Abstract
-dependent fluid secretion by the corneal endothelium controls corneal hydration and maintains corneal transparency. Recently, it has been shown that mRNA for the cystic fibrosis transmembrane conductance regulator (CFTR) is expressed in the corneal endothelium; however, protein expression, functional localization, and a possible role in transport have not been reported. Immunoblotting for CFTR showed a single band at ~170 kDa for both freshly isolated and primary cultures of bovine corneal endothelial cells. Indirect immunofluorescence confocal microscopy indicated that CFTR locates to the apical membrane. Relative changes in apical and basolateral chloride permeability were estimated by measuring the rate of fluorescence quenching of the halide-sensitive indicator 6-methoxy-N-ethylquinolinium iodide during Cl− influx in the absence and presence of forskolin (FSK). Apical and basolateral Cl− permeability increased 10- and 3-fold, respectively, in the presence of 50 µM FSK. FSK-activated apical chloride permeability was unaffected by H2DIDs (250 µM); however, 5-nitro-2-(3-phenylpropyl-amino)-benzoic acid (NPPB; 50 µM) and glibenclamide (100 µM) inhibited activated Cl− fluxes by 45% and 30%, respectively. FSK-activated basolateral Cl− permeability was insensitive to NPPB, glibenclamide, or furosemide but was inhibited 80% by H2DIDS. permeability was estimated by measuring changes in intracellular pH in response to quickly lowering bath . FSK (50 µM) increased apical permeability by twofold, which was inhibited 42% by NPPB and 65% by glibenclamide. Basolateral permeability was unaffected by FSK. Genistein (50 µM) significantly increased apical and Cl− permeability by 1.8- and 16-fold, respectively. When 50 [µM genistein was combined with 50 µM FSK there was no further increase in Cl− permeability; however, permeability was reduced to the control level. In summary, we conclude that CFTR is present in the apical membrane of bovine corneal endothelium and could contribute to transendothelial Cl− and transport. Furthermore, there is a cAMP-activated Cl− pathway on the basolateral membrane that is not CFTR.
Keywords: cornea, endothelium, chloride permeability, MEQ, bicarbonate permeability, intracellular pH, BCECF, forskolin, cAMP, genistein
Corneal Transparency and thus good vision are dependent on the hydration of the corneal stromal connective tissue. When the stroma becomes edematous (i.e., tissue hydration >78%), there is increased light scatter from collagen fibers, which degrades the retinal image and gives the cornea a hazy appearance. The glycos-aminoglycans of the stroma exert a fluid imbibition pressure or “leak” that must be opposed by a cellular ion “pump” to control corneal hydration. Damage to the anterior corneal epithelium or posterior corneal endothelium can produce stromal edema; however, it is the endothelial cells, a thin monolayer of “leaky epithelium,” that provides most of the ion-coupled fluid transport activity or pump function for the cornea (32). Thus disorders of the corneal endothelium (e.g., Fuchs’ endothelial dystrophy) produce corneal edema and loss of vision (1).
Corneal endothelial fluid transport is dependent on the presence of both (11, 16, 36) and Cl− (50). In addition, fluid transport is slowed by stilbene derivatives (26, 50) and carbonic anhydrase inhibitors (17, 23, 36). More recently, it has been shown that bumetanide can induce corneal edema (22), indicating roles for both Cl− and transporters. and Cl− are loaded into corneal endothelial cells, to levels above electrochemical equilibrium (5), on the basolateral (stromal) side by the cotransporter (NBC-1) (45) and the Na+-K+-2Cl− cotransporter (NKCC1) (20, 22), respectively. What is less clear, however, is the mechanism for apical anion efflux.
Three possible mechanisms for secretion across the apical membrane of corneal endothelial cells have been postulated: 1) exchange, 2) CO2 efflux and conversion to by a membrane-bound carbonic anhydrase (CAIV), and 3) conductive flux via anion channels. The anion exchanger (AE2) is expressed in freshly isolated corneal endothelial cells (7, 46); however, its apical or basolateral localization has not been determined. Primary cultures of bovine corneal endothelial cells (BCEC) do not express AE2 (46) and show little if any anion exchange activity (7). Nevertheless, cultured endothelial cells can transport fluid at the same level as the complete cornea (34), indicating that anion exchange may not have a role in fluid secretion. Apical membrane CAIV has been shown to enhance apical CO2 diffusion (4); however, whether this can significantly contribute to transendothelial flux has not been demonstrated. Corneal endothelial fluid transport is stimulated by increasing cytosolic [cAMP] (12, 37). Furthermore, cAMP activates Cl− transport in cultured corneal endothelial cells (5), and recently it has been shown that mRNA for the cystic fibrosis (CF) transmembrane regulator (CFTR) is present in the corneal endothelium (46). Because CFTR has significant permeability to as well as to Cl− (8, 35, 44), it could serve as a possible apical anion efflux pathway.
In this study, we examine CFTR protein expression in fresh and cultured corneal endothelial cells. We used primary cultures of endothelial cells to determine the physical and functional localization of CFTR. We show that CFTR protein is expressed and exclusively locates to the apical membrane. This localization corresponds with forskolin (FSK)-stimulated Cl− and fluxes being inhibited by 5-nitro-2-(3-phenylpropyl-amino)-benzoic acid (NPPB) and glibenclamide only on the apical membrane.
MATERIALS AND METHODS
Cell culture
BCEC were cultured to confluence onto 13-mm Anodiscs or T-25 flasks as previously described (6, 29). Briefly, primary cultures from fresh cow eyes were established in T-25 flasks with 3 ml DMEM, 10% bovine calf serum, and antibiotic-antimycotic (100 U/ml penicillin, 100 U/ml streptomycin, and 0.25 µ]g/ml Fungizone) gassed with 5% CO2–95% air at 37°C and fed every 2–3 days. Primary cultures were subcultured to three T-25 flasks and grown to confluence in 5–7 days. The resulting second passage cultures were then further subcultured onto Anodisc membranes and allowed to reach confluence within 2 wk.
Immunoprecipitation
Fresh BCEC were scraped from dissected cow corneas that had been kept on ice for 2–3 h since death. The cell scrapings were placed into ice-cold PBS containing a protease inhibitor cocktail (Complete, BoehringerMannheim) and washed twice. Cell pellets were obtained by low-speed centrifugation and resuspended in immunoprecipitation (IP) buffer [1.0% Nonidet P-40, 150 mM NaCl, 0.1% SDS, 0.5% sodium deoxycholate, and 50 mM Tris-HCl (pH 8.0) containing a protease inhibitor cocktail]. Cultured cells in T-25 flasks were washed with PBS and dissolved directly in IP buffer. Both preparations were sonicated on ice. Sonicated samples were centrifuged at 10,000 g for 10 min at 4°C. The supernatant was transferred and then incubated for 16–18 h with a monoclonal antibody (2 µg antibody mg protein−1,ml IP buffer−1) directed against the COOH terminus of CFTR (R&D Systems; Minneapolis, MN). Immobilized protein A agarose was added to the solution during the final 2 h of incubation. The immune complexes were collected by centrifugation at 10,000 g for 15 s at 4°C and washed three times with ice-cold IP buffer (1 ml). The immune complexes were resuspended with 50 µl Laemmli sample buffer [2% SDS, 10% glycerol, 100 mM dithiothreitol, 60 mM Tris (pH 6.8), and 0.01% bromophenol blue] and heated to 80°C for 10 min before loading. After being separated by 8% SDS-PAGE, samples were transferred to a polyvinylidene fluoride membrane. The membrane was blocked with 5% nonfat dry milk for 1 h at room temperature and then probed with the anti-CFTR antibody (1:1,000) in PBS containing 5% nonfat dry milk for 1 h at room temperature with shaking. Next, the blots were washed five times for 5 min each with PBS-Tween 20, incubated with goat anti-mouse secondary antibody coupled to horseradish peroxidase (Sigma) for 1 h at room temperature, washed with PBS-Tween 20 five times for 5 min each, and then developed by enhanced chemiluminescence. Films were scanned to produce digital images that were then assembled and labeled using Microsoft Powerpoint software.
Immunofluorescence and confocal microscopy
Cultured cells grown to confluence on coverslips were washed three to four times with PBS and fixed for 30 min in PLP fixation solution [2% paraformaldehyde, 75 mM lysine, 10 mM sodium periodate, and 45 mM sodium phosphate (pH 7.4)] on a rocker. After fixation, the cells were washed three to four times with PBS. Coverslips were then kept in PBS for 20 min containing 0.01% saponin to permeablize the cell membranes and washed three times in PBS. Cells were blocked for 1 h in PBS containing 0.2% BSA, 5% goat serum, 0.01% saponin, and 50 mM NH4Cl. To aid in CFTR membrane localization, indirect double immunofluorescence staining for CFTR and ZO-1 was performed. A mixture of mouse anti-human monoclonal CFTR antibody diluted 1:10 and rat anti-ZO-1 monoclonal antibody diluted 1:100 in PBS-goat serum (1:1) was applied onto coverslips at room temperature for 1 h. Cover-slips were washed three times for 15 min in PBS containing 0.01% saponin. The mixture of secondary antibodies conjugated to Oregon green (CFTR) and Texas red (ZO-1) (1:500, Molecular Probes; Eugene, OR) was then applied for 1 h at room temperature. Coverslips were washed and mounted with Prolong anti-fade medium according to the manufacturer’s (Molecular Probes) instructions. Fluorescence was observed at X40 with a standard epifluorescence microscope equipped with a cooled charge-coupled device camera. Fluorescence of selected specimens was documented with a Bio-Rad laser scanning confocal microscope to determine membrane localization.
Microscope perfusion
For measurement of Cl− and fluxes using fluorescent dyes, cells cultured to confluence on Anodisc membranes were placed in a double-sided perfusion chamber designed for independent perfusion of the apical and basolateral sides as previously described (7). The chamber was placed on a water-jacketed (37°C) brass collar held on the stage of an inverted microscope (Nikon Diaphot) and viewed with a long working distance (1.2 mm) water immersion objective (×40, Zeiss). Apical and basolateral compartments were connected to hanging syringes containing Ringer solutions in a Plexiglas warming box (37°C) using Phar-Med tubing. The flow of the perfusate (~0.5 ml/min) was achieved by gravity. Two independent eight-way valves were employed to select the desired perfusate for the apical and basolateral chambers.
