Abstract
Meat animals are raised for their carcasses, and carcasses are composed from muscle, fat and bone. Enhancing muscle growth and reducing fat accumulation improve the efficiency of animal production. Fetal stage is crucial for skeletal muscle development. Due to extensive efforts to increase lean growth, marbling (intramuscular fat) is reducing in beef, pork and chicken breast, which impairs the eating quality of meat. Because fat is the major contributor to meat flavor, the presence of intramuscular fat is indispensible for the high eating quality of meat. However, up to now, our understanding of adipogenesis (formation of fat cells) in skeletal muscle is limited. Adipocyte differentiation in skeletal muscle initiates from mesenchymal multipotent cells, which are abundant in skeletal muscle at early developmental stages. In this review, the known cellular mechanisms regulating adipogenesis from multipotent cells are summarized, which include hedgehog, Wingless and Int (Wnt)/α-catenin, and bone morphogenesis protein (BMP) mediated signaling pathways, as well as AMP-activated protein kinase. Promoting adipogenesis inside skeletal muscle will dramatically increase intramuscular fat, improving the quality of meat.
Keywords: adipogenesis, meat, skeletal muscle, mesenchymal stem cells, signaling, marbling
INTRODUCTION
Animal carcasses are composed of muscle, fat, connective tissue, and bone, all of which are largely derived from mesenchymal multipotent cells (MC) during the early developmental stage. Among these tissues, skeletal muscle apparently is the most important. Fetal stage is especially important for skeletal muscle development because there is no net increase in the muscle fiber number after birth (Du et al., 2009; Stickland, 1978). Moreover, lean meat itself is composed of muscle fibers, fat cells and fibroblasts which embed in connective tissue. Thus, skeletal muscle development involves myogenesis, adipogenesis and fibrogenesis. Promoting myogenesis from MC increases lean mass and lean/fat ratio; enhancing intramuscular adipogenesis increases intramuscular fat and improves eating quality of pork but fat deposition elsewhere leads to waste; promoting fibrogenesis leads to the accumulation of connective tissue which contributes to the background toughness of meat and is undesirable. Hence, it is essential to regulate MC differentiation in order to enhance lean growth and improve meat quality.
Excessive fat accumulation accounts for huge waste in animal production. For this reason, livestock have been selected for lean growth for generations. Accompanying such selection is the unwanted decline in intramuscular fat which is essential for the eating quality of meat. It will be ideal to reduce fat deposition in visceral, subcutaneous and intramuscular depots while enhancing intramuscular fat deposition. Maternal obesity impacts fetal adipogenesis. However, to date, our understanding of mechanisms regulating sequential fat deposition remains largely unclear. In this review, we first discussed fetal programming and its impact on carcass composition, followed by discussion of mechanisms regulating adipogenesis and fat deposition.
FETAL PROGRAMMING
The concept of fetal programming, or fetal origins of adult diseases, known as the Barker hypothesis, is based on the epidemiological data linking long-term effects for adult health of low birth weight (Barker et al., 2002). The fetal origins hypothesis states that fetal nutrient deficiency leads to disproportionate fetal growth and programs later predisposition to chronic disease states such as coronary heart diseases, stroke, diabetes and hypertension (Barker, 2001). Low birth weight has been linked to adult diseases. The prevalence of non-insulin dependent diabetes increases for 3 fold for men who had weighed 5.5 lb at birth when compared to those who had birth weights around 9.5 lb. This relationship has been confirmed by several studies in Britain, United States and Sweden (Barker, 2003, 1995; Hales et al., 1991).
The concept of fetal programming can be equally applied to animal production. Failure of the fetus to achieve its optimal growth potential is the major unsolved production problem. Maternal nutrient deficiency is the leading cause of poor fetal growth and development. Besides, many disorders including those of genetic, metabolic, vascular, autoimmune and infective origins, limit the cross-placental nutrient delivery and reduce nutrients available for proper fetal development (Cetin et al., 2004). Offspring delivered by dams that experienced maternal nutrient deficiency during pregnancy are fatter than control animals (Bispham et al., 2003; Desai et al., 2005; Symonds et al., 2004). Impairment of fetal skeletal muscle development may be one of the main reasons for the predisposition of offspring to obesity and diabetes (Zhu et al., 2004).
Sheep is the livestock species which has been used extensively used for fetal programming studies, mainly as a model for human pregnancy. Compared to rodents, which are born highly immature, sheep is born mature which is similar to humans. In addition, the number of fetuses and the ratio of fetal/maternal body mass are also very similar to human pregnancy, which makes sheep one of the ideal models for human pregnancy studies. Thus, much of our understanding about fetal programming in livestock, as well as in human health, is derived from sheep studies.
Beef cattle production is the main component of agriculture in Rocky Mountain states, where are characterized with arid and semi-arid conditions (NWS, 1988–1989; USGS, 2004). A persistent drought has been observed since 2000 in the states of Wyoming, Colorado, Utah, New Mexico, Nevada, Arizona, and part of California (USGS, 2004). Drought condition limits availability and protein contents of forage, affecting animal production (Du and Zhu, 2009). Due to the seasonal nature of cow reproduction, cows in the Rocky Mountain areas frequently experience nutrient restriction, especially protein deficiency. Such nutrient deficiency during gestation likely programs fetal skeletal muscle development, reducing the growth performance of offspring.
MATERNAL NUTRITION AND OFFSPRING BODY COMPOSITION AND MEAT QUALITY
Pigs
Run piglets experienced nutrient restriction compared to normal littermates during pregnancy. Therefore, runt piglets are good models for studying the impact of maternal nutrient restriction on fetal development, offspring carcass composition and meat quality. Existing data clearly show that maternal nutrient restriction reduces birth weight, increases offspring carcass fatness. In early studies, runt pigs were compared to normal pigs for their growth performance and muscle fiber numbers (Hegarty and Allen, 1978). Runt pigs required 23 more days to reach 106 kg slaughter weight compared to their littermates. Runt pigs had fewer muscle fiber numbers (Hegarty and Allen, 1978). These observations were confirmed by several later studies (Powell and Aberle, 1980). Lower birth weight littermates had 14% greater cross-sectional areas in LM muscle (Gondret et al., 2006). The mean fiber area tended to be larger in light littermates compared to heavy littermates (Bee, 2004). In addition, several studies show that maternal nutrient deficiency affects the composition of muscle fibers. The percentage of slow oxidative fibers in the low birth weight pigs were higher than large birth weight pigs (birth weight, 1544 g) compared to runt pigs (776 g) (Handel and Stickland, 1987).
