Abstract
Study Design
Prospective cohort study
Objective
To assess the correlation between central motor conduction time (CMCT) and various subjective and objective clinical assessment measures in patients with cervical spondylotic myelopathy (CSM) undergoing decompressive surgery
Summary of Background Data
CSM can cause a spectrum of neurological deficits across individuals. Subjective clinical assessments of disease severity may lack the sensitivity of objective tests. Transcranial magnetic stimulation (TMS) provides objective electrophysiologic data on the integrity of the corticospinal tracts, which may be useful for monitoring disease progression or neurological improvement after surgery.
Methods
Patients undergoing surgical decompression for CSM performed subjective and objective testing before surgery and at 1, 3, 6 and 12 months after surgery. Subjective measures included modified Japanese Orthopaedic Assessment (mJOA), Neck Disability Index (NDI), Nurick grade, and visual analog scale (VAS) score. Objective measures included CMCT as measured using TMS, the 10-meter walk test (10MWT), the 9-hole peg task (9HPT), and grip and release test (GRT). Primary outcome was the correlation between CMCT and subjective or objective measures at preoperative and postoperative time points. Secondary outcome was the correlation between preoperative CMCT and performance in subjective or objective testing after surgical intervention.
Results
Improvement in both subjective and objective measures was observed after surgery. CMCT correlated with other objective measures (10MWT, 9HPT, and GRT) both at baseline and after decompressive surgery in these 17 patients with CSM. Patients with high baseline CMCTs were associated with poor performance on the 10MWT, 9HPT, and GRT. mJOA correlated with CMCT at baseline but not after surgical intervention. CMCT was not associated with other subjective measures, such as NDI, Nurick grade, and VAS, at preoperative or postoperative time points.
Conclusion
CMCT as measured by TMS is a responsive objective assessment of CSM. It can be used to monitor disease severity and neurological function before and after surgical intervention. Prolonged baseline CMCT may be associated with worse surgical outcomes.
Keywords: Transcranial magnetic stimulation, central motor conduction time, Cervical spondylotic myelopathy, cervical spine, cervical surgery, modified Japanese Orthopaedic Assessment (mJOA), 10-meter walk test, 9-hole peg task, grip and release test, outcome, prospective study
Introduction
Current surgical decision making in cervical spondylotic myelopathy (CSM) is based on a combination of subjective and objective clinical assessments and knowledge of the patient’s disease progression. Most patients with moderate to severe myelopathy who present with symptoms, such as gait disturbance, difficulty with fine motor skills, and bowel or bladder dysfunction, and who have concordant imaging findings are recommended for surgical decompression. For patients with subtle and early cervical myelopathy and milder symptoms, surgical decision making is less straightforward. Several subjective and objective measures have been correlated with outcome after decompressive surgery in patients with CSM.1–3 Transcranial magnetic stimulation (TMS), a noninvasive neurophysiologic measure of the integrity of the corticospinal tracts, has been used as an objective measure of disease severity in patients with CSM.1,4–12
TMS allows for direct characterization of the integrity of the anterior columns in an awake patient.13 Magnetic flux induces an activating pulse in the axons in the motor cortex, generating direct and indirect potentials in the corticospinal tract without sensation of electrical shocks. Previous studies have evaluated the correlation between TMS and clinical and radiographic parameters in CSM.1,11 Patients with CSM have a prolonged central motor conduction time (CMCT), the time needed for nerve impulses to reach the cervical spinal roots after stimulation of the motor cortex, which is caused by a reduction in number of intact corticospinal neurons.9,10 We investigated the correlation between CMCT and several subjective and objective clinical assessment measures in patients with CSM undergoing decompressive surgery. Identifying the association between CMCT and these measures may help surgeons to more accurately evaluate disease severity and response to treatment in patients with CSM. Furthermore, understanding the correlation between baseline CMCT results and postoperative testing performance may help surgeons to counsel patients on the extent of neurological improvement that could be expected after decompressive surgery.
Methods
Patients
After approval by the Institutional Review Board, we enrolled patients undergoing anterior, posterior, or combined cervical decompression for CSM between March 2011 and March 2012 in a prospective cohort study. CSM was defined by the presence of any of the following criteria: long-tract signs localized to the cervical spinal cord (spasticity, hyperreflexia, ankle clonus, Babinski and/or Hoffman signs); impairment of fine motor activities or gait; bowel or bladder dysfunction; abnormal spinal cord signal from compression due to spondylotic changes on T2-weighted MRI
Exclusion criteria included age less than 18 years; obesity (body mass index >30); pregnancy; CSM secondary to ossification of the posterior longitudinal ligament; previous brain lesions or history of brain surgery; and the presence of other disease that would interfere with central motor and sensory conduction, such as diabetes, amyotrophic lateral sclerosis, or multiple sclerosis.