Measurement of intracellular [Cl−]
Relative intracellular [Cl−] changes in cultured BCEC were assessed with the halide-sensitive fluorescent dye 6-methoxy-N-ethylquino-linium iodide (MEQ). Corneal endothelial cells on Anodiscs were exposed to the nonfluorescent cell-permeant reduced quinoline derivative of MEQ (diH-MEQ) (3, 51), which is oxidized to MEQ within the cytoplasm. Cells were exposed to 10 µM diH-MEQ for 10 min at room temperature in Cl−-free Ringer solution and washed for 30 min with Cl−-free Ringer solution. Cellular fluorescence was measured with a microscope spot fluorimeter (DeltaRam, Photon Technology International; Monmouth Junction, NJ). Fluorescence was excited at 365 ± 10 nm and emission was collected at 420–450 nm. Synchronization of excitation with emission measurement and data collection (1 s−1) were controlled by Felix software (PTI). Relative differences in Cl− permeability between control and experimental conditions in the same cells were determined by comparing the percent change in MEQ fluorescence (F/F0) after addition of Cl− to either the apical or basolateral bath, where F0 is the fluorescence in the absence of Cl−. The maximum slope of the fluorescence change was determined by calculating the first derivative using Felix software.
Measurement of intracellular pH
BCEC cultured onto permeable Anodisc filters were loaded with the pH-sensitive fluorescent dye 2’,7’-bis(2-carboxyethyl)-5(6)-carboxyfluorescein (BCECF) by incubation in -free Ringer solution containing 1–5 µM BCECF-AM at room temperature for 30–60 min. Dye-loaded cells were then kept in -free Ringer solution for at least 30 min before use. Fluorescence was excited alternately at 495 ± 10 and 440 ± 10 nm at 1 ratio (F495/F440) s−1, and ratios were calibrated against intracellular pH (pHi) by the high K+-nigericin technique (47). Anodiscs were perfused on both sides with -rich Ringer (BR) solution. Apical or basolateral efflux was then induced by introduction of a Ringer (LB) solution. This was then repeated in the presence of agonists and inhibitors. The maximum slope of the change in pHi over time (dpHi/dt) after introduction of LB solution was determined by calculating the first derivative using Felix software. Data are expressed as means ± SE. Student’s t-test was used to determine significance (P < 0.05).
Solutions and chemicals
The composition of the BR solution used throughout this study was (in mM) 150 Na+, 4 K+, 0.6 Mg2+, 1.4 Ca2+, 118 Cl−, 1 , 10 HEPES, 28.5 , 2 gluconate−, and 5 glucose. Ringer solutions were equilibrated with 5% CO2, and pH was adjusted to 7.50 at 37°C. LB solution (2.85 mM, pH 6.5) was prepared by replacing 25.65 mM NaHCO3 with sodium gluconate. Cl−-rich, -free Ringer solution (pH 7.5) was prepared by equimolar substitution of NaHCO3 with sodium gluconate. Cl−-free Ringer solution was prepared by equimolar replacement of NaCl and KCl with sodium nitrate and potassium nitrate. In some experiments, gluconate salts were used. Osmolarity was adjusted to 295 ± 5 mosM with sucrose.
MEQ, BCECF-AM, and H2DIDS were obtained from Molecular Probes. FSK and genistein were obtained from LC Laboratories (Woburn, MA). Glibenclamide and NPPB were from Sigma (St. Louis, MO).
RESULTS
IP and indirect immunofluorescence
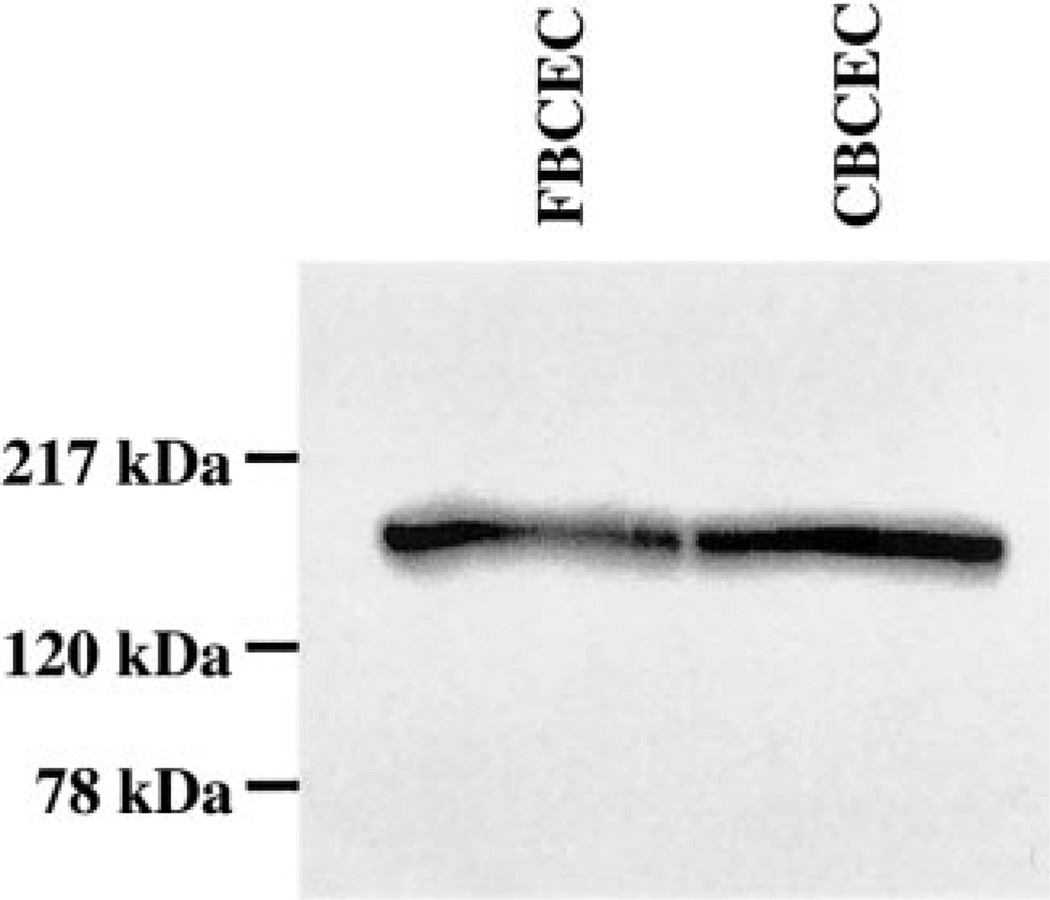
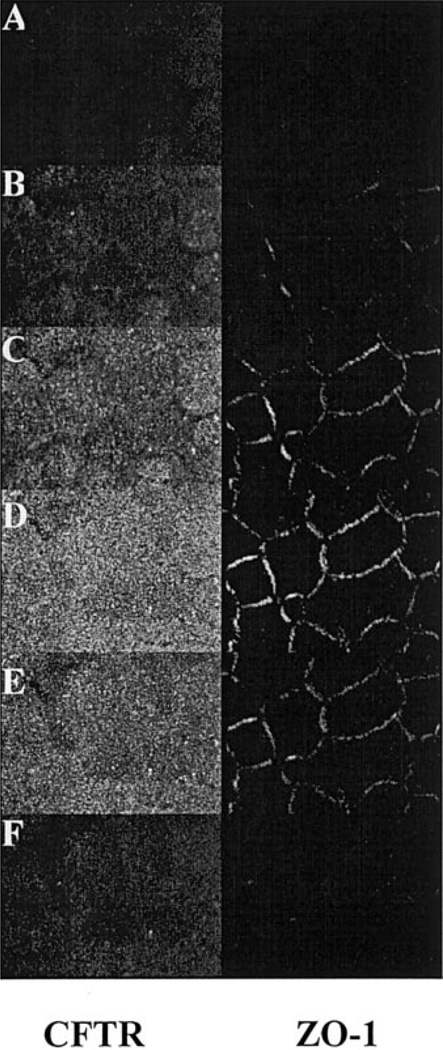
To demonstrate the expression of CFTR protein in bovine corneal endothelium, CFTR was immunoprecipitated from cultured and fresh BCEC lysates with mouse anti-human CFTR antibody. Figure 1 shows that the CFTR antibody produced strong positive bands for both cultured and fresh corneal endothelium at −170 kDa, which is the expected range for mature CFTR (30). Further evidence for the expression of CFTR in BCEC is provided by indirect immunofluorescence confocal micrographs, as shown in Fig. 2. Cultured BCEC were stained for both CFTR and the tight junction protein ZO-1. CFTR fluorescence was apparent just apical to ZO-1 and at the same level as ZO-1, but not basolateral to ZO-1. This result indicates that CFTR is predominately located at the apical membrane of BCEC.
Fig. 1.
CFTR immunoprecipitation analysis of cultured (CBCEC) and fresh bovine corneal endothelial cells (FBCEC). CFTR was precipitated by mouse anti-human CFTR monoclonal antibody and protein A-linked agarose beads from whole cell extracts. The immu-noprecipitates were separated by polyacrylamide gel electrophoresis, transferred to polyvinylidene fluoride membranes, and then probed with an antibody to CFTR.
Fig. 2.