In addition, lower birth weight pigs have a fatter carcass and enlarged subcutaneous adipocytes (Gondret et al., 2006; Powell and Aberle, 1980). Runt pigs had higher marbling and intramuscular fat content (Powell and Aberle, 1980). The tenderness of pork is impaired in runt piglets (Gondret et al., 2006), which could be due to increased collagen content in muscle. Collagen contributes to the background toughness of meat. Compared to their counterparts, grown runt pigs have higher concentration of collagen in skeletal muscle (Karunaratne et al., 2005).
The speed of postmortem pH decline affects meat quality. However, there is no difference in postmortem pH decline between heavy and light birth weight piglets (Gondret et al., 2006).
Cattle and sheep
Consistent with pig studies, maternal nutrient deficiency also reduces the growth performance of ruminant animals. In our previous study, ewes were fed to 50% (nutrient restricted) or 100% (control fed) of total digestible nutrients (NRC requirement) from Days 28 to 78 of gestation when ewes were euthanized for fetal longissimus dorsi muscle sampling. Fetuses of nutrient restricted ewes showed retarded muscle and skeleton development. Muscle from nutrient restricted fetuses contained fewer secondary myofibers than control fetuses, and the average area of fasciculi was smaller (Zhu et al., 2004). In addition, maternal nutrient deficiency demonstrated long-term effects on offspring performance. Maternal nutrient restriction during early to mid-gestation decreased the number of myofibers in the offspring compared with offspring of ad libitum fed ewes. Intramuscular triglyceride and visceral fat contents were increased in skeletal muscle of nutrient restricted lambs (Zhu et al., 2006). These data were confirmed by another study (Daniel et al., 2007).
Similarly, nutrient restriction in cows impairs the growth performance of offspring steers. Crossbred beef cows were placed on improved pasture (IP) or native range (NR) pasture from 120 to 150 through 180 to 210 days of gestation. Esophageal extrusa samples collected by cows grazing IP varied from 11.1% crude protein of organic matter early in the test period to 6.0% crude protein of organic matter at the end of the grazing period; whereas, extrusa samples of cows grazing NR ranged from 6.5% crude protein of organic matter during early grazing to 5.4 % crude protein of organic matter at the end of the grazing period. Steers were slaughtered around 460 days of age and carcass characteristics were collected. Steers born to mothers grazed on IP had heavier live weights and hot carcass weights than NR steers. Tenderness was greater in IP compared to NR steers. The 12th rib fat thickness and adjusted 12th rib fat thickness were greater for IP than for NR steers. Subcutaneous adipose tissue of IP steers tended to have a greater number of cells per field of view than that of NR steers. Data show improving nutritional status of cows during mid to late gestation affects tenderness, adipose tissue deposition and growth in steers (Underwood et al., 2010). In another study, we hypothesized that maternal protein supplementation enhances fetal muscle development. Early to mid gestation is as important period for muscle and adipose tissue development in beef cattle, and hence nutrition during this time is expected to affect muscle and adipose tissue development and resulting carcass characteristics of steers. In this study, thirty six crossbred beef cows were randomly placed on a control diet (100% NRC requirements, n = 12, C), nutrient restricted (70% of requirements, n = 12, NR), or a nutrient restricted diet with protein supplement (NRP, n = 12) designed to equal flow of amino acids to the small intestine of C diet from d 45 to 185 of gestation. Then, both groups of cows were placed together, managed to meet requirements and allowed to calve. Calves were weaned at 210 d of age and back-grounded. Steers were placed in feedlot and provided a high energy diet for 195 d. Steers were slaughtered at 405 d of age. Twelfth rib fat thickness and adjusted 12th rib fat thickness of NR steers was less (P ≤ 0.02) than C steers and tended to be less (P ≤ 0.08) than NRP steers. Kidney, pelvic and heart fat percentage was lower (P ≤ 0.05) for NRP steers compared to C and NR steers. Adipocyte diameter tended to be larger (P = 0.10) for NR steers than for NRP steers, and NRP steers tended (P = 0.09) to have a greater number of adipocytes per field of view. Steers born to NRP dams had larger semitendinosus muscles than C steers (P = 0.008) and NR steers (P = 0.07). Muscle fiber diameter was similar (P ≥ 0.43) between treatments, but total muscle fiber number in LM area was higher (P = 0.02) in NR steers than C steers. These data show maternal nutrition and protein supplementation during gestation affects muscle and adipose tissue development in subsequent beef steers (K. R. Underwood, J. F. Tong, P. L. Price, B. W. Hess, S. I. Paisley, W. J. Means, and M. Du, unpublished data).
Dr. Greenwood’s lab in Australia did series of studies on the impact of maternal nutrient deficiency on growth performance of beef cattle (Greenwood and Cafe, 2007). Impaired early development due to maternal undernutrition results in smaller animals at any given age (Cafe et al., 2009). Retail yield of severe retarded offspring was reduced (Greenwood et al., 2009). These data support our results. However, no difference in carcass composition was observed in these studies, which should be due to feeding a forage based diet and a lack of a fattening period before slaughter in these cattle. In our studies in mice, the difference in body composition was exacerbated when fed a high energy diet (J. F. Tong, and M. Du, unpublished data). In sheep, without the challenge of high energy diet, there is no difference in body composition between control and treated animals, and dramatic difference was observed only after feeding high energy diet (Long et al., 2010).
MESENCHYMAL STEM CELL DIFFERENTATION
Above studies clearly show that maternal nutrition affects fetal development. Livestock carcasses are composed of muscle, fat, connective tissue, and bone, all of which are largely derived from mesenchymal multipotent cells (MC) during the early developmental stage and there is no net increase in the muscle fiber number after birth (Du et al., 2009; Stickland, 1978). Moreover, lean meat is composed of muscle fibers, fat cells and fibroblasts which embed in connective tissue. Thus, skeletal muscle development itself involves myogenesis, adipogenesis and fibrogenesis. Promoting myogenesis from MC increases lean mass and lean/fat ratio; enhancing intramuscular adipogenesis increases intramuscular fat and improves eating quality of pork but fat deposition elsewhere leads to waste; promoting fibrogenesis leads to the accumulation of connective tissue which contributes to the background toughness of meat and is undesirable. Hence, it is essential to regulate MC differentiation in order to enhance lean growth and improve meat quality. However, to date, mechanisms governing MC differentiation has not been well studied, and most studies are in rodents.
