Abstract
Background
The BRAF V600E (BRAF+) mutation activates the MAPK/ERK pathway and may confer an aggressive phenotype in papillary thyroid cancer (PTC). However, clinically BRAF+PTC behavior varies from indolent to aggressive. SPRY2 is a negative feedback regulator of the MAPK/ERK pathway. We hypothesize that the level of SPRY2 expression contributes to MAPK/ERK pathway output and accounts for BRAF+ clinical heterogeneity.
Methods
A tissue microarray with BRAF+PTCs was constructed and analyzed for SPRY2 expression and MAPK/ERK output. Data were studied in the context of clinicopathologic factors to develop a risk stratification system predictive of tumor biology. SPRY2 function was studied by silencing SPRY2 in BRAF+PTC cells. These cells were treated with MAPK/ERK pathway inhibitors and assessed for growth effects.
Results
BRAF+PTCs with an intact MAPK/ERK feedback pathway, do not exhibit lymph node metastases. BRAF+PTCs with dysregulated feedback pathways have nodal metastasis. When SPRY2 is silenced the BRAF+PTC cells are significantly more sensitive to MAPK/ERK inhibition.
Conclusions
PTC behavior likely is dependent on both the driver of the MAPK/ERK pathway and its regulatory feedback. When the feedback pathway is intact the tumor phenotype seems to be less aggressive. This has a direct and important clinical implication and may alter our treatment strategies.
Background
This year there will be more than 50,000 new cases of thyroid cancer in the United States. The incidence of thyroid cancer is increasing at a rate far greater than any other cancer in this country 1. Papillary thyroid cancer (PTC) accounts for over 80% of all thyroid cancers and can be effectively managed by surgery with or without radioactive iodine (RAI) ablation with excellent clinical outcomes. However, 5–10% of cases display aggressive behavior, hallmarked by early metastasis and increased mortality 2, 3. These tumors are often RAI resistant. Clinical factors alone cannot accurately predict which tumors may behave in an aggressive fashion making it difficult to tailor the extent of surgery and RAI ablation to maximize patient benefit and avoid overtreatment. By better understanding the biologic mechanisms controlling the behavior of PTC, treatment plans can be individualized to the patient. This will help us select patients requiring aggressive treatment and more importantly, it will minimize risk for those patients with indolent tumors, who might not even require surgery.
Activating mutations of the mitogen activated protein kinase (MAPK/ERK) pathway are the most common genetic aberrations in thyroid cancer. Among these, the BRAF V600E (BRAF+) mutation is the most common and is present in 20 – 80% of PTCs 4, 5. This mutation constitutively activates the MAPK/ERK pathway and is thought to confer an aggressive phenotype 5. However, the clinical presentation of BRAF+ PTC varies from indolent to aggressive 6–9. This suggests that other biological factors regulating the phenotype are involved.
The MAPK/ERK pathway is regulated by feedback factors, which govern pathway output. One of these factors Sprouty 2 (SPRY2), is an inducible inhibitor of MAPK/ERK signaling. SPRY2 has been studied in multiple tumor systems and results demonstrate that MAPK/ERK pathway activation can lead to increased SPRY2 expression, which regulates pathway output and downstream processes such as proliferation, survival, and motility 10–14 (Figure 1).
Figure 1.
