Abstract
BACKGROUND & AIMS
Little is known about the effects of family history of hepatocellular carcinoma (HCC) on hepatitis B progression or risk of HCC. We examined how family HCC history and presence or stage of hepatitis B virus (HBV) infection affect risk for HCC.
METHODS
We performed a population-based cohort study of 22,472 participants from 7 townships in Taiwan who underwent evaluation for liver disease from 1991 through 1992. Those who received a first diagnosis of HCC from January 1, 1991, to December 31, 2008, were identified from the Taiwanese cancer registry.
RESULTS
There were 374 cases of incident HCC over 362,268 person-years of follow-up evaluation. The cumulative risk of HCC in hepatitis B surface antigen (HBsAg)-seronegative patients without a family history of HCC was 0.62%, in those with a family history of HCC the cumulative risk was 0.65%, in HBsAg-seropositive patients without a family history of HCC the cumulative risk was 7.5%, and in HBsAg-seropositive patients with a family history of HCC the cumulative risk was 15.8% (P < .001). The multivariate-adjusted hazard ratio for HBsAg-seropositive individuals with family history, compared with HBsAg-seronegative individuals without a family history of HCC, was 32.33 (95% confidence interval, 20.8–50.3; P < .001). The relative excess risk owing to interaction was 19, the attributable proportion was 0.59, and the synergy index value was 2.54. These findings indicate synergy between family HCC history and HBsAg serostatus. The synergy between these factors remained significant in stratification analyses by HBeAg serostatus and serum level of HBV DNA.
CONCLUSIONS
Family history of HCC multiplies the risk of HCC at each stage of HBV infection. Patients with a family history of HCC require more intensive management of HBV infection and surveillance for liver cancer.
Keywords: Cirrhosis, Liver Disease, Epidemiology, Cancer Risk Factor
Hepatocellular carcinoma (HCC) is now the third leading cause of cancer mortality worldwide.1,2 The prevalence and incidence of HCC is expected to continue to increase in the coming decades.3,4 Despite advances in the pathogenesis and treatment of HCC, the death rates resulting from HCC continue to parallel the incidence rates.5–8 Hepatitis B virus (HBV), a carcinogenic virus, is one of the leading causes of HCC worldwide.9,10
Patients infected with HBV are known to be at increased risk of HCC over their lifetime.11–15 Previous studies have reported familial aggregation of HCC,16,17 and recent meta-analyses have suggested that family history of HCC increases the risk of HCC in patients with viral hepatitis.18 However, the interaction between family history of HCC and presence of hepatitis B surface antigen (HBsAg), HBV DNA levels, and presence or absence of hepatitis B e antigen (HBeAg) has not been elucidated systematically. Detailed epidemiologic investigation of interaction between familial risk and various stages of natural history of HBV infection would improve our understanding of pathways that lead to the development of HCC as well as prognostication for HCC within patients with HBV. Understanding prognostic factors and their interaction is especially of immense clinical importance in patients with HBV infection because they can develop HCC at each stage of HBV infection and do not necessarily need to have cirrhosis to develop HCC.19 Risk stratification of HBV patients by such an approach would lead to refinement of HCC surveillance guidelines that could be personalized based on the status of family history of HCC and stage of HBV infection.
By using a large, well-characterized, prospective, community-dwelling cohort of participants with and without HBV infection, we conducted this study to examine whether the interaction between family history of HCC and various stages of HBV infection is synergistic in increasing the risk of incident HCC over 16 years of follow-up evaluation.
Methods
Study Participants
This study cohort was derived from the Risk Evaluation of Viral Load Elevation and Associated Liver Disease/ Cancer-Hepatitis B Virus (REVEAL) study, which is a prospective cohort study that examines risk factors that increase the risk of incident HCC in participants with and without viral hepatitis.20–22 Briefly, 47,079 male and 42,214 female residents (total, 89,293 residents) from 7 townships in Taiwan were invited to participate in a cancer screening program between 1991 and 1992. Among them, a total of 11,973 men (25%) and 11,847 women (28%) agreed to participate and provided written informed consent. Information on demographic and lifestyle factors was collected via a structured personal interview by well-trained public health nurses. Overnight fasting blood samples were collected at baseline, and tests on serum HBsAg, HBeAg, HBV DNA, and alanine aminotransferase levels were performed using commercial kits, and detailed laboratory procedures were followed as described previously.11,20,21 The detailed description of the REVEAL cohort, study procedures, and protocol has been published previously.11,20,21,23 Alcohol consumption was ascertained with the help of a questionnaire at the baseline visit.22 Participants who tested positive for anti–hepatitis C antibodies were excluded. Participants were asked about their alcohol use (ever: yes/no), age at first use of alcohol, and present alcohol use (yes/no). Duration of alcohol use (in years) also was documented. Alcohol drinkers were defined as those who consumed alcohol at least 4 days per week for at least 1 year. All participants provided informed consent to participate in this study, and the data collection procedures were reviewed and approved by the Institutional Review Board of the College of Public Health at the National Taiwan University.
Exposure: Family History of Hepatocellular Carcinoma Status
Presence of family history was defined as having a first-degree relative with HCC. The family history status was ascertained with the help of a standardized questionnaire that was given to all participants at the baseline visit.
Hepatitis B Virus Infection Status
All participants underwent testing for HBsAg and those who were positive were classified as having HBV infection. To assess the effect of stages of natural history of hepatitis B, we further subclassified patients based on 2 key effect modifiers of HCC risk based on previous studies published using the REVEAL cohort: HBeAg status and the presence of high HBV DNA levels (≥10,000 copies/mL).11,20 These 2 modifiers were chosen because HBeAg as well as HBV DNA independently and robustly predict HCC in patients with HBV.
We also assessed the presence of HBV genotype (whether genotype B vs genotype B/C or C) and also the presence of a basal core promoter mutation (wild type vs basal core promoter mutant) or the presence of a precore mutation (wild type vs precore mutation).