MSC differentiation--- a crucial step regulating fetal SM development
Adipogenesis
The formation of adipocytes begins around the mid-gestation (Feve, 2005; Gnanalingham et al., 2005; Muhlhausler et al., 2006). Adipogenesis inside muscle during the fetal stage has a dominant effect on the number of intramuscular adipocytes, an event linked to IR due to their paracrine effects and close proximity (Aguiari et al., 2008; Morino et al., 2005; Petersen and Shulman, 2002). The total number of adipocytes is set when reaching adolescence (Spalding et al., 2008). Thus, it is important to prevent excessive adipogenesis inside fetal and early postnatal SM. Up to now, mechanisms controlling adipogenesis in fetal and postnatal SM in vivo are poorly defined, though there are numerous in vitro cell culture studies (Rosen and MacDougald, 2006). These studies have demonstrated that PPARγ and CCAAT-enhancer-binding proteins (C/EBPs) are crucial factors controlling adipogenesis. Their expression induces adipogenesis from MSC (Rosen et al., 1999).
Fibrogenesis
Fibrogenesis in fetal SM has not been systemically studied and seems ongoing during the whole gestation period but is more active after mid-gestation, coinciding with adipogenesis (Hausman and Poulos, 2009). Fibrogenesis is mainly mediated by TGF-α signaling pathway (Chen et al., 2005; Salvadori et al., 2005), which promotes fibrosis via activation of the Smad signaling pathway, specifically phosphorylation of Smad2 and Smad3 which then oligomerize with Smad4 and translocate into the nucleus to initiate transcription of TGFα target genes (Decologne et al., 2007; Gosselin et al., 2004; Tu and Luo, 2007), including fibronectin and type I collagen (Foidart et al., 1981; Kennedy et al., 2008).
Myogenesis
Compared to adipogenesis and fibrogenesis, mechanisms associated with myogenesis are better studied. During SM development, primary myofibers are first formed in the embryonic stage, followed by the formation of secondary myofibers in the mid and late gestation in humans, and late and neonatal stages in mice (Du et al., 2009). The formation of secondary myofibers overlaps with adipogenesis which is initiated at mid-gestation in humans and late gestation in rodents. Myogenesis are regulated by a series of transcription factors, including Pax 3, Pax 7, Gli, and four myogenic regulatory factors including MyoD, Myf-5, myogenin and MRF-4 (Relaix et al., 2005).
In the bovine fetus, primary muscle fibers form within two months of gestation (Russell and Oteruelo, 1981), and the secondary myogenesis occurs between 2 to 7 months of gestation (Russell and Oteruelo, 1981). Adipogenesis is initiated around mid-gestation in ruminant animals (Feve, 2005; Gnanalingham et al., 2005; Muhlhausler et al., 2006), which partially overlaps with the period of secondary myogenesis (Du and Zhu, 2009). Tissues and organs are most susceptible to maternal physiological stress when they are at the initial stages of active development. Thus, maternal nutrient deficiency at different gestational stage is expected to have different effects on fetal development and offspring performance (Du et al., 2010).
SIGNALING PATHWAYS REGULATING MYOGENESIS AND ADIPOGENESIS
Complicated signaling pathways regulating the differentiation of MC into myocytes and adipocytes. Based on our current understanding, several extracellular morphogens appear very important in these processes.
Morphogens are crucial for pattern development during fetal development. Morphogens, including hedgehog, Wingless and Int (Wnt), and bone morphogenic proteins (BMP). The detailed mechanisms for these morphogens in the regulation of myogenesis and adipogenesis has been previously reviewed (Du et al.) and thus we will only briefly review here.
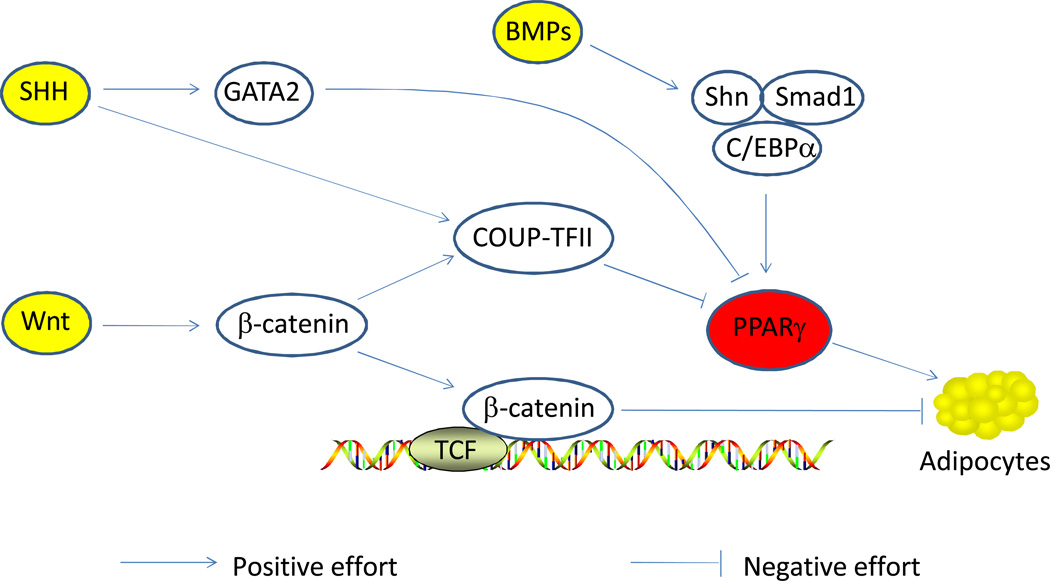
Three different hedgehog proteins are present in mammals, which are Sonic, India and Desert hedgehogs, with Sonic hedgehog (SHH) of the best studied. Activation of SHH signaling inhibits adipogenesis in 3T3-L1 and C3H10T1/2 cells (Cousin et al., 2007; Spinella-Jaegle et al., 2001; Suh et al., 2006; Zehentner et al., 2000). A decrease in Hedgehog signaling is necessary but not sufficient to trigger adipocyte differentiation (Fontaine et al., 2008). SHH may elicit antiadipogenic effects by enhancing chicken ovalbumin upstream promoter-transcription factor II (Okamura et al., 2009; Xu et al., 2008) and GATA binding protein 2 (GATA2) expression (Suh et al., 2006), which mediates the expression of PPARγ and C/EBPα (Hu and Davies, 2009; Schupp et al., 2009) and inhibits adipogenesis.