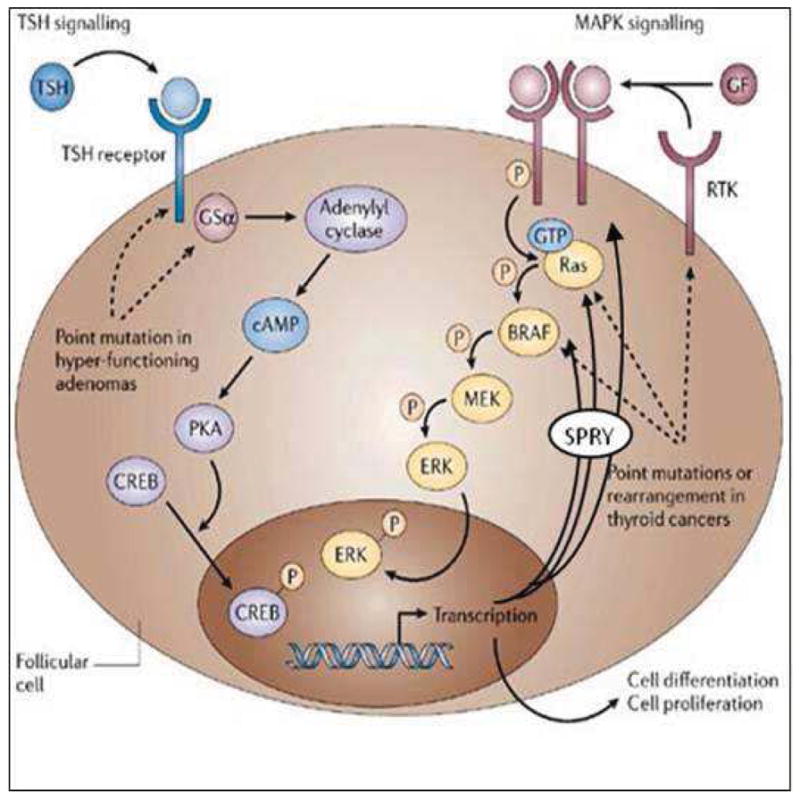
Diagram of MAPK/ERK signaling and potential SPRY feedback inhibition sites.
Adapted from: Nature Reviews Cancer 6, 292–306 (April 2006). Pathogenetic mechanisms in thyroid follicular-cell neoplasia. Tetsuo Kondo, Shereen Ezzat & Sylvia L. Asa.
We have shown that SPRY2 expression does reflect BRAF mutation status in PTC, however this expression is variable 6. The current study was undertaken to evaluate the hypothesis that the level of SPRY2 expression contributes to MAPK/ERK pathway output and accounts for the clinical heterogeneity in BRAF+ PTCs.
Methods
Thyroid cancer samples
The Division of Endocrine Surgery at New York University Langone Medical Center houses all tissue samples from all thyroid tumors greater than one centimeter in an IRB approved Tissue Banking and Acquisition Facility (NYU Langone Medical Center, New York, NY). Tumor samples are linked to a clinical database that is updated regularly by the Division of Endocrine Surgery and holds over sixty data points. The quality of our specimens has been highlighted in our prior publication 6.
We analyzed 30 consecutive classical PTCs from patients undergoing total thyroidectomy with elective central node dissection. Tumors were utilized to create the tissue microarray. All samples were reviewed by a dedicated pathologist.
DNA extraction
A 10-μm frozen section was taken from each sample and was subjected to Genomic DNA extraction per the manufacturer’s protocol using the DNeasy Blook and Tissue Kit (Qiagen).
Detection of BRAFV600E mutation
Exon 15 of the BRAF gene was amplified with 2 primers that annealed to the introns flanking it. Our technique has been previously described 6.
Cell Lines and reagents
Human thyroid carcinoma cell line KHM5M (BRAF+) was used for the experiments. It was grown in RPMI + nonessential amino acids + 10% FCS with 100-U/mL penicillin G and 100ug/mL streptomycin sulfate. It was maintained in a 5% CO2-95% air humidified incubator at 37°C. PD 184352 is a noncompetitive MEK ½ inhibitor. PLX 4720 is a BRAFV600E inhibitor.
RNA interference
The constructs for shRNA (short hairpin) were generated by inserting annealed oligos into lentiviral vector 6. The oligonucleotides used for cloning SPRY2 shRNA constructs were generated in accordance with Promega’s protocol for DNA-directed RNAi system and contained the loop sequence of 5′-TTCAAGAGA-3′ and the target sequence of 5′-GTGTGAAAGGTCTCTTCTA-3′, matching on nt 662–680 at human SPRY2 open reading frame.
MTT Assay
SPRY2 silenced and control thyroid cancer cells were seeded in a 96-well plate at a density of 500 cells per well and cultured for 24 hours at 37°C. They were treated separately with PD 184352, PLX 4720 and dimethyl sufoxide (DMSO) and cultured for 72 hours. MTT assay was performed in the standard fashion to assess for cell proliferation. The absorbance used was 570nm. Softmax Pro v.5 was used to analyze the data.
