Abstract
Background
BRAF V600E mutation is the most common genetic alteration in papillary thyroid cancer (PTC). We utilized a mutation-specific antibody for immunohistochemical (IHC) detection of the BRAF V600E mutation and correlated expression with clinicopathological features. The study was designed to validate the accuracy and determine the significance of IHC detection of the BRAF V600E mutation in PTC.
Methods
Direct sequencing and IHC for BRAF V600E mutation was performed in 37 consecutive PTCs. IHC was scored on an intensity, proportion scale. IHC positive tumors were stratified into intensity categories. The categories were assessed for clinicopathologic variables including age, extrathyroidal extension, lymphovascular invasion, and lymph node metastases.
Results
25 PTCs were BRAF V600E positive and 12 were BRAF mutation negative on IHC. The BRAF V600E mutation-specific antibody showed a sensitivity of 89% and specificity of 100% for detecting the mutation. Tumors with high intensity staining were significantly more likely to have extrathyroidal extension.
Conclusions
IHC is an accurate method for the detection of the BRAF V600E mutation in PTC and its ability to quantify the mutation expression may serve as a better predictor of tumor behavior than molecular sequencing. It provides a potentially rapid, easily applicable and economical alternative to current techniques.
Introduction
Thyroid cancer is increasing at a rate faster than any other cancer in this country. This year there will be more than 50,000 new cases of thyroid cancer in the United States 1. Papillary thyroid cancer (PTC) is the most common, comprising over 80% of all thyroid cancers. Activating mutations of the mitogen activated protein kinase (MAPK/ERK) pathway are the most common genetic aberrations in PTC, of which BRAF V600E mutation (BRAF+) is the most prevalent and almost exclusively found in PTC. It constitutively activates the MAPK/ERK pathway and is present in at least 45% of all PTCs, with recent studies showing even higher prevalence 2–4. A recent meta-analysis of 14 articles concluded that BRAF+PTCs were more likely to recur, have lymph node metastasis, extrathyroidal extension, and advanced stage 5. The aggressive behavior associated with the BRAF mutation, potentially makes the BRAF V600E mutation an important prognostic parameter.
Many different methods for BRAF mutation analyses with different sensitivities have been developed. A major drawback of these methods is that they are expensive, labor and time intensive, not always available, and may be difficult to implement in clinical settings. These methods include single-strand conformation polymorphism, direct gene sequencing, mutation specific PCR, and colorimetric mutation analysis. Furthermore, these techniques may be hampered by difficulty in preserving DNA and the presence of non-neoplastic tissue diluting the cells of interest. They also lack a structural representation of BRAF positive cells within a tumor and lack the ability to quantify mutation expression 6–8.
Recently, a mutation-specific antibody was developed, which allows immunohistochemical (IHC) visualization of the BRAF V600E protein with high sensitivity and specificity (Springer-Bio, VE1). This antibody has been shown to be reliable in many neoplastic tissues including thyroid cancer 9–14. VE1 is a monoclonal mouse antibody whose immunogen involves a synthetic peptide representing the BRAF V600E mutated amino acid sequence from amino acid 596 to 606 (GLATEKSRWSG). This antibody has been shown to be specific for the V600E mutation with negligible cross reactivity with other BRAF codon 600 hotspot mutations or BRAF genotypes, such as V600D, V600K, K601E, K601Q or T599dup. The aim of this study was to confirm the accuracy of this antibody in detecting the BRAF V600E aberrant protein in PTC and determine if the extent of aberrant protein staining correlates with clinicopathologic features of the tumor.
Methods
Thyroid cancer samples
The Division of Endocrine Surgery at New York University Langone Medical Center houses all tissue samples from all thyroid tumors greater than one centimeter in an IRB approved Tissue Banking and Acquisition Facility (NYU Langone Medical Center, New York, NY). Tumor samples are linked to a clinical database that is updated regularly by the Division of Endocrine Surgery and holds over sixty data points. The quality of our specimens has been highlighted in our prior publication 15.