Transcranial magnetic stimulation
TMS was performed with a MagStim Rapid device (MagStim Company Ltd, UK) using a MagStim HP 90-mm coil (model 9784-00), which has been FDA approved for peripheral nerve stimulation (FDA Reference # K992911). The peak magnetic field output of the coil was 2 Tesla with a maximum duration of 250 msec. The maximum pulse rate is 20 Hz, but did not exceed 1 Hz for this study. Single-pulse TMS was used.
Motor-evoked potentials were made with adhesive surface electrodes placed in the first dorsal interosseous (FDI) and abductor pollicis brevis (APB) muscles bilaterally. The magnetic coil was positioned over the vertex in the posterior-anterior direction. Pulse stimulation was performed, starting at 10% of maximum TMS output, to locate where three out of five responses in the FDI or APB could be observed at an amplitude of ≥50 mV. Onset latency, peak to peak amplitude, CMCT, and wave complexity (polyphasic, triphasic, biphasic, monophasic, or absent) was recorded.
CMCT is an estimate of conduction time of corticospinal fibers between motor cortex and spinal motor neurons. It includes the time of excitation of cortical cells, conduction via the corticospinal tract and excitation of the motor neuron sufficient to exceed its firing threshold.14 CMCT is measured by subtracting spinal motor neuron–to-muscle latency from head-to-muscle latency.
Attempts were made to obtain TMS results from the lower extremities. Insufficient data, however, were collected to provide an accurate representation of the study population. No reliable TMS response was recorded in half of the lower extremities tested. Consequently, our analysis included only data obtained from upper extremity TMS results. Because CMCT results correct for lower motor neuron latency, results obtained from the upper extremities can be used to evaluate impairment in both the upper and lower extremities.
Subjective measures of disease severity
Clinical assessment indices were used as subjective measures of the patient’s disease severity, including the modified Japanese Orthopaedic Association Score (mJOA),15 the Neck Disability Index (NDI),16 the Nurick grade,17 and the Visual Analog Scale (VAS) score18 to assess neck and arm pain. These assessments have been recommended for screening and follow-up assessment for patients with CSM based on their common use or psychometrics, although no validity, reliability or responsiveness studies have been performed for the NDI and VAS in this patient population.19
Objective measures of disease severity
Reliable tests20–22 of physical and functional activity targeting gait and fine motor abilities were used as objective measures of disease severity. These included a 10-meter walk test (10MWT),23 a 9-hole peg task (9HPT),21 and a grip and release test (GRT).24 The 10MWT is a gait measure used in patients with various causes of neurological impairment.20 As disability increases, the time required to complete the test increases and the patient requires more steps because of a shorter stride. In the 9HPT, patients were timed to evaluate how fast they could take small pegs, one by one, and place them in holes in a board. The 9HPT was completed with each hand and timed separately. In the GRT, patients were asked to make a fist grip and then open and close the hand as many times as possible in 20 seconds.
Study design
Study participants underwent prospective diagnostic testing using TMS, clinical assessment indices, and physical activity tests during their preoperative and 1-, 3-, 6-, and 12-month postoperative clinic visits. Data collected at the preoperative visit served as baseline results.
The primary study outcome was the correlation between TMS results and subjective and objective measures of CSM severity at each time point. The secondary outcome was the correlation between preoperative TMS results and performance in subjective and objective testing after cervical decompressive surgery.
Statistical analysis
The side with the longest CMCT reading for the upper extremity was used as the baseline measurement. Spearman correlation between objective or subjective variables and CMCT were obtained. A repeated measures analysis of variance (ANOVA) was performed to determine the relationship between the baseline CMCT and the variables in the study. An autoregressive correlation structure was used since the current reading should be correlated with the previous reading. ROC analysis was used to determine the threshold CMCT value at which clinical improvement could be predicted. The threshold was selected at the point that sensitivity and 1 minus specificity were maximized. A p-value <0.05 was considered to be statistically significant.
Results
Patient characteristics and demographics
Seventeen patients with CSM were included (mean 60 years, range 42–78 years) (Table 1). Ten patients underwent anterior surgery, 4 had posterior surgery, and 3 had combined anterior/posterior surgery. There were fewer subject measurements at the later time periods: One participant withdrew voluntarily from the study after the 1-month postoperative visit, one withdrew voluntarily after the 3-month visit, one was lost to follow-up by 6 months, and 4 others were lost to follow-up by the 12-month visit. One participated in all testing but did not contribute reliable TMS data.
