Abstract
We describe a method to construct water-soluble porphyrinic nanospheres with enhanced photo-physical properties as a result of precluding (via intra-molecular host-guest interactions) the individual porphyrins units from aromatic-aromatic stacking.
Porphyrins and their congeners are being intensely investigated as building blocks for the construction of functional nanomaterials.1 This interest is due to the attractive photo-physical properties of porphyrin assemblies leading to potential applications, inter alia, in solar energy harvesting, sensing, photoconductance, and catalysis.2 Indeed, porphyrinic nanostructures of diverse architectures (including rings,2a,3 rods,3,4 wires,5 tubes6, and sheets7) have been explored. Particular attention has focused on the development of water compatible porphyrin nanospheres8 (NSs) since such systems can be used as agents for biological imaging and photodynamic therapy (PDT).9 However, a significant problem in the development of water compatible porphyrinic nanostructures (especially for imaging and PDT applications) is that the porphyrin macrocycles tend to aggregate via aromatic-aromatic stacking leading to diminished photo-physical properties including decreased photon absorption and fluorescence.2e,8a,c,d,10
Herein we describe a facile method to construct water-soluble porphyrinic NSs wherein the individual porphyrin units have significantly attenuated stacking. In particular, we show, for the first time, that the concept of self-inclusion via double intra-molecular β-cyclodextrin (β-CD) based host-guest interactions, can be used in tandem with NS forming conditions (via self-organization of the porphyrin units) leading to well-defined porphyrin NSs (Figure 1). Importantly, these NSs display superior fluorescence and singlet-oxygen generation properties compared to analogously prepared NSs composed of porphyrins that lack the self-encapsulating β-CD units and therefore do undergo stacking.
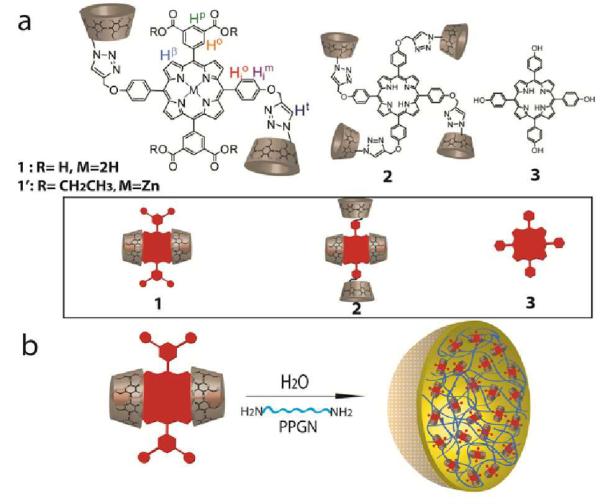
Figure 1.
(a) Structures and self-inclusion complexes (inset) of porphyrins 1, 2 and 3. (b) Assembly scheme for porphyrin containing nanospheres.
It is well-known that permethylated β-cyclodextrins (PMβCDs) can form inclusion complexes with tetraphenyl porphyrins in water via an induced-fit mechanism (wherein the β-CD units bind to the meso-phenyl position and a portion of the porphyrin core11). Further, the groups of Kano and Liu have shown that when the β-CD is covalently attached, in a para fashion, to the meso-phenyl positions of porphyrins via its primary face, the glucopyranose unit directly linked to the porphyrin can rotate about its glycosidic linkages resulting in self-inclusion wherein the larger secondary rim of the PMβCD is directed onto the macrocycle core.12 Since inclusion complex formation/molecular encapsulation of dyes typically leads to prevention of dye-based aggregation,13 we were eager to explore whether porphyrins flanked with PMβCD units could be used as precursors to develop porphyrin NSs with attenuated aromatic-aromatic stacking.
In particular, we focused on porphyrin 1 (Figure 1a) that contains two PMβCD arms at the 5 and 15 meso-phenyl positions and two dicarboxylatophenyl arms at the 10 and 20 meso-phenyl positions. The PMβCD arms of 1 were expected to self-encapsulate the macrocycle from opposite ends (Figure 1a, inset) whilst the meta-substituted dicarboxylatophenyl units would provide steric hindrance thereby minimizing any potential inter-molecular host-guest interactions.12a In addition, we investigated porphyrin 2 appended with four PMβCD units that was also expected to form a double self-inclusion complex in water.12b The synthesis of porphyrins 1 and 2 are provided in the ESI. Commercially available porphyrin 3 lacking PMβCD arms was included in our studies as a control as it should readily undergo stacking in water.