Laser scanning confocal immunofluorescence microscopy of ZO-1 (Texas red-linked secondary antibody) and CFTR-stained (Oregon green-linked secondary antibody) cultured bovine corneal endothelium. The 6 images are sequential from the most basolateral section (A) to the most apical (F). The z-axis separation between images is 0.5 µm. Sections more basal to F did not contain fluorescence.
cAMP increases apical and basolateral Cl− permeability
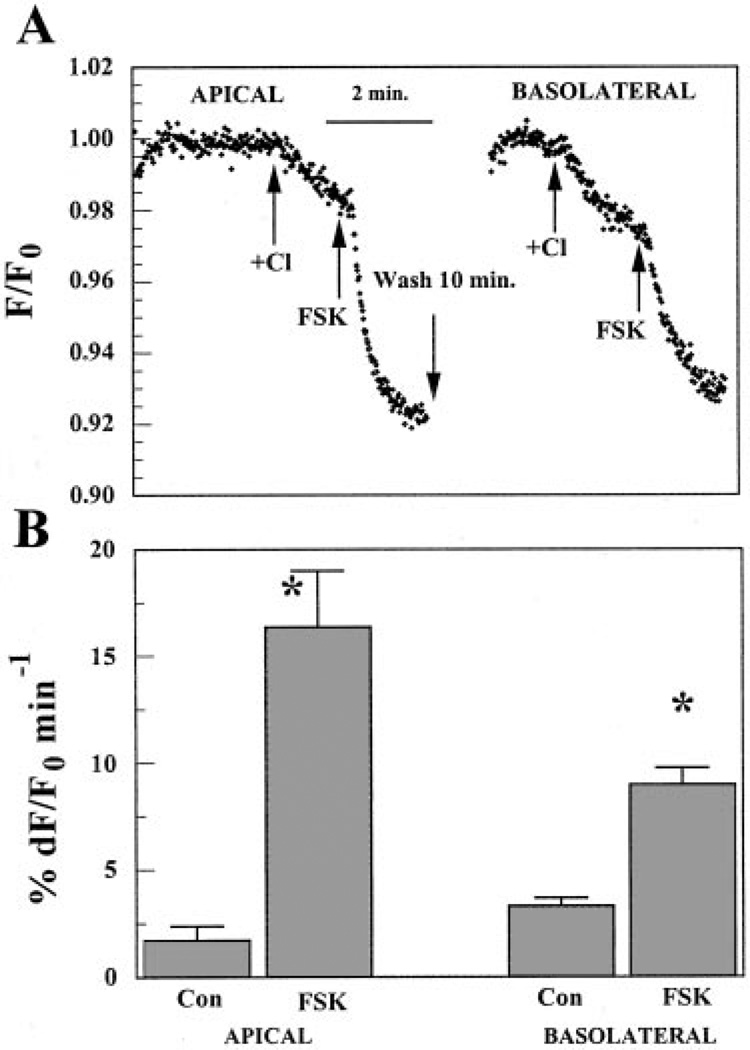
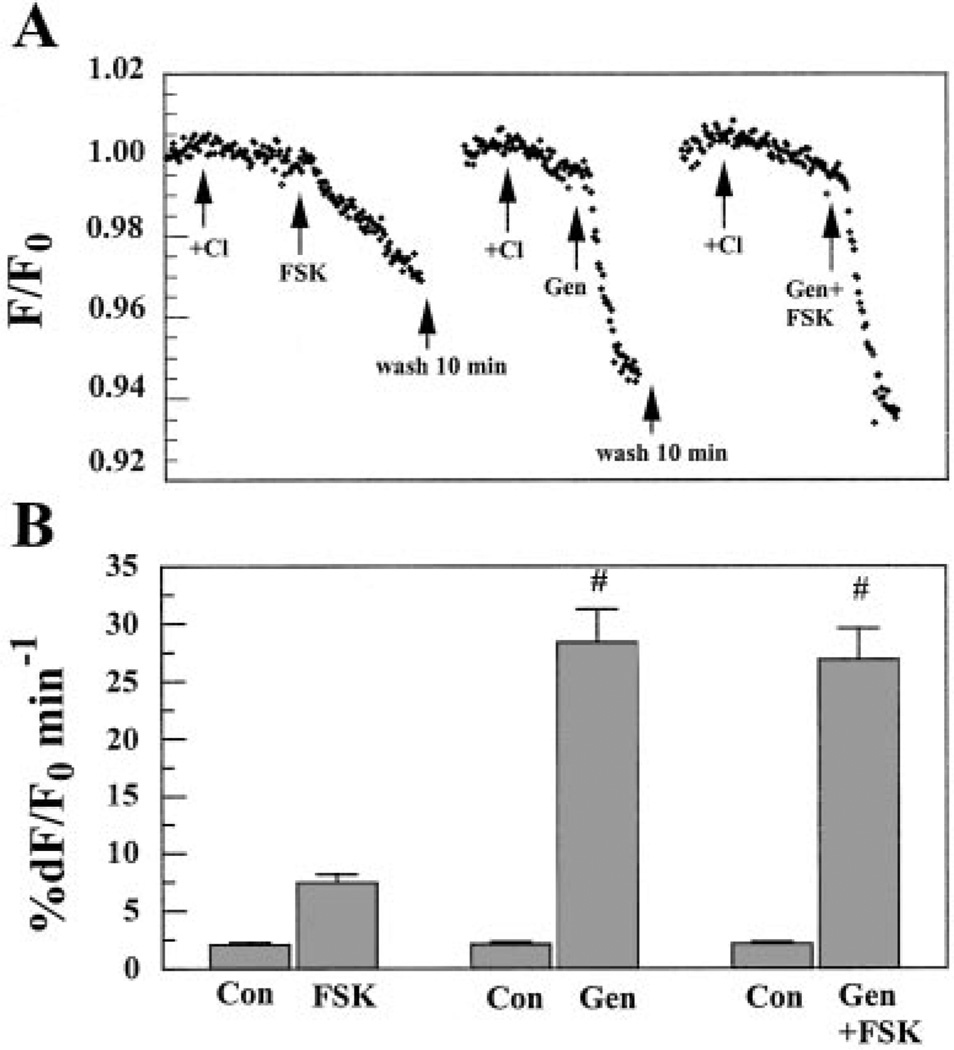
If CFTR contributes to Cl− permeability and is localized to the apical membrane in BCEC, then increasing cellular cAMP should enhance apical Cl− permeability. This was tested by measuring the relative change in MEQ fluorescence quenching due to Cl− influx in the absence and then presence of FSK (Fig. 3). Both apical and basolateral sides were initially perfused with Cl−-free (nitrate substituted) Ringer solution. When Cl− was added to the apical side for 90 s, Cl− entry caused a small slow decrease in MEQ fluorescence (1.6% min−1). Immediately after this 90-s exposure to Cl−, 50 µMFSK was introduced in the continued presence of Cl− for ~1 min. As shown in Fig. 3, this dramatically accelerated the decrease in MEQ fluorescence by ~ 11-fold (16.3% min−1) relative to the control, indicating that cAMP produced a significant increase in apical Cl− permeability. The same procedure was then performed on the basolateral side to test whether cAMP could enhance basolateral Cl− permeability. After a 10-min wash with Cl−-free Ringer solution on the apical side, Cl− was introduced for 90 s on the basolateral side. This caused a relatively faster and larger decrease in MEQ fluorescence (3.3% min−1) than on the apical side. This is consistent with previous studies and is contributed primarily by the basolateral Na+-K+-2Cl− cotransporter (NKCC1) (20, 22). The application of 50 µM FSK also accelerated the decrease in MEQ fluorescence during basolateral Cl− influx by 2.7-fold (9% min−1) relative to the controls. Similar results were obtained if the sequence of Cl− addition/ FSK exposure was first basolateral and then followed by apical, indicating that the ~15-min wash between FSK pulses was sufficient time to reduce cAMP to control levels. Figure 3B summarizes the results, indicating that apical and basolateral Cl− permeability was increased by FSK ~10- and 3-fold, respectively.
Fig. 3.
Changes in 6-methoxy-N-ethylquinolinium iodide (MEQ) fluorescence (F/F0) after apical or basolateral Cl− addition in the absence and presence of forskolin (FSK). BCEC were cultured to confluence on Anodisc permeable membranes. They were loaded with MEQ in the absence of Cl− (nitrate substituted), washed, and placed in a microscope-fluorimeter two-sided perfusion chamber. A: both apical and basolateral compartments were initially perfused with Cl−-free (nitrate substituted) Ringer solution. Arrows indicate when Cl−-rich Ringer solution and 50 µM FSK were applied from the apical or basolateral side. B: percent change in maximum F/F0 (%dF/F0) per minute. Values are means ± SE. *Significantly different from controls (Con; n = 6, P < 0.05).