Tissue Microarray (TMA)
Thirty consecutive cases of BRAF+ papillary thyroid cancers and three cases of normal thyroid tissue were retrieved from our Tissue Banking and Acquistion Service. All sections were reviewed by a dedicated pathologist prior to sectioning. The TMA was constructed based on previously described methods 15, 16. Three cores from each sample were used to control for variance in the tissue. Three samples of normal thyroid tissue (each with three cores) were incorporated into the TMA as controls. The TMA was scored by a dedicated pathologist using the standard Allred-Scoring, intensity (0–3), proportion (0–100%), scale. Level of protein expression was determined by comparing each specimen’s mean Allred score to the expression level found in control normal thyroid tissue.
Immunohistochemistry
Immunohistochemistry was performed on 4 micron formalin fixed, paraffin embedded thyroid tissue microarray slides using the following antibodies: rabbit anti-human phosphorylated (Ser 201) MEK 1,2 (clone 166F8), rabbit anti-human phosphorylated (Thr202/Tyr204) ERK 1,2 (clone20G11) (both from Cell Signaling Technologies Danvers, MA USA) and rabbit polyclonal anti-human Sprouty-2 (Upstate Cell Signaling/Millipore Billerica, MA USA). Specificity, phosphorylated ERK 1,2 was verified using sections from paraffin-embedded NIH/3T3 cells, treated with either a phosphorylation inhibitor (U0126) or inducer (TPA) and supplied by the manufacture (Cell Signaling Danvers, MA USA). Control slides for both phosphorylated ERK and MEK were also incubated with lambda phosphatase (New England Biolabs Ipswich, MA USA) for 2 hours at 37° C to demonstrate the loss of phospho-specific labeling.
In brief, sections were deparaffinized in xylene, rehydrated through graded alcohol changes and rinsed in distilled water. Heat induced epitope retrieval was performed in a 1200-Watt microwave oven at 100% power in 10 mM Citrate buffer, pH 6.0 for 10 minutes (phosphorylated ERK) and 20 minutes (phosphorylated MEK and SPRY2), respectively. Sections were allowed to cool for 30 minutes and then rinsed in distilled water. Antibody incubation and detection were carried out at 40°C on a NexES instrument (Ventana Medical Systems Tucson, Arizona USA) using Ventana’s reagent buffer and detection kits unless otherwise noted. Endogenous peroxidase activity was blocked with hydrogen peroxide. Anti-phosphorylated MEK was diluted 1:25 and anti-phosphorylated ERK 1:50, both in diluent supplied by the manufacture (Cell Signaling Danvers, MA USA). Anti-sprouty-2 was diluted 1:300 in Dulbecco’s phosphate buffered saline (Life Technologies Grand Island, New York USA). All antibodies were incubated overnight at room temperature. Primary antibodies were detected with iView biotinylated goat anti-rabbit followed by application of streptavidin-horseradish-peroxidase conjugate. The complex was visualized with 3,3 diaminobenzidene and enhanced with copper sulfate. Slides were washed in distilled water, counterstained with hematoxylin, dehydrated and mounted with permanent media. Positive and negative controls were included with the study sections.
Statistical Analysis
A statistical analysis was performed using SPSSv18 software (IBM)©. Where applicable Pearson Chi-Square or ANOVA were performed. This study was approved by the New York University School of Medicine Institutional Review Board (IRB).