Table 1.
Demographic information in 17 patients with cervical spondylotic myelopathy who underwent surgical decompression
| Variable | Measurement |
|---|---|
| No. males (%) | 9 (53) |
| Mean age (years), range | 60 (42–78) |
| No. (%) with comorbidities | |
| Smoking | 5 (29) |
| Heart disease | 12 (71) |
| Diabetes | 3 (18) |
| Depression | 1 (6) |
| No. (%) undergoing each procedure | |
| Anterior | 10 (59) |
| Posterior | 4 (23) |
| Combined anterior/posterior | 3 (18) |
Transcranial magnetic stimulation
Mean and standard deviation (SD) for upper extremity CMCTs at the preoperative visit was 9.7±4.9 msec. Baseline CMCTs were obtained in at least one upper extremity in 16 patients. After surgery, mean CMCTs decreased to a low of 8.5±3.5 msec at the 6-month postoperative visit (Table 2).
Table 2.
Mean and standard deviation (range) of objective variables by visit
| Visit | 10m walk time (sec) | 10m walk no. steps | Grasp/release | 9 hole peg right time (sec) | 9 hole peg left time (sec) | Upper extremity CMCT |
|---|---|---|---|---|---|---|
| Pre-operative | 10.6±4.2 (5.9–24.4) | 17.9±5.0 (12–31) | 17.2±7.8 (8–34) | 18.6±7.5 (10–40.8) | 31.3±29.5 (9.3–105.8) | 9.7±4.9 (3.3–20.3) |
| 1 month | 10.3±3.3 (6.6–18.8) | 17.7±4.1 (13–27) | 20.3±7.1 (8–32) | 20.2±7.6 (12.2–36.6) | 35.9±41.9 (11.7–137.7) | 8.7±2.5 (3.7–12.4) |
| 3 month | 8.8±1.7 (5.7–11.5) | 16.3±3.6 (11–24) | 19.9±7.2 (6–30) | 19.6±5.0 (13.1–28.0) | 33.1±32.0 (10.9–109.8) | 8.8±3.2 (4.4–13.6) |
| 6 month | 9.6±2.0 (6.2–12.8) | 16.7±3.8 (12–22) | 21.6±7.9 (8–35) | 20.1±4.7 (13.1–28.4) | 21.5±7.2 (14.6–37.9) | 8.5±3.5 (4.0–13.3) |
| 12 month | 9.7±1.3 (7.8–10.8) | 16.8±2.2 (14–19) | 16.8±3.4 (12–20) | 21.2±6.0 (16.8–30.1) | 17.4±2.4 (14.7–19.8) | 9.4±3.8 (5.7–13.6) |
CMCT, central motor conduction time
Objective measures of CSM severity
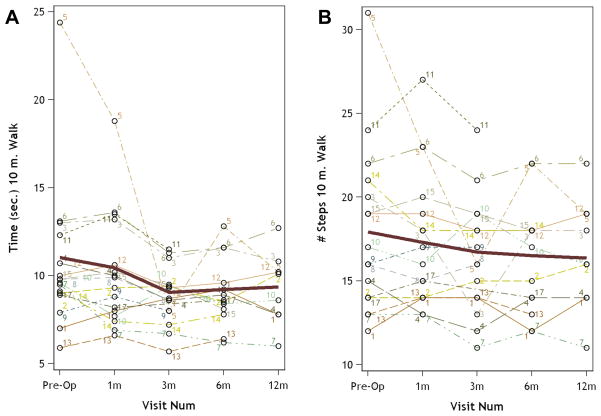
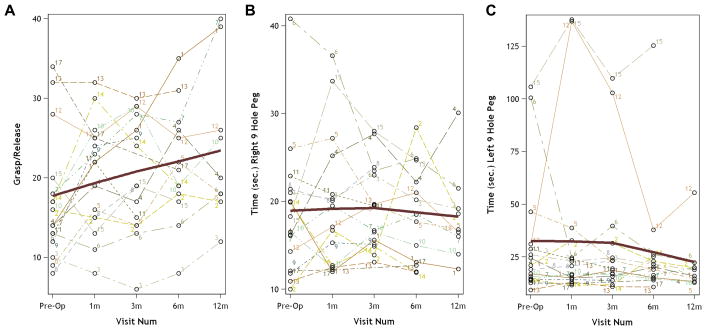
Baseline results for objective measures included time (10.6±4.2 sec) and number of steps (17.9±5.0) required to complete the 10MWT, number of hand repetitions during the GRT (17.2±7.8), and the time required to complete the 9HPT with the right hand (18.6±7.5 sec) and left hand (31.3±29.5 sec) (Table 2). Study participants demonstrated postoperative improvement in each of these measures of gait and hand function. The greatest improvement after surgery was seen at 3 months for 10MWT (8.8±1.7 sec, 16.3±3.6 steps) (Figure 1) and 6 months for GRT (21.6±7.9 repetitions) and left-handed 9HPT (17.2±2.4 sec) (Figure 2).