Wnt proteins are secreted glycoproteins (Johnson and Rajamannan, 2006). Activation of Wnt signaling stabilizes β-catenin, which regulates the expression of transcription factors Pax3 as well as Gli (Borycki et al., 2000; Capdevila et al., 1998). Pax3 is essential for skeletal myogenesis and acts upstream of MyoD during skeletal muscle development, while Gli factors induces Myf-5 expression (Gustafsson et al., 2002; Ridgeway and Skerjanc, 2001). Myf5 is a direct target of Wnt/α-catenin (Borello et al., 2006). Activation of the Wnt signaling pathway enhances myogenesis and inhibits adipogenesis in cultured mesenchymal stem cells (Shang et al., 2007). Blocking the β-catenin pathway reduces the total number of myocytes (Pan et al., 2005; Yamanouchi et al., 2007). Over-expression of β-catenin results in increased myoblast proliferation and enhanced muscle repair following ischaemia-induced muscle damage (Kim et al., 2006; Otto et al., 2008).
BMPs belong to TGF-α super-family. Upon binding to BMPs, Smad 1, 5 and/or 8, so called regulatory Smads, are activated, which partners with Smad 4 to enhance adipogenesis. There are about 15 BMPs identified in mammals. BMP-2 and 4 regulates adipogenesis and osteogenesis, while BMP-7 promotes brown adipogenesis (Tseng et al., 2008). As previously discussed, TGF-α signaling pathway promotes fibrogenesis via Smad 2 or 3, which also binds to Smad4 to induce the expression of genes associated with fibrosis. The shared signaling pathways clearly show the similarity in fibrogenesis and adipogenesis. Because fibrogenesis leads to accumulation of connective tissue which increases meat toughness while adipogenesis is crucial for meat quality, it may be possible to enhance adipogenesis and inhibit fibrogenesis by manipulating these shared pathways. In order to do it, of course, we need further understand underlying mechanisms. In a recent study, a transcription factor, Zfp423, was identified to be critical in determining whether fibrogenic cells go to adipogenic differentiation (Gupta et al.).
Above morphogens, or additional unidentified morphogens, may also be responsible for depot specific adipose tissue development. Up to now, the reason why fat is easy to accumulate under the skin and surrounding muscle bundles, but difficult to form inside muscle bundles (intramuscular fat) is unclear. Highly possibly, this is due to the presence of gradient concentration of these morphogens (Figure 1). Higher concentration of morphogens inside muscle bundles promotes myogenesis but outside of muscle adipogenesis is enhanced due to the absence of these morphogens due to their short traveling distance.
Figure 1. Hedgehog signaling, Wingless and Int (Wnt) signaling, bone morphogenesis proteins (BMPs) and adipogenesis.
C/EBPα: CAAT/enhancer binding protein α; COUP-TFII: chicken ovalbumin upstream promoter-transcription factor II; GATA2: GATA binding protein 2; Shn: Schnurri protein; PPARγ: peroxisome proliferator-activated receptor γ.
EPIGENETIC MODIFICATIONS
Since myogenesis, adipogenesis and fibrogenesis from mesenchymal stem cells are controlled by the expression of one or more crucial genes, maternal nutrition might change fetal muscle development through epigenetic modifications. The development of the human body starts from one fertilized cell, which generates an increasing body of diversified cells sharing the same copy of genes. Silencing genes while allowing other genes to express is essential for maintaining the diversity of cells which are accomplished through epigenetic modifications (Schuettengruber et al., 2007). By definition, epigenetic heritance occurs when the modifications, such as cytosine methylation and histone modifications, pass from parent cells to daughter cells (Bernstein et al., 2007). Depending on the nature of modifications, epigenetic modifications have different degree of plasticity. Histone modification usually only passes for several cell generations (Rando and Verstrepen, 2007), but histone modifications can guide DNA methylation leading to stable alterations in gene expression (Vire et al., 2006). DNA methylation passes on for more than 100 cell generations; considering that only forty-four cell generations are needed to form a human body, epigenetic modification through DNA methylation is very stable (Reik, 2007). Polycomb group proteins (PcG) and trithorax (trxG) group proteins regulate histone methylation, which leads to other epigenetic modifications during cell differentiation (Reik, 2007). PcG and trxG regulate the methylation of histone H3 through binding to PcG and trxG response elements in genome. PcG group proteins possess H3K27-specific trimethylase activity which mediates gene expression repression, whereas trxG complexes have H3K4 trimethylase activity which mediates activation of genes (Schuettengruber et al., 2007). The crucial development is the demonstration that PcG-mediated gene repression leads to DNA methylation of the targeted genes (Vire et al., 2006). The PcG protein EZH2 (Enhancer of Zeste homolog 2) interacts with DNA methyltransferases and serves as a recruitment platform for DNA methyltransferases, thus converting plastic histone modifications to stable DNA methylation (Vire et al., 2006). DNA methylation leads to the silence of genes through several mechanisms: 1) recruitment of histone deacetylases, which removes histone acetylation. Since acetylation of the lysine residues at the histone neutralizes its positive charges, de-acetylation increases the affinity between histones and DNA, inhibiting gene expression; 2) DNA methylation can directly interfere with the binding of transcription factors; 3) DNA methylation leads to the formation of inactive chromatin structure.
Currently, no studies are available linking maternal nutrition to epigenetic modifications in fetal muscle. However, indirect evidences support epigenetic modification in key genes controlling fetal muscle development. Maternal under-nutrition permanently changed the insulin/insulin-like growth factor-1 signaling in fetal muscle (Ozanne et al., 1996), very likely through epigenetic modifications. Maternal diet alters the expression of PPARs in fetal muscle through DNA methylation (Rees et al., 2008). In another study, maternal cocaine administration causes epigenetic modification of a key protein kinase gene in rat heart (Zhang et al., 2007).
Histone deacetylases (HDAC) are becoming increasingly important for epigenetic modifications associated with cell differentiation and tissue development (Martin et al., 2009). HDACs are further separated into several groups, of which class IIa (HDAC4, 5, 7 and 9), may be very important for the regulation of MC differentiation. Class IIa HDAC need to interact with myocyte enhancer factor 2 (MEF2) to identify targets (Martin et al., 2007).
p300 is a necessary co-activator of PPARγ, and AMPK phosphorylates p300 at Ser 89 which reduces its function as a co-activator of several examined nuclear receptors (Yang et al., 2001). Here, we hypothesize that MO reduces AMPK activity, which decreases p300 phosphorylation and promotes the expression of PPARγ target genes, increasing adipogenesis in fetal SM (Fig. 1).
Taken together, p300 regulates both fibrogenesis and adipogenesis, and AMPK is likely to regulate both processes through phosphorylation of p300 (Fig. 1). In our obese sheep fetal SM, we observed down-regulation of AMPK activity and enhancement of both adipogenesis and fibrogenesis in fetal SM (Huang et al., 2010; Yan et al.), strongly supporting this notion.