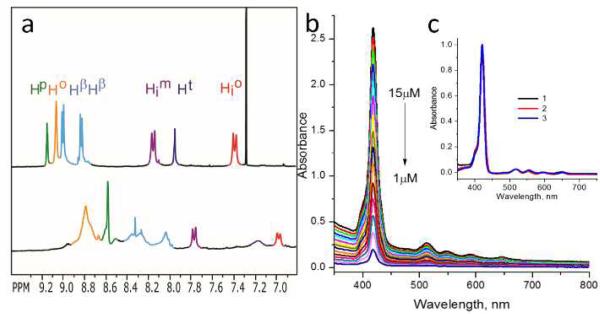
1H-NMR experiments were performed to determine whether the PMβCD linked porphyrins form self-inclusion complexes. We first investigated the tetra ethyl ester version of porphyrin 1 (i.e., 1’, which is a Zn containing synthetic precursor of 1) in CDCl3, since the bulky ester appendages and the non-polar nature of CDCl3 were expected to constrain 1’ into a non self-encapsulated conformation. As illustrated in Figure 2a top, porphyrin 1’ exhibits sharp and well-resolved resonances corresponding to the porphyrin β-pyrrole protons (Hβ), phenyl protons (Hp, Ho, H oi, and Hmi), and the triazole protons (Ht). In marked contrast, the spectra of hydrolyzed porphyrin 1 in D2O (Figure 2a, bottom) shows significant up-field shifts with most of the resonances being substantially broadened. These NMR shifts indicate that the chemical environment of the low-field protons are drastically changed when going from 1’ in CDCl3 to 1 in D2O, suggesting the formation of a self-inclusion complex for porphyrin 1 in D2O. In addition, for the case of 1 in D2O, new proton signals appear in the 2 – 3 ppm region (ESI-S1) that are ascribed to the PMβCD protons (via 2D ROESY experiments, ESI-S6) that are close to the porphyrin and benzene rings. These results are consistent with other porphyrins linked to PMβCDs via the para-position of the phenyl rings, wherein the larger rim of the PMβCD toroid engulfs the porphyrin macrocycle.12a
Figure 2.
(a) Downfield region 1H-NMR spectra of (top) porphyrin 1’ in CDCl3, and (bottom) porphyrin 1 in D2O (0.1 M phosphate buffer at pH = 7.0). (b) UV-vis spectra of porphyrins 1 at varying concentrations in H2O. (c) Normalized UV-vis spectra of porphyrins 1-3 in THF (λmax = 421 nm).
Furthermore, UV-vis spectroscopy (Figure 2b) showed that porphyrin 1 does not undergo appreciable stacking in water as the Soret band is sharp and the λmax (418 nm) is close to the λmax for the monomeric porphyrin in organic solvents (such as THF (Figure 2c) and CHCl3). Additionally, the concentration dependent UV-vis showed that the λmax traced linearly with increasing concentration (1 -15 μM) suggesting no appreciable stacking of 1 under these concentrations (ESI-S9). 1H-NMR and UV-vis characterization of free-base porphyrin 2 were also consistent with inclusion complex formation in aqueous medium (See ESI-S7 and ESI-S10).12b In contrast to 1 and 2, porphyrin 3 forms an essentially colorless solution with black precipitates in water (even at 5 μM concentration) suggesting poor aqueous solubility as a result of aggregation. Further, when the precipitate of 3 is filtered off, the remaining solution containing soluble porphyrin 3 displays a slight red shift in the Soret band (λmax = 424 nm) and, moreover, the whole spectrum is significantly broadened (ESI-S11), indicating that porphyrin 3 readily stacks in water.