We analyzed 37 consecutive classical PTCs from patients only undergoing total thyroidectomy with elective central node dissection. Patients who also had a lateral neck dissection were excluded. Tumors were analyzed for the BRAF V600E mutation by direct sequencing and IHC. All samples were reviewed by a dedicated pathologist (FMD).
DNA extraction
A 10 μm section was taken from each frozen tissue block and was subjected to Genomic DNA extraction per the manufacturer’s protocol using the DNeasy Blood and Tissue Kit (Qiagen).
Detection of BRAFV600E mutation
Exon 15 of the BRAF gene was amplified with 2 primers that annealed to the introns flanking it. Our technique has been previously described 15.
Immunohistochemistry
We used a novel commercially available BRAF V600E mutation specific antibody (Springer-Bio, VE1). Immunohistochemical staining was performed on the paraffin embedded TMA using the mouse anti-human BRAF V600E hybridoma supernatant clone VE1. Sections were deparaffinized in xylene (3 changes), rehydrated through graded alcohols (3 changes 100% ethanol, 3 changes 95% ethanol) and rinsed in distilled water. Heat induced epitope retrieval was performed in a 1200-Watt microwave oven at 100% power in 1.0 mM Tris-EDTA buffer, pH 8.5 for 10 minutes. Sections were allowed to cool for 30 minutes and then rinsed in distilled water. Antibody incubation and detection were carried out at 40°C on a NexES instrument (Ventana Medical Systems Tucson, Arizona USA) using Ventana’s reagent buffer and detection kits unless otherwise noted. Endogenous peroxidase activity was blocked with hydrogen peroxide. Anti-BRAF was diluted 1:2 in Dulbecco’s Phosphate Buffered Saline, (Life Technologies Grand Island, New York USA) and incubated overnight at room temperature. Primary antibody was detected with iView biotinylated goat anti-mouse followed by application of streptavidin-horseradish-peroxidase conjugate. The complex was visualized with 3,3 diaminobenzidene and enhanced with copper sulfate. Slides were washed in distilled water, counterstained with hematoxylin, dehydrated and mounted with permanent media. Known PCR BRAF mutation positive and negative were included with the study sections.
Tissue Microarray (TMA)
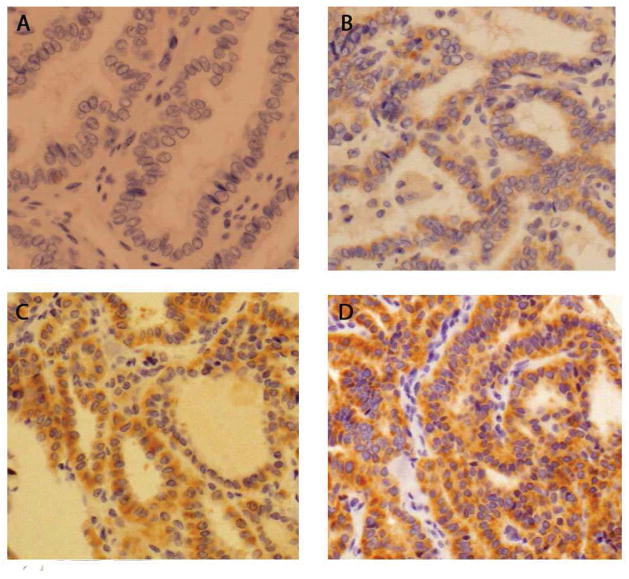
The same PTC samples that were used for direct sequencing were used to create tissue microarrays, with 3 cores from each sample. TMAs were analyzed using the novel BRAF V600E mutant-specific antibody for IHC as described. TMAs were scored on a standard intensity (0–3), proportion (0–100%) scale by a single pathologist who was blinded to the BRAF status, determined by sequencing, of each sample. Tumors with intensity <1 and staining proportion <20% or non-specific staining were considered negative. Positively staining tumors (majority displayed >80% proportion) were then stratified into 3 intensity categories: +1, +2, +3 (Figure 1). The 3 categories were grouped and correlated with clinicopathologic variables including age, extrathyroidal extension, lymphovascular invasion, and regional lymph node metastases.