CONCLUSION
Current studies identified that extracellular morphogens, including hedgehog, Wnt/α-catenin, and BMP signaling pathways regulate adipogenesis. AMPK, an intercellular kinase, also has crucial roles in the regulation of adipogenesis. Future efforts in the elucidation of the mechanisms regulating adipogenesis should be focused on: 1) deepening our understanding of identified pathways on adipogenesis; 2) further efforts to identify additional pathways regulating adipogenesis; 3) examining the integration of these identified signaling pathways and their cross-talks to regulate adipogenesis; and 4) up to now, most of these knowledge about adipogenesis is obtained from rodent and cell line studies, studies using primary cultures of farm animals and in vivo studies are apparently needed, especially those studies on the impact of physiological factors at the critical stage of adipogenesis of farm animals are apparently needed.
ACKNOWLEDGEMENT
This work was supported by Research Initiative Grants 2008-35206-18826 and 2006-55618-16914 from the USDA Cooperative State Research, Education and Extension Service.
LITERATURE CITED
- Aguiari P, Leo S, Zavan B, Vindigni V, Rimessi A, Bianchi K, Franzin C, Cortivo R, Rossato M, Vettor R, Abatangelo G, Pozzan T, Pinton P, Rizzuto R. High glucose induces adipogenic differentiation of muscle-derived stem cells. Proc Natl Acad Sci U S A. 2008;105:1226–1231. doi: 10.1073/pnas.0711402105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker DJ. Fetal origins of coronary heart disease. Bmj. 1995;311:171–174. doi: 10.1136/bmj.311.6998.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker DJ. A new model for the origins of chronic disease. Med Health Care Philos. 2001;4:31–35. doi: 10.1023/a:1009934412988. [DOI] [PubMed] [Google Scholar]
- Barker DJ. Coronary heart disease: A disorder of growth. Horm Res. 2003;59(Suppl 1):35–41. doi: 10.1159/000067843. [DOI] [PubMed] [Google Scholar]
- Barker DJ, Eriksson JG, Forsen T, Osmond C. Fetal origins of adult disease: Strength of effects and biological basis. Int J Epidemiol. 2002;31:1235–1239. doi: 10.1093/ije/31.6.1235. [DOI] [PubMed] [Google Scholar]
- Bee G. Effect of early gestation feeding, birth weight, and gender of progeny on muscle fiber characteristics of pigs at slaughter. J Anim Sci. 2004;82:826–836. doi: 10.2527/2004.823826x. [DOI] [PubMed] [Google Scholar]
- Bernstein BE, Meissner A, Lander ES. The mammalian epigenome. Cell. 2007;128:669–681. doi: 10.1016/j.cell.2007.01.033. [DOI] [PubMed] [Google Scholar]
- Bispham J, Gopalakrishnan GS, Dandrea J, Wilson V, Budge H, Keisler DH, Broughton Pipkin F, Stephenson T, Symonds ME. Maternal endocrine adaptation throughout pregnancy to nutritional manipulation: Consequences for maternal plasma leptin and cortisol and the programming of fetal adipose tissue development. Endocrinology. 2003;144:3575–3585. doi: 10.1210/en.2003-0320. [DOI] [PubMed] [Google Scholar]
- Borello U, Berarducci B, Murphy P, Bajard L, Buffa V, Piccolo S, Buckingham M, Cossu G. The wnt/beta-catenin pathway regulates gli-mediated myf5 expression during somitogenesis. Development. 2006;133:3723–3732. doi: 10.1242/dev.02517. [DOI] [PubMed] [Google Scholar]
- Borycki A, Brown AM, Emerson CP., Jr Shh and wnt signaling pathways converge to control gli gene activation in avian somites. Development. 2000;127:2075–2087. doi: 10.1242/dev.127.10.2075. [DOI] [PubMed] [Google Scholar]
- Cafe LM, Hennessy DW, Hearnshaw H, Morris SG, Greenwood PL. Consequences of prenatal and preweaning growth for feedlot growth, intake and efficiency of piedmontese- and wagyu-sired cattle. Animal Production Science. 2009;49:461–467. [Google Scholar]
- Capdevila J, Tabin C, Johnson RL. Control of dorsoventral somite patterning by wnt-1 and beta-catenin. Dev Biol. 1998;193:182–194. doi: 10.1006/dbio.1997.8806. [DOI] [PubMed] [Google Scholar]
- Cetin I, Foidart JM, Miozzo M, Raun T, Jansson T, Tsatsaris V, Reik W, Cross J, Hauguel-de-Mouzon S, Illsley N, Kingdom J, Huppertz B. Fetal growth restriction: A workshop report. Placenta. 2004;25:753–757. doi: 10.1016/j.placenta.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Chen YW, Nagaraju K, Bakay M, McIntyre O, Rawat R, Shi R, Hoffman EP. Early onset of inflammation and later involvement of tgfbeta in duchenne muscular dystrophy. Neurology. 2005;65:826–834. doi: 10.1212/01.wnl.0000173836.09176.c4. [DOI] [PubMed] [Google Scholar]
- Cousin W, Fontaine C, Dani C, Peraldi P. Hedgehog and adipogenesis: Fat and fiction. Biochimie. 2007;89:1447–1453. doi: 10.1016/j.biochi.2007.08.012. [DOI] [PubMed] [Google Scholar]
- Daniel ZC, Brameld JM, Craigon J, Scollan ND, Buttery PJ. Effect of maternal dietary restriction during pregnancy on lamb carcass characteristics and muscle fiber composition. J Anim Sci. 2007;85:1565–1576. doi: 10.2527/jas.2006-743. [DOI] [PubMed] [Google Scholar]
- Decologne N, Kolb M, Margetts PJ, Menetrier F, Artur Y, Garrido C, Gauldie J, Camus P, Bonniaud P. Tgf-beta1 induces progressive pleural scarring and subpleural fibrosis. J Immunol. 2007;179:6043–6051. doi: 10.4049/jimmunol.179.9.6043. [DOI] [PubMed] [Google Scholar]
- Desai M, Gayle D, Babu J, Ross MG. Programmed obesity in intrauterine growth-restricted newborns: Modulation by newborn nutrition. Am J Physiol Regul Integr Comp Physiol. 2005;288:R91–R96. doi: 10.1152/ajpregu.00340.2004. [DOI] [PubMed] [Google Scholar]