Figure 1.
Graphs showing improvement in time (left) and number of steps (right) required to complete the 10m walk test (10MWT) after decompressive surgery for cervical spondylotic myelopathy. Mean is indicated by the dark line.
Figure 2.
Graphs showing improvement in hand function after decompressive surgery for cervical spondylotic myelopathy as measured by increase in the number of repetitions completed in the grasp and release test (GRT) (A) and decrease in time to complete the 9-hole peg task (9HPT) with right (B) and left (C) hands. Mean is indicated by the dark line.
Subjective measures of CSM severity
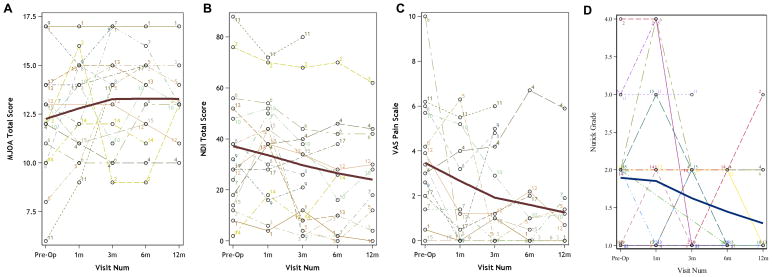
Baseline results for subjective measures included mJOA score (12.1±2.8), NDI score (35.1±24.1), Nurick grade (1.8±0.9), and VAS (3.8±2.7) (Table 3). Study participants demonstrated improvement in each of these subjective measures of pain and disability after surgery. The greatest improvement after surgery was seen at 3 months for mJOA score (13.6±2.7) and at 6 months for Nurick grade (1.3±0.5), NDI score (24.0±24.4), and VAS (1.4±2.3) (Figure 3).
Table 3.
Mean and standard deviation (range) of subjective variables measured by visit
| Visit | Nurick grade | mJOA score | NDI score | VAS |
|---|---|---|---|---|
| Pre-operative | 1.8±0.9 (1–4) | 12.1±2.8 (6–17) | 35.1±24.1 (2–88) | 3.8±2.7 (0–10) |
| 1 month | 2.1±1.2 (1–4) | 12.7±2.3 (9–16) | 39.9±18.6 (6–72) | 2.3±2.4 (0–6.3) |
| 3 month | 1.6±0.6 (1–3) | 13.6±2.7 (9–17) | 27.4±24.5 (2–80) | 2.1±2.4 (0–6.0) |
| 6 month | 1.3±0.5 (1–2) | 13.4±2.7 (9–17) | 24.0±24.4 (0–70) | 1.4±2.3 (0–6.7) |
| 12 month | 1.8±1.0 (1–3) | 13.0±2.2 (10–15) | 30.5±27.2 (4–62) | 2.4±3.1 (0–5.9) |
mJOA, modified Japanese Orthopaedic Association Score; NDI, Neck Disability Index; (VAS) Visual Analog Scale (VAS)
Figure 3.
Graphs showing improvement in subjective measures after decompressive surgery for cervical spondylotic myelopathy. Mean is indicated by the dark line. (A) Modified Japanese Orthopaedic Association Score; (B) Neck Disability Index; (C) Visual Analog Scale; (D) Nurick grade.
Correlation between TMS and subjective and objective measures
Subjective and objective measures correlated with CMCT at several time points (asterisks in Table 4). At baseline, CMCT correlated with number of steps (r=0.549, p=0.028) and time required to complete the 10MWT (r=0.801, p<0.001), mJOA score (r=−0.614, p=0.011), 9HPT with left (r=0.626, p=0.009) and right (r=0.471, p=0.066) hands, and GRT (r=−0.442, p=0.086).
Table 4.