Figure 1.
Representative images of BRAF V600E immunohistochemical staining in PTC.
A− No staining
B− 1+ staining
C− 2+ staining
D− 3+ staining
Statistical analysis
A statistical analysis was performed using SPSS 20.0 software (IBM)©. Where applicable Pearson Chi-Square, t-test or Fisher exact test were performed. This study was approved by the New York University School of Medicine Institutional Review Board
Results
Immunohistochemistry utilizing the novel mutation specific antibody accurately identifies the BRAF V600E mutation in PTC
Of the 37 tumors 28 (76%) were found to be BRAF V600E mutation positive by direct sequencing. 25 of these 28 (89%) were found to be positive by IHC. The staining was uniformly cytoplasmic. There were no false positives and 3 false negatives, for an IHC sensitivity of 89% and a specificity of 100%. The positive predictive value was 100% and the negative predictive value was 75%.
Of the tumors that stained positive, the median percentage of positive staining cells within the tumor was 86% (range 20% – 100%). 80% of the positive cases (20/25) had greater than 80% positive cells. Most of the tumors had homogeneous staining as defined by analogous labeling intensity in ≥ 80% of the tumor cells.
Papillary thyroid cancers with increased BRAF V600E expression as detected by IHC are associated with extrathyroidal extension
Patient data and clinicopathologic characteristics of the tumor with respect to BRAF V600E expression are listed in Table 1. The median number of central lymph nodes removed in each group, BRAF V600E + vs. BRAF V600E − was not statistically significant (5 vs. 3 respectively). Tumors were considered positive for LN metastases when at least one central node was positive. Overall, BRAF mutation as detected by IHC did not correlate with patient age, gender, size of tumor, multifocality, extrathyroidal extension, lymphovascular invasion, or central node positivity.
Table 1.
Clinicopathologic features of all papillary thyroid cancers with respect to BRAF V600E mutation status based on IHC. LVI- lymphovascular invasion, LN metastasis- central nodal disease.
| BRAF+ | BRAF− | p value | |
|---|---|---|---|
| Total | 25 | 12 | |
| Male (%) | 8(32) | 4(33) | 1 |
| Age | 44 | 41 | 0.5 |
| Size (mm) | 19 | 30 | 0.188 |
| Multifocal (%) | 15(60) | 6(50) | 0.726 |
| Extrathyroidal extension (%) | 13(52) | 5(42) | 0.728 |
| LVI (%) | 19(76) | 8(67) | 0.696 |
| LN metastasis (%) | 17(68) | 6(50) | 0.47 |
All BRAF mutation positive staining tumors were then stratified into 3 IHC intensity categories: <1, 1–2, >2 by our pathologist. When these 3 categories were compared there was no association with clinicopathologic signs of aggressive behavior. When the BRAF+ tumors were stratified into two IHC intensity categories (≤2, >2), 8/25 (32%) of our positive samples displayed high intensity (>2) and 17/25 (68%) displayed low intensity (≤2) staining. When these 2 groups were compared, there was a significant difference in the proportion of cases with extrathyroidal extension. Tumors with a high intensity stain had significantly more extrathyroidal extension, 7/8 (88%), than tumors with lower intensity stain, 6/17 (33%). No correlation was seen with patient age, gender, size of tumor, multifocality, lymphovascular invasion, or central node positivity (Table 2).
Table 2.
Clinicopathologic features of all BRAF V600E mutation positive papillary thyroid cancers with respect to intensity of IHC staining. LVI- lymphovascular invasion, LN metastasis- central nodal disease.