- Du M, Tong J, Zhao J, Underwood KR, Zhu M, Ford SP, Nathanielsz PW. Fetal programming of skeletal muscle development in ruminant animals. J Anim Sci. 2010;88:E51–E60. doi: 10.2527/jas.2009-2311. [DOI] [PubMed] [Google Scholar]
- Du M, Yan X, Tong JF, Zhao J, Zhu MJ. Maternal obesity, inflammation, and fetal skeletal muscle development. Biol Reprod. 2009 doi: 10.1095/biolreprod.109.077099. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du M, Yin J, Zhu MJ. Cellular signaling pathways regulating the initial stage of adipogenesis and marbling of skeletal muscle. Meat Science. 86:103–109. doi: 10.1016/j.meatsci.2010.04.027. [DOI] [PubMed] [Google Scholar]
- Du M, Zhu MJ. Fetal programming of skeletal muscle development. In: Du M, McCormick RJ, editors. Applied muscle biology and meat science No. Boca Raton, FL: CRC press; 2009. In press. [Google Scholar]
- Feve B. Adipogenesis: Cellular and molecular aspects. Best Pract Res Clin Endocrinol Metab. 2005;19:483–499. doi: 10.1016/j.beem.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Foidart M, Foidart JM, Engel WK. Collagen localization in normal and fibrotic human skeletal muscle. Arch Neurol. 1981;38:152–157. doi: 10.1001/archneur.1981.00510030046006. [DOI] [PubMed] [Google Scholar]
- Fontaine C, Cousin W, Plaisant M, Dani C, Peraldi P. Hedgehog signaling alters adipocyte maturation of human mesenchymal stem cells. Stem Cells. 2008;26:1037–1046. doi: 10.1634/stemcells.2007-0974. [DOI] [PubMed] [Google Scholar]
- Gnanalingham MG, Mostyn A, Symonds ME, Stephenson T. Ontogeny and nutritional programming of adiposity in sheep: Potential role of glucocorticoid action and uncoupling protein-2. Am J Physiol Regul Integr Comp Physiol. 2005;289:R1407–R1415. doi: 10.1152/ajpregu.00375.2005. [DOI] [PubMed] [Google Scholar]
- Gondret F, Lefaucheur L, Juin H, Louveau I, Lebret B. Low birth weight is associated with enlarged muscle fiber area and impaired meat tenderness of the longissimus muscle in pigs. J Anim Sci. 2006;84:93–103. doi: 10.2527/2006.84193x. [DOI] [PubMed] [Google Scholar]
- Gosselin LE, Williams JE, Deering M, Brazeau D, Koury S, Martinez DA. Localization and early time course of tgf-beta 1 mrna expression in dystrophic muscle. Muscle Nerve. 2004;30:645–653. doi: 10.1002/mus.20150. [DOI] [PubMed] [Google Scholar]
- Greenwood PL, Cafe LM. Prenatal and pre-weaning growth and nutrition of cattle: Longterm consequences for beef production. Animal. 2007;1:1283–1296. doi: 10.1017/S175173110700050X. [DOI] [PubMed] [Google Scholar]
- Greenwood PL, Cafe LM, Hearnshaw H, Hennessy DW, Morris SG. Consequences of prenatal and preweaning growth for yield of beef primal cuts from 30-month-old piedmontese- and wagyu-sired cattle. Animal Production Science. 2009;49:468–478. [Google Scholar]
- Gupta RK, Arany Z, Seale P, Mepani RJ, Ye L, Conroe HM, Roby YA, Kulaga H, Reed RR, Spiegelman BM. Transcriptional control of preadipocyte determination by zfp423. Nature. 464:619–623. doi: 10.1038/nature08816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson MK, Pan H, Pinney DF, Liu Y, Lewandowski A, Epstein DJ, Emerson CP., Jr Myf5 is a direct target of long-range shh signaling and gli regulation for muscle specification. Genes Dev. 2002;16:114–126. doi: 10.1101/gad.940702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hales CN, Barker DJ, Clark PM, Cox LJ, Fall C, Osmond C, Winter PD. Fetal and infant growth and impaired glucose tolerance at age 64. Bmj. 1991;303:1019–1022. doi: 10.1136/bmj.303.6809.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handel SE, Stickland NC. The growth and differentiation of porcine skeletal muscle fibre types and the influence of birthweight. J Anat. 1987;152:107–119. [PMC free article] [PubMed] [Google Scholar]
- Hausman GJ, Poulos SP. Adipose tissue development in extramuscular and intramuscular depots. In: Du M, McCormick RJ, editors. Applied muscle biology and meat science. Boca Raton, FL: CRC Press; 2009. [Google Scholar]
- Hegarty PV, Allen CE. Effect of pre-natal runting on the post-natal development of skeletal muscles in swine and rats. J Anim Sci. 1978;46:1634–1640. doi: 10.2527/jas1978.4661634x. [DOI] [PubMed] [Google Scholar]
- Hu Y, Davies GE. Berberine increases expression of gata-2 and gata-3 during inhibition of adipocyte differentiation. Phytomedicine. 2009;16:864–873. doi: 10.1016/j.phymed.2009.03.002. [DOI] [PubMed] [Google Scholar]
- Huang Y, Yan X, Zhu MJ, McCormick RJ, Ford SP, Nathanielsz PW, Du M. Enhanced transforming growth factor beta (tgf-beta) signaling and fibrogenesis in ovine fetal skeletal muscle of obese dams at late gestation. American Journal of Physiology-Endocrinology and Metabolism Submitted. 2010 doi: 10.1152/ajpendo.00015.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson ML, Rajamannan N. Diseases of wnt signaling. Rev Endocr Metab Disord. 2006;7:41–49. doi: 10.1007/s11154-006-9003-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karunaratne JF, Ashton CJ, Stickland NC. Fetal programming of fat and collagen in porcine skeletal muscles. J Anat. 2005;207:763–768. doi: 10.1111/j.1469-7580.2005.00494.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy L, Shi-Wen X, Carter DE, Abraham DJ, Leask A. Fibroblast adhesion results in the induction of a matrix remodeling gene expression program. Matrix Biol. 2008;27:274–281. doi: 10.1016/j.matbio.2008.01.004. [DOI] [PubMed] [Google Scholar]