The following step-wise assessment was performed to assess the effect of each category and then all combined. Association between a family history of HCC, and (1) the presence (or absence) of HBsAg, (2) the presence (or absence) of HBeAg, and (3) the presence (or absence) of high HBV DNA levels.
Duration of Follow-up Evaluation
All of the 22,472 Taiwanese residents from 7 townships in Taiwan were followed up for a mean ± standard deviation of 16.12 ± 2.89 years (median ± interquartile range, 16.86 ± 0.83 y) for a first diagnosis HCC from January 1, 1991, to December 31, 2008.
Primary Outcome Measure: Hepatocellular Carcinoma
All participants who consented to participate in the study and underwent a baseline examination were followed up for development of HCC and were accounted for in the analyses. Participants with a prior diagnosis of HCC that was identified either by the national cancer registry or diagnosed with HCC during the baseline visit or from personal interview before or at enrollment were excluded from the analysis. All participants had a unique national identification number that was used to link with the computerized national cancer registry profiles in Taiwan to identify all the new HCC cases between January 1, 1991, and December 31, 2008. The Taiwan nationwide cancer registry system was initiated in 1978 and contains updated, accurate, and complete information on HCC. Because histologic confirmation of the HCC diagnosis was not reported in all the cases identified by the national cancer registry, adjudication of HCC diagnosis was performed by experts via chart reviews in those participants. This chart review was performed on 164 HCC cases in HBsAg-positive carriers to further confirm and validate the primary HCC diagnosis derived from the national cancer registry. All HCC cases included in this study must have met at least one of the following criteria endorsed by the American Association for the Study of Liver Diseases guidelines24: histopathologically confirmed HCC, detection and confirmation of HCC by at least 2 imaging tools (ultrasonography, angiography, or computed tomography), or detection of HCC via one imaging diagnostic modality and with a serum a-fetoprotein level of 400 ng/mL or greater.
Measures of Interaction
To test the null hypothesis, we performed a comprehensive assessment of interaction using relative excess risk caused by interaction (RERI), attributable proportion (AP) caused by interaction, and Rothman's synergy index (SI) and their 95% confidence interval (CI).25 RERI is an estimate of excess risk that is attributable to the interaction between 2 exposures: family history of HCC and HBV status in this case. AP is defined as the proportion of risk that is attributable to the interaction. SI is a ratio that estimates whether a synergistic (SI, >1) or antagonistic (SI, <1) interaction exists between 2 exposures.26,27
Statistical Analysis
Person-years of follow-up evaluation for each participant were calculated as the time from study enrollment until the date of HCC diagnosis, the date of death, or December 31, 2008, whichever came first. Idividuals who were not diagnosed with HCC by death or by the last follow-up date were censored. The Kaplan–Meier curve was used to examine and estimate the cumulative incidence of HCC, and survival curves were compared using the log-rank test. Univariate and multivariate-adjusted hazard ratios (HRs) (with 95% CIs) of HCC were calculated using the Cox proportional hazards model. Significance levels were set at a P value of less than .05, and all statistical analyses were performed using SAS version 9.2 (SAS Institute, Cary, NC).
Results
Baseline Characteristics
The study included 22,472 participants (50% women) who were followed up for a mean of 16.12 years (SD, 2.89 y). The mean age and body mass index was 47.2 years (SD, ± 10 y) and 24 kg/m2 (SD, ± 3.4 kg/m2), respectively. The prevalence of HBsAg was 17.5%, and among HBsAg-positive individuals 15.5% were HBeAg-positive. Family history of HCC was present in 2.4% of the entire cohort. Baseline characteristics of the cohort stratified by family history of HCC (yes or no) and presence (or absence) of HBsAg status are shown in Table 1.
Table 1.
Baseline Characteristics of the Cohort Stratified by the Family History of HCC and HBsAg
| Subgroup |
|||||
|---|---|---|---|---|---|
| Characteristics | All | Family history of HCC negative and HBsAg negative | Family history of HCC positive and HBsAg negative | Family history of HCC negative and HBsAg positive | Family history of HCC positive and HBsAg positive |
| N | 22,472 | 18,194 | 347 | 3751 | 180 |
| HCC cases, n | 374 | 100 | 2 | 246 | 26 |
| Mean age ± SD, y | 47.2 ± 10.0 | 47.5 ± 10.1 | 45.5 ± 9.4 | 45.9 ± 9.8 | 45.4 ± 9.2 |
| Mean BMI ± SD, kg/m2 a | 24.0 ± 3.4 | 24.0 ± 3.4 | 24.0 ± 3.5 | 24.0 ± 3.4 | 23.7 ± 3.3 |
| Alcohol use, n (%)b | |||||
| No | 19,999 (89.2) | 16,242 (89.5) | 302 (87.0) | 3298 (88.1) | 157 (87.7) |
| Yes | 2422 (10.8) | 1908 (10.5) | 45 (13.0) | 447 (11.9) | 22 (12.3) |
| Smoking, n (%)c | |||||
| No | 15,917 (71.0) | 13,025 (71.7) | 245 (70.6) | 2528 (67.5) | 119 (66.1) |
| Yes | 6517 (29.1) | 5134 (28.3) | 102 (29.4) | 1220 (32.6) | 61 (33.9) |
| ALT level, U/L | |||||
| <45 | 21,859 (97.3) | 17,815 (97.9) | 339 (97.7) | 3537 (94.3) | 168 (93.3) |
| ≥45 | 613 (2.7) | 379 (2.1) | 8 (2.3) | 214 (5.7) | 12 (6.7) |
| Diabetesd | |||||
| No | 21,867 (97.6) | 17,693 (97.5) | 341 (98.3) | 3657 (97.7) | 176 (97.8) |
| Yes | 545 (2.4) | 448 (2.5) | 6 (1.7) | 87 (2.3) | 4 (2.2) |
| Genotype | |||||
| B | 1923 (66.5) | N/A | N/A | 1844 (66.9) | 79 (57.7) |
| BC and C | 970 (33.5) | 912 (33.1) | 58 (42.3) | ||
| Precore mutant (1896 G/A) | |||||
| Wild type | 703 (38.2) | N/A | N/A | 672 (38.3) | 31 (35.6) |
| Mutant + mixed type | 1138 (61.8) | 1082 (61.7) | 56 (64.4) | ||
| BCP mutant (1762/1764) | |||||
| Wild type | 1031 (56.9) | N/A | N/A | 990 (57.3) | 41 (48.8) |
| Mutant + mixed type | 782 (43.1) | 739 (42.7) | 43 (51.2) | ||
| HBeAg | |||||
| Seronegative | 3155 (84.5) | N/A | N/A | 3014 (84.7) | 141 (81.5) |
| Seropositive | 577 (15.5) | 545 (15.3) | 32 (18.5) | ||
| HBV DNA level, copies/mL | |||||
| HBV DNA level, <10,000 | 2035 (55.7) | N/A | N/A | 1941 (55.7) | 94 (54.7) |
| HBV DNA level, ≥10,000 | 1621 (44.3) | 1543 (44.3) | 78 (45.4) | ||
ALT, alanine aminotransferase; BCP, basal core promoter mutation; BMI, body mass index.