| BRAF >2 | BRAF ≤2 | p value | |
|---|---|---|---|
| Total | 8 | 17 | |
| Male (%) | 1(13) | 7(41) | 0.205 |
| Age | 50 | 41 | 0.228 |
| Size (mm) | 23 | 17 | 0.138 |
| Multifocal (%) | 5(63) | 10(59) | 1 |
| Extrathyroidal extension (%) | 7(88) | 6(35) | 0.03 |
| LVI (%) | 8(100) | 11(65) | 0.129 |
| LN metastasis (%) | 6(75) | 11(65) | 1 |
Discussion
BRAF V600E mutation is the most common genetic alteration in PTC and maybe associated with an aggressive phenotype. However, a high variation of the incidence of BRAF mutations in PTC (20% to 80%) has been observed in the literature 4. This variation may reflect regional and/or temporal differences in the pathogenesis of PTC but may also be due to different materials (eg, frozen tissue vs. paraffin embedded tissue) and/or different mutation detection techniques (macrodissection, microdissection, direct sequencing, pyrosequencing, high-resolution melting curve analysis etc). Each of these techniques has differing sensitivities (80% to 99%), specificities, and costs. Furthermore, they require rigorous quality control and use of specialized, expensive equipment which may not be readily available in most surgical pathology laboratories. In routine clinical practice tumor tissue often needs to be sent to specialized laboratories for molecular testing. Immunohistochemical (IHC) staining is a technique that is widely utilized in routine diagnostic pathology laboratories and in contrast to the molecular techniques, it is more rapid and potentially cheaper. Importantly, current techniques lack the ability to quantify the extent of BRAF V600E mutation expression, which may help explain why some studies associate the mutation with aggressive behavior while others do not. IHC staining provides the ability to easily quantify BRAF V600E mutation expression and has the added advantage of allowing visualization of individual antigen-bearing tumor cells and assessing for tumor homogeneity and heterogeneity 12.
We utilized a mutation-specific antibody for IHC detection of the BRAF V600E mutation and correlated this with clinicopathological features. Our study was designed to validate the accuracy of IHC detection of the BRAF V600E mutation in PTC and determine the clinical significance of the extent of staining. Our data show that the BRAF mutation-specific antibody is highly reliable in identifying PTC harboring the BRAF V600E mutation, with a sensitivity of 89% and specificity of 100%. 76% of our PTCs were BRAF V600E positive by direct sequencing and 68% positive by IHC. Papillary thyroid cancers that were BRAF mutation positive on IHC had a very homogeneous, cytoplasmic staining pattern throughout the tumor. This indicates that the BRAF V600E mutation is likely a clonal event in PTC. 80% of the IHC positive cases had greater than 80% positive cells. This corroborates previous studies that have tested the antibody on other tissues 9–14.
Although many authors have found an association between BRAF mutation and aggressive tumor behavior, some could not demonstrate this association 16–18. In our study, the presence of mutated BRAF did not correlate with clinicopathologic signs of aggressive tumor behavior such as lymphovascular invasion, extrathyroidal extension or lymph node metastases. However, when we analyzed the tumors with respect to intensity of BRAF V600E staining, tumors with high intensity staining displayed increased extrathyroidal extension. This may help explain the observation why some studies correlate the BRAF V600E mutation with aggressive behavior while others do not. The extent of mutated protein expression (IHC staining) may be a better predictor of PTC behavior than direct sequencing (BRAF V600E + vs. BRAF V600E −). This potentially provides prognostic value to the IHC technique.
There have been several studies assessing the use of BRAF mutation testing preoperatively on fine needle aspiration biopsy (FNAB) of thyroid nodules. They have shown that BRAF mutation is associated with higher risk of extrathyroidal extension, lymph node metastasis, and disease persistence/recurrence 19–22. It has been shown that using molecular testing preoperatively can help distinguish benign from malignant disease and can help limit the cost of thyroid care by limiting the number of completion thyroidectomies 23. IHC for BRAF V600E might be an interesting diagnostic tool for FNAB of thyroid nodules. Its detection in FNAB of thyroid nodules has been proposed as an additional diagnostic tool, improving the specificity of the cytodiagnostic procedure and potentially guiding treatment strategies 11.
This study has several limitations. Staining intensity can be affected by antigen preservation and poor tissue quality. However, our tissue banking protocol allows for rapid tumor collection, processing and cryopreservation with a median time of 12 minutes. Although it is possible the false negative cases may represent tumors where the section for genetic testing was different from the section used for IHC, care was taken to use the same sections and avoid this potential source of error. In cases where ambiguous weak or focal immunostaining is seen, additional genetic analysis may be required to clarify the BRAF mutation status.
