Abstract
Infliximab, a chimeric monoclonal antibody against tumor necrosis factor-α, has shown activity against steroid refractory acute graft-versus-host disease (GVHD). We conducted a prospective trial of infliximab for the prophylaxis of acute GVHD. Patients older than 20 years undergoing myeloablative allogeneic stem cell transplantation for hematologic malignancies were eligible. GVHD prophylaxis consisted of infliximab given one day prior to conditioning and then on days 0, +7, +14, +28 and +42, together with standard cyclosporine and methotrexate. Nineteen patients with a median age of 53 years were enrolled. All patients received peripheral blood allografts from matched sibling (n=14) or unrelated donors (n=5). Results were compared with a matched historical control group (n=30) treated contemporaneously at our institution. The cumulative incidences of grades II–IV acute GVHD in the infliximab and control groups were 36.8% and 36.6% respectively (p=0.77). Rates of chronic GVHD were 78% and 61% respectively (p=0.22). Significantly more bacterial and invasive fungal infections were observed in the infliximab group (p=0.01 and p=0.02 respectively). Kaplan-Meier estimates of 2 year overall survival and progression free survival for patients receiving infliximab were 42% and 36% respectively. The corresponding numbers for patients in the control group were 46% and 43% respectively. The addition of infliximab to standard GVHD prophylaxis did not lower the risk of GVHD and was associated with an increased risk of bacterial and invasive fungal infections.
Keywords: Hematopoietic stem cell transplantation, Allogeneic, Graft-versus-Host Disease, Steroid refractory, Infliximab, Tumor necrosis factor, unrelated donor
Introduction
Acute graft-versus-host disease (GVHD) is one of the most frequent complications after allogeneic hematopoietic stem cell transplantation (HSCT) (1). Overproduction of tumor necrosis factor-α (TNF-α) is implicated in the pathophysiology of acute GVHD through several mechanisms including up-regulation of the expression of major histocompatibility complex antigens, endothelial cell and leukocyte adhesion molecules, induction of target tissues apoptosis through TNF-α receptor, activation of macrophages, neutrophils, eosinophils, B-cells and T-cells and increased production of additional inflammatory cytokines (2–4). Elevated serum levels of TNF-α are seen in patients with acute GVHD and may be predictive of the severity of GVHD (5,6).
Infliximab (Remicade, Centocor, Malvern, PA, USA) is a murine-human chimeric IgG1κ monoclonal antibody that binds with high affinity to the soluble and transmembrane forms of TNF-α, and inhibits their binding with the cellular receptors (7). A number of retrospective studies have shown activity of this drug in the treatment of steroid refractory acute GVHD (8–10) generally employing high doses (10mg/kg/week). Although no prospective clinical trial of infliximab for the prophylaxis of acute GVHD has been reported, neutralizing anti-TNF-α monoclonal antibodies administered prior to TBI in murine models have shown significantly delayed mortality and improved body weight in treated mice (11). We theorized that infliximab may decrease the risk of acute GVHD and therefore conducted a prospective trial to evaluate the efficacy and toxicity of infliximab prophylaxis prior to allogeneic stem cell transplant.
Patients, materials and Methods
Patient population
Beginning in April 2004, 19 patients were enrolled at the time of allogeneic transplant for acute myeloid leukemia, myelodysplastic syndrome, acute lymphoblastic leukemia/lymphoma, non-Hodgkin’s lymphoma, Hodgkin’s lymphoma or chronic myeloid leukemia (CML) in accelerated phase or blast crisis. Patients undergoing HSCT for CML in chronic phase or aplastic anemia were not eligible. CML patients in chronic phase were excluded since these patients generally have favorable outcomes with standard GVHD prophylaxis (cyclosporine + methotrexate), and more intense GVHD prophylaxis in this group may increase the risk of disease relapse post transplantation (12). Patients with acute leukemia in first complete remission, myelodysplastic syndrome with refractory anemia or refractory anemia with ringed sideroblasts were considered to have low-risk disease. All other patients were placed in the high-risk disease category. Patients with human immunodeficiency virus seropositivity, Karnofsky performance status less than 60%, and those receiving reduced intensity conditioning were excluded. The study was approved by the Ohio State University Institutional Review Board and all patients underwent informed consent.