Results
Intact feedback pathway correlates to decreased lymph node metastasis in BRAF + papillary thyroid cancer
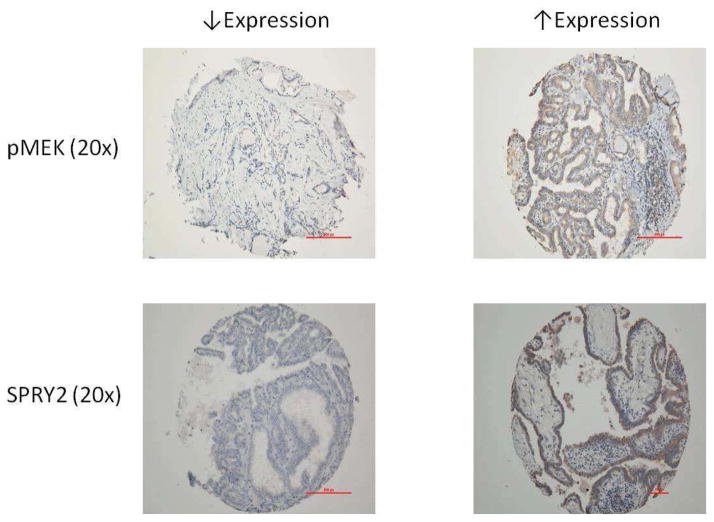
An example of tumors stained for SPRY2, pMEK and pERK is shown in Figure 2. Using the TMA Allred scores, tumors were placed in groups based on expression level of SPRY2 and pMEK, representing functional and dysfunctional pathway feedback (Figure 3). Four tumors displayed increased expression of SPRY2 and decreased expression of pMEK reflecting intact feedback regulation. Ten specimens displayed increased SPRY2 and pMEK expression representing feedback dysregulation. Eight tumors demonstrated decreased SPRY2 and pMEK expression representing feedback dysregulation and eight tumors showed decreased SPRY2 and increased pMEK expression again representing feedback dysregulation. Clinicopathologic variables were then compared between groups (Table 1). Tumors with intact negative feedback regulation had no lymph node metastases. All other groups with dysregulation had a high incidence of lymph node metastases.
Figure 2.
Example of TMA staining pattern for SPRY2 and pMEK in BRAF+PTC. Expression of pMEK reflects MAPK/ERK pathway output.
Figure 3.
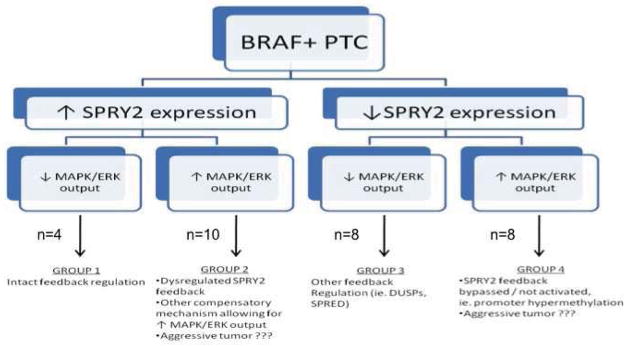
Schematic depicting tumor stratification based on SPRY2 expression and MAPK/ERK output (pMEK expression). Groups represent functional and dysfunctional pathway feedback.
Table 1.
Clinicopathologic Variables and MAPK/ERK expression
| Categories | SPRY2
 pMEK pMEK
 (n=4) (n=4) |
SPRY2
 pMEK pMEK
 (n=10) (n=10) |
SPRY2
 pMEK pMEK
 (n=8) (n=8) |
SPRY2
 pMEK pMEK
 (n=8) (n=8) |
p Value |
|---|---|---|---|---|---|
| Female (%) | 1 (25) | 7 (70) | 4 (50) | 6 (75) | 0.16 |
| Multifocal (%) | 1 (25) | 6 (60) | 6 (75) | 5 (63) | 0.18 |
| Bilateral (%) | 1 (25) | 5 (50) | 5 (63) | 4 (50) | 0.11 |
| Extrathyroidal extension (%) | 0 (0) | 5 (50) | 4 (50) | 3 (38) | 0.12 |
| Vascular Invasion (%) | 1 (25) | 8 (80) | 8 (100) | 4 (50) | 0.09 |
| Positive Lymph Nodes (%) | 0 (0) | 7 (70) | 8 (100) | 5 (63) | 0.01 |
| Age | 28.5 | 38.2 | 32.7 | 58.4 | 0.01 |
| Size (mm) | 18.5 | 16.0 | 17.3 | 19.8 | 0.85 |
SPRY2 silencing increases sensitivity of BRAF + thyroid cancer cells to BRAF and MEK inhibition
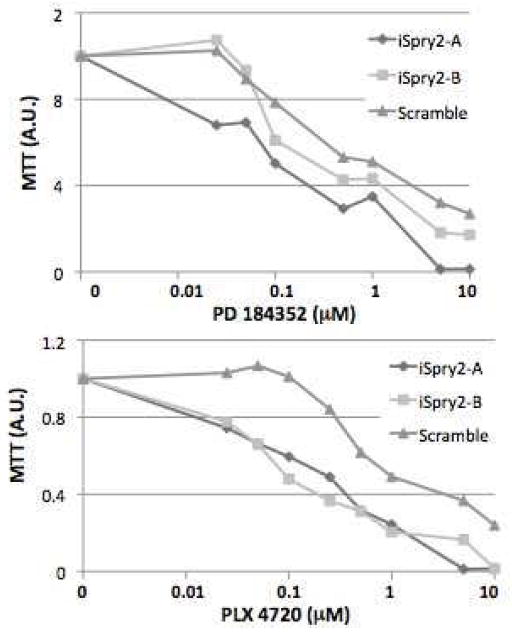
It has been shown that BRAF+ cells have increased MAPK/ERK pathway output and they are sensitive to BRAF and MEK inhibition 17–20. We have shown that SPRY2 silencing enhances MAPK/ERK activity in BRAF +TC cells demonstrating intact feedback regulation 6. To assess for a potential functional role we silenced SPRY2 and found that when SPRY2 is silenced, BRAF+ TC cells are significantly more sensitive (10x) to MAPK/ERK inhibition (Figure 4).