Fifty-three subjects were excluded because of missing data on body mass index.
Fifty-one subjects were excluded because of missing data on alcohol use.
Thirty-eight subjects were missing data on smoking.
Sixty subjects were missing data on history of diabetes.
Risk of Hepatocellular Carcinoma
There were a total of 374 HCC cases over 362,268 person-years of follow-up evaluation (Table 2). The cumulative rates of HCC in the 4 exposure categories including family history of HCC-negative and HBsAg-negative, family history of HCC-positive and HBsAg-negative, family history of HCC-negative and HBsAg-positive, and family history of HCC-positive and HBsAg-positive were 0.62%, 0.65%, 7.5%, and 15.8% (P < .001), respectively (Figure 1A).
Table 2.
Unadjusted and Multivariate Adjusted HRs for Incident HCC
| Characteristic | N | HCC cases, n | Person-years of follow-up evaluation | Incidence rate per 100,000 person-years | Crude HR (95% CI) | Multivariate-adjusted HR (95% CI) |
|---|---|---|---|---|---|---|
| Age, y | 1.06 (1.05-1.08) | 1.08 (1.07-1.09) | ||||
| BMI, kg/m2 | 1.05 (1.02-1.08) | 1.04 (1.01-1.07) | ||||
| Sex | ||||||
| Female | 11,081 | 91 | 182,624.8 | 49.83 | Referent | Referent |
| Male | 11,391 | 283 | 179,643.5 | 157.53 | 3.18 (2.51-4.02) | 2.09 (1.58-2.77) |
| Smoking habit | ||||||
| No | 15,917 | 207 | 260,559.5 | 79.44 | Referent | Referent |
| Yes | 6517 | 165 | 101,085.5 | 163.23 | 2.07 (1.68-2.54) | 1.11 (0.87-1.42) |
| Alcohol consumption | ||||||
| No | 19,999 | 294 | 324,238.9 | 90.67 | Referent | Referent |
| Yes | 2422 | 78 | 37,222.8 | 209.55 | 2.32 (1.81-2.98) | 1.46 (1.12-1.91) |
| Diabetes | ||||||
| No | 21,867 | 350 | 353,976.7 | 98.88 | Referent | Referent |
| Yes | 545 | 23 | 7329.5 | 313.80 | 1.12 (0.98-1.28) | 1.19 (1.02-1.39) |
| ALT level, U/L | ||||||
| ≤45 | 21,859 | 321 | 353,039.6 | 90.93 | Referent | Referent |
| >45 | 613 | 53 | 9228.7 | 574.30 | 6.32 (4.73-8.46) | 3.66 (2.72-4.94) |
| Family history of HCC | ||||||
| Negative | 21,945 | 346 | 353,905.6 | 97.77 | Referent | Referent |
| Positive | 527 | 28 | 8362.7 | 334.82 | 3.42 (2.33-5.03) | 1.29 (0.32-5.24) |
| HBsAg serostatus | ||||||
| Negative | 18,541 | 102 | 300,596.8 | 33.93 | Referent | Referent |
| Positive | 3931 | 272 | 61,671.5 | 441.05 | 13.01 (10.36-16.33) | 13.04 (10.28-16.54) |
| Family history of HCC/HBsAg | ||||||
| No/HBsAg-negative | 18,194 | 100 | 294,950.6 | 33.90 | Referent | Not included |
| Yes/HBsAg-negative | 347 | 2 | 5646.2 | 35.42 | 1.04 (0.26-4.22) | |
| No/HBsAg-positive | 3751 | 246 | 58,954.9 | 417.27 | 12.32 (9.76-15.54) | |
| Yes/HBsAg-positive | 180 | 26 | 2716.6 | 958.10 | 28.33 (18.40-43.62) |
ALT, alanine aminotransferase; BMI, body mass index.
Figure 1.
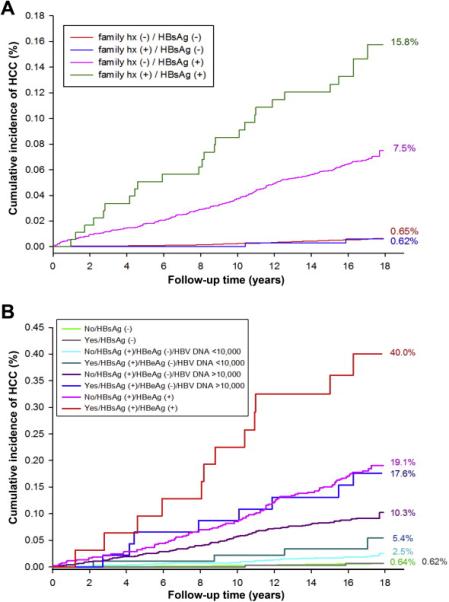
(A) Family history of HCC, HBsAg serostatus, and risk of incident HCC. (B) Family history of HCC, HBsAg and HBeAg serostatus, HBV DNA level, and risk of incident HCC.