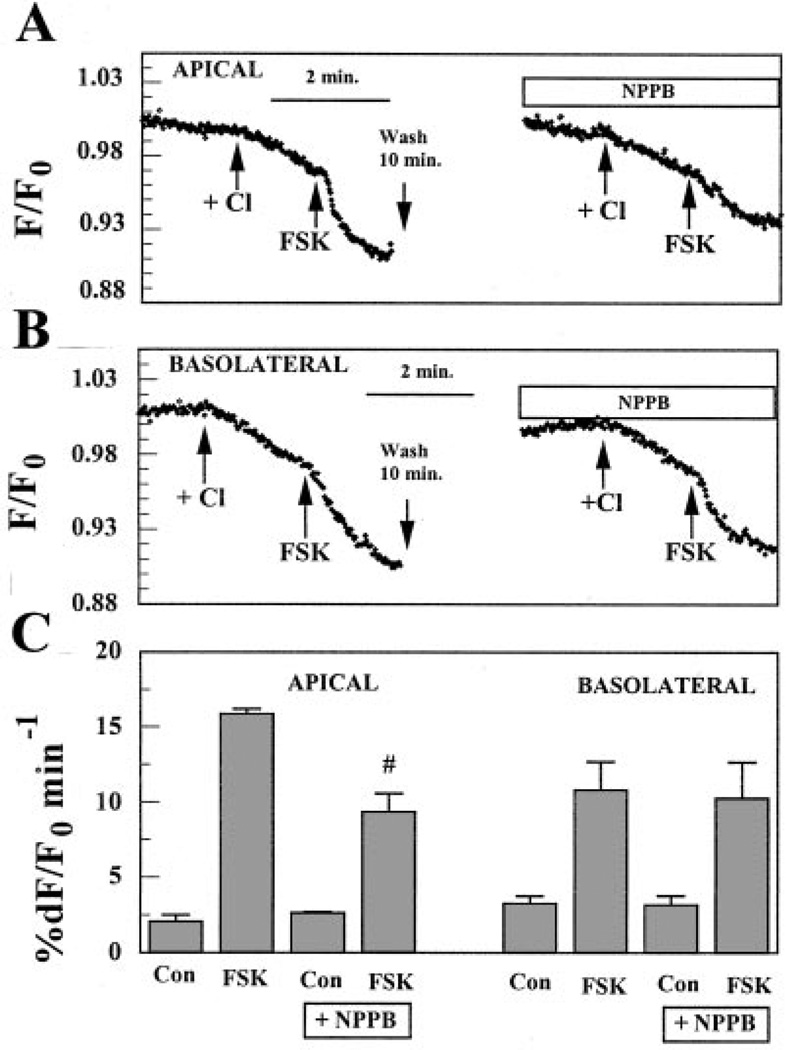
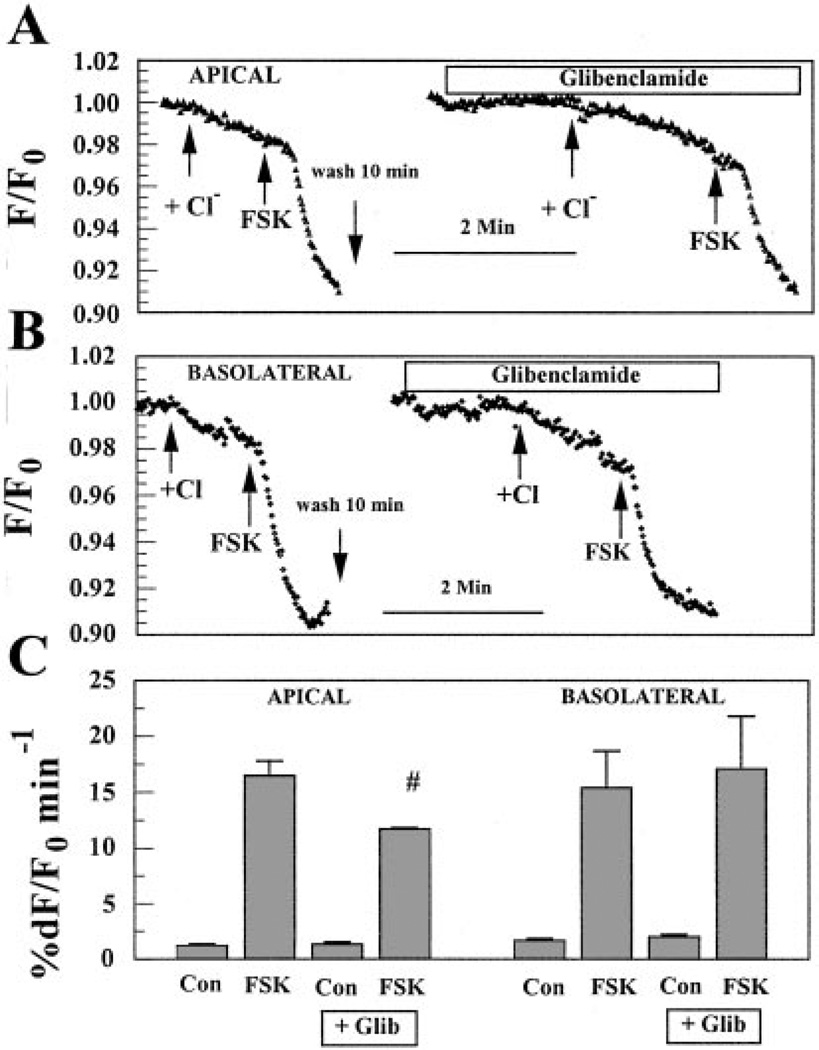
If the changes in Cl− permeability induced by FSK on the apical and basolateral sides were contributed by activation of CFTR, then the activated Cl− fluxes should be sensitive to the Cl− channel inhibitors NPPB and glibenclamide, which are known (in some systems) to inhibit CFTR, but should not be inhibited by DIDS. We found that H2DIDS (200 µM) had no effect on the FSK-activated apical Cl− flux but did reduce FSK-activated basolateral flux by 80 ± 20% (n = 4, data not shown). Conversely, Fig. 4A shows that when Cl− was added on the apical side in the presence of 50 µM FSK and 50 µM NPPB, the rate of MEQ fluorescence quenching was reduced by ~45% relative to FSK alone. On the other hand, Fig. 4b shows that when Cl− was introduced on the basolateral side in the presence of 50 µM FSK and 50 µM NPPB, the acceleration in the decrease of MEQ fluorescence caused by FSK did not change. These results are summarized in Fig. 4C, which shows that NPPB significantly reduced FSK-activated Cl− permeability on the apical side but not on the basolateral side.
Fig. 4.
Effects of 5-nitro-2-(3-phenylpropyl-amino)benzoic acid (NPPB) on FSK-activated Cl− flux on the apical or basolateral side. A: effect of NPPB on the apical side. Both apical and basolateral compartments were initially perfused with Cl−-free (nitrate substituted) Ringer solution as in Fig. 3. Arrows indicate the applications of Cl−-rich Ringer solution and 50 µM FSK on the apical side. Before the addition of NPPB, there was a 10-min wash with Cl−-free Ringer solution on the apical side. B: effect of NPPB on the basolateral side. C: maximum %dF/F0 per minute. Values are means ± SE. #Significantly less than FSK alone (n = 5, P < 0.05).
Figure 5A shows the effect of glibenclamide on FSK-activated Cl− flux at the apical side. When Cl− was applied on the apical side in the presence of 50 µM FSK together with 100 µM glibenclamide, the rate of MEQ fluorescence quenching was reduced by ~30% relative to FSK alone. On the other hand, Figure. 5B shows that on the basolateral side the addition of glibenclamide did not cause any inhibition in the rate of fluorescence change induced by FSK. These results are summarized in Fig. 5C, which shows that glibenclamide, like NPPB, inhibited FSK-activated Cl− flux only on the apical side.
Fig. 5.
Effects of glibenclamide (Glib) on FSK-activated Cl− flux on the apical or basolateral side. A: effect of Glib on the apical side. Both apical and basolateral compartments were initially perfused with Cl−-free (nitrate substituted) Ringer solution as in Figs. 3 and 4. Arrows indicate Cl− introduction and FSK addition. Before the addition of Glib, there was a 10-min wash with Cl−-free Ringer solution on the apical side.B: effect of Glib on the basolateral side. C: maximum %dF/F0 per minute. Values are means ± SE. #Significantly less than FSK alone (n = 8, P < 0.05).
The Na+-K+-2Cl− cotransporter (NKCC1) provides significant basolateral Cl− permeability in BCEC (20, 22). In some systems, NKCC1 can be activated by cAMP (10, 13, 15, 27, 31, 41), so we tested if FSK-activated basolateral Cl− permeability could be provided by NKCC1. We found that 100 µM furosemide (bumetanide was not used due to its fluorescence at 360 nm), which strongly inhibits basolateral Na+-K+-2Cl− cotransport in endothelial cells (20), had no effect on the basolateral FSK-activated Cl− flux (n = 4, data not shown).
In summary, FSK-activated apical Cl− permeability was inhibited by NPPB and glibenclamide but not H2DIDS, consistent with CFTR having an apical location. On the other hand, FSK-activated basolateral Cl− permeability was inhibited by H2DIDS but not NPPB, glibenclamide, or furosemide, indicating that CFTR is not on the basolateral membrane and that an unidentified cAMP-activated, DIDS-sensitive Cl− permeability is present on the basolateral membrane.
cAMP increases apical but not basolateral permeability
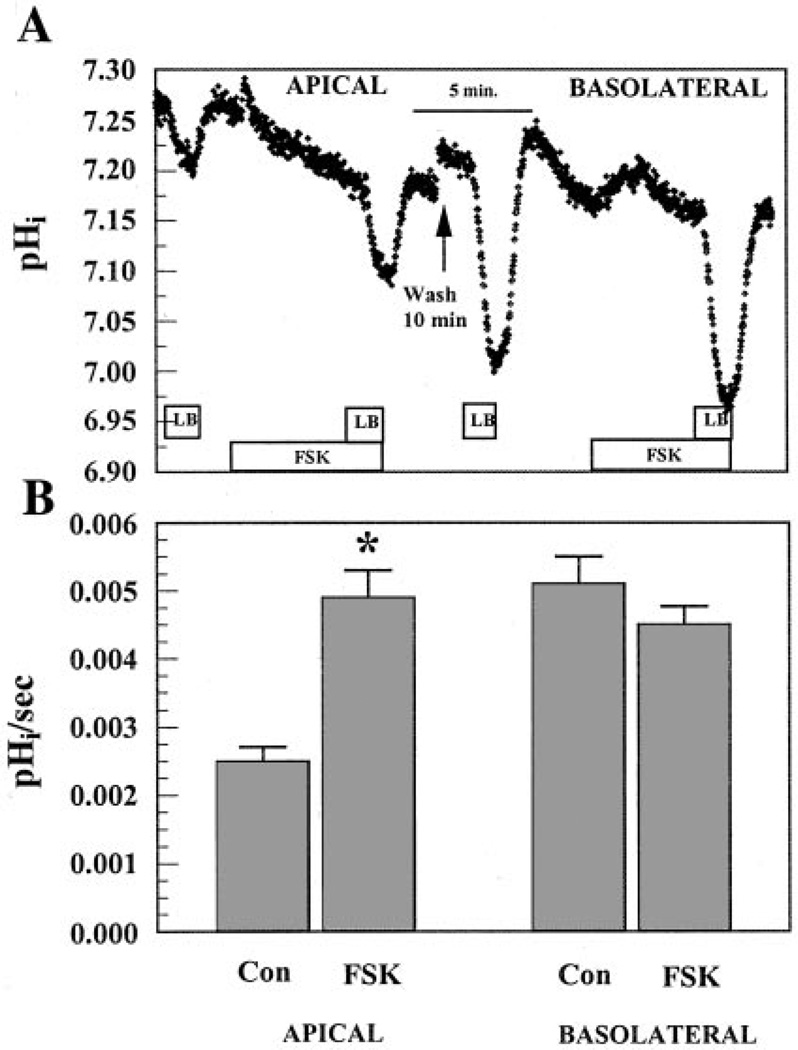
Previous investigations have shown that CFTR is permeable to (8, 35, 44). Because transport is important for fluid transport in BCEC, we examined whether CFTR could enhance permeability. A constant-CO2 protocol described previously (4) was used to examine apical and basolateral permeability of cultured corneal endothelial cells. Perfusate [] concentration was reduced from 28.5 mM at pH 7.5 (BR solution) to 2.85 mM at pH 6.5 (LB solution) while both apical and basolateral solutions were continually gassed with 5% CO2. The decreased [] and lower pH of the bath can both contribute to a drop in pHi. H+ fluxes, however, were only 18% of the initial dpHi/dt (4) and were unaffected by FSK (data not shown). Figure 6 shows that there was a small drop in pHi when LB solution was introduced on the apical side. After cells were returned to BR solution perfusion on both sides, introduction of FSK caused a small but rapid alkalinization followed by a new steady-state pHi that was slightly below the baseline. In the continued presence of FSK for 5 min, introduction of LB solution caused a faster and deeper acidification than control. Figure 6B summarizes the results from 14 experiments and shows that apical permeability was increased approximately twofold by FSK. After a 10-min wash on the apical side with BR solution, the basolateral side was then exposed to LB solution (Fig. 6A). Because of the presence of the cotransporter on the basolateral side (45), there was a much greater acidification than on the apical side. Cells were returned to BR solution and FSK was then introduced for 5 min, followed by LB solution on the basolateral side. The rate of acidification was unaffected by FSK. Again, reversing the order of LB solution exposure to basolateral followed by apical produced similar results. Figure 6B summarizes these results and indicates that FSK caused a small but statistically insignificant decrease in permeability on the basolateral side. Taken together, these results indicate that apical, but not basolateral, [] permeability is increased by cAMP.