After determining that porphyrins 1 and 2 form self-inclusion complexes in water whilst porphyrin 3 forms stacked aggregates and precipitates, we next investigated nanoparticle formation by introducing the porphyrins dissolved in THF into an aqueous solution containing an agglomeration inhibitor (Figure 1b). Such a mixed solvent method is known to be effective in preparing porphyrinic nanoparticles, albeit where the porphyrins are typically aggregated.8c We chose poly(propylene glycol) bis(2-aminopropyl ether) with a molecular weight of 2000 (PPGN) as the agglomeration inhibitor because this polymer is (a) water soluble as it includes repeating ether units and terminal amines, and (b) cannot be threaded into the PMβCD arms14 and thus should not compete for PMβCD inclusion. Briefly, 50 μL of 3 mM porphyrins 1-3 in THF were injected into a 2 mL deionized water solution containing one equivalent of PPGN, while stirring vigorously. The resultant yellow-green solution was stirred for 4 hours, at which point THF was removed by evaporation under reduced pressure. The resulting aqueous solution was filtered (using a 0.45 μm filter) and collected.
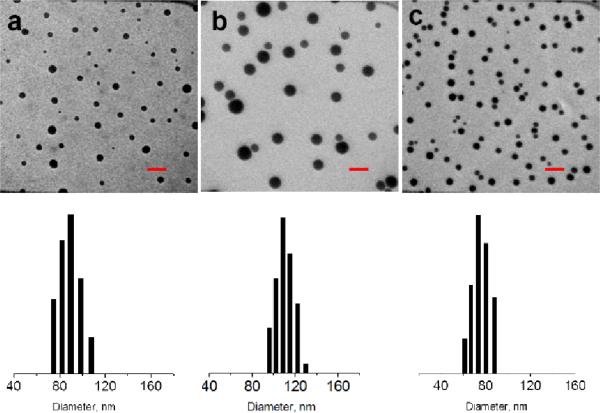
The porphyrin containing NSs were first probed by transmission electron microscopy (TEM). The TEM samples were prepared by placing a drop of aqueous solution containing porphyrin NSs ([porphyrin] ≈ 0.1 mM) onto a carbon coated copper grid, followed by evaporation of the aqueous solvent. As shown in the TEM images and confirmed via scanning electron microscopy (ESI-S12), all three porphyrins formed spherical nanoparticles with diameters in the 100 nm range. Interestingly, the smaller size porphyrins formed NSs with slightly shorter diameters than the larger porphyrins.15 In order to gather more quantitative information of the nanoparticle sizes, solution based dynamic light scattering studies (DLS) were conducted. These experiments, are consistent with the microscopy images, and provided an average diameter of 88, 110, and 80 nm for NSs formed by porphyrins 1, 2, and 3, respectively.
The absorption characteristics of the nanoparticles were next probed. Interestingly, the NSs formed by porphyrins 1 and 2 showed sharp absorption profiles (Figure 4a). Not surprisingly, the NSs formed by porphyrin 3 showed a more broadened and red-shifted spectrum (although not as broadened as 3 in the absence of PPGN). More importantly, when the NSs are excited at an absorbance matched wavelength (427 nm), the fluorescence from the porphyrin 3 containing NSs is substantially lower than the fluorescence of NSs composed of porphyrins 1 or 2 (Figure 4b). The fluorescence quantum yield for NSs composed of porphyrins 1, 2 and 3 were estimated to be 0.029, 0.024 and 0.002 in water respectively, when tetraphenylporphyrin sulfonate (0.08) was used as the standard.
Figure 4.
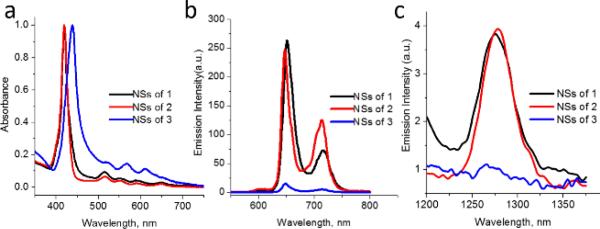
(a) Normalized UV-vis spectra of NSs composed of 1, 2 and 3 in water. (b) Fluorescence spectra of NSs composed of 1-3 in water (excited at 427 nm; absorbance matched). (c) Emission spectra of singlet oxygen generated by nanospheres containing 1-3 in D2O in air saturated solution (excited at 419 nm; absorbance matched).
While enhancing the fluorescence properties of porphyrinic NSs is salient for imaging applications, another important photo-physical property of porphyrin NSs that needs to be optimized is the photo-induced generation of singlet oxygen, as this can lead to nanoparticles with therapeutic properties. To determine the singlet oxygen generation capacities of the porphyrin containing NSs, the luminescence of singlet oxygen was directly investigated. As shown in Figure 4c, the NSs composed of porphyrins 1 and 2 clearly induce the production of singlet oxygen as observed by an emission peak at 1275 nm. On the other hand, no significant luminescence at 1275 nm is observed when the NSs containing porphyrin 3 was probed. Taken together, these emission experiments provide strong evidence that precluding porphyrin-based stacking by intra-molecular self-inclusion can lead to porphyrinic NSs with enhanced photo-physical properties.