The patients enrolled in this study were compared with a historical control group of 30 patients who had undergone allogeneic HSCT at our center at approximately the same time as the study group (between January 2003 and October 2005) and who had either not consented to the study or were not eligible due to insurance denial. Matching criteria for control group selection included diagnosis, pretransplant disease status and risk-category, age, stem cell source, myeloablative conditioning with TBI or busulfan containing regimen, GVHD prophylaxis, donor type and degree of HLA match.
HLA typing and Donor matching
In patients with sibling donors HLA typing for class I antigens was performed using standard serologic techniques. Typing for Class II alleles (HLA-DRB1) was resolved with sequence-specific oligonucleotide primers for hybridization of amplified DNA, followed by high-resolution typing in all patients and donors. Unrelated donors were matched for HLA-A, -B, and –DRB1 by high-resolution typing.
Treatment protocol
Infliximab was administered as a 120 minute infusion at 10mg/kg the day before the conditioning regimen. Five subsequent doses were given on days 0 (after hematopoietic stem cell infusion), +7, +14, +28 and +42. No dose modifications were done for renal or hepatic impairment. All patients received standard prophylaxis of acute GVHD with cyclosporine (3mg/kg/day IV, commencing on day −1) and short course methotrexate (15mg/m2 day 1, and 10mg/m2 days 3, 6 and 11). Cyclosporine levels were maintained between 150–450ng/ml. From day +100 onwards cyclosporine was tapered at the discretion of the treating physician.
Transplantation procedure and supportive care
All patients received myeloablative conditioning regimens using either TBI (1200cGy) or busulfan (16mg/kg oral or 12.8mg/kg IV) in combination with other drugs. Donor cells were infused on day 0. Patients were monitored weekly for cytomegalovirus (CMV) reactivation with Digene™ hybrid capture assay. Gancyclovir or foscarnet were used at the discretion of the treating physician for patients with evidence of CMV reactivation. Filgastrim (G-CSF) was not routinely administered. CMV negative products were used for CMV seronegative patients. Neutrophil engraftment was defined as the first of three successive days after transplantation, with ANC ≥ 0.5 × 109/L. Platelet engraftment was considered to have occurred on the first of three consecutive days with platelet count 20 × 109/L or higher, in the absence of platelet transfusion.
Assessment of GVHD
Staging and grading of acute GVHD were scored according to consensus criteria (13). Biopsies of all involved organs were required to corroborate the clinical diagnosis of acute GVHD. Exception was patients with multi-organ acute GVHD where liver biopsy was preferred but not required. Liver-only GVHD required biopsy confirmation. Patients were evaluable for chronic GVHD if engraftment occurred and the patient survived for 100 days post-transplantation. Assessments were made according to previously described criteria (14).
Adverse events
Adverse events were assessed and recorded after HSCT for an evaluation of safety. Veno-occlusive disease (VOD) was diagnosed and graded according to McDonald (15) and Bearman (16) criteria respectively. Infections were documented as “proven” if an organism was isolated or confirmed by serological, molecular, culture or histological evidence, or “suspected” if patients developed fevers and radiological or clinical evidence of infection without organism identified. Urine and/or serum BK-virus PCR was obtained in all suspected cases of hemorrhagic cystitis. Primary cause of death and treatment related (non-relapse) mortality (TRM) were defined according to National Marrow Donor Program criteria (17).
Statistical analysis
The primary efficacy outcome for this study was cumulative incidence of grade II–IV acute GVHD within 100 days of allogeneic HSCT. Secondary objectives included incidence of chronic GVHD, TRM, overall survival (OS), progression free survival (PFS), adverse events and infectious complications. Competing risk analysis between acute GVHD and TRM were used to estimate the cumulative incidence. PFS rates were calculated using death and disease progression as events. Actuarial survival after transplantation was evaluated by the Kaplan-Meier method. Predictors of response were evaluated by logistic regression. Predictors of survival were evaluated by Cox proportional hazards model. Categorical variables and acute GVHD incidence between the study group and the historical control group were compared by using the chi-square test or Fisher exact test, as appropriate; continuous variables were compared by using the Mann-Whitney test, and OS and PFS data were analyzed by the log-rank test. Cumulative incidence of acute GVHD between infliximab and historical controls was compared using Gray’s test. All analyses were run using Stata 10.0 (Stata Corporation, College Station, Texas).