In summary, our study showed that the immunohistochemical detection of the mutated BRAF V600E protein is a reliable, highly specific method for detection of the BRAF V600E mutation in PTC. High expression of mutated BRAF protein showed strong correlation with extrathyroidal extension and provides potential prognostic significance. These data are being validated on a larger patient cohort. In addition, FNAB immunostaining for BRAF V600E might be an interesting diagnostic tool in identifying malignant thyroid nodules and potentially guiding treatment. It may be applicable for use in detecting the mutation in microcarcinomas and lymph node metastasis. IHC is an accurate, rapid, easily applicable and potentially cost effective alternative to standard molecular techniques in the detection of the BRAF V600E mutation. It may serve as a better predictor of tumor behavior than current techniques. Findings from our study support the use of IHC for detection of the BRAF V600E mutation as a diagnostic and potentially prognostic tool for papillary thyroid cancer.
Acknowledgments
Sources of Financial Support: NYUCI Center Support Grant, “NIH/NCI 5 P30CA16087-31.” This project was funded in whole or in part by the National Institutes of Health’s National Center for Advancing Translational Sciences through its Clinical and Translational Science Awards Program (CTSA), grant # UL1 TR000038.
Footnotes
Disclosure of Financial Interests and Potential Conflicts of Interest
None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Davies L, Welch HG. Increasing incidence of thyroid cancer in the United States, 1973–2002. Jama. 2006;295(18):2164–7. doi: 10.1001/jama.295.18.2164. [DOI] [PubMed] [Google Scholar]
- 2.Nikiforov YE, Nikiforova MN. Molecular genetics and diagnosis of thyroid cancer. Nat Rev Endocrinol; 7(10):569–80. doi: 10.1038/nrendo.2011.142. [DOI] [PubMed] [Google Scholar]
- 3.Nikiforova MN, Nikiforov YE. Molecular genetics of thyroid cancer: implications for diagnosis, treatment and prognosis. Expert Rev Mol Diagn. 2008;8(1):83–95. doi: 10.1586/14737159.8.1.83. [DOI] [PubMed] [Google Scholar]
- 4.Mathur A, Moses W, Rahbari R, et al. Higher rate of BRAF mutation in papillary thyroid cancer over time: a single-institution study. Cancer; 117(19):4390–5. doi: 10.1002/cncr.26072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li C, Lee KC, Schneider EB, Zeiger MA. BRAF V600E mutation and its association with clinicopathological features of papillary thyroid cancer: a meta-analysis. J Clin Endocrinol Metab; 97(12):4559–70. doi: 10.1210/jc.2012-2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim SK, Kim DL, Han HS, et al. Pyrosequencing analysis for detection of a BRAFV600E mutation in an FNAB specimen of thyroid nodules. Diagn Mol Pathol. 2008;17(2):118–25. doi: 10.1097/PDM.0b013e31815d059d. [DOI] [PubMed] [Google Scholar]
- 7.Kim HS, Kim JO, Lee DH, et al. Factors influencing the detection of the BRAF T1799A mutation in papillary thyroid carcinoma. Oncol Rep; 25(6):1639–44. doi: 10.3892/or.2011.1225. [DOI] [PubMed] [Google Scholar]
- 8.Lee HJ, Choi J, Hwang TS, et al. Detection of BRAF mutations in thyroid nodules by allele-specific PCR using a dual priming oligonucleotide system. Am J Clin Pathol; 133(5):802–8. doi: 10.1309/AJCPO3F2ENKMDTUS. [DOI] [PubMed] [Google Scholar]
- 9.Capper D, Preusser M, Habel A, et al. Assessment of BRAF V600E mutation status by immunohistochemistry with a mutation-specific monoclonal antibody. Acta Neuropathol; 122(1):11–9. doi: 10.1007/s00401-011-0841-z. [DOI] [PubMed] [Google Scholar]
