers appeared very thick and smooth (Figure 5C). In contrast, in the TMO+VB group, collagen fibers exhibited a disrupted morphology when compared to those in the TMO group (Figure 5D).
Figure 5.
Examination of PDL collagen fibers on the tension side by two-photon microscopy. In the VBO group (B) there was slight fiber thickening (arrow, white) compared to the CTRL group (A) to accommodate the compressive cyclical force. In the TMO group (C), fibers exhibited thick, uniform, and smooth morphology. In the TMO+VB group (D), fibers were thinner, wavy, and exhibited disrupted morphology when compared to the TMO group.
DISCUSSION
The reason why 30 Hz was chosen for this study was based on our preliminary results that found that other frequencies (60 Hz, 100 Hz, and 200Hz) caused approximately the same amount of tooth movement as the TMO group. The magnitude of cyclical force applied in our experiments (0.4 N) may have influenced some of the results. Other studies did not delineate well the force used.2,17 We had initially surmised that in rats, due to the periodontal ligament, forces would need to be at a higher magnitude than whole-body vibration forces18,19 in order for the forces to be transmitted to the surrounding alveolar bone. From this study, a force of this magnitude could be considered harmful to the periodontal ligament, but forces at this frequency were still not translated well to the alveolar bone. However, this assumption could be erroneous, and future studies are needed to examine the effect of lower cyclical forces on tooth movement.
It is important to use caution in extrapolating this rodent data to humans due to the magnitude of force used and also because of the direction of force applied in this loading model. It is possible that this vertical application on the occlusal surface of the first molar could cause additional damage to the periodontal ligament. This may require that alternative points for application of cyclical force be used (eg, vibrating the alveolar bone instead of the tooth crown or applying the external cyclical forces in the direction of the orthodontic force).
The exact reason why cyclical forces inhibited OTM is uncertain. One possible explanation is that during OTM on the tension side, there is an alignment of the periodontal ligament fibers20 that is disturbed in rats exposed to vibration. Further, alignment of periodontal ligament fibers may be important in the activation of osteoclasts.21 Another explanation could be that 30-Hz cyclical forces inhibit osteoclastogenesis and bone formation that normally occur during the tooth movement, which was indicated by an increase in the alveolar BVF and a decrease in osteoclasts in the TMO+VB group.
Regardless of the mechanism, this pilot study shows that cyclical forces decrease the rate of OTM in rats. Furthermore, the effects of vibration on tooth movement may be both frequency and dose dependent. Although animal experimentation results do not always directly apply and translate to humans, prudent use of vibration is needed in orthodontics, and further research is necessary to evaluate the effect of cyclical forces on tooth movement.
CONCLUSIONS
Although the results were obtained from groups with a small sample size, this pilot study showed that cyclical forces inhibit OTM.
The effects of cyclical forces on OTM may cause opposite effects depending on force magnitude, frequency, or point of application.
REFERENCES
- 1.Henneman S, Von den Hoff JW, Maltha JC. Mechanobiology of tooth movement. Eur J Orthod. 2008;30:299–306. doi: 10.1093/ejo/cjn020. [DOI] [PubMed] [Google Scholar]
- 2.Nishimura M, Chiba M, Ohashi T, et al. Periodontal tissue activation by vibration: intermittent stimulation by resonance vibration accelerates experimental tooth movement in rats. Am J Orthod Dentofacial Orthop. 2008;133:572–583. doi: 10.1016/j.ajodo.2006.01.046. [DOI] [PubMed] [Google Scholar]
- 3.Liu D. Moving teeth faster, better and painless. Is it possible. Dent Press J Orthod. 2010;15:14–17. [Google Scholar]
- 4.Miles P, Smith H, Weyant R, Rinchuse DJ. The effects of a vibrational appliance on tooth movement and patient discomfort: a prospective randomised clinical trial. Aust Orthod J. 2012;28:213–218. [PubMed] [Google Scholar]