Fig. 6.
Effects of FSK on apical and basolateral fluxes. A: both apical and basolateral compartments were initially perfused with Ringer (BR; 28.5 mM) solution. Boxes indicate when BR was changed to low bicarbonate (LB; 2.8 mM) solution on the apical or basolateral side. FSK (50 µ.M) was preincubated 5 min in BR solution before the LB solution pulse. The break in the data indicates a 10-min wash with BR solution on the apical side. B: maximum change in intracellular pH (pHi) per second. Values are means ± SE. *Significantly different from the control (apical: n = 14, P < 0.05; basolateral: n = 5).
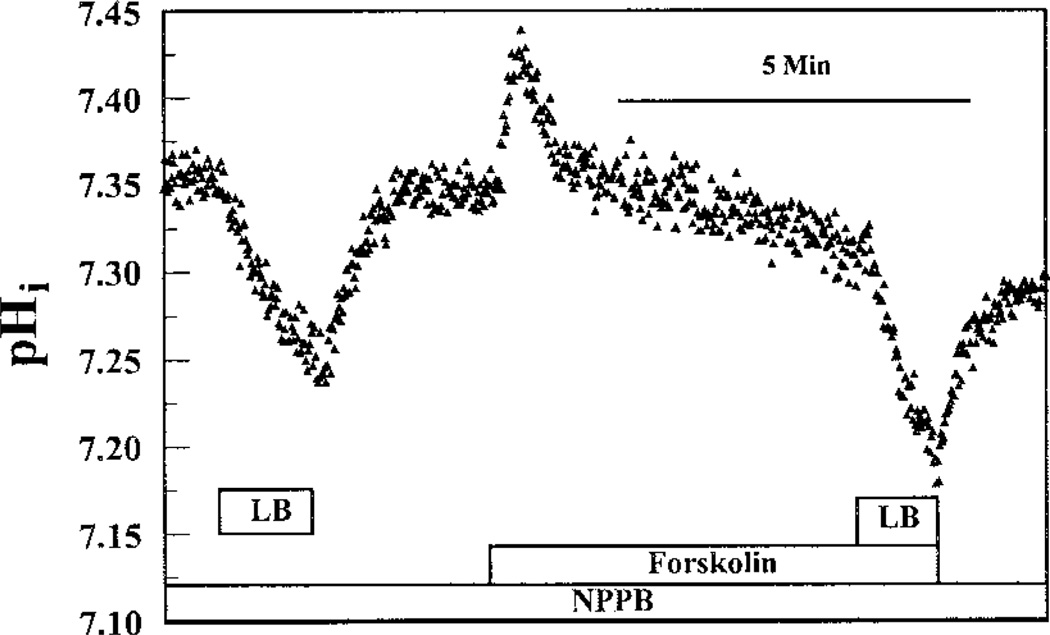
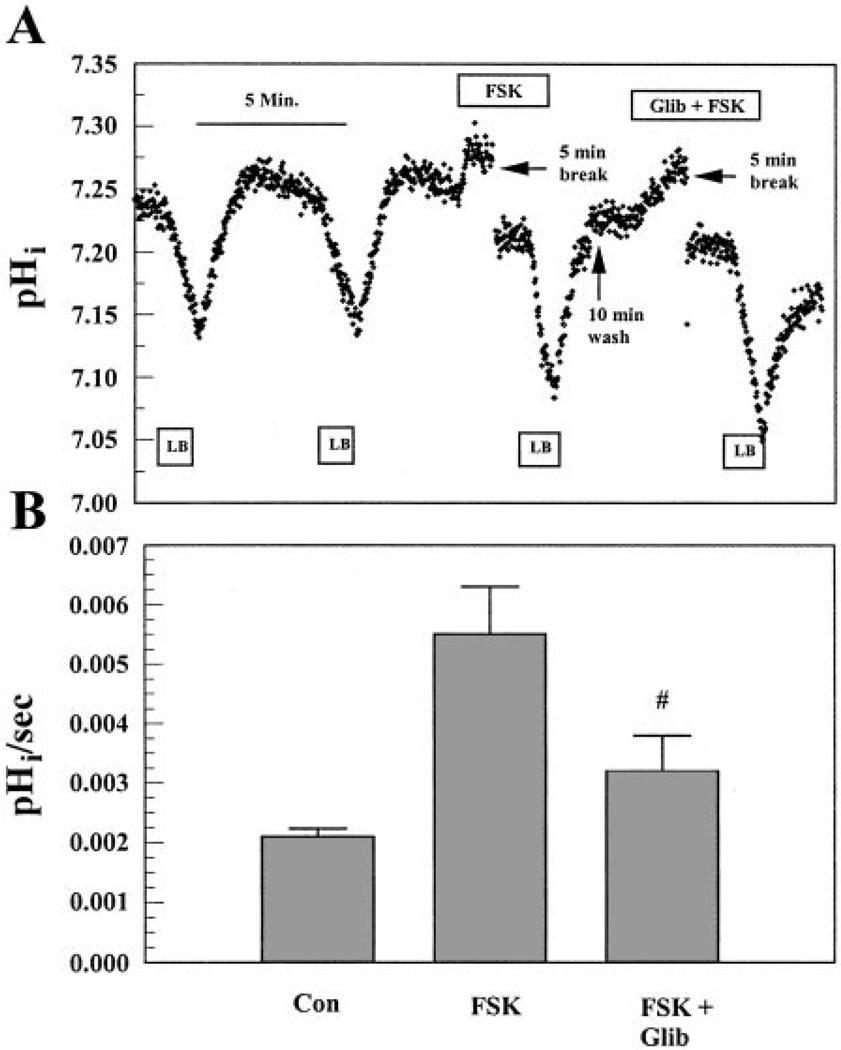
To further confirm that FSK-activated apical permeability is caused by CFTR, the Cl− channel blockers NPPB and glibenclamide were applied in the presence of FSK. Figure 7 shows the effect of 50 µM NPPB on FSK-activated flux on the apical side. Because NPPB can partially absorb BCECF fluorescence excitation at 440 nm, which induces an increase in F495/F440, paired experiments were not possible. Therefore, we applied 50 µM NPPB during the entire experiment. Figure 7 shows that FSK in the presence of 50 µM NPPB only increased apical flux by ~38%. This represents a 59 ± 15% (n = 6, P < 0.05) inhibition of FSK-induced apical flux by NPPB relative to FSK alone. Figure 8A shows the inhibition of FSK-induced flux on the apical side by glibenclamide. After a 5-min preincubation with 50 µM FSK, LB solution was introduced on the apical side, which caused a rapid acidification of 1.6-fold greater than controls. After cells were returned to BR solution on both sides, 100 µM glibenclamide + FSK was added to the apical perfusate and preincubated for 10 min. LB solution was then introduced in the continued presence of FSK and glibenclamide, which produced a 52% decrease in permeability relative to FSK alone. These results are summarized in Fig. 8B and suggest that FSK-activated apical permeability was caused, at least partially, by the activation of apical CFTR.
Fig. 7.
Effect of NPPB on FSK-induced apical flux. Both apical and basolateral compartments were initially perfused with BR solution. NPPB (50 µM) was applied on the apical side in the entire experiment. LB solution was first pulsed on the apical side in the absence of FSK. After the reintroduction of BR solution, 50 µM FSK was applied and preincubated for 5 min. LB solution was then pulsed in the presence of FSK.
Fig. 8.
Effect of Glib on FSK-induced apical flux. A: both apical and basolateral compartments were initially perfused with BR solution. LB solution was introduced on the apical side and repeated. After the reintroduction of BR solution, 50 µM FSK was applied and preincubated for 5 min. LB solution with FSK was then introduced. After a 10-min wash with BR solution, cells were preincubated with 100 µM Glib together with 50 µM FSK for 5 min in BR solution followed by the LB solution pulse. B: maximum change in pHi per second. Values are means ± SE. #Significantly different from FSK alone (n = 7, P < 0.05).
Genistein increases apical Cl− and permeability
Recently, many reports have demonstrated that the isoflavone genistein stimulates CFTR Cl− channel activity (2, 14, 18, 19, 28, 40, 49). Here, we investigated the effect of 50 µM genistein on Cl− and fluxes in BCEC. Figure 9 shows the effect of genistein on apical Cl− permeability. Both sides were initially perfused with Cl−-free Ringer solution. When Cl− was added on the apical side, there was a small drop in MEQ fluorescence that was strongly accelerated by the addition of FSK. After a 10-min wash with Cl−-free Ringer solution on the apical side, Cl− was added again to the apical side, inducing a small drop in MEQ fluorescence. In the continued presence of Cl−, addition of 50 |jiM genistein produced a sharp decrease in MEQ fluorescence, consistent with genistein activating CFTR. When genistein was combined with FSK and added to the apical side, there was no further increase in Cl− permeability over genistein alone. Figure 9B shows that 50 µM genistein significantly increased apical Cl− permeability by 16-fold. Interestingly, Figure 9B also shows that in these paired experiments the genistein-induced increase in Cl− permeability was over fourfold stronger than that of FSK alone, which is consistent with previous reports of genistein activation of CFTR (40).
Fig. 9.