Conclusions
In summary, we have described a straightforward method to prepare water-soluble porphyrinic NSs wherein porphyrin based aromatic-aromatic stacking is inhibited as a result of accessing intra-molecular host-guest interactions. Preventing stacking significantly enhances the fluorescence and singlet oxygen generation capabilities of the porphyrin NSs. We are currently investigating these porphyrin NSs for biological imaging and therapeutic applications.
Supplementary Material
Figure 3.
TEM images (top) and DLS histograms (bottom) of nanospheres formed by porphyrins (a) 1, (b) 2, and (c) 3. Red scale bar is 200 nm.
Acknowledgments
This work was funded by grants from the NSF (CHE-1112091), the NIH (R01GM097571), and the ACS PRF (50272-DNI4) to JJ.
Footnotes
Electronic Supplementary Information (ESI) available: [details of any supplementary information available should be included here]. See DOI: 10.1039/c000000x/
Notes and references
- 1.a Beletskaya I, Tyurin VS, Tsivadze AY, Guilard R, Stern C. Chem. Rev. 2009;109:1659–1713. doi: 10.1021/cr800247a. [DOI] [PubMed] [Google Scholar]; b Medforth CJ, Wang Z, Martin KE, Song Y, Jacobsen JL, Shelnutt JA. Chem. Commun. 2009:7261–7277. doi: 10.1039/b914432c. [DOI] [PubMed] [Google Scholar]; c Drain CM, Varotto A, Radivojevic I. Chem. Rev. 2009;109:1630–1658. doi: 10.1021/cr8002483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.a Aratani N, Kim D, Osuka A. Acc. Chem. Res. 2009;42:1922–1934. doi: 10.1021/ar9001697. [DOI] [PubMed] [Google Scholar]; b Balaban TS. Acc. Chem. Res. 2005;38:612–623. doi: 10.1021/ar040211z. [DOI] [PubMed] [Google Scholar]; c Sgobba V, Giancane G, Conoci S, Casilli S, Ricciardi G, Guldi DM, Prato M, Valli L. J. Am. Chem. Soc. 2007;129:3148–3156. doi: 10.1021/ja0655789. [DOI] [PubMed] [Google Scholar]; d Monti D, Nardis S, Stefanelli M, Paolesse R, Di Natale C, Amico AD'. J. Sens. 2009:2009. [Google Scholar]; e Dini F, Martinelli E, Pomarico G, Paolesse R, Monti D, Filippini D, D'Amico A, Lundström I, Natale CD. Nanotechnology. 2009;20:055502. doi: 10.1088/0957-4484/20/5/055502. [DOI] [PubMed] [Google Scholar]; f Wang H, Song Y, Medforth CJ, Shelnutt JA. J. Am. Chem. Soc. 2006;128:9284–9285. doi: 10.1021/ja0619859. [DOI] [PubMed] [Google Scholar]; g Nam YS, Shin T, Park H, Magyar AP, Choi K, Fantner G, Nelson KA, Belcher AM. J. Am. Chem. Soc. 2010;132:1462–1463. doi: 10.1021/ja908812b. [DOI] [PubMed] [Google Scholar]
- 3.Doan SC, Shanmugham S, Aston DE, McHale JL. J. Am. Chem. Soc. 2005;127:5885–5892. doi: 10.1021/ja0430651. [DOI] [PubMed] [Google Scholar]
- 4.a Schwab AD, Smith DE, Rich CS, Young ER, Smith WF, de Paula JC. J. Phys. Chem. B. 2003;107:11339–11345. [Google Scholar]; b Guo P, Chen P, Liu M. ACS Appl. Mater. Interfaces. 2013;5:5336–5345. doi: 10.1021/am401260n. [DOI] [PubMed] [Google Scholar]