Joint Effects of Family History of Hepatocellular Carcinoma and Hepatitis B Surface Antigen on Incident Hepatocellular Carcinoma Risk
Combined effects of family history of HCC and HBsAg showed a statistically significant and synergistic association with HCC risk, with the highest risk among those who had both a positive family history of HCC and HBsAg positivity in both unadjusted (HR, 28.33; 95% CI, 18.40–43.62; P < .001; Table 2) and multivariate-adjusted analyses (HR, 32.33; 95% CI, 20.78–50.30; P < .01), as shown in Table 3.
Table 3.
Crude and Multivariate-Adjusted HRs of Incident HCC
| Characteristic | All (N = 22,472) |
HBsAg negative (n = 18,541) |
HBsAg positive (n = 3931) |
HBsAg-positive subset (n = 1767) |
||
|---|---|---|---|---|---|---|
| Crude HR (95% CI) | Age/sex-adjusted HR (95% CI) | Multivariate adjusted HR (95% CI) | Multivariate adjusted HR (95% CI) | Multivariate adjusted HR (95% CI) | Multivariate adjusted HR (95% CI) | |
| Sex | ||||||
| Female | Referent | Referent | Referent | Referent | Referent | Referent |
| Male | 3.18 (2.51-4.02) | 2.47 (1.95-3.13) | 2.09 (1.58-2.77) | 1.05 (0.61-1.80) | 2.85 (2.01-4.04) | 3.02 (1.99-4.58) |
| Age, y | 1.06 (1.05-1.08) | 1.08 (1.06-1.09) | 1.08 (1.07-1.09) | 1.09 (1.06-1.11) | 1.08 (1.06-1.09) | 1.09 (1.07-1.11) |
| BMI, kg/m2 | 1.05 (1.02-1.08) | 1.04 (1.01-1.07) | 1.05 (0.99-1.11) | 1.04 (1.00-1.08) | 1.04 (1.00-1.09) | |
| Smoking habit | ||||||
| No | Referent | Referent | Referent | Referent | Referent | |
| Yes | 2.07 (1.68-2.54) | 1.11 (0.87-1.42) | 1.42 (0.83-2.45) | 1.06 (0.81-1.38) | 1.22 (0.88-1.71) | |
| Alcohol consumption | ||||||
| No | Referent | Referent | Referent | Referent | Referent | |
| Yes | 2.32 (1.81-2.98) | 1.46 (1.12-1.91) | 1.32 (0.75-2.34) | 1.50 (1.11-2.04) | 1.26 (0.86-1.84) | |
| Diabetes | ||||||
| No | Referent | Referent | Referent | Referent | Referent | |
| Yes | 1.12 (0.98-1.28) | 1.19 (1.02-1.39) | 1.20 (0.99-1.46) | 1.17 (0.92-1.49) | 1.08 (0.82-1.44) | |
| ALT level, U/L | ||||||
| ≤45 | Referent | Referent | Referent | Referent | Referent | |
| >45 | 6.32 (4.73-8.46) | 3.66 (2.72-4.94) | 3.19 (1.46-6.95) | 3.65 (2.64-5.05) | 2.17 (1.48-3.18) | |
| Genotypea | ||||||
| B | Referent | Referent | ||||
| BC and C | 2.53 (1.95-3.28) | 1.88 (1.37-2.60) | ||||
| Precore mutant (1896 G/A)a | ||||||
| Wild type | Referent | Referent | ||||
| Mutant + mixed type | 0.49 (0.37-0.65) | 0.49 (0.36-0.67) | ||||
| BCP mutant (1762/1764)a | ||||||
| Wild type | Referent | Referent | ||||
| Mutant + mixed type | 2.47 (1.83-3.32) | 1.78 (1.29-2.45) | ||||
| Family history of HCC/HBsAg | ||||||
| No/HBsAg negative | Referent | Referent | Referent | Referent | ||
| Yes/HBsAg negative | 1.04 (0.26-4.22) | 1.26 (0.31-5.09) | 1.29 (0.32-5.24) | 1.26 (0.31-5.14) | ||
| No/HBsAg positive | 12.32 (9.76-15.54) | 13.38 (10.59-16.91) | 13.04 (10.28-16.54) | Referent | Referent | |
| Yes/HBsAg positive | 28.33 (18.40-43.62) | 32.79 (21.26-50.58) | 32.33 (20.78-50.30) | 2.46 (1.63-3.72) | 2.33 (1.40-3.88) | |
ALT, alanine aminotransferase; BCP, basal core promoter mutation; BMI, body mass index.
Fifty-three subjects were excluded because of missing data on body mass index.
Assessment of Interaction Between Family History of Hepatocellular Carcinoma and Hepatitis B Surface Antigen
We then examined the RERI, AP, and SI; measures of synergistic interaction between family history of HCC (positive or negative) and HBsAg (positive or negative) in their association with incident HCC risk. We found that the RERI, AP, and SI were 19 (95% CI, 5.56–32.43), 0.59 (95% CI, 0.18–0.99), and 2.54 (95% CI, 1.62–3.99), respectively. These findings suggest a synergistic interaction between family history of HCC and HBsAg in their joint association in increasing HCC risk.
Sensitivity Analyses
We further assessed the association between family history of HCC and the stage of HBV infection by performing subgroup analyses in patients who were HBsAg positive. Previous studies from the REVEAL cohort have shown that the presence of HBeAg and high HBV DNA level, defined as 10,000 copies/mL or greater, are both associated with increased risk of HCC.