- 5.Milne TJ, Ichim I, Patel B, McNaughton A, Meikle MC. Induction of osteopenia during experimental tooth movement in the rat: alveolar bone remodelling and the mechanostat theory. Eur J Orthod. 2009;31:221–231. doi: 10.1093/ejo/cjp032. [DOI] [PubMed] [Google Scholar]
- 6.Chang HW, Huang HL, Yu JH, Hsu JT, Li YF, Wu YF. Effects of orthodontic tooth movement on alveolar bone density. Clin Oral Invest. 2012;16:679–688. doi: 10.1007/s00784-011-0552-9. [DOI] [PubMed] [Google Scholar]
- 7.Rubin C, Turner AS, Bain S, Mallinckrodt C, McLeod K. Anabolism. Low mechanical signals strengthen long bones. Nature. 2001;412:603–604. doi: 10.1038/35088122. [DOI] [PubMed] [Google Scholar]
- 8.Rubin C, Turner AS, Muller R, et al. Quantity and quality of trabecular bone in the femur are enhanced by a strongly anabolic, noninvasive mechanical intervention. J Bone Miner Res. 2002;17:349–357. doi: 10.1359/jbmr.2002.17.2.349. [DOI] [PubMed] [Google Scholar]
- 9.Xie L, Jacobson JM, Choi ES, et al. Low-level mechanical vibrations can influence bone resorption and bone formation in the growing skeleton. Bone. 2006;39:1059–1066. doi: 10.1016/j.bone.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 10.Xie L, Rubin C, Judex S. Enhancement of the adolescent murine musculoskeletal system using low-level mechanical vibrations. J Appl Physiol. 2008;104:1056–1062. doi: 10.1152/japplphysiol.00764.2007. [DOI] [PubMed] [Google Scholar]
- 11.Flieger J, Karachalios T, Khaldi L, Raptou P, Lyritis G. Mechanical stimulation in the form of vibration prevents postmenopausal bone loss in ovariectomized rats. Calcif Tissue Int. 1998;63:510–514. doi: 10.1007/s002239900566. [DOI] [PubMed] [Google Scholar]
- 12.Kopher RA, Mao JJ. Suture growth modulated by the oscillatory component of micromechanical strain. J Bone Miner Res. 2003;18:521–528. doi: 10.1359/jbmr.2003.18.3.521. [DOI] [PubMed] [Google Scholar]
- 13.Othman H, Thonar EJ, Mao JJ. Modulation of neonatal growth plate development by ex vivo intermittent mechanical stress. J Biomech. 2007;40:2686–2693. doi: 10.1016/j.jbiomech.2006.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alikhani M, Khoo E, Alyami B, et al. Osteogenic effect of high-frequency acceleration on alveolar bone. J Dent Res. 2012;91:413–419. doi: 10.1177/0022034512438590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suzuki N, Kitamura K, Nemoto T, et al. Effect of vibration on osteoblastic and osteoclastic activities: analysis of bone metabolism using goldfish scale as a model for bone. Adv Space Res. 2007;40:1711–1721. [Google Scholar]
- 16.Wu SH, Zhong ZM, Chen JT. Low-magnitude high-frequency vibration inhibits RANKL-induced osteoclast differentiation of RAW264.7 cells. Int J Med Sci. 2012;9:801–807. doi: 10.7150/ijms.4838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Darendeliler MA, Zea A, Shen G, Zoellner H. Effects of pulsed electromagnetic field vibration on tooth movement induced by magnetic and mechanical forces: a preliminary study. Aust Dent J. 2007;52:282–287. doi: 10.1111/j.1834-7819.2007.tb00503.x. [DOI] [PubMed] [Google Scholar]
- 18.Sandhu E, Miles JD, Dahners LE, Keller BV, Weinhold PS. Whole body vibration increases area and stiffness of the flexor carpi ulnaris tendon in the rat. J Biomech. 2011;44:1189–1191. doi: 10.1016/j.jbiomech.2011.02.017. [DOI] [PubMed] [Google Scholar]
- 19.Yang P, Jia B, Ding C, Wang Z, Qian A, Shang P. Whole-body vibration effects on bone before and after hind-limb unloading in rats. Aviat Space Environ Med. 2009;80:88–93. doi: 10.3357/asem.2368.2009. [DOI] [PubMed] [Google Scholar]
- 20.Naveh GR, Brumfeld V, Shahar R, Weiner S. Tooth periodontal ligament: direct 3D microCT visualization of the collagen network and how the network changes when the tooth is loaded. J Struct Biol. 2013;181:108–115. doi: 10.1016/j.jsb.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 21.Li X, Zhang L, Wang N, Feng X, Bi L. Periodontal ligament remodeling and alveolar bone resorption during orthodontic tooth movement in rats with diabetes. Diabetes Technol Ther. 2010;12:65–73. doi: 10.1089/dia.2009.0085. [DOI] [PubMed] [Google Scholar]