- 5.a Koepf M, Conradt J, Szmytkowski J, Wytko JA, Allouche L, Kalt H, Balaban TS, Weiss J. Inorg. Chem. 2011;50:6073–6082. doi: 10.1021/ic2001255. [DOI] [PubMed] [Google Scholar]; b Lee SJ, Hupp JT, Nguyen ST. J. Am. Chem. Soc. 2008;130:9632–9633. doi: 10.1021/ja801733t. [DOI] [PubMed] [Google Scholar]; c Jintoku H, Sagawa T, Takafuji M, Ihara H. Org. Biomol. Chem. 2009;7:2430–2434. doi: 10.1039/b818358a. [DOI] [PubMed] [Google Scholar]; d Fathalla M, Neuberger A, Li S-C, Schmehl R, Diebold U, Jayawickramarajah J. J. Am. Chem. Soc. 2010;132:9966–9967. doi: 10.1021/ja1030722. [DOI] [PubMed] [Google Scholar]
- 6.Wang Z, Medforth CJ, Shelnutt JA. J. Am. Chem. Soc. 2004;126:15954–15955. doi: 10.1021/ja045068j. [DOI] [PubMed] [Google Scholar]
- 7.Wang Z, Li Z, Medforth CJ, Shelnutt JA. J. Am. Chem. Soc. 2007;129:2440–2441. doi: 10.1021/ja068250o. [DOI] [PubMed] [Google Scholar]
- 8.a Guo P, Chen P, Liu M. Langmuir. 2012;28:15482–15490. doi: 10.1021/la3033594. [DOI] [PubMed] [Google Scholar]; b Xing C, Xu Q, Tang H, Liu L, Wang S. J. Am. Chem. Soc. 2009;131:13117–13124. doi: 10.1021/ja904492x. [DOI] [PubMed] [Google Scholar]; c Gong X, Milic T, Xu C, Batteas JD, Drain CM. J. Am. Chem. Soc. 2002;124:14290–14291. doi: 10.1021/ja027405z. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Ou Z, Yao H, Kimura K. J. Photochem. Photobiol. Chem. 2007;189:7–14. [Google Scholar]
- 9.a Wu C, Bull B, Christensen K, McNeill J. Angew. Chem. Int. Ed. 2009;48:2741–2745. doi: 10.1002/anie.200805894. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Marrache S, Tundup S, Harn DA, Dhar S. ACS Nano. 2013;7:7392–7402. doi: 10.1021/nn403158n. [DOI] [PubMed] [Google Scholar]; c Ng KK, Lovell JF, Vedadi A, Hajian T, Zheng G. ACS Nano. 2013;7:3484–3490. doi: 10.1021/nn400418y. [DOI] [PubMed] [Google Scholar]; d Kuruppuarachchi M, Savoie H, Lowry A, Alonso C, Boyle RW. Mol. Pharm. 2011;8:920–931. doi: 10.1021/mp200023y. [DOI] [PubMed] [Google Scholar]; e Shen X, He F, Wu J, Xu GQ, Yao SQ, Xu Q-H. Langmuir. 2011;27:1739–1744. doi: 10.1021/la104722q. [DOI] [PubMed] [Google Scholar]
- 10.De Napoli M, Nardis S, Paolesse R, Vicente MGH, Lauceri R, Purrello R. J. Am. Chem. Soc. 2004;126:5934–5935. doi: 10.1021/ja0494757. [DOI] [PubMed] [Google Scholar]
- 11.Kano K, Nishiyabu R, Doi R. J. Org. Chem. 2005;70:3667–3673. doi: 10.1021/jo0500535. [DOI] [PubMed] [Google Scholar]
- 12.a Nishiyabu R, Kano K. Eur. J. Org. Chem. 2004:4985–4988. [Google Scholar]; b Liu Y, Ke C-F, Zhang H-Y, Cui J, Ding F. J. Am. Chem. Soc. 2008;130:600–605. doi: 10.1021/ja075981v. [DOI] [PubMed] [Google Scholar]
- 13.a Zill AT, Licha K, Haag R, Zimmerman SC. New J. Chem. 2012;36:419–427. [Google Scholar]; b Arunkumar E, Forbes CC, Smith BD. Eur. J. Org. Chem. 2005:4051–4059. [Google Scholar]
- 14.Okada M, Kamachi M, Harada A. J. Phys. Chem. B. 1999;103:2607–2613. [Google Scholar]
- 15.Drain CM, Smeureanu G, Patel S, Gong X, Garno J, Arijeloye J. New J. Chem. 2006;30:1834–1843. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.