- Kim KI, Cho HJ, Hahn JY, Kim TY, Park KW, Koo BK, Shin CS, Kim CH, Oh BH, Lee MM, Park YB, Kim HS. Beta-catenin over expression augments angiogenesis and skeletal muscle regeneration through dual mechanism of vascular endothelial growth factor-mediated endothelial cell proliferation and progenitor cell mobilization. Arterioscler Thromb Vasc Biol. 2006;26:91–98. doi: 10.1161/01.ATV.0000193569.12490.4b. [DOI] [PubMed] [Google Scholar]
- Long NM, George LA, Uthlaut AB, Smith DT, Nijland MJ, Nathanielsz PW, Ford SP. Maternal obesity and high nutrient intake before and during gestation in the ewe results in altered growth, adiposity and glucose tolerance in adult offspring. Journal of Animal Science. 2010 doi: 10.2527/jas.2010-3083. In press. [DOI] [PubMed] [Google Scholar]
- Martin M, Kettmann R, Dequiedt F. Class iia histone deacetylases: Regulating the regulators. Oncogene. 2007;26:5450–5467. doi: 10.1038/sj.onc.1210613. [DOI] [PubMed] [Google Scholar]
- Martin M, Kettmann R, Dequiedt F. Class iia histone deacetylases: Conducting development and differentiation. Int J Dev Biol. 2009;53:291–301. doi: 10.1387/ijdb.082698mm. [DOI] [PubMed] [Google Scholar]
- Morino K, Petersen KF, Dufour S, Befroy D, Frattini J, Shatzkes N, Neschen S, White MF, Bilz S, Sono S, Pypaert M, Shulman GI. Reduced mitochondrial density and increased irs-1 serine phosphorylation in muscle of insulin-resistant offspring of type 2 diabetic parents. J Clin Invest. 2005;115:3587–3593. doi: 10.1172/JCI25151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhlhausler BS, Duffield JA, McMillen IC. Increased maternal nutrition stimulates peroxisome proliferator activated receptor-{gamma} (ppar{gamma}), adiponectin and leptin mrna expression in adipose tissue before birth. Endocrinology. 2006 doi: 10.1210/en.2006-1115. [DOI] [PubMed] [Google Scholar]
- NWS. National water summary--floods and droughts: Wyoming. 1988–1989 [Google Scholar]
- Okamura M, Kudo H, Wakabayashi K, Tanaka T, Nonaka A, Uchida A, Tsutsumi S, Sakakibara I, Naito M, Osborne TF, Hamakubo T, Ito S, Aburatani H, Yanagisawa M, Kodama T, Sakai J. Coup-tfii acts downstream of wnt/beta-catenin signal to silence ppargamma gene expression and repress adipogenesis. Proc Natl Acad Sci U S A. 2009;106:5819–5824. doi: 10.1073/pnas.0901676106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto A, Schmidt C, Luke G, Allen S, Valasek P, Muntoni F, Lawrence-Watt D, Patel K. Canonical wnt signalling induces satellite-cell proliferation during adult skeletal muscle regeneration. J Cell Sci. 2008;121:2939–2950. doi: 10.1242/jcs.026534. [DOI] [PubMed] [Google Scholar]
- Ozanne SE, Wang CL, Coleman N, Smith GD. Altered muscle insulin sensitivity in the male offspring of protein-malnourished rats. Am J Physiol. 1996;271:E1128–E1134. doi: 10.1152/ajpendo.1996.271.6.E1128. [DOI] [PubMed] [Google Scholar]
- Pan W, Jia Y, Wang J, Tao D, Gan X, Tsiokas L, Jing N, Wu D, Li L. Beta-catenin regulates myogenesis by relieving i-mfa-mediated suppression of myogenic regulatory factors in p19 cells. Proc Natl Acad Sci U S A. 2005;102:17378–17383. doi: 10.1073/pnas.0505922102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen KF, Shulman GI. Pathogenesis of skeletal muscle insulin resistance in type 2 diabetes mellitus. Am J Cardiol. 2002;90:11G–18G. doi: 10.1016/s0002-9149(02)02554-7. [DOI] [PubMed] [Google Scholar]
- Powell SE, Aberle ED. Effects of birth weight on growth and carcass composition of swine. J Anim Sci. 1980;50:860–868. doi: 10.2527/jas1980.505860x. [DOI] [PubMed] [Google Scholar]
- Rando OJ, Verstrepen KJ. Timescales of genetic and epigenetic inheritance. Cell. 2007;128:655–668. doi: 10.1016/j.cell.2007.01.023. [DOI] [PubMed] [Google Scholar]
- Rees WD, McNeil CJ, Maloney CA. The roles of ppars in the fetal origins of metabolic health and disease. PPAR Res. 2008;2008:459030. doi: 10.1155/2008/459030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reik W. Stability and flexibility of epigenetic gene regulation in mammalian development. Nature. 2007;447:425–432. doi: 10.1038/nature05918. [DOI] [PubMed] [Google Scholar]
- Relaix F, Rocancourt D, Mansouri A, Buckingham M. A pax3/pax7-dependent population of skeletal muscle progenitor cells. Nature. 2005;435:948–953. doi: 10.1038/nature03594. [DOI] [PubMed] [Google Scholar]
- Ridgeway AG, Skerjanc IS. Pax3 is essential for skeletal myogenesis and the expression of six1 and eya2. J Biol Chem. 2001;276:19033–19039. doi: 10.1074/jbc.M011491200. [DOI] [PubMed] [Google Scholar]
- Rosen ED, MacDougald OA. Adipocyte differentiation from the inside out. Nat Rev Mol Cell Biol. 2006;7:885–896. doi: 10.1038/nrm2066. [DOI] [PubMed] [Google Scholar]
- Rosen ED, Sarraf P, Troy AE, Bradwin G, Moore K, Milstone DS, Spiegelman BM, Mortensen RM. Ppar gamma is required for the differentiation of adipose tissue in vivo and in vitro. Mol Cell. 1999;4:611–617. doi: 10.1016/s1097-2765(00)80211-7. [DOI] [PubMed] [Google Scholar]
- Russell RG, Oteruelo FT. An ultrastructural study of the differentiation of skeletal muscle in the bovine fetus. Anat Embryol (Berl) 1981;162:403–417. doi: 10.1007/BF00301866. [DOI] [PubMed] [Google Scholar]
- Salvadori C, Peters IR, Day MJ, Engvall E, Shelton GD. Muscle regeneration, inflammation, and connective tissue expansion in canine inflammatory myopathy. Muscle Nerve. 2005;31:192–198. doi: 10.1002/mus.20252. [DOI] [PubMed] [Google Scholar]
- Schuettengruber B, Chourrout D, Vervoort M, Leblanc B, Cavalli G. Genome regulation by polycomb and trithorax proteins. Cell. 2007;128:735–745. doi: 10.1016/j.cell.2007.02.009. [DOI] [PubMed] [Google Scholar]