Spearman correlations of subjective and objective variables with upper extremity CMCT by visit
| Preoperative | 1 month | 3 months | 6 months | 12 months | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sp. corr. | p value | Sp. corr. | p value | Sp. corr. | p value | Sp. corr. | p value | Sp. corr. | p value | |
| No. steps 10m walk | 0.549 | 0.028* | 0.370 | 0.159 | −0.023 | 0.934 | 0.660 | 0.014* | 0.489 | 0.181 |
| Grasp/release | −0.442 | 0.086 | −0.612 | 0.012* | −0.630 | 0.012* | −0.459 | 0.114 | −0.644 | 0.061 |
| mJOA score | −0.614 | 0.011* | −0.456 | 0.087 | −0.329 | 0.251 | −0.299 | 0.321 | −0.104 | 0.789 |
| NDI score | 0.074 | 0.786 | 0.102 | 0.707 | 0.034 | 0.904 | 0.237 | 0.435 | 0.050 | 0.898 |
| Nurick grade | 0.090 | 0.740 | 0.370 | 0.174 | 0.025 | 0.934 | 0.258 | 0.443 | 0.050 | 0.899 |
| Time (sec) 10m walk | 0.801 | <0.001* | 0.756 | <0.001* | 0.306 | 0.267 | 0.666 | 0.013* | −0.571 | 0.108 |
| Time (sec) Left 9 hole peg | 0.626 | 0.009* | 0.524 | 0.037* | 0.493 | 0.062 | 0.511 | 0.074 | 0.250 | 0.516 |
| Time (sec) Right 9 hole peg | 0.471 | 0.066 | 0.703 | 0.002* | 0.679 | 0.005* | 0.505 | 0.078 | 0.717 | 0.030* |
| VAS | 0.467 | 0.108 | 0.260 | 0.391 | −0.189 | 0.556 | 0.238 | 0.507 | 0.122 | 0.774 |
<0.05
mJOA, modified Japanese Orthopaedic Association Score; NDI, Neck Disability Index; (VAS) Visual Analog Scale (VAS); CMCT, central motor conduction time
One month postoperatively, correlations between measures and CMCT were significant for the GRT (r=−0.612, p=0.012), timed 10MWT (r=0.756, p≤0.001), and 9HPT with left (r=0.524, p=0.037) and right (r=0.703, p=0.002) hands. At 6 months postoperatively, correlations between measures and CMCT retained significance for the number of steps (r=0.660, p=0.014) and time required to complete the 10MWT (r=0.666, p=0.013).
The correlation was not significant between CMCT and NDI score, Nurick grade, or VAS at any of the time points.
Predictive value of baseline TMS
Repeated measures ANOVA demonstrated that baseline CMCT is associated with postoperative performance on the GRT, timed 10MWT, and 9HPT (Table 5). Patients with higher baseline CMCT in the upper extremity required more time to complete the 10MWT and NHPT on postoperative visits. Higher baseline CMCT was also associated with fewer repetitions on the GRT. The association was not statistically significant between baseline CMCT and postoperative mJOA score, NDI score, Nurick grade, VAS scale, number of steps needed to complete the 10MWT, and the time required to complete the 9HPT with the right hand.
Table 5.
Repeated measures ANOVA to assess association of CMCT with objective and subjective measures
| Variable | Parameter estimate | Standard error | p value |
|---|---|---|---|
| No. steps 10m walk | 0.286 | 0.182 | 0.139 |
| Grasp/release | −0.821 | 0.273 | 0.010* |
| mJOA score | −0.149 | 0.094 | 0.137 |
| NDI score | −0.423 | 1.033 | 0.688 |
| Nurick grade | −0.003 | 0.032 | 0.926 |
| Time (sec) 10m walk | 0.310 | 0.110 | 0.014* |
| Time (sec) Left 9 hole peg | 0.579 | 0.224 | 0.022* |
| Time (sec) Right 9 hole peg | 0.579 | 1.330 | 0.672 |
| VAS | 0.075 | 0.099 | 0.463 |
<0.05
mJOA, modified Japanese Orthopaedic Association Score; NDI, Neck Disability Index; (VAS) Visual Analog Scale (VAS); CMCT, central motor conduction time
Positive values for the parameter estimates indicate higher baseline CMCT is associated with higher readings of the given variable in postoperative visits. Negative values for the parameter estimates indicate that higher baseline CMCT is associated with lower readings of the given variable in postoperative visits.
Threshold value of baseline TMS for Clinical Improvement
Logistic regression was used to determine the threshold preoperative CMCT value that could predict postoperative clinical improvement. Clinical improvement was defined as a ≥50% increase in number of repetitions completed on the GRT at 3 months after surgery compared with baseline. The GRT was chosen because it produced the most responsive testing results among participants. Three months was chosen because it is a reasonable time point at which neurological improvement after surgery could be observed, and 15 of 17 patients provided follow-up data. ROC curve analysis revealed a threshold preoperative CMCT value of 7.2 msec (probability = 0.45, AUC = 0.65).