Figure 4.
KHM5M BRAF + TC cells with and without silencing of SPRY2. iSpry2-A and iSpry2-B represent two distinct shRNAs designed to silence SPRY2. Scramble is the control shRNA. MTT assay was performed showing increased inhibition (10x) of cell proliferation with MEK and BRAF inhibitors compared to the control based on standard AU (absorbance unit).
Discussion
In normal cells, MAPK/ERK signaling is accompanied by rapid and complex feedback inhibition, resulting in a pulse of pathway activation followed by its rapid down-regulation 21, 22. In transformed cells, increased MAPK/ERK activity is associated with increased output of the pathway, leading to enhanced expression of transcription factors and negative feedback regulators such as SPRY2. Constitutive activation of MAPK/ERK signaling by activated oncoproteins, such as BRAF V600E, is likely to result in constitutive negative feedback. However, the feedback mechanism may be functional in some tumors and dysfunctional in others. Dysfunctional feedback may be caused by either insensitivity of the mutant protein to normal regulation or second hits such as posttranslational modification of the feedback regulator 23. Our data show that MAPK/ERK pathway feedback by SPRY2 likely plays a role in determining the clinical phenotype of BRAF+PTC tumors.
It has been shown that high levels of pERK do not correlate with dependence of the tumor cell on the MAPK/ERK pathway for proliferation and pERK levels are a poor surrogate marker for MAPK/ERK pathway activation 24. Conversely, the level of pMEK and the expression of feedback regulator SPRY2 does correlate with BRAF V600E status in PTC and pMEK can be a surrogate marker for MAPK/ERK pathway activation 6. Based on this we used pMEK expression to represent MAPK/ERK pathway activation.
Our model suggests that PTC behavior is dependent on both the driver of the MAPK/ERK pathway (BRAF+) and the functionality of the regulatory feedback (SPRY2). When the feedback pathway is intact the tumor phenotype seems to be less aggressive. In our study, none of the four patients with an intact feedback pathway had nodal metastases. Dysfunctional feedback pathways were associated with central lymph node metastasis in 20/26 patients. Tumors in which the feedback pathway was dysfunctional, as determined by decreased SPRY2 expression and increased MAPK/ERK activity, were significantly more common in older patients. This suggests that the mechanism of feedback dysfunction may be patient age related and may help explain age related changes seen in thyroid cancer. This model offers a novel explanation for the observed clinical heterogeneity in BRAF+PTCs and has the potential to identify an unrecognized role of feedback regulation in PTC behavior and progression.
Negative feedback seems to play a critical role in targeted therapy of cancer 25. Drugs targeting the MAPK/ERK pathway (BRAF, MEK inhibitors) are predicted to inhibit the pathway but also to relieve the negative feedback. This loss of negative feedback can itself promote oncogenic signals and cancer cell survival. Drug-induced relief of feedback may be viewed as one of the major consequences of targeted therapy and a key contributor to therapeutic resistance 25. To assess for a direct functional role of feedback regulation in BRAF+PTC we treated SPRY2 silenced and control thyroid cancer cells with MEK and BRAF inhibitors. Interestingly, SPRY2 silenced BRAF+ thyroid cancer cells were 10x more sensitive to pathway inhibition than control cells. This indicates that SPRY2 silencing of BRAF+PTCs may sensitize the cells to pathway inhibition establishing a potential therapeutic role for SPRY2.