- 10.Capper D, Berghoff AS, Magerle M, et al. Immunohistochemical testing of BRAF V600E status in 1,120 tumor tissue samples of patients with brain metastases. Acta Neuropathol; 123(2):223–33. doi: 10.1007/s00401-011-0887-y. [DOI] [PubMed] [Google Scholar]
- 11.Koperek O, Kornauth C, Capper D, et al. Immunohistochemical detection of the BRAF V600E-mutated protein in papillary thyroid carcinoma. Am J Surg Pathol; 36(6):844–50. doi: 10.1097/PAS.0b013e318246b527. [DOI] [PubMed] [Google Scholar]
- 12.Long GV, Wilmott JS, Capper D, et al. Immunohistochemistry is highly sensitive and specific for the detection of V600E BRAF mutation in melanoma. Am J Surg Pathol; 37(1):61–5. doi: 10.1097/PAS.0b013e31826485c0. [DOI] [PubMed] [Google Scholar]
- 13.Preusser M, Capper D, Berghoff AS, et al. Expression of BRAF V600E Mutant Protein in Epithelial Ovarian Tumors. Appl Immunohistochem Mol Morphol; 21(2):162–7. doi: 10.1097/PAI.0b013e31825d7402. [DOI] [PubMed] [Google Scholar]
- 14.Preusser M, Berghoff AS, Capper D, et al. No Evidence for BRAF-V600E Mutations in Gastroeosophageal Tumors: Results From a High-throughput Analysis of 534 Cases Using a Mutation-specific Antibody. Appl Immunohistochem Mol Morphol. doi: 10.1097/PAI.0b013e31827ce693. [DOI] [PubMed] [Google Scholar]
- 15.Xu L, Zhou JL, Cohen M, et al. Spry2 expression correlates with BRAF mutation in thyroid cancer. Surgery. 148(6):1282–7. doi: 10.1016/j.surg.2010.09.028. discussion 1287. [DOI] [PubMed] [Google Scholar]
- 16.Xing M. BRAF mutation in papillary thyroid cancer: pathogenic role, molecular bases, and clinical implications. Endocr Rev. 2007;28(7):742–62. doi: 10.1210/er.2007-0007. [DOI] [PubMed] [Google Scholar]
- 17.Lee KC, Li C, Schneider EB, et al. Is BRAF mutation associated with lymph node metastasis in patients with papillary thyroid cancer? Surgery; 152(6):977–83. doi: 10.1016/j.surg.2012.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nam JK, Jung CK, Song BJ, et al. Is the BRAF(V600E) mutation useful as a predictor of preoperative risk in papillary thyroid cancer? Am J Surg; 203(4):436–41. doi: 10.1016/j.amjsurg.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 19.Nikiforov YE, Ohori NP, Hodak SP, et al. Impact of mutational testing on the diagnosis and management of patients with cytologically indeterminate thyroid nodules: a prospective analysis of 1056 FNA samples. J Clin Endocrinol Metab; 96(11):3390–7. doi: 10.1210/jc.2011-1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nikiforov YE, Yip L, Nikiforova MN. New Strategies in Diagnosing Cancer in Thyroid Nodules: Impact of Molecular Markers. Clin Cancer Res. doi: 10.1158/1078-0432.CCR-12-1253. [DOI] [PubMed] [Google Scholar]
- 21.Howell GM, Nikiforova MN, Carty SE, et al. BRAF V600E mutation independently predicts central compartment lymph node metastasis in patients with papillary thyroid cancer. Ann Surg Oncol; 20(1):47–52. doi: 10.1245/s10434-012-2611-0. [DOI] [PubMed] [Google Scholar]
- 22.Melck AL, Yip L, Carty SE. The utility of BRAF testing in the management of papillary thyroid cancer. Oncologist; 15(12):1285–93. doi: 10.1634/theoncologist.2010-0156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yip L, Farris C, Kabaker AS, et al. Cost impact of molecular testing for indeterminate thyroid nodule fine-needle aspiration biopsies. J Clin Endocrinol Metab; 97(6):1905–12. doi: 10.1210/jc.2011-3048. [DOI] [PMC free article] [PubMed] [Google Scholar]