- Schupp M, Cristancho AG, Lefterova MI, Hanniman EA, Briggs ER, Steger DJ, Qatanani M, Curtin JC, Schug J, Ochsner SA, McKenna NJ, Lazar MA. Re-expression of gata2 cooperates with peroxisome proliferator-activated receptor-gamma depletion to revert the adipocyte phenotype. J Biol Chem. 2009;284:9458–9464. doi: 10.1074/jbc.M809498200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang YC, Zhang C, Wang SH, Xiong F, Zhao CP, Peng FN, Feng SW, Yu MJ, Li MS, Zhang YN. Activated beta-catenin induces myogenesis and inhibits adipogenesis in bm-derived mesenchymal stromal cells. Cytotherapy. 2007;9:667–681. doi: 10.1080/14653240701508437. [DOI] [PubMed] [Google Scholar]
- Spalding KL, Arner E, Westermark PO, Bernard S, Buchholz BA, Bergmann O, Blomqvist L, Hoffstedt J, Naslund E, Britton T, Concha H, Hassan M, Ryden M, Frisen J, Arner P. Dynamics of fat cell turnover in humans. Nature. 2008;453:783–787. doi: 10.1038/nature06902. [DOI] [PubMed] [Google Scholar]
- Spinella-Jaegle S, Rawadi G, Kawai S, Gallea S, Faucheu C, Mollat P, Courtois B, Bergaud B, Ramez V, Blanchet AM, Adelmant G, Baron R, Roman-Roman S. Sonic hedgehog increases the commitment of pluripotent mesenchymal cells into the osteoblastic lineage and abolishes adipocytic differentiation. J Cell Sci. 2001;114:2085–2094. doi: 10.1242/jcs.114.11.2085. [DOI] [PubMed] [Google Scholar]
- Stickland NC. A quantitative study of muscle development in the bovine foetus (bos indicus) Anat Histol Embryol. 1978;7:193–205. doi: 10.1111/j.1439-0264.1978.tb00795.x. [DOI] [PubMed] [Google Scholar]
- Suh JM, Gao X, McKay J, McKay R, Salo Z, Graff JM. Hedgehog signaling plays a conserved role in inhibiting fat formation. Cell Metab. 2006;3:25–34. doi: 10.1016/j.cmet.2005.11.012. [DOI] [PubMed] [Google Scholar]
- Symonds ME, Pearce S, Bispham J, Gardner DS, Stephenson T. Timing of nutrient restriction and programming of fetal adipose tissue development. Proc Nutr Soc. 2004;63:397–403. doi: 10.1079/pns2004366. [DOI] [PubMed] [Google Scholar]
- Tseng YH, Kokkotou E, Schulz TJ, Huang TL, Winnay JN, Taniguchi CM, Tran TT, Suzuki R, Espinoza DO, Yamamoto Y, Ahrens MJ, Dudley AT, Norris AW, Kulkarni RN, Kahn CR. New role of bone morphogenetic protein 7 in brown adipogenesis and energy expenditure. Nature. 2008;454:1000–1004. doi: 10.1038/nature07221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu AW, Luo K. Acetylation of smad2 by the co-activator p300 regulates activin and transforming growth factor beta response. J Biol Chem. 2007;282:21187–21196. doi: 10.1074/jbc.M700085200. [DOI] [PubMed] [Google Scholar]
- Underwood KR, Tong JF, Price PL, Roberts AJ, Grings EE, Hess BW, Means WJ, Du M. Nutrition during mid to late gestation affects growth, adipose tissue deposition, and tenderness in cross-bred beef steers. Meat Science. 2010 doi: 10.1016/j.meatsci.2010.04.008. In press. [DOI] [PubMed] [Google Scholar]
- USGS. Climatic fluctuations, drought, and flow in the colorado river basin. 2004 [Google Scholar]
- Vire E, Brenner C, Deplus R, Blanchon L, Fraga M, Didelot C, Morey L, Van Eynde A, Bernard D, Vanderwinden JM, Bollen M, Esteller M, Di Croce L, de Launoit Y, Fuks F. The polycomb group protein ezh2 directly controls DNA methylation. Nature. 2006;439:871–874. doi: 10.1038/nature04431. [DOI] [PubMed] [Google Scholar]
- Xu Z, Yu S, Hsu CH, Eguchi J, Rosen ED. The orphan nuclear receptor chicken ovalbumin upstream promoter-transcription factor ii is a critical regulator of adipogenesis. Proc Natl Acad Sci U S A. 2008;105:2421–2426. doi: 10.1073/pnas.0707082105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanouchi K, Hosoyama T, Murakami Y, Nishihara M. Myogenic and adipogenic properties of goat skeletal muscle stem cells. J Reprod Dev. 2007;53:51–58. doi: 10.1262/jrd.18094. [DOI] [PubMed] [Google Scholar]
- Yan X, Zhu MJ, Xu W, Tong JF, Ford SP, Nathanielsz PW, Du M. Up-regulation of toll-like receptor 4/nuclear factor-kappab signaling is associated with enhanced adipogenesis and insulin resistance in fetal skeletal muscle of obese sheep at late gestation. Endocrinology. 151:380–387. doi: 10.1210/en.2009-0849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W, Hong YH, Shen XQ, Frankowski C, Camp HS, Leff T. Regulation of transcription by amp-activated protein kinase: Phosphorylation of p300 blocks its interaction with nuclear receptors. J Biol Chem. 2001;276:38341–38344. doi: 10.1074/jbc.C100316200. [DOI] [PubMed] [Google Scholar]
- Zehentner BK, Leser U, Burtscher H. Bmp-2 and sonic hedgehog have contrary effects on adipocyte-like differentiation of c3h10t1/2 cells. DNA Cell Biol. 2000;19:275–281. doi: 10.1089/10445490050021186. [DOI] [PubMed] [Google Scholar]
- Zhang H, Darwanto A, Linkhart TA, Sowers LC, Zhang L. Maternal cocaine administration causes an epigenetic modification of protein kinase cepsilon gene expression in fetal rat heart. Mol Pharmacol. 2007;71:1319–1328. doi: 10.1124/mol.106.032011. [DOI] [PubMed] [Google Scholar]
- Zhu MJ, Ford SP, Means WJ, Hess BW, Nathanielsz PW, Du M. Maternal nutrient restriction affects properties of skeletal muscle in offspring. J Physiol. 2006;575:241–250. doi: 10.1113/jphysiol.2006.112110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu MJ, Ford SP, Nathanielsz PW, Du M. Effect of maternal nutrient restriction in sheep on the development of fetal skeletal muscle. Biol Reprod. 2004;71:1968–1973. doi: 10.1095/biolreprod.104.034561. [DOI] [PubMed] [Google Scholar]