Family History of Hepatocellular Carcinoma and Hepatitis B Virus DNA Levels in Hepatitis B Surface Antigen–Positive Patients
Combined effects of family history of HCC and HBV DNA showed a statistically significant and synergistic association with HCC risk, with the highest risk among those who had both a positive family history of HCC and high HBV DNA levels (≥10,000 copies/mL) in both unadjusted (HR, 13.75; 95% CI, 8.05–23.47; P < .001) and multivariate-adjusted analyses (HR, 15.72; 95% CI, 9.06–27.27; P < .01) (Supplementary Tables 1 and 2).
We then examined the RERI, AP, and SI between family history of HCC (positive or negative) and HBV DNA levels (≥10,000 or <10,000 copies/mL) in their association with incident HCC risk and found that the RERI, AP, and SI were 8.79 (95% CI, 0.89–16.69), 0.56 (95% CI, 0.18–0.93), and 2.48 (95% CI, 1.33–4.64), respectively, showing statistically significant synergistic interaction.
Family History of Hepatocellular Carcinoma and Hepatitis B e Antigen in Hepatitis B Surface Antigen–Positive Patients
Combined effects of family history of HCC and HBeAg showed a statistically significant and synergistic association with HCC risk, with the highest risk among those who had both a positive family history of HCC and were HBeAg positive in both unadjusted (HR, 10.86; 95% CI, 6.01–19.62; P < .001) and multivariate-adjusted analyses (HR, 20.14; 95% CI, 10.71–37.87; P < .01) (Supplementary Tables 3 and 4).
We then examined the RERI, AP, and SI between family history of HCC (positive or negative) and HBeAg (positive or negative) in their association with incident HCC risk and found that the RERI, AP, and SI were 12.87 (95% CI, 0.44–25.29), 0.64 (95% CI, 0.37–0.91), and 3.05 (95% CI, 1.53–6.10), respectively, showing statistically significant synergistic interaction.
Assessing the Effect of Family History of Hepatocellular Carcinoma, and Stages of Hepatitis B Virus Infection: Hepatitis B Surface Antigen–Positive, High or Low Hepatitis B Virus DNA Level, and Presence or Absence of Hepatitis B e Antigen
When analyses were stratified by family history of HCC (yes/no), and HBsAg (yes/no), HBeAg-negative status with or without high HBV DNA level (>10,000 copies/mL), and HBeAg-positive status, the risk of incident HCC synergistically increased dose-dependently in those with a positive family history of HCC across all stages of HBV infection, and was highest in those with a family history of HCC and HBeAg positivity in multivariate-adjusted analyses (HR, 174.61; 95% CI, 92.2–330.8; P < .01), as shown in Table 4. The effect of the presence of family history of HCC remained significant and synergistic in each stage of HBV infection and multiplied the risk several-fold in those who already were at high risk for HCC (Table 4). Figure 1B shows that the presence of both a positive family history and HBeAg positivity increased the cumulative risk of HCC to 40%, underscoring clinical significance of these statistically significant results.
Table 4.
Multivariate-Adjusted HRs of HCC by Family History Status and the Stage of HBV Infection Including HBsAg, HBeAg, and HBV DNA Level
| Characteristic | Age/sex-adjusted HR (95% CI) | All (N = 22,472) |
HBsAg positive only (n = 3931) |
|---|---|---|---|
| Multivariate adjusted HR (95% CI) | Multivariate adjusted HR (95% CI) | ||
| Sex, male vs female | 2.16 (1.69-2.75) | 1.78 (1.33-2.39) | 2.36 (1.63-3.41) |
| Age, y | 1.09 (1.08-1.11) | 1.09 (1.08-1.11) | 1.10 (1.09-1.11) |
| BMI, kg/m2 | 1.05 (1.01-1.08) | 1.05 (1.01-1.09) | |
| Smoking habit, yes vs no | 1.25 (0.97- 1.61) | 1.24 (0.93-1.65) | |
| Alcohol consumption, yes vs no | 1.40 (1.06-1.86) | 1.41 (1.03-1.94) | |
| Diabetes, yes vs no | 1.12 (0.96-1.31) | 1.06 (0.83-1.36) | |
| ALT level, >45 vs ≤45 U/L | 2.23 (1.62-3.06) | 2.04 (1.45-2.89) | |
| Family history of HCC/HBsAg/HBeAG/HBV DNA level | |||
| No/HBsAg negative | Referent | Referent | N/A |
| Yes/HBsAg negative | 1.30 (0.32-5.26) | 1.31 (0.32-5.33) | N/A |
| No/HBsAg positive/HBeAg negative/HBV DNA <10,000 copies/mL | 3.75 (2.57-5.47) | 3.87 (2.65-5.65) | Referent |
| Yes/HBsAg positive/HBeAg negative/HBV DNA <10,000 copies/mL | 9.27 (3.41-25.20) | 9.42 (3.46-25.63) | 2.44 (0.87-6.84) |
| No/HBsAg positive/HBeAg negative/HBV DNA ≥ 10,000 copies/mL | 17.22 (12.94-22.91) | 17.37 (13.02-23.18) | 4.43 (3.02-6.50) |
| Yes/HBsAg positive/HBeAg negative/HBV DNA ≥10,000 copies/mL | 36.62 (17.80-75.35) | 39.04 (18.91-80.61) | 9.90 (4.59-21.37) |
| No/HBsAg positive/HBeAg positive | 60.21 (44.79-80.95) | 52.33 (38.36-71.37) | 13.91 (9.31-20.77) |
| Yes/HBsAg positive/HBeAg positive | 178.38 (96.83-328.63) | 174.61 (92.16-330.83) | 45.52 (22.86-90.63) |
NOTE. We did not adjust for genotype and mutants because these data were available only in patients with a DNA level greater than 10,000.