Results
Patient and disease characteristics
Twenty-four consecutive patients provided informed consent for enrollment in the infliximab group. Five patients did not receive infliximab for GVHD prophylaxis secondary to insurance denial, and are not included in the final analysis. The remaining 19 patients constitute the infliximab group, while 30 matched historical controls were selected. Demographics and transplant characteristics of both groups are summarized in Table 1. Compared to patients receiving infliximab, the control group was slightly younger (median age 43.5 vs. 53 years, p-value = 0.05). All patients in the control group received acute GVHD prophylaxis with short course methotrexate and cyclosporine. No patient in either group received a T-cell depleted graft.
Table 1.
Baseline characteristics of patients in the clinical trial and their matched controls.
| Clinical trial (N=19) |
Control group (N=30) |
p-value† | |
|---|---|---|---|
| N (%) | N (%) | ||
| Median age, y (range) | 53 (27–64) | 43.5 (21–64) | 0.05 |
| Sex | |||
| Male | 13 (68) | 20 (67) | 0.99 |
| Female | 6 (32) | 10 (33) | |
| Stem cell source | |||
| PBSC | 19 (100) | 30 (100) | - |
| BM | 0 | 0 | |
| Donor source | |||
| HLA-matched related | 14 (74) | 25 (83) | 0.49 |
| HLA-mismatched related | 0 (0) | 1 (3) | |
| HLA-matched unrelated | 4 (21) | 2 (7) | |
| HLA-mismatched unrelated | 1 (5) | 2 (7) | |
| Diagnosis | |||
| MDS/AML | 11 (58) | 21 (70) | 0.26 |
| Non-Hodgkin’s lymphoma | 4 (21) | 3 (10) | |
| ALL | 4 (21) | 3 (10) | |
| HOD | 0 | 3 (10) | |
| Disease Risk | |||
| Standard-risk | 8 | 14 | 0.75 |
| High-risk | 11 | 16 | |
| Gender match | |||
| Sex matched | 9 (47) | 16 (54) | 0.86 |
| Male to female | 4 (22) | 7 (23) | |
| Female to male | 6 (31) | 7 (23) | |
| ABO matched/mismatched | |||
| Matched | 11 (58) | 20 (67) | 0.14 |
| Major mismatch | 5 (26) | 2 (7) | |
| Minor mismatch | 3 (16) | 8 (26) | |
| Median CD34+ cell dose (106 cells/kg recipient wt.) | 4.95 | 5.22 | 0.70 |
| Median CD3+ cell dose (107 cells/kg recipient wt.) | 2.94 | 3.02 | 0.72 |
| G-CSF used | |||
| Yes | 12 (63) | 13 (43) | 0.24 |
| No | 7 (37) | 17 (57) | |
| Conditioning regimen | |||
| TBI-containing | 4 (21) | 3 (10) | 0.41 |
| Bu/Cy | 15 (79) | 27 (90) | |
ALL: acute lymphoblastic lymphoma; AML: acute myeloid leukemia, BM: bone marrow; Bu/Cy: busulphan and cyclophosphamide; G-CSF: granulocyte colony stimulating factor; HOD: Hodgkin’s lymphoma; MDS: myelodysplastic syndrome; PBSC: peripheral blood stem cells; TBI: total body irradiation.
p-values are based on Wilcoxon rank-sum for medians and Fisher’s Exact test for categorical data.
Engraftment
There were no engraftment failures in either group. The median time to neutrophil engraftment was 16 days (range 12–27 days) for patients in the infliximab groups and 14 days (range 11–23 days) in the control group (p-value = 0.06). Two and three patients in infliximab and control group failed to meet the criteria for platelet engraftment respectively. The median time to platelet engraftment in infliximab group (26 days, range 14–99 days) was significantly longer (p-value = 0.02) compared to the control group (16 days, range 13–47 days).