Effect of genistein (Gen) on apical Cl− flux as measured by MEQ fluorescence (F/F0). Both sides were initially perfused with Cl−-free (nitrate substituted) Ringer solution. Arrows indicate the applications of Cl−-rich Ringer solution, 50 µM FSK, and 50 µM Gen as well as the combination of FSK and Gen on the apical side from the same Anodisc culture. B: maximum %dF/F0 per minute. Values are means ± SE. #Significantly different from FSK alone (n= 6, P< 0.05).
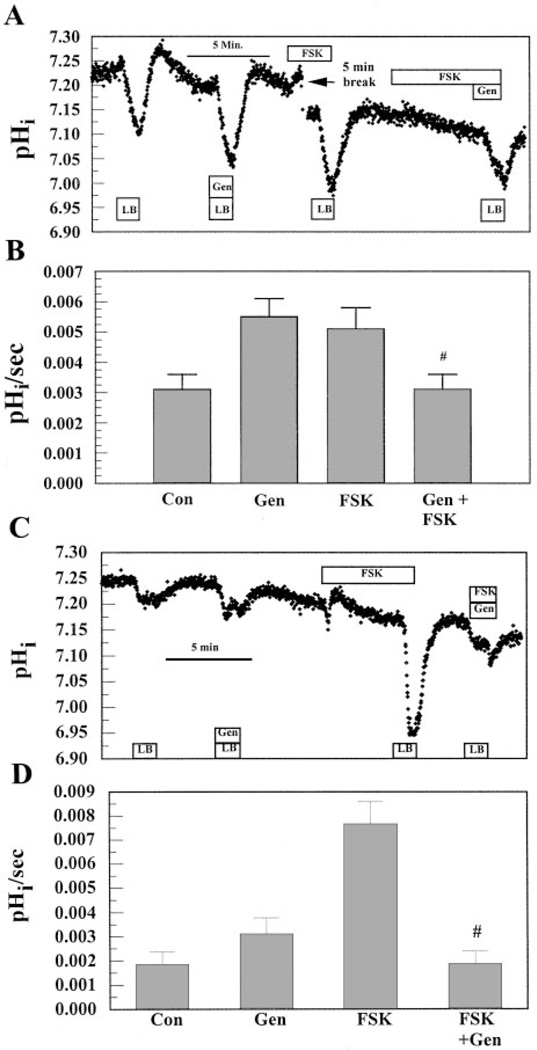
Figure 10 shows the effect of genistein on apical flux under the constant-CO2 protocol. Both sides were initially perfused with BR solution. When the apical side was perfused with LB solution in the presence of 50 µM genistein, acidification was significantly faster relative to controls. In these same cells, LB solution perfusion induced an acidification in the presence of 50 µM FSK that was approximately the same as that with genistein. Figure 10A also shows that, surprisingly, when 50 µM genistein was combined with 50 µM FSK, flux was reduced to control levels. These results are summarized in Fig. 10B, which shows that genistein alone can increase permeability to the same extent as FSK but that there is a negative synergistic effect on permeability by the two drugs.
Fig. 10.
Effect of Gen on apical flux. A: both apical and basolateral compartments were initially perfused with BR solution. LB solution was then pulsed on the apical side. After the reintroduc-tion of BR solution, LB solution was pulsed in the presence of 50 µM Gen. After a 10-min wash, cells were preincubated with 50 µM FSK for 5 min before the LB solution pulse. Finally, FSK was preincubated for 5 min, followed by 50 µM Gen in LB solution. B: maximum change in pHi per second. Values are means ± SE. #Significantly less than FSK or Gen alone (n = 7, P < 0.05). C: same experiment as A except in Cl−-free (gluconate substituted) Ringer solution and no preincubation with FSK just before the last LB solution pulse with FSK and Gen combined. D: maximum change in pHi per second. Values are means ± SE. #Significantly less than FSK or Gen alone (n = 9, P< 0.05)
Phosphorylation of CFTR can increase activation by flavonoids such as genistein (18). However, at concentrations exceeding 50 µM flavonoids, prephosphorylation can be inhibitory. Considering the possibility that the 5-min preexposure to FSK could sensitize CFTR to being inhibited by genistein, the experiment was repeated except that FSK, genistein, and LB solution were introduced simultaneously, (i.e., no preexposure to FSK, which is the same protocol as Cl− flux experiments shown in Fig. 3–Fig. 5 and Fig. 9). The result, however, was the same; permeability in the combined presence of FSK and genistein was reduced to control levels even though there was no preexposure to FSK (n = 8, data not shown). Finally, we considered the possibility that this negative drug interaction on permeability may be influenced by the presence of Cl− because Cl− permeability is increased to such a large extent by genistein. To test this possibility, the experiment was repeated in the absence of Cl− (gluconate substituted). Figure 9C shows that again when FSK and genistein were added together, permeability was reduced to control levels. Figure 9D summarizes these experiments. In the absence of Cl−, genistein alone increased permeability by 1.67-fold, which is not significantly different than in the presence of Cl− (1.77-fold; Fig. 9B). On the other hand, in the absence of Cl−, FSK increased permeability 4.2-fold compared with only 1.7-fold in the presence of Cl−. Presumably, this is due to removal of a competitive inhibitor. Nevertheless, the absence of Cl− had no effect on the inhibitory effect of the FSK-genistein combination.
DISCUSSION
CFTR is expressed on the apical membrane of bovine corneal endothelium
Previously, we have shown by RT-PCR analysis that CFTR mRNA is present in corneal endothelial cells (46). Immunoblotting (Fig. 1) confirmed the expression of CFTR protein in both fresh and cultured corneal endothelial cells. Indirect immunofluorescence confocal images (Fig. 2) further confirmed the expression of CFTR and showed that CFTR is at the same level as ZO-1 and not basolateral to ZO-1, indicating an exclusively apical localization.
FSK-activated CFTR increases apical Cl− and permeability
Functional analysis from a wide variety of cell types has demonstrated that CFTR can be activated by at least two different mechanisms. One is a cAMP-dependent activation of protein kinase A, leading to the phosphorylation of the R domain of CFTR and activation of the channel (19, 24). Another is a cAMP-independent mechanism, which is mediated by the addition of such channel openers as genistein (a specific inhibitor of protein tyrosine kinases), which directly interact with CFTR, activating and prolonging the open channel conformation (14, 28, 40).
We examined FSK-activated Cl− permeability across apical and basolateral membranes. Interestingly, FSK increased Cl− permeability on both sides; however, the augmentation on the basolateral side (~3-fold) is significantly lower than that on the apical side (~ 10-fold; Fig. 3). FSK-stimulated apical Cl− fluxes were inhibited by NPPB and glibenclamide (Fig. 4 and Fig. 5), whereas H2DIDS had no effect, consistent with an apically located CFTR. The negative effect of H2DIDS on stimulated apical Cl− flux also suggests that the enhanced Cl− flux is not via the DIDS-sensitive outwardly rectifying Cl− channels, which can be secondarily stimulated by activated CFTR (9, 21, 39). FSK-activated basolateral Cl− permeability was unaffected by NPPB or glibenclamide but significantly inhibited by H2DIDS, consistent with CFTR not being present on the basolateral membrane. FSK-activated basolateral Cl− permeability may be caused by NKCC1 because it has been reported that NKCC1 can be activated by cAMP (10, 13, 15, 27, 31, 41). Furosemide, however, had no effect on the FSK-activated basolateral Cl− flux. This result together with the H2DIDS sensitivity on the basolateral side indicates that NKCC1 is unlikely to be involved in FSK-activated basolateral Cl− permeability. These data are similar to those found in airway epithelium, which showed a basolateral cAMP activated inwardly rectifying Cl− channel that is not CFTR (48). Further studies are needed to fully characterize this basolateral cAMP-dependent Cl− flux in corneal endothelial cells.
Because corneal endothelial fluid transport is dependent and CFTR is permeable to both and Cl− in a variety of cells (35, 42, 52), we examined the effect of cAMP on apical and basolateral permeability. We found that FSK-stimulated flux was only present on the apical side and that this was inhibited by NPPB and glibenclamide, consistent with the flux being facilitated by CFTR. The increased permeability shown here is not due to an anion exchanger because anion exchange could not be demonstrated in cultured corneal endothelium (7) and apical efflux is unchanged in Cl−-free, gluconate-substituted solutions (4).
FSK did not enhance basolateral efflux on exposure to LB solution, suggesting that the cotransporter, which is the major flux pathway on the basolateral side (4), is not directly stimulated by cAMP. In fact, there was a small but not statistically significant decrease in basolateral efflux in the presence of FSK. This may be due to the slightly lower baseline pHi caused by FSK (i.e., lower intracellular [] and slightly reduced driving force for efflux). On the other hand, in the presence of BR solution on both sides, FSK alone produced a transient alkalinization quickly followed by acidification to below resting pHi (see Fig. 6). The initial alkalinization may be explained by the membrane potential (Em) depolarization produced by FSK in BCEC (5), which would increase influx via basolateral cotransport. We speculate that the initial burst of influx is then offset by increasing apical efflux, leading to a new lower steady-state pHi. These findings suggest that cAMP can indirectly stimulate via Em depolarization from activation of apical CFTR (and possibly basolateral cAMP-dependent channels), as suggested for the pancreatic duct (43).
Genistein-stimulated Cl− and permeability
Further evidence for the expression and functionality of CFTR in corneal endothelial cells is the potent stimulation of Cl− and fluxes by genistein (Fig. 9 and Fig. 10). Genistein (50 µM) alone produced a 16-fold increase in Cl− permeability relative to control. This stimulation was unaffected by addition of FSK, indicating that CFTR had been maximally stimulated. Genistein alone also increased apical permeability. However, most interestingly, the combination of genistein and FSK (50 µM each) completely abolished any increase in apical permeability. That this block occurred for and not Cl− permeability is puzzling. The protocols were slightly different in that for Cl− flux FSK and genistein were added acutely, whereas for flux cells were preincubated for 5 min with FSK. Earlier studies (18) have shown that phosphorylated CFTR is more easily activated by flavonoids, including genistein. However, high concentrations of flavonoids (>50 µM) can inhibit phosphorylated CFTR. Thus we tested whether the preincubation with FSK may contribute to the inhibitory effect of genistein. Without preincubation, the FSK-genistein combination still reduced permeability to control levels (Fig. 10). Finally, we considered the possibility that this negative drug interaction on permeability may be influenced by the presence of a competitive anion. Cl− flux experiments were performed in the absence of , and there was no inhibition by the FSK-genistein combination (Fig. 9). Therefore, we repeated the flux experiments in the absence of Cl−. Activation of permeability by genistein alone was unaffected; however, FSK-activated permeability was significantly increased (Fig. 10), consistent with the removal of a competitive inhibitor. Nevertheless, the FSK-genistein combination still reduced permeability to control levels. Thus we conclude that the FSK-genistein combination can inhibit CFTR, consistent with earlier findings(18), but that the sensitivity to this combination may be anion dependent.