ALT, alanine aminotransferase; BMI, body mass index.
Association Between Risk of Hepatocellular Carcinoma and Number of Hepatocellular Carcinoma Cases Among First-Degree Relatives
Although there were trends suggesting that familial aggregation of HCC increased the risk of HCC, they were not significant in this cohort (Tables 5 and 6).
Table 5.
Multivariate-Adjusted HRs of HCC According to the Number of First-Degree Relatives With HCC
| Characteristic | All with positive family history (N = 527) |
HBsAg positive only (N = 180) |
|---|---|---|
| Age/sex-adjusted HR (95% CI) | Multivariate adjusted HR (95% CI) | |
| Sex, male vs female | 2.40 (0.86-6.68) | 1.81 (0.56-5.88) |
| Age, y | 1.05 (1.01-1.10) | 1.06 (1.02-1.10) |
| BMI, kg/m2 | 0.98 (0.88-1.10) | 0.97 (0.84-1.12) |
| Smoking habit, yes vs no | 1.26 (0.49-3.25) | 1.76 (0.65-4.74) |
| Alcohol consumption, yes vs no | 0.62 (0.17-2.20) | 0.78 (0.21-2.94) |
| Diabetes, yes vs no | 1.79 (0.24- 13.59) | 1.44 (0.18-11.80) |
| ALT level, >45 vs ≤45 U/L | 9.16 (3.53-23.76) | 6.71 (2.36-19.02) |
| First-degree relatives with HCC, n | ||
| 1 | 1.0 | 1.0 |
| ≥1 | 1.83 (0.53-6.32) | 1.57 (0.43-5.72) |
NOTE. We could not look at genotype or mutant data because there were no data for more than 80% of these individuals because the majority of people were HBsAg negative. There were only 2 HCC cases among HBsAg-negative participants, so it was not possible to perform this analyses in this subset.
ALT, alanine aminotransferase; BMI, body mass index.
Table 6.
Distribution of HCC Cases and Number of Relatives With HCC
| Characteristic | HCC non-case | HCC case |
|---|---|---|
| 1 first-degree relative with HCC | 465 | 25 |
| ≥1 first-degree relative with HCC | 34 | 3 |
Discussion
Main Findings
In this large, well-characterized, prospective, community-dwelling cohort study, we showed that family history of HCC and HBsAg synergistically increases the risk of incident HCC among Taiwanese men and women. Family history of HCC multiplies the risk of HCC at each stage of HBV infection. Because family history of HCC effect modification of HCC risk in each stage of HBV infection was statistically significant and clinically meaningful, we therefore speculate that this suggests a significant shared host gene effect as the underlying mechanistic pathway to explain these findings. Future studies are warranted to examine whether more intensive HCC surveillance strategies should be considered in highest-risk HBV patients based on family history status similar to colon cancer.
By using RERI, AP, and SI, we showed that the interaction between family history of HCC and HBsAg is synergistic. Furthermore, we report that the risk approaches 40% when both family history of HCC and HBeAg are positive. These findings underscore the need for routine use of family history of HCC in risk assessment of patients with HBV for their HCC risk.28 These data would form the basis for considering more stringent surveillance studies in these high-risk groups of patients with HBV. Future studies are needed to confirm whether the effect of family history of HCC on increasing the HCC risk in HBV patients is caused by shared gene effects or, perhaps, shared environment.29
Strengths and Limitations
Strengths of this study included a large prospective cohort comprising 24,272 participants who were followed up during 16 years, family history of HCC that was ascertained using a standardized questionnaire that was given to all the participants and obtained by self-report, and availability of potential confounders or effect modifiers as well as key HBV markers (such as HBsAg, HBeAg, and HBV DNA levels) associated with HCC at baseline for adjustment in the multivariate analyses. However, we acknowledge the following limitations of this study, including that the study population was exclusively Taiwanese. We did not have information on the age at diagnosis of HCC in family members, age at HBV infection in cases, and presence of family history of cirrhosis or HBV infection status in family members. We did not adjust for delta hepatitis and anti–hepatitis B core antibodies in this study. However, we believe that these issues, although noteworthy, do not reflect a systematic bias that differentially favors the results in the opposite direction. Hence, these limitations are not likely to have influenced the study findings. Furthermore, the study was restricted to the association between family history of HCC and HBV infection, and these data are not general-izable to other liver diseases such as chronic hepatitis C infection, primary biliary cirrhosis, or nonalcoholic fatty liver disease. Family history was ascertained by self-report, and there was a possibility of recall bias. However, given that family history was recorded at baseline and we followed up all participants for incident HCC, it is unlikely that cases were likely to report (recall) family history of HCC, as is likely to happen in a case-control study.29 Previous studies have shown that family history of cancer in first-degree relatives is likely to have 70% to 80% accuracy based on self-report.30 Therefore, we included a positive family history in those who reported a first-degree relative with HCC.
In Context With Published Literature
Our results are consistent with previous reports showing that family history of HCC is an important risk factor for HCC.16,31–33 Turati et al18 conducted a case-control study (229 HCC patients vs 431 controls) and showed that the odds ratio of HCC was significantly higher in patients who had a family history of HCC and viral hepatitis B or C. They did not find a multiplicative interaction in their study. However, they did not examine RERI, AP, and SI to assess interaction. A basal core promoter mutation was associated with increased risk of HCC. However, the presence of a precore mutation was associated with a reduced risk of HCC. Neither of these factors showed a significant effect on modification of HCC risk with family history status. Our study used a large, prospective cohort design and assessed incident risk of HCC using Cox proportional hazards analyses and examined the presence of interaction using validated tools such as RERI, AP, and SI.26 Therefore, our results provide robust prospective data and a comprehensive interaction assessment that will inform future research in this area.