Acute GVHD
The cumulative incidence of grade II–IV acute GVHD (Figure 1) was 36.8% in the infliximab group (n = 7) and 36.6% in the control group (n = 11) (p-value = 0.77). The distribution of acute GVHD grades in both groups is presented in Table 2. No difference in the incidence of acute GVHD was seen among the two groups, across all severity grades (p-value = 0.22). On multivariate logistic regression analysis adjusted for patient age, sex, degree of HLA-compatibility, donor type and conditioning regimen, no difference in odds of developing acute GVHD between the infliximab and control group was found (p-value = 0.94, 95% CI 0.13–3.55). Median time to onset of acute GVHD in the infliximab and control groups was 30 and 32 days respectively (p-value = 0.4). Within the infliximab group no difference (p-value = 0.70) was seen in the median time to the onset of acute GVHD in patients conditioned with TBI-containing regimens (29 days) versus those receiving non-TBI based conditioning (31 days). Distribution of organ involvement was similar between the two groups. Among patients undergoing transplantation from sibling donors the rates of grade II–IV acute GVHD in the infliximab and control groups were 35.7% (n=5) and 30.7% (n=8) respectively (p-value = 0.75), while the corresponding rates of grade II–IV acute GVHD in recipients of allografts from unrelated donors in the infliximab and control groups were 40% (n=2) and 75% (n=3) respectively (p-value = 0.29). Interestingly subgroup analysis of patients in the infliximab group according to disease risk-categories revealed that 7 out of 11 patients with high-risk disease (63%) developed grade II–IV acute GVHD, while none of the eight patients with standard risk disease developed grade II–IV acute GVHD (p-value = 0.004). Five out of 16 patients with high-risk disease in the control group (31%) developed grade II–IV acute GVHD. This was not significantly different from rates seen in high risk patients in the infliximab group (p-value = 0.09). However among patients with standard risk disease infliximab produced significantly better control of grade II–IV acute GVHD (0%) compared to the control group (42%) (p-value = 0.02).
Figure 1.
Cumulative incidence of acute grade II–IV GVHD (black and grey curves depict infliximab treated and control groups respectively) Gray’s test p-value = 0.77.
Table 2.
Distribution of acute GVHD grades
| Grade of acute GVHD | Infliximab Group (%) (N=19) |
Control Group (%) (N=30) |
|---|---|---|
| 0 | 9 (47.3) | 10 (33.3) |
| I | 3 (15.7) | 9 (30) |
| II | 1 (5.2) | 7 (23.3) |
| III | 3 (15.7) | 2 (6.6) |
| IV | 3 (15.7) | 2 (6.6) |
Chronic GVHD
Fourteen patients in the infliximab group and 26 in the control group were evaluable for chronic GVHD. In patients receiving infliximab prophylaxis, the incidence of chronic GVHD was 78% (n = 11), compared to 61% (n = 16) in the control group (p-value = 0.22). Nine patients in the infliximab group, while 11 in the control group had extensive chronic GVHD (p-value = 0.44).
Toxicity and infections
Four patients did not receive all planned six doses of infliximab. Reasons of discontinuation included: development of grade III–IV acute GVHD while still receiving infliximab prophylaxis (n=2), disease progression in central nervous system in a patient with Burkitt lymphoma (n=1) and overwhelming sepsis (n=1). No allergic or infusion related adverse events were attributed to infliximab. Similarly no neurologic or cardiac complications attributable to infliximab developed. No cases of post-transplant lymphoproliferative disease, second malignancies, tuberculosis or atypical mycobacterium developed. Non-infectious complications possibly related to infliximab included: nausea/vomiting (n=1), fatigue (n=1), diarrhea (n=1), night sweats (n=1), pulmonary fibrosis (n=1), acute respiratory distress syndrome (n=2), transient renal insufficiency (n=2), shortness of breath (n=1), pleural effusion (n=1) and gastric bleeding (n = 1). Infectious complications were frequent. Table 3 summarizes proven infections among patients in the infliximab and control group. Five patients developed invasive fungal infections including; invasive pulmonary aspergillosis (n = 2), candidemia (n = 1), pulmonary candidiasis (n = 1) and candida urinary tract infection (n = 1) in infliximab group. Compared to the control group, invasive fungal infections were significantly more frequent in the infliximab group (p-value = 0.03). Viral infections in the infliximab group included CMV reactivation (n = 13), BK-virus associated hemorrhagic cystitis (n = 4), and VZV reactivation (n = 1). One patient developed CMV pneumonitis. In contrast in the control group 18 patients developed CMV reactivation and 3 had BK-virus associated hemorrhagic cystitis. In addition one episode each of influenza A and EBV reactivation was seen in the control group. No significant difference between the two groups was present in terms of viral infections (p-value = 0.90). In the infliximab group eighteen patients (95%) developed a total of 40 bacterial infectious events (including 26 episodes of bacteremias, 3 urinary tract infections, 7 episodes of Clostridium difficile colitis and 4 respiratory tract infections). In the control group 19 patients (63%) developed a total of 39 bacterial infections (p-value = 0.01).
Table 3.