Physiological implications
Increased cAMP levels stimulate rabbit corneal endothelial fluid secretion (12, 37). Thus an apical CFTR could play a significant role in stimulated ion-coupled fluid transport. In unstimulated cells, apical Cl− and permeability are approximately two and three times lower than basolateral permeability, respectively (4, 20). Thus the rate-limiting step in transendothelial anion transport is at the apical membrane. Basolateral Cl− and permeability are predominantly due to the Na+-K+-2Cl− cotransporter and the cotransporter (45, 46), respectively. Here, we show that FSK stimulation increases both apical and basolateral Cl− permeability so that apical is now twice basolateral permeability. FSK stimulation also increases apical permeability but not basolateral, so that apical and basolateral permeability become essentially equal. These results suggest that cAMP enhanced fluid transport in corneal endothelium could be due directly or indirectly to activation of CFTR.
The relative contributions of Cl− and fluxes to baseline (i.e., unstimulated) endothelial fluid transport are not precisely known. Tracer flux experiments and electrophysiology are hampered by the extreme leakiness of the fresh or cultured corneal endothelial preparation (R = 25 Ω cm−2, transendothelial potential = −0.5 mV). Nevertheless, we do know that both Cl− (50) and (11, 17, 23, 36) are needed to maintain corneal hydration. Removal of , application of DIDS, or carbonic anhydrase inhibitors (36) prevent dehydration of edematous corneas, whereas in the presence of bumetanide corneal hydration can return to normal (38, 50). Previously, it has been shown that bumetanide has no effect on normally hydrated corneas (38); however, this conflicts with a recent report indicating that bumetanide can cause a small amount of swelling (~6%) of normally hydrated corneas (22). Furthermore, furosemide has no effect on intracellular [Cl−] (20) unless cells are stimulated by cAMP (5). On balance, these findings do not favor a strong role for Na+-K+-2Cl− cotransport; however, they do not exclude a direct contribution of Cl− flux to corneal endothelial fluid transport. Cl− fluxes could also contribute indirectly to supporting transport. For example, conductive efflux of Cl−, either in the resting or cAMP stimulated condition, will depolarize Em, thereby dissipating the hyperpolarizing effects of the basolateral cotransporter and thus promoting continuous uptake of . In fact, we have shown that flux through the cotransporter is slowed in gluconate-substituted Cl−-free solutions (4). At the apical membrane, either anion has the potential to contribute to net transendothelial flux; however, the greater sensitivity of fluid transport to transport inhibitors favors a role for over Cl−. Interestingly, bicarbonate-activated adenylyl cyclase, which is not activated by FSK, can increase [cAMP] in BCEC by 56% compared with that in the absence of (33). Thus it is conceivable that CFTR has a role in baseline fluid transport as well.
In general, CF patients do not have visual or ocular problems. Corneal transparency and function appear to be normal. One study (25) has shown that CF patients have slightly thicker corneas, ~4% greater than normal. This may represent a small level of edema, but it is not enough to effect corneal transparency. Interestingly, there are corneal endothelial morphological differences between normal and CF patients. CF patients have a significantly higher cell density (18%) than normal patients (25). This may be a sign of compensation for the CFTR defect. For the same surface area of the cornea, an endothelial layer with higher cell density will have greater lateral membrane surface area. Thus the lateral membrane may have a greater concentration of Na+-K+-ATPase or co-transporters. It is not clear how this could compensate for an apical anion channel defect, but it indicates that the endothelial cells have responded to the defect. To determine whether the morphological change is related to transport function will require further investigation.
Acknowledgments
This study was supported by National Institutes of Health Grant EY-08834.
REFERENCES
- 1.Adamis A, Filatov V, Tripathi B, Tripathi R. Fuchs’ endothelial dystrophy of the cornea. Surv Ophthalmol. 1993;38:149–168. doi: 10.1016/0039-6257(93)90099-s. [DOI] [PubMed] [Google Scholar]
- 2.Al-Nakkash L, Hu S, Li M, Hwang T-C. A common mechanism for cystic fibrosis transmembrane conductance regulator protein activation by genistein and benzimidazolone analogs. J Pharmacol Exp Ther. 2001;296:464–472. [PubMed] [Google Scholar]
- 3.Biwersi J, Verkman A. Cell-permeable fluorescent indicator for cytosolic chloride. Biochemistry. 1991;30:7879–7883. doi: 10.1021/bi00246a001. [DOI] [PubMed] [Google Scholar]
- 4.Bonanno J, Guan Y, Jelamskii S, Kang X. Apical and basolateral permeability in cultured bovine corneal endothelial cells. Am J Physiol Cell Physiol. 1999;277:C545–C553. doi: 10.1152/ajpcell.1999.277.3.C545. [DOI] [PubMed] [Google Scholar]
- 5.Bonanno J, Srinivas S. Cyclic AMP activates anion channels in cultured bovine corneal endothelial cells. Exp Eye Res. 1997;64:953–962. doi: 10.1006/exer.1997.0290. [DOI] [PubMed] [Google Scholar]
- 6.Bonanno JA, Giasson C. Intracellular pH regulation in fresh and cultured bovine corneal endothelium I Na/H exchange in the absence and presence of . Invest Ophthalmol Vis Sci. 1992;33:3058–3067. [PubMed] [Google Scholar]
- 7.Bonanno JA, Yi G, Kang XJ, Srinivas SP. Reevaluation of exchange in cultured bovine corneal endothelial cells. Invest Ophthalmol Vis Sci. 1998;39:2713–2722. [PubMed] [Google Scholar]
- 8.Devor D, Singh A, Lambert L, DeLuca A, Frizzell R, Bridges R. Bicarbonate and chloride secretion in Calu-3 human airway epithelial cells. J Gen Physiol. 1999;113:743–760. doi: 10.1085/jgp.113.5.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Egee S, Lapaix F, Cossins A, Thomas S. The role of anion and cation channels in volume regulatory responses in trout red blood cells. Bioelectrochemistry. 2000;52:133–149. doi: 10.1016/s0302-4598(00)00096-9. [DOI] [PubMed] [Google Scholar]
- 10.Farokhzad OC, Sagar GD, Mun EC, Sicklick JK, Lotz M, Smith JA, Song JC, O’Brien TC, Sharma CP, Kinane TB, Hodin RA, Matthews JB. Protein kinase C activation downregulates the expression and function of the basolateral Na+/K+/2Cl− cotransporter. J Cell Physiol. 1999;181:489–498. doi: 10.1002/(SICI)1097-4652(199912)181:3<489::AID-JCP13>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 11.Fischbarg J, Lim J. Role of cations, anions, and carbonic anhydrase in fluid transport across rabbit corneal endothelium. J Physiol. 1974;241:647–675. doi: 10.1113/jphysiol.1974.sp010676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fischbarg J, Lim J, Bourguet J. Adenosine stimulation of fluid transport across rabbit corneal endothelium. J Membr Biol. 1977;35:95–112. doi: 10.1007/BF01869942. [DOI] [PubMed] [Google Scholar]
- 13.Flagella M, Clarke LL, Miller ML, Erway LC, Giannella RA, Andringa A, Gawenis LR, Kramer J, Duffy JJ, Doetschman T, Lorenz JN, Yamoah EN, Cardell EL, Shull GE. Mice lacking the basolateral Na-K-2Cl cotransporter have impaired epithelial chloride secretion and are profoundly deaf. J Biol Chem. 1999;274:26946–26955. doi: 10.1074/jbc.274.38.26946. [DOI] [PubMed] [Google Scholar]
- 14.Goddard CA, Evans MJ, Colledge WH. Genstein activates CFTR-mediated Cl− secretion in the murine trachea and colon. Am J Physiol Cell Physiol. 2000;279:C383–C392. doi: 10.1152/ajpcell.2000.279.2.C383. [DOI] [PubMed] [Google Scholar]
- 15.Hecht G, Koutsouris A. Myosin regulation of NKCC1: effects on cAMP-mediated Cl− secretion in intestinal epithelia. Am J Physiol Cell Physiol. 1999;277:C441–C447. doi: 10.1152/ajpcell.1999.277.3.C441. [DOI] [PubMed] [Google Scholar]
- 16.Hodson S. The regulation of corneal hydration by a salt pump requiring the presence of sodium and bicarbonate ions. J Physiol. 1974;236:271–302. doi: 10.1113/jphysiol.1974.sp010435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hodson S, Miller F. The bicarbonate ion pump in the endothelium which regulates the hydration of rabbit cornea. J Physiol. 1976;263:563–577. doi: 10.1113/jphysiol.1976.sp011645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Illek B, Fischer H. Flavonoids stimulate Cl conductance of human airway epithelium in vitro and in vivo. Am J Physiol Lung Cell Mol Physiol. 1998;275:L902–L910. doi: 10.1152/ajplung.1998.275.5.L902. [DOI] [PubMed] [Google Scholar]