Discussion
TMS is quick, noninvasive, and painless and can be used in patients with severe gait and/or limb disturbances who may not be able to participate in other objective assessments. The use of TMS to evaluate CSM has been well described. Patients with cervical spondylosis who have electrophysiologic abnormalities are more likely to develop myelopathy than those who do not.25 Symptoms of myelopathy and radiculopathy frequently co-exist and can be difficult to distinguish. TMS, however, may provide useful objective data to differentiate patients with CSM12,14,26 and can identify patients early in their disease course by detecting cord compression before the onset of myelopathic signs or symptoms.5,26,27
In CSM, the severity of cord compression and the deficits that result are highly variable across individuals. Such heterogeneity creates difficulty when attempting to quantify and compare clinical symptoms and subjective patient assessments. TMS provides objective measurements of the integrity of the corticospinal tracts that are easily compared across individuals. Previous studies have correlated TMS results with radiographic parameters 11 and JOA scores1. This is the first prospective study to validate the use of TMS in comparison to several established subjective and objective measures of CSM severity before and after decompressive surgery.
Subjective and objective measures of CSM severity
We found that CMCT positively correlates with the 10MWT, 9HPT, and GRT at preoperative and postoperative time points. That is, patients with prolonged CMCT tended to have poor performance on hand and gait testing before and after surgery. Unlike functional subjective assessments, such as the mJOA, NDI, and Nurick grade that focus on impairments in everyday activities. The 10MWT, 9HPT, and GRT directly test the impairments that are often considered to be the most important sequelae of CSM—impairment of ambulation and gait.28–30 These tests have the advantage of objectivity and are characterized by high intra- and inter-observer reliability.20–22 Other researchers have reported similar positive correlations between surgical outcomes and the 10MWT,25,31 9HPT,21 and GRT.1
The use of subjective functional outcome measures, such as the mJOA, has been recommended in the assessment of patients undergoing surgery for CSM.32 Subjective measures are useful for recognizing the severity of neurological status, but they may be influenced by patients’ expectations and psychological state. Subjective assessments tend to be poorly quantitative and contain few broad categories in their inventory. Furthermore, the use of subjective measures can be problematic because of a disconnect between objective measurements and patient-reported functional outcomes, such as pain and activity tolerance. Good objective measures are highly quantitative, reproducible, and more sensitive to change. The identification of valid, reliable, and responsive measures of improvement after surgical treatment of CSM is valuable.
We found a correlation between lower preoperative mJOA scores and higher baseline CMCT. That is, patients with severe disease had higher levels of disability and impaired conduction via corticospinal tracts before surgery. The mJOA score has been shown to be a predictor of outcomes after decompressive surgery for CSM.33 In one study, severe myelopathy (JOA <7) was associated with a good outcome in 55% of patients versus 70% in patients with mild disease.34 Others have reported that CMCT correlates with mJOA at both preoperative and postoperative time points in patients with severe CSM1,2,12. Many surgeons use a mJOA score of 12 (separating moderate from severe disease) as a threshold below which there is a negative impact on surgical outcome.29 The mean mJOA in our cohort was 12.1 at baseline.
Although we found that baseline CMCT correlated with several postoperative objective measures (10MWT, GRT, 9HPT), we did not identify a similar association between CMCT and postoperative subjective measures (NDI, mJOA, VAS). Given that patients in our cohort demonstrated clinical improvement in both subjective and objective measures after surgery, this discrepancy suggests that surgical decompression for CSM may produce quantifiable improvement in physiologic parameters that can be detected using objective tests that may be more responsive, sensitive to change and reproducible than traditional subjective assessments. For these reasons, we favor the use of CMCT, 10MWT, GRT, and 9HPT over the mJOA, NDI, Nurick grade, and VAS when evaluating outcomes of patients with CSM undergoing surgery.
Several studies have used neurophysiologic testing to provide prognostic information regarding outcome after decompressive surgery for CSM.3,35–37 Our findings demonstrate that preoperative CMCT may have a role in identifying patients who do not improve clinically after surgery, since patients who had high preoperative CMCT tended to perform poorly on postoperative testing for the 10MWT, 9HPT, and GRT. Our calculated threshold CMCT value (7.2 msec) for predicting clinical improvement is within the upper range of published normal results.38 Only 2 of 9 patients with preoperative CMCT values greater than 7.2 msec had >50% postoperative improvement on GRT whereas 4 of 6 patients with CMCT values less than this threshold demonstrated clinical improvement.