Infections in patients receiving infliximab prophylaxis and control group
| Clinical trial | Control group | |
|---|---|---|
| Type of infections | No of patients (%) | No of patients (%) |
| Bacterial | ||
| Gram-positive | ||
| Staphylococcus aureus | 6 (31) | 3 (10) |
| Staphylococcus (not aureus) | 11 (58) | 11 (37) |
| Enterococcus | 3 (16) | 5 (16) |
| Clostridium difficile | 5 (26) | 7 (23) |
| Other | 3 (16) | - |
| Gram-negative | 8 (42) | 10 (33) |
| Fungal | ||
| Candida glabrata | 2 (10) | 0 |
| Candida spp | 1 (5) | 0 |
| Aspergillus spp | 2 (10) | 1 (3) |
| Viral | ||
| Cytomegalovirus | 13 (68) | 18 (60) |
| BK-virus | 4 (21) | 3 (10) |
| Adenovirus | - | - |
| Epstein-Barr virus | - | 1 (3) |
| Others | 1 (5) | 1 (3) |
Outcomes
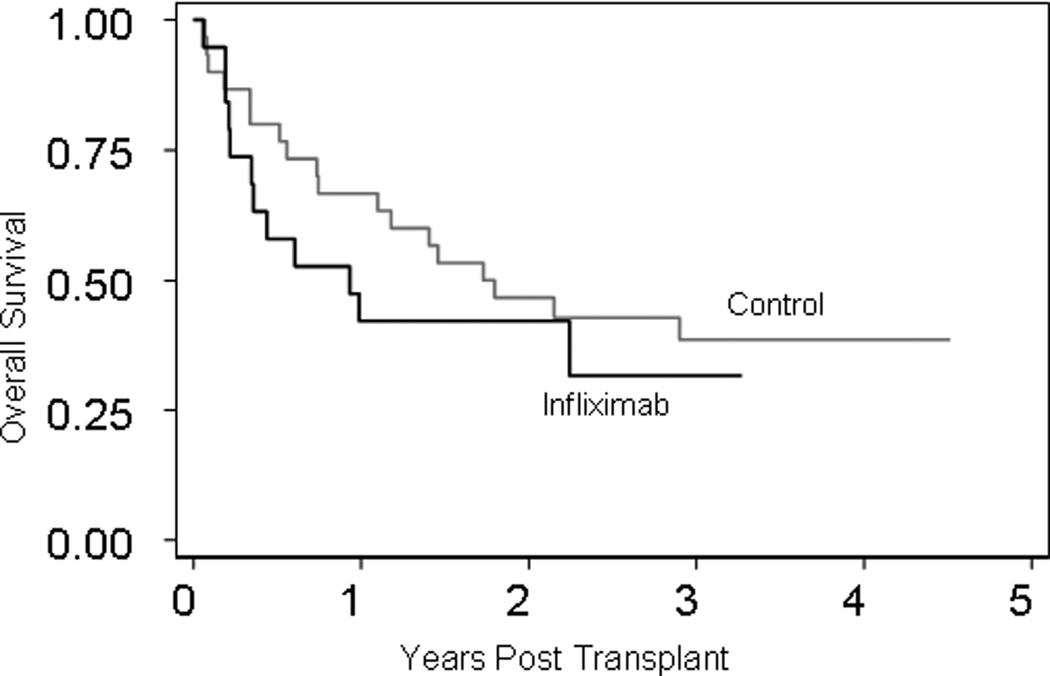
Seven patients in the infliximab group are alive at a median follow-up of 34 months (range 21–42 months). All surviving patients have chronic GVHD (six patients have extensive chronic GVHD), and display no evidence of disease progression. Twelve of 19 enrolled patients died. Causes of death included disease relapse (n = 6), GVHD (n = 3), pulmonary invasive fungal infections in patients with GVHD (n = 2) and sepsis with multi-organ failure (n = 1). The Kaplan-Meier estimates of overall survival at 2 years after transplantation were 42% in the infliximab group and 46% in the control group (p-value = 0.49) (Figure 2). Similarly estimates of the 2 year progression free survival between the two groups were 36% and 43% respectively (p-value = 0.50). Six patients in the infliximab group experienced disease relapse (31%) compared to 10 patients (33%) in the control group (p-value = 0.89). No significant difference in the day 100 TRM rates between infliximab (21%) and control (13%) groups was seen (p-value = 0.47). Two year TRM rates were 31% (n = 6) and 26% (n = 8) in the infliximab and control groups respectively (p-value = 0.76).