- 19.Illek B, Fischer H, Santos GF, Widdicombe JH, Machen TE, Reenstra WW. cAMP-independent activation of CFTR Cl channels by the tyrosine kinase inhibitor genistein. Am J Physiol Cell Physiol. 1995;268:C886–C893. doi: 10.1152/ajpcell.1995.268.4.C886. [DOI] [PubMed] [Google Scholar]
- 20.Jelamskii S, Sun XC, Herse P, Bonanno JA. Basolateral Na+-K+-2Cl− cotransport in cultured and fresh bovine corneal endothelium. Invest Ophthalmol Vis Sci. 2000;41:488–495. [PubMed] [Google Scholar]
- 21.Jovov B, Shlyonsky VG, Berdiev BK, Ismailov II, Benos DJ. Purification and reconstitution of an outwardly rectified Cl− channel from tracheal epithelia. Am J Physiol Cell Physiol. 1998;275:C449–C458. doi: 10.1152/ajpcell.1998.275.2.C449. [DOI] [PubMed] [Google Scholar]
- 22.Kuang K, Li Y, Wen Q, Wang Z, Li J, Yang Y, Iserovich P, Reinach PS, Sparrow J, Diecke FP, Fischbarg J. Corneal endothelial NKCC: molecular identification, location, and contribution to fluid transport. Am J Physiol Cell Physiol. 2001;280:C491–C499. doi: 10.1152/ajpcell.2001.280.3.C491. [DOI] [PubMed] [Google Scholar]
- 23.Kuang K, Xu M, Koniarek J, Fischbarg J. Effects of ambient bicarbonate, phosphate and carbonic anhydrase inhibitors on fluid transport across rabbit endothelium. Exp Eye Res. 1990;50:487–493. doi: 10.1016/0014-4835(90)90037-u. [DOI] [PubMed] [Google Scholar]
- 24.Lader AS, Wang Y, Jackson GR, Jr, Borkan SC, Can-tiello HF. cAMP-activated anion conductance is associated with expression of CFTR in neonatal mouse cardiac myocytes. Am J Physiol Cell Physiol. 2000;278:C436–C450. doi: 10.1152/ajpcell.2000.278.2.C436. [DOI] [PubMed] [Google Scholar]
- 25.Lass JH, Spurney RV, Dutt RM, Andersson H, Kochar H, Rodman HM, Stern RC, Doershuk CF. A morphologic and fluorophotometric analysis of the corneal endothelium in type I diabetes mellitus and cystic fibrosis. Am J Ophthalmol. 1985;100:783–788. doi: 10.1016/s0002-9394(14)73367-7. [DOI] [PubMed] [Google Scholar]
- 26.Liebovitch L, Fischbarg J. Effects of inhibitors of passive Na+ and HCO3 fluxes on electrical potential and fluid transport across rabbit corneal endothelium. Curr Eye Res. 1982;2:183–186. doi: 10.3109/02713688208997692. [DOI] [PubMed] [Google Scholar]
- 27.Liedtke CM, Cody D, Cole TS. Differential regulation of Cl− transport proteins by PKC in Calu-3 cells. Am J Physiol Lung Cell Mol Physiol. 2001;280:L739–L747. doi: 10.1152/ajplung.2001.280.4.L739. [DOI] [PubMed] [Google Scholar]
- 28.Luo J, Zhu T, Evagelidis A, Pato MD, Hanrahan JW. Role of protein phosphatases in the activation of CFTR (ABCC7) by genistein and bromotetramisole. Am J Physiol Cell Physiol. 2000;279:C108–C119. doi: 10.1152/ajpcell.2000.279.1.C108. [DOI] [PubMed] [Google Scholar]
- 29.MacCallum D, Lillie J, Scaletta L, Occhino J, Frederick W, Ledbetter S. Bovine corneal endothelium in vitro. Exp Cell Res. 1982;139:1–13. doi: 10.1016/0014-4827(82)90313-5. [DOI] [PubMed] [Google Scholar]
- 30.Marino C, Matovcik L, Gorelick F, Cohn J. Localization of the cystic fibrosis transmembrane conductance regulator in pancreas. J Clin Invest. 1991;88:712–716. doi: 10.1172/JCI115358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matthews JB, Hassan I, Meng S, Archer SY, Hrnjez BJ, Hodin RA. Na-K-2Cl cotransporter gene expression and function during enterocyte differentiation Modulation of Cl− secretory capacity by butyrate. J Clin Invest. 1998;101:2072–2079. doi: 10.1172/JCI1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maurice D. The location of the fluid pump in the cornea. J Physiol. 1972;221:43–54. doi: 10.1113/jphysiol.1972.sp009737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mittag T, Guo W-B, Kobayashi K. Bicarbonate-activated adenylyl cyclase in fluid-transporting tissues. Am J Physiol Renal Fluid Electrolyte Physiol. 1993;264:F1060–F1064. doi: 10.1152/ajprenal.1993.264.6.F1060. [DOI] [PubMed] [Google Scholar]
- 34.Narula P, Xu M, Kuang K, Akiyama R, Fischbarg J. Fluid transport across cultured bovine corneal endothelial cell monolayers. Am J Physiol Cell Physiol. 1992;262:C98–C113. doi: 10.1152/ajpcell.1992.262.1.C98. [DOI] [PubMed] [Google Scholar]
- 35.Poulsen JH, Fischer H, Illek B, Machen TE. Bicarbonate conductance and pH regulatory capability of cystic fibrosis transmembrane conductance regulator. Proc Natl Acad Sci USA. 1994;91:5340–5344. doi: 10.1073/pnas.91.12.5340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Riley M, Winkler B, Czajkowski C, Peters M. The roles of bicarbonate and CO2 in transendothelial fluid movement and control of corneal thickness. Invest Ophthalmol Vis Sci. 1995;36:103–112. [PubMed] [Google Scholar]
- 37.Riley M, Winkler B, Starnes C, Peters M. Adenosine promotes regulation of corneal hydration through cyclic adenosine monophosphate. Invest Ophthalmol Vis Sci. 1996;37:1–10. [PubMed] [Google Scholar]
- 38.Riley M, Winkler B, Starnes C, Peters M. Fluid and ion transport in corneal endothelium: insensitivity to modulators of Na-K-2Cl cotransport. Am J Physiol Cell Physiol. 1997;273:C1480–C1486. doi: 10.1152/ajpcell.1997.273.5.C1480. [DOI] [PubMed] [Google Scholar]
- 39.Schwiebert E, Flotte T, Cutting G, Guggino W. Both CFTR and outwardly rectifying chloride channels contribute to cAMP-stimulated whole cell chloride currents. Am J Physiol Cell Physiol. 1994;266:C1464–C1477. doi: 10.1152/ajpcell.1994.266.5.C1464. [DOI] [PubMed] [Google Scholar]
- 40.Sears C, Firoozmand F, Mellander A, Chambers F, Eromar I, Bot A, Scholte B, Jonge HD, Donowitz M. Genistein and tyrphostin 47 stimulate CFTR-mediated Cl- secretion in T84 cell monolayers. Am J Physiol Gastrointest Liver Physiol. 1995;269:G874–G882. doi: 10.1152/ajpgi.1995.269.6.G874. [DOI] [PubMed] [Google Scholar]
- 41.Selvaraj NG, Prasad R, Goldstein JL, Rao MC. Evidence for the presence of cGMP-dependent protein kinase-II in human distal colon and in T84, the colonic cell line. Biochim Biophys Acta. 2000;1498:32–43. doi: 10.1016/s0167-4889(00)00075-6. [DOI] [PubMed] [Google Scholar]
- 42.Sheppard D, Welsh M. Structure and function of the CFTR chloride channel. Physiol Rev. 1999;79:23–45. doi: 10.1152/physrev.1999.79.1.S23. [DOI] [PubMed] [Google Scholar]
- 43.Shumaker H, Amlal H, Frizzell R, Ulrich CD, 2nd, Soleimani M. CFTR drives cotransport in pancreatic duct cells: a basis for defective secretion in CF. Am J Physiol Cell Physiol. 1999;276:C16–C25. doi: 10.1152/ajpcell.1999.276.1.C16. [DOI] [PubMed] [Google Scholar]
- 44.Smith J, Welsh M. cAMP stimulates bicarbonate secretion across normal, but not cystic fibrosis airway epithelia. J Clin Invest. 1992;89:1148–1153. doi: 10.1172/JCI115696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sun XC, Bonanno JA, Jelamskii S, Xie Q. Expression and localization of NaHCO3 cotransporter in bovine corneal endothelium. Am J Physiol Cell Physiol. 2000;279:C1648–C1655. doi: 10.1152/ajpcell.2000.279.5.C1648. [DOI] [PubMed] [Google Scholar]
- 46.Sun XC, McCutheon C, Bertram P, Xie Q, Bonanno JA. Studies on the expression of mRNA for anion transport related proteins in corneal endothelial cells. Curr Eye Res. 2001;22:1–7. doi: 10.1076/ceyr.22.1.1.6981. [DOI] [PubMed] [Google Scholar]
- 47.Thomas J, Buchsbaum R, Zimniak A, Racker E. Intra-cellular pH measurements in ehrlich ascites tumor cells utilizing spectroscopic probes generated in situ. Biochemistry. 1979;18:2210–2218. doi: 10.1021/bi00578a012. [DOI] [PubMed] [Google Scholar]
- 48.Uyekubo S, Fischer H, Maminishkis A, Illek B, Miller S, Widdicombe J. cAMP-dependent absorption of chloride across airway epithelium. Am J Physiol Lung Cell Mol Physiol. 1998;275:L1219–L1227. doi: 10.1152/ajplung.1998.275.6.L1219. [DOI] [PubMed] [Google Scholar]
- 49.Wang F, Zeltwanger S, Yang IC-H, Nairn AC, Hwang T-C. Actions of genistein on cystic fibrosis transmembrane conductance regulator channel gating Evidence for two binding sites with opposite effects. J Gen Physiol. 1998;111:477–490. doi: 10.1085/jgp.111.3.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Winkler B, Riley M, Peters M, Williams F. Chloride is required for fluid transport by the rabbit corneal endothelium. Am J Physiol Cell Physiol. 1992;262:C1167–C1174. doi: 10.1152/ajpcell.1992.262.5.C1167. [DOI] [PubMed] [Google Scholar]
- 51.Woll E, Gschwentner M, Furst J, Hofer S, Buemberger G, Jungwirth A, Frick J, Deetjen P, Paulmichl M. Fluorescence-optical measurements of chloride movements in cells using the membrane-permeable dye diH-MEQ. Pflu¨gers Arch. 1996;432:486–93. doi: 10.1007/s004240050160. [DOI] [PubMed] [Google Scholar]
- 52.Zegarra-Moran O, Porcelli A, Rugolo M. The phorbol ester PMA and cyclic AMP activate different Cl− and fluxes in C127 cells expressing CFTR. Biochim Biophys Acta. 2001;1535:120–127. doi: 10.1016/s0925-4439(00)00089-2. [DOI] [PubMed] [Google Scholar]