This study is limited by its small sample size, lack of follow-up, the inability to use TMS to assess the laterality of symptom severity, and technical considerations since each attempt to use TMS did not yield CMCT readings in our cohort. Despite these limitations, within our cohort we found several objective measures that had statistically significant correlations with CMCT. Our findings reflect the use of CMCT in preoperative and postoperative assessment of CSM severity. None of the patients in our cohort underwent nonoperative treatment, where the role of CMCT in monitoring patients has not been determined. Whether an anterior or posterior surgical approach affects the amount of change in CMCT should be a consideration in future studies.
High-quality evidence to guide surgical decision making in CSM is lacking. A recent Cochrane Review identified only two randomized controlled trials, both of which showed no significant difference in outcome between non-surgical and surgical interventions.39 TMS may be useful in providing reliable objective data to monitor disease progression and identify candidates who may benefit from surgical intervention. Larger prospective trials are needed to confirm the association between CMCT and objective and subjective measures of disease severity and to verify the preoperative CMCT threshold value that can predict the patients with CSM who are likely to experience clinical improvement after surgical decompression.
Conclusion
CMCT correlates with several objective measures both at baseline and after decompressive surgery in patients with CSM. Patients with high baseline CMCTs were associated with poor performance on the 10MWT, 9HPT, and GRT both before and after surgery. mJOA correlated with CMCT at baseline but not after surgery. CMCT was not associated with other subjective measures, such as NDI, Nurick grade, and VAS. CMCT is a responsive objective assessment of CSM that can be used to monitor disease severity and neurological function before and after surgery. High baseline CMCTs may be associated with worse surgical outcomes.
Key Points.
Central motor conduction time as measured by transcranial magnetic stimulation is a responsive objective assessment of cervical spondylotic myelopathy.
Central motor conduction time correlates with objective measures of neurologic function, such as the 10-meter walk test, 9-hole peg task, and grip and release test, both at baseline and after decompressive surgery.
Central motor conduction time can be used to monitor disease severity and neurological improvement after surgical decompression in patients with cervical spondylotic myelopathy.
Acknowledgments
AANS/CNS Section on Spine and Peripheral Nerve Disorders Sanford Larson Award and the University of Utah Study Design and Biostatistics Center, with funding in part from the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant 8UL1TR000105 (formerly UL1RR025764), funds were received to support this work.
Relevant financial activities outside the submitted work: payment for development of educational presentations.
The authors thank Kristin Kraus, M.Sc., for help in preparation of this paper.
Footnotes
The device(s)/drug(s) is/are FDA-approved or approved by corresponding national agency for this indication
Level of Evidence: 2
References
- 1.Takahashi J, Hirabayashi H, Hashidate H, et al. Assessment of cervical myelopathy using transcranial magnetic stimulation and prediction of prognosis after laminoplasty. Spine (Phila Pa 1976) 2008;33:E15–20. doi: 10.1097/BRS.0b013e31815e5dae. [DOI] [PubMed] [Google Scholar]
- 2.Capone F, Tamburelli FC, Pilato F, et al. The role of motor-evoked potentials in the management of cervical spondylotic myelopathy. Spine J. 2013;13:1077–9. doi: 10.1016/j.spinee.2013.02.063. [DOI] [PubMed] [Google Scholar]
- 3.Jaskolski DJ, Laing RJ, Jarratt JA, et al. Pre- and postoperative motor conduction times, measured using magnetic stimulation, in patients with cervical spondylosis. Br J Neurosurg. 1990;4:187–92. doi: 10.3109/02688699008992722. [DOI] [PubMed] [Google Scholar]
- 4.Jaskolski DJ, Jarratt JA, Jakubowski J. Clinical evaluation of magnetic stimulation in cervical spondylosis. Br J Neurosurg. 1989;3:541–8. doi: 10.3109/02688698909002845. [DOI] [PubMed] [Google Scholar]