Figure 2.
Kaplan-Meier estimates of overall survival (black and grey curves depict infliximab treated and control groups respectively) Log-rank p-value = 0.43.
Discussion
Despite recent advances, GVHD remains a significant barrier towards broader and safer application of allogeneic HSCT. Effective prophylaxis of acute GVHD remains crucial for improving allogeneic transplant outcomes. The role of TNF-α in the pathogenesis of acute GVHD is supported by successful prevention of murine GVHD by neutralizing polyclonal TNF-α antibody before TBI and allografting (3,11). Although infliximab has shown activity in steroid refractory acute GVHD (8–10,18,19), it has not been formally evaluated as a prophylactic agent for acute GVHD.
Holler et al. reported delayed onset and severity of acute GVHD disease (especially in patients receiving TBI-based conditioning) with MAK 195F, a murine monoclonal antibody neutralizing human TNF-α (not available in U.S.A) compared to historic controls (20). In contrast we did not detect any significant difference in the incidence, severity or time to onset of acute GVHD following incorporation of infliximab into a standard GVHD prophylaxis regimen. Standard acute GVHD prophylaxis used in both studies was identical (cyclosporine and methotrexate). These paradoxical GVHD-related outcomes reported in our study compared to those reported by Holler et al, might be secondary to unknown biological differences between these two TNF-α blocking agents. Patient selection may have a role. Interestingly the MAK 195F trial included younger patients (median age 45.2 years) with matched sibling donors and the majority of enrolled patients had CML in first chronic phase. In contrast our patients were older, the majority had high-risk disease (n=11) and included those receiving unrelated donor grafts.
TNF-α has shown no protective effects on development of chronic GVHD in murine models (3). No statistically significant impact of TNF-α antibody on chronic GVHD was seen in MAK 195F study (20). In our trial a statistically non-significant trend towards increased GVHD was seen with infliximab use. Increased incidence of chronic GVHD in 90% of patients receiving infliximab for steroid refractory acute GVHD has been reported previously (8).
Infliximab use in our study was associated with significantly delayed engraftment of platelets and a trend towards delayed neutrophil engraftment as well. TNF-α has been shown to strongly augment interleukin-3 induced short-term proliferation of human CD34+ hematopoietic progenitor cells (21). Abrogation of this effect might lead to delayed engraftment seen in our study. Delayed neutrophil and platelet engraftment was also apparent in MAK 195F study, especially in cohorts receiving higher doses of TNF-α antibody (20). There has been an increase in infectious complications with use of infliximab in patients with steroid refractory acute GVHD (8,18,19). Marty et al. (22) reported significantly increased risk of invasive fungal infections with infliximab (45%) in patients with steroid refractory GVHD compared to those not exposed to the drug (12%). However patients in these studies with steroid refractory GVHD were profoundly immunocompromised. Nevertheless, infliximab use in our study, as prophylaxis of acute GVHD, was associated with a significantly increased incidence of fungal and bacterial infections.
The dose of infliximab employed in our study for GVHD prophylaxis (10mg/kg) was selected based on the dose used in majority of the studies reporting infliximab’s efficacy in steroid-refractory acute GVHD and is higher compared to the infliximab dose (5mg/kg) recommended for rheumatologic indications (8,9,18,19,23–26). We did not perform any pharmacokinetic sampling and therefore it is possible that other dosing schemes might be more effective in this setting. The frequent infectious complications seen in our study might be secondary to the dose and administration schedule of infliximab; however such infectious events are well documented, even with standard (5mg/kg) infliximab dosing schedules (23,25,27). This study has several limitations. Prospectively enrolled patients who received infliximab for acute GVHD prophylaxis were compared to matched historical-controls previously treated in our institution. Although the control group was well-matched, this type of analysis is confounded by inherent selection bias. Nevertheless, important conclusions can be drawn. Infliximab use did not produce a significant improvement in the cumulative incidence of acute GVHD, may have delayed platelet engraftment, and was associated with frequent infectious complications. Infliximab is unlikely to make a major impact in controlling and preventing acute GVHD following myeloablative conditioning at least in patients with high-risk hematological malignancies.
Figure 3.
Kaplan-Meier estimates of progression free survival (black and grey curves depict infliximab treated and control groups respectively) Log-rank p-value = 0.50.