Impact on Clinical Practice
Routine use of family history of HCC can further improve the HCC risk stratification of patients with hepatitis B. The cumulative risk of HCC when both family history and HBeAg are present is 40%. These patients should be considered at extremely high risk and more stringent screening and surveillance of HCC may be considered in this subset of patients because management of HCC is improving with transplantation, locoregional therapies, as well as newer chemo-therapeutic agents.6,24 High-risk HCC subgroups may be identified in emerging centers of excellence in HCC care that may lead to early identification and prompt institution of therapies to manage HCC and improve morbidity and mortality caused by HCC.
Future Research Directions
Familial risk has been used in other cancers to perform earlier screening and surveillance such as in breast and colon carcinoma. Improved understanding of mechanisms underlying synergism between familial risk and HBV may refine prognostication for HCC risk and may lead to earlier and more stringent screening and surveillance strategies.34 We challenge the paradigm that family history does not have an interaction with HBV status and propose a multiplicative effect of family history and HBV on HCC risk. Further research is needed in a multi-ethnic population with and without viral hepatitis to assess whether this synergism is appreciated in diverse cohorts. Finally, more research is needed regarding whether anti-HBV therapies can reduce the risk of HCC in this subset of patients, especially those who have a family history of HCC.
Conclusions
By using a prospective cohort study design, we showed that family history of HCC multiplies the risk of HCC in patients with hepatitis B. This risk dose-dependently multiplied by the presence of HBeAg and higher levels of HBV DNA. Antiviral therapy should be considered in patients who are at highest risk of HCC such as those who have a family history of HCC, are HBeAg positive, and have an increased ALT level. Future studies are warranted to examine whether more intensive HCC surveillance strategies should be considered in highest-risk HBV patients based on family history status similar to other cancers such as colon cancer. We propose that shared gene effects along with shared environment,29 albeit a lesser contribution, increases susceptibility to HCC. Exact underlying mechanisms remain to be elucidated. Whether screening and surveillance guidelines for HCC need to be revised based on familial risk and HBV status remains to be investigated in future studies.
Supplementary Material
Acknowledgments
Funding
Supported in part by the American Gastroenterological Association Foundation – Sucampo – ASP Designated Research Award in Geriatric Gastroenterology, a T. Franklin Williams Scholarship Award; Atlantic Philanthropies, Inc; the John A. Hartford Foundation, the Association of Specialty Professors, the American Gastroenterological Association (R.L.); the National Institute for Diabetes and Digestive Kidney Diseases (K23 DK090303 to R.L.); and a National Cancer Institute grant (P30CA23100-27 to R.L.). The REVEAL-Hepatitis B Virus study was supported by a grant from the Department of Health, Academia Sinica, National Health Research Institutes in Taiwan, and by Bristol-Myers Squibb Company, Wallingford, CT.
Abbreviations used in this paper
- AP
attributable proportion
- CI
confidence interval
- HBeAg
hepatitis B e antigen
- HBsAg
hepatitis B surface antigen
- HBV
hepatitis B virus
- HCC
hepatocellular carcinoma
- HR
hazard ratio
- RERI
relative excess risk caused by interaction
- REVEAL
Risk Evaluation of Viral Load Elevation and Associated Liver Disease/Cancer-Hepatitis B Virus
- SI
synergy index
Footnotes
Supplementary Material
Note: To access the supplementary material accompanying this article, visit the online version of Clinical Gastroenterology and Hepatology at www.cghjournal.org, and at http://dx.doi.org/10.1016/j.cgh.2013.04.043.
Conflicts of interest
The authors disclose no conflicts.
References
- 1.Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J Clin Oncol. 2009;27:1485–1491. doi: 10.1200/JCO.2008.20.7753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.The global burden of disease: 2004 update. WHO Press; Oct, 2008. [2011]. Available at: http://www.who.int/evidence/bod. [Google Scholar]
- 3.Everhart JE, Ruhl CE. Burden of digestive diseases in the United States part III: liver, biliary tract, and pancreas. Gastroenterology. 2009;136:1134–1144. doi: 10.1053/j.gastro.2009.02.038. [DOI] [PubMed] [Google Scholar]
- 4.El-Serag HB, Davila JA, Petersen NJ, et al. The continuing increase in the incidence of hepatocellular carcinoma in the United States: an update. Ann Intern Med. 2003;139:817–823. doi: 10.7326/0003-4819-139-10-200311180-00009. [DOI] [PubMed] [Google Scholar]
- 5.Kim WR, Gores GJ, Benson JT, et al. Mortality and hospital utilization for hepatocellular carcinoma in the United States. Gastroenterology. 2005;129:486–493. doi: 10.1016/j.gastro.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 6.Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 7.Lopez PM, Villanueva A, Llovet JM. Systematic review: evidence-based management of hepatocellular carcinoma–an updated analysis of randomized controlled trials. Aliment Pharmacol Ther. 2006;23:1535–1547. doi: 10.1111/j.1365-2036.2006.02932.x. [DOI] [PubMed] [Google Scholar]
- 8.Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362:1907–1917. doi: 10.1016/S0140-6736(03)14964-1. [DOI] [PubMed] [Google Scholar]