This study has several limitations, the most significant being the relatively small sample size. We are currently validating these results on a larger cohort of patients with BRAF+PTC tumors with matched normal thyroid tissue from each patient. Our data suggest that when the feedback pathway is intact there is a trend towards decreased vascular invasion and extrathyroidal extension, which does not reach significant. With a larger sample size these differences may prove to be significant. Furthermore, we are expanding our in-vitro studies by utilizing more thyroid cancer cell lines to assess the functional role of SPRY2. It is likely that future work will reveal that negative feedback regulators mediate important aspects of cell cycle proliferation and constitute potential new predictive and therapeutic targets for tumors harboring BRAF mutations.
Acknowledgments
Sources of Financial Support: NYUCI Center Support Grant, “NIH/NCI 5 P30CA16087-31.” This project was funded in whole or in part by the National Institutes of Health’s National Center for Advancing Translational Sciences through its Clinical and Translational Science Awards Program (CTSA), grant # UL1 TR000038.
Footnotes
Disclosure of Financial Interests and Potential Conflicts of Interest
None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Davies L, Welch HG. Increasing incidence of thyroid cancer in the United States, 1973–2002. Jama. 2006;295(18):2164–7. doi: 10.1001/jama.295.18.2164. [DOI] [PubMed] [Google Scholar]
- 2.Nikiforova MN, Nikiforov YE. Molecular genetics of thyroid cancer: implications for diagnosis, treatment and prognosis. Expert Rev Mol Diagn. 2008;8(1):83–95. doi: 10.1586/14737159.8.1.83. [DOI] [PubMed] [Google Scholar]
- 3.Nikiforov YE, Nikiforova MN. Molecular genetics and diagnosis of thyroid cancer. Nat Rev Endocrinol. 7(10):569–80. doi: 10.1038/nrendo.2011.142. [DOI] [PubMed] [Google Scholar]
- 4.Xing M. BRAF mutation in papillary thyroid cancer: pathogenic role, molecular bases, and clinical implications. Endocr Rev. 2007;28(7):742–62. doi: 10.1210/er.2007-0007. [DOI] [PubMed] [Google Scholar]
- 5.Xing M, Westra WH, Tufano RP, et al. BRAF mutation predicts a poorer clinical prognosis for papillary thyroid cancer. J Clin Endocrinol Metab. 2005;90(12):6373–9. doi: 10.1210/jc.2005-0987. [DOI] [PubMed] [Google Scholar]
- 6.Xu L, Zhou JL, Cohen M, et al. Spry2 expression correlates with BRAF mutation in thyroid cancer. Surgery. 148(6):1282–7. doi: 10.1016/j.surg.2010.09.028. discussion 1287. [DOI] [PubMed] [Google Scholar]
- 7.Lee KC, Li C, Schneider EB, et al. Is BRAF mutation associated with lymph node metastasis in patients with papillary thyroid cancer? Surgery. 152(6):977–83. doi: 10.1016/j.surg.2012.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Soares P, Sobrinho-Simoes M. Cancer: Small papillary thyroid cancers--is BRAF of prognostic value? Nat Rev Endocrinol. 7(1):9–10. doi: 10.1038/nrendo.2010.213. [DOI] [PubMed] [Google Scholar]
- 9.Nam JK, Jung CK, Song BJ, et al. Is the BRAF(V600E) mutation useful as a predictor of preoperative risk in papillary thyroid cancer? Am J Surg. 203(4):436–41. doi: 10.1016/j.amjsurg.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 10.Edwin F, Anderson K, Ying C, Patel TB. Intermolecular interactions of Sprouty proteins and their implications in development and disease. Mol Pharmacol. 2009;76(4):679–91. doi: 10.1124/mol.109.055848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim HJ, Taylor LJ, Bar-Sagi D. Spatial regulation of EGFR signaling by Sprouty2. Curr Biol. 2007;17(5):455–61. doi: 10.1016/j.cub.2007.01.059. [DOI] [PubMed] [Google Scholar]