References
- 1.Deeg HJ. How I treat refractory acute GVHD. Blood. 2007;109(10):4119–4126. doi: 10.1182/blood-2006-12-041889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferrara JL, Levy R, Chao NJ. Pathophysiologic mechanisms of acute graft-vs.-host disease. Biol Blood Marrow Transplant. 1999;5(6):347–356. doi: 10.1016/s1083-8791(99)70011-x. [DOI] [PubMed] [Google Scholar]
- 3.Piguet PF, Grau GE, Allet B, Vassalli P. Tumor necrosis factor/cachectin is an effector of skin and gut lesions of the acute phase of graft-vs.-host disease. J Exp Med. 1987;166(5):1280–1289. doi: 10.1084/jem.166.5.1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jasinski M, Wieckiewicz J, Ruggiero I, Pituch-Noworolska A, Zembala M. Isotype-specific regulation of MHC class II gene expression in human monocytes by exogenous and endogenous tumor necrosis factor. J Clin Immunol. 1995;15(4):185–193. doi: 10.1007/BF01541088. [DOI] [PubMed] [Google Scholar]
- 5.Holler E, Kolb HJ, Hintermeier-Knabe R, Mittermuller J, Thierfelder S, Kaul M, et al. Role of tumor necrosis factor alpha in acute graft-versus-host disease and complications following allogeneic bone marrow transplantation. Transplant Proc. 1993;25(1 Pt 2):1234–1236. [PubMed] [Google Scholar]
- 6.Holler E, Kolb HJ, Moller A, Kempeni J, Liesenfeld S, Pechumer H, et al. Increased serum levels of tumor necrosis factor alpha precede major complications of bone marrow transplantation. Blood. 1990;75(4):1011–1016. [PubMed] [Google Scholar]
- 7.Knight DM, Trinh H, Le J, Siegel S, Shealy D, McDonough M, et al. Construction and initial characterization of a mouse-human chimeric anti-TNF antibody. Mol Immunol. 1993;30(16):1443–1453. doi: 10.1016/0161-5890(93)90106-l. [DOI] [PubMed] [Google Scholar]
- 8.Couriel D, Saliba R, Hicks K, Ippoliti C, de Lima M, Hosing C, et al. Tumor necrosis factor-alpha blockade for the treatment of acute GVHD. Blood. 2004;104(3):649–654. doi: 10.1182/blood-2003-12-4241. [DOI] [PubMed] [Google Scholar]
- 9.Jacobsohn DA, Hallick J, Anders V, McMillan S, Morris L, Vogelsang GB. Infliximab for steroid-refractory acute GVHD: a case series. Am J Hematol. 2003;74(2):119–124. doi: 10.1002/ajh.10392. [DOI] [PubMed] [Google Scholar]
- 10.Yamane T, Yamamura R, Aoyama Y, Nakamae H, Hasegawa T, Sakamoto C, et al. Infliximab for the treatment of severe steroid refractory acute graft-versus-host disease in three patients after allogeneic hematopoietic transplantation. Leuk Lymphoma. 2003;44(12):2095–2097. doi: 10.1080/1042819031000123483. [DOI] [PubMed] [Google Scholar]
- 11.Hattori K, Hirano T, Miyajima H, Yamakawa N, Tateno M, Oshimi K, et al. Differential effects of anti-Fas ligand and anti-tumor necrosis factor alpha antibodies on acute graft-versus-host disease pathologies. Blood. 1998;91(11):4051–4055. [PubMed] [Google Scholar]
- 12.Devergie A, Blaise D, Attal M, Tigaud JD, Jouet JP, Vernant JP, et al. Allogeneic bone marrow transplantation for chronic myeloid leukemia in first chronic phase: a randomized trial of busulfan-cytoxan versus cytoxan-total body irradiation as preparative regimen: a report from the French Society of Bone Marrow Graft (SFGM) Blood. 1995;85(8):2263–2268. [PubMed] [Google Scholar]
- 13.Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15(6):825–828. [PubMed] [Google Scholar]
- 14.Atkinson K, Horowitz MM, Gale RP, Lee MB, Rimm AA, Bortin MM. Consensus among bone marrow transplanters for diagnosis, grading and treatment of chronic graft-versus-host disease. Committee of the International Bone Marrow Transplant Registry. Bone Marrow Transplant. 1989;4(3):247–254. [PubMed] [Google Scholar]