- 9.McGlynn KA, London WT. The global epidemiology of hepatocellular carcinoma: present and future. Clin Liver Dis. 2011;15:223–243. vii–x. doi: 10.1016/j.cld.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Venook AP, Papandreou C, Furuse J, et al. The incidence and epidemiology of hepatocellular carcinoma: a global and regional perspective. Oncologist. 2010;15(Suppl 4):5–13. doi: 10.1634/theoncologist.2010-S4-05. [DOI] [PubMed] [Google Scholar]
- 11.Yang HI, Lu SN, Liaw YF, et al. Hepatitis B e antigen and the risk of hepatocellular carcinoma. N Engl J Med. 2002;347:168–174. doi: 10.1056/NEJMoa013215. [DOI] [PubMed] [Google Scholar]
- 12.Sherman M. Hepatocellular carcinoma: epidemiology, risk factors, and screening. Semin Liver Dis. 2005;25:143–154. doi: 10.1055/s-2005-871194. [DOI] [PubMed] [Google Scholar]
- 13.Lok AS. Prevention of hepatitis B virus-related hepatocellular carcinoma. Gastroenterology. 2004;127:S303–S309. doi: 10.1053/j.gastro.2004.09.045. [DOI] [PubMed] [Google Scholar]
- 14.Omata M, Lesmana LA, Tateishi R, et al. Asian Pacific Association for the Study of the Liver consensus recommendations on hepatocellular carcinoma. Hepatol Int. 2010;4:439–474. doi: 10.1007/s12072-010-9165-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mair RD, Valenzuela A, Ha NB, et al. Incidence of hepatocellular carcinoma among US patients with cirrhosis of viral or nonviral etiologies. Clin Gastroenterol Hepatol. 2012;10:1412–1417. doi: 10.1016/j.cgh.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu MW, Chang HC, Liaw YF, et al. Familial risk of hepatocellular carcinoma among chronic hepatitis B carriers and their relatives. J Natl Cancer Inst. 2000;92:1159–1164. doi: 10.1093/jnci/92.14.1159. [DOI] [PubMed] [Google Scholar]
- 17.Lok AS, Lai CL. Factors determining the development of hepato-cellular carcinoma in hepatitis B surface antigen carriers. A comparison between families with clusters and solitary cases. Cancer. 1988;61:1287–1291. doi: 10.1002/1097-0142(19880401)61:7<1287::aid-cncr2820610702>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 18.Turati F, Edefonti V, Talamini R, et al. Family history of liver cancer and hepatocellular carcinoma. Hepatology. 2012;55:1416–1425. doi: 10.1002/hep.24794. [DOI] [PubMed] [Google Scholar]
- 19.Yang JD, Kim WR, Coelho R, et al. Cirrhosis is present in most patients with hepatitis B and hepatocellular carcinoma. Clin Gastroenterol Hepatol. 2011;9:64–70. doi: 10.1016/j.cgh.2010.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen CJ, Yang HI, Su J, et al. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA. 2006;295:65–73. doi: 10.1001/jama.295.1.65. [DOI] [PubMed] [Google Scholar]
- 21.Chen CL, Yang HI, Yang WS, et al. Metabolic factors and risk of hepatocellular carcinoma by chronic hepatitis B/C infection: a follow-up study in Taiwan. Gastroenterology. 2008;135:111–121. doi: 10.1053/j.gastro.2008.03.073. [DOI] [PubMed] [Google Scholar]
- 22.Loomba R, Yang HI, Su J, et al. Synergism between obesity and alcohol in increasing the risk of hepatocellular carcinoma: a prospective cohort study. Am J Epidemiol. 2013;177:333–342. doi: 10.1093/aje/kws252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Loomba R, Yang HI, Su J, et al. Obesity and alcohol synergize to increase the risk of incident hepatocellular carcinoma in men. Clin Gastroenterol Hepatol. 2010;8:891–898. e1–2. doi: 10.1016/j.cgh.2010.06.027. [DOI] [PubMed] [Google Scholar]
- 24.Bruix J, Sherman M. American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rothman KJ, Greenland S, Walker AM. Concepts of interaction. Am J Epidemiol. 1980;112:467–470. doi: 10.1093/oxfordjournals.aje.a113015. [DOI] [PubMed] [Google Scholar]
- 26.de Mutsert R, Jager KJ, Zoccali C, et al. The effect of joint exposures: examining the presence of interaction. Kidney Int. 2009;75:677–681. doi: 10.1038/ki.2008.645. [DOI] [PubMed] [Google Scholar]
- 27.Richardson DB, Kaufman JS. Estimation of the relative excess risk due to interaction and associated confidence bounds. Am J Epidemiol. 2009;169:756–760. doi: 10.1093/aje/kwn411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Volk ML, Lok AS. Is family history of liver cancer a risk factor for hepatocellular carcinoma? J Hepatol. 2009;50:247–248. doi: 10.1016/j.jhep.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 29.Berg AO, Baird MA, Botkin JR, et al. National Institutes of Health State-of-the-Science Conference Statement: family history and improving health. Ann Intern Med. 2009;151:872–877. doi: 10.7326/0003-4819-151-12-200912150-00165. [DOI] [PubMed] [Google Scholar]
- 30.Bravi F, Bosetti C, Negri E, et al. Family history of cancer provided by hospital controls was satisfactorily reliable. J Clin Epidemiol. 2007;60:171–175. doi: 10.1016/j.jclinepi.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 31.Chan AO, Yuen MF, Lam CM, et al. Prevalence and characteristics of familial hepatocellular carcinoma caused by chronic hepatitis B infection in Hong Kong. Aliment Pharmacol Ther. 2004;19:401–406. doi: 10.1046/j.1365-2036.2004.01855.x. [DOI] [PubMed] [Google Scholar]
- 32.Hassan MM, Spitz MR, Thomas MB, et al. The association of family history of liver cancer with hepatocellular carcinoma: a case-control study in the United States. J Hepatol. 2009;50:334–341. doi: 10.1016/j.jhep.2008.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Donato F, Gelatti U, Chiesa R, et al. A case-controlstudy onfamily history of liver cancer as a risk factor for hepatocellular carcinoma in North Italy. Brescia HCC Study. Cancer Causes Control. 1999;10:417–421. doi: 10.1023/a:1008989103809. [DOI] [PubMed] [Google Scholar]
- 34.Sherman M, Bruix J, Porayko M, et al. for the APGC. Screening for hepatocellular carcinoma: the rationale for the American Association for the Study of Liver Diseases recommendations. Hepatology. 2012;56:793–796. doi: 10.1002/hep.25869. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