- 12.Kim HJ, Bar-Sagi D. Modulation of signalling by Sprouty: a developing story. Nat Rev Mol Cell Biol. 2004;5(6):441–50. doi: 10.1038/nrm1400. [DOI] [PubMed] [Google Scholar]
- 13.Mason JM, Morrison DJ, Basson MA, Licht JD. Sprouty proteins: multifaceted negativefeedback regulators of receptor tyrosine kinase signaling. Trends Cell Biol. 2006;16(1):45–54. doi: 10.1016/j.tcb.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 14.Lito P, Mets BD, Kleff S, et al. Evidence that sprouty 2 is necessary for sarcoma formation by H-Ras oncogene-transformed human fibroblasts. J Biol Chem. 2008;283(4):2002–9. doi: 10.1074/jbc.M709046200. [DOI] [PubMed] [Google Scholar]
- 15.Watanabe A, Cornelison R, Hostetter G. Tissue microarrays: applications in genomic research. Expert Rev Mol Diagn. 2005;5(2):171–81. doi: 10.1586/14737159.5.2.171. [DOI] [PubMed] [Google Scholar]
- 16.Hewitt SM. Design, construction, and use of tissue microarrays. Methods Mol Biol. 2004;264:61–72. doi: 10.1385/1-59259-759-9:061. [DOI] [PubMed] [Google Scholar]
- 17.Leboeuf R, Baumgartner JE, Benezra M, et al. BRAFV600E Mutation Is Associated with Preferential Sensitivity to Mitogen-Activated Protein Kinase Kinase Inhibition in Thyroid Cancer Cell Lines. The Journal of clinical endocrinology and metabolism. 2008;93(6):2194–2201. doi: 10.1210/jc.2007-2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schweppe RE, Kerege AA, Sharma V, et al. Distinct genetic alterations in the mitogen-activated protein kinase pathway dictate sensitivity of thyroid cancer cells to mitogen-activated protein kinase kinase 1/2 inhibition. Thyroid : official journal of the American Thyroid Association. 2009;19(8):825–835. doi: 10.1089/thy.2008.0362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Joseph EW, Pratilas CA, Poulikakos PI, et al. The RAF inhibitor PLX4032 inhibits ERK signaling and tumor cell proliferation in a V600E BRAF-selective manner. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(33):14903–14908. doi: 10.1073/pnas.1008990107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lito P, Pratilas CA, Joseph EW, et al. Relief of profound feedback inhibition of mitogenic signaling by RAF inhibitors attenuates their activity in BRAFV600E melanomas. Cancer Cell. 22(5):668–82. doi: 10.1016/j.ccr.2012.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Amit I, Citri A, Shay T, et al. A module of negative feedback regulators defines growth factor signaling. Nature Genetics. 2007;39(4):503–512. doi: 10.1038/ng1987. [DOI] [PubMed] [Google Scholar]
- 22.Traverse S, Gomez N, Paterson H, et al. Sustained activation of the mitogen-activated protein (MAP) kinase cascade may be required for differentiation of PC12 cells. Comparison of the effects of nerve growth factor and epidermal growth factor. The Biochemical journal. 1992;288 ( Pt 2):351–355. doi: 10.1042/bj2880351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brady SC, Coleman ML, Munro J, et al. Sprouty2 association with B-Raf is regulated by phosphorylation and kinase conformation. Cancer Res. 2009;69(17):6773–81. doi: 10.1158/0008-5472.CAN-08-4447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pratilas CA, Taylor BS, Ye Q, et al. (V600E)BRAF is associated with disabled feedback inhibition of RAF-MEK signaling and elevated transcriptional output of the pathway. Proc Natl Acad Sci U S A. 2009;106(11):4519–24. doi: 10.1073/pnas.0900780106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chandarlapaty S. Negative feedback and adaptive resistance to the targeted therapy of cancer. Cancer Discov. 2(4):311–9. doi: 10.1158/2159-8290.CD-12-0018. [DOI] [PMC free article] [PubMed] [Google Scholar]