- 5.Maertens de Noordhout A, Remacle JM, Pepin JL, et al. Magnetic stimulation of the motor cortex in cervical spondylosis. Neurology. 1991;41:75–80. doi: 10.1212/wnl.41.1.75. [DOI] [PubMed] [Google Scholar]
- 6.Di Lazzaro V, Restuccia D, Colosimo C, et al. The contribution of magnetic stimulation of the motor cortex to the diagnosis of cervical spondylotic myelopathy. Correlation of central motor conduction to distal and proximal upper limb muscles with clinical and MRI findings. Electroencephalogr Clin Neurophysiol. 1992;85:311–20. doi: 10.1016/0168-5597(92)90107-m. [DOI] [PubMed] [Google Scholar]
- 7.Tavy DL, Wagner GL, Keunen RW, et al. Transcranial magnetic stimulation in patients with cervical spondylotic myelopathy: clinical and radiological correlations. Muscle Nerve. 1994;17:235–41. doi: 10.1002/mus.880170215. [DOI] [PubMed] [Google Scholar]
- 8.Ofuji A, Kaneko K, Taguchi T, et al. New method to measure central motor conduction time using transcranial magnetic stimulation and T-response. J Neurol Sci. 1998;160:26–32. doi: 10.1016/s0022-510x(98)00160-9. [DOI] [PubMed] [Google Scholar]
- 9.Kalupahana NS, Weerasinghe VS, Dangahadeniya U, et al. Abnormal parameters of magnetically evoked motor-evoked potentials in patients with cervical spondylotic myelopathy. Spine J. 2008;8:645–9. doi: 10.1016/j.spinee.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 10.Kaneko K, Taguchi T, Morita H, et al. Mechanism of prolonged central motor conduction time in compressive cervical myelopathy. Clin Neurophysiol. 2001;112:1035–40. doi: 10.1016/s1388-2457(01)00533-8. [DOI] [PubMed] [Google Scholar]
- 11.Lo YL, Chan LL, Lim W, et al. Systematic correlation of transcranial magnetic stimulation and magnetic resonance imaging in cervical spondylotic myelopathy. Spine (Phila Pa 1976) 2004;29:1137–45. doi: 10.1097/00007632-200405150-00017. [DOI] [PubMed] [Google Scholar]
- 12.Kameyama O, Shibano K, Kawakita H, et al. Transcranial magnetic stimulation of the motor cortex in cervical spondylosis and spinal canal stenosis. Spine (Phila Pa 1976) 1995;20:1004–10. doi: 10.1097/00007632-199505000-00006. [DOI] [PubMed] [Google Scholar]
- 13.Barker AT, Jalinous R, Freeston IL. Non-invasive magnetic stimulation of human motor cortex. Lancet. 1985;1:1106–7. doi: 10.1016/s0140-6736(85)92413-4. [DOI] [PubMed] [Google Scholar]
- 14.Chen R, Cros D, Curra A, et al. The clinical diagnostic utility of transcranial magnetic stimulation: report of an IFCN committee. Clin Neurophysiol. 2008;119:504–32. doi: 10.1016/j.clinph.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 15.Benzel EC, Lancon J, Kesterson L, et al. Cervical laminectomy and dentate ligament section for cervical spondylotic myelopathy. J Spinal Disord. 1991;4:286–95. doi: 10.1097/00002517-199109000-00005. [DOI] [PubMed] [Google Scholar]
- 16.Vernon H, Mior S. The Neck Disability Index: a study of reliability and validity. J Manipulative Physiol Ther. 1991;14:409–15. [PubMed] [Google Scholar]
- 17.Nurick S. The natural history and the results of surgical treatment of the spinal cord disorder associated with cervical spondylosis. Brain. 1972;95:101–8. doi: 10.1093/brain/95.1.101. [DOI] [PubMed] [Google Scholar]
- 18.Scott J, Huskisson E. Graphic representation of pain. Pain. 1976;2:175–84. [PubMed] [Google Scholar]
- 19.Kalsi-Ryan S, Singh A, Massicotte EM, et al. Ancillary outcome measures for assessment of individuals with cervical spondylotic myelopathy. Spine (Phila Pa 1976) 2013;38:S111–22. doi: 10.1097/BRS.0b013e3182a7f499. [DOI] [PubMed] [Google Scholar]
- 20.Rossier P, Wade DT. Validity and reliability comparison of 4 mobility measures in patients presenting with neurologic impairment. Arch Phys Med Rehabil. 2001;82:9–13. doi: 10.1053/apmr.2001.9396. [DOI] [PubMed] [Google Scholar]
- 21.Olindo S, Signate A, Richech A, et al. Quantitative assessment of hand disability by the Nine-Hole-Peg test (9-HPT) in cervical spondylotic myelopathy. J Neurol Neurosurg Psychiatry. 2008;79:965–7. doi: 10.1136/jnnp.2007.140285. [DOI] [PubMed] [Google Scholar]
- 22.Hosono N, Sakaura H, Mukai Y, et al. A simple performance test for quantifying the severity of cervical myelopathy. J Bone Joint Surg Br. 2008;90:1210–3. doi: 10.1302/0301-620X.90B9.20459. [DOI] [PubMed] [Google Scholar]
- 23.Watson MJ. Refining the ten-metre walking test for use with