- 15.McDonald GB, Hinds MS, Fisher LD, Schoch HG, Wolford JL, Banaji M, et al. Veno-occlusive disease of the liver and multiorgan failure after bone marrow transplantation: a cohort study of 355 patients. Ann Intern Med. 1993;118(4):255–267. doi: 10.7326/0003-4819-118-4-199302150-00003. [DOI] [PubMed] [Google Scholar]
- 16.Bearman SI. The syndrome of hepatic veno-occlusive disease after marrow transplantation. Blood. 1995;85(11):3005–3020. [PubMed] [Google Scholar]
- 17.Copelan E, Casper JT, Carter SL, van Burik JA, Hurd D, Mendizabal AM, et al. A scheme for defining cause of death and its application in the T cell depletion trial. Biol Blood Marrow Transplant. 2007;13(12):1469–1476. doi: 10.1016/j.bbmt.2007.08.047. [DOI] [PubMed] [Google Scholar]
- 18.Patriarca F, Sperotto A, Damiani D, Morreale G, Bonifazi F, Olivieri A, et al. Infliximab treatment for steroid-refractory acute graft-versus-host disease. Haematologica. 2004;89(11):1352–1359. [PubMed] [Google Scholar]
- 19.Sleight BS, Chan KW, Braun TM, Serrano A, Gilman AL. Infliximab for GVHD therapy in children. Bone Marrow Transplant. 2007;40(5):473–480. doi: 10.1038/sj.bmt.1705761. [DOI] [PubMed] [Google Scholar]
- 20.Holler E, Kolb HJ, Mittermuller J, Kaul M, Ledderose G, Duell T, et al. Modulation of acute graft-versus-host-disease after allogeneic bone marrow transplantation by tumor necrosis factor alpha (TNF alpha) release in the course of pretransplant conditioning: role of conditioning regimens and prophylactic application of a monoclonal antibody neutralizing human TNF alpha (MAK 195F) Blood. 1995;86(3):890–899. [PubMed] [Google Scholar]
- 21.Caux C, Saeland S, Favre C, Duvert V, Mannoni P, Banchereau J. Tumor necrosis factor-alpha strongly potentiates interleukin-3 and granulocyte-macrophage colony-stimulating factor-induced proliferation of human CD34+ hematopoietic progenitor cells. Blood. 1990;75(12):2292–2298. [PubMed] [Google Scholar]
- 22.Marty FM, Lee SJ, Fahey MM, Alyea EP, Soiffer RJ, Antin JH, et al. Infliximab use in patients with severe graft-versus-host disease and other emerging risk factors of non-Candida invasive fungal infections in allogeneic hematopoietic stem cell transplant recipients: a cohort study. Blood. 2003;102(8):2768–2776. doi: 10.1182/blood-2003-01-0267. [DOI] [PubMed] [Google Scholar]
- 23.Bongartz T, Sutton AJ, Sweeting MJ, Buchan I, Matteson EL, Montori V. Anti-TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies: systematic review and meta-analysis of rare harmful effects in randomized controlled trials. JAMA. 2006;295(19):2275–2285. doi: 10.1001/jama.295.19.2275. [DOI] [PubMed] [Google Scholar]
- 24.Bruner RJ, Farag SS. Monoclonal antibodies for the prevention and treatment of graft-versus-host disease. Semin Oncol. 2003;30(4):509–519. doi: 10.1016/s0093-7754(03)00250-1. [DOI] [PubMed] [Google Scholar]
- 25.Selby LA, Hess D, Shashidar H, de Villiers WJ, Selby LA. Crohn's disease, infliximab and idiopathic thrombocytopenic purpura. Inflamm Bowel Dis. 2004;10(5):698–700. doi: 10.1097/00054725-200409000-00033. [DOI] [PubMed] [Google Scholar]
- 26.Smith CH, Jackson K, Bashir SJ, Perez A, Chew AL, Powell AM, et al. Infliximab for severe, treatment-resistant psoriasis: a prospective, open-label study. Br J Dermatol. 2006;155(1):160–169. doi: 10.1111/j.1365-2133.2006.07316.x. [DOI] [PubMed] [Google Scholar]
- 27.Vidal F, Fontova R, Richart C. Severe neutropenia and thrombocytopenia associated with infliximab. Ann Intern Med. 2003;139(3):W–W63. doi: 10.7326/0003-4819-139-3-200308050-00021-w4. [DOI] [PubMed] [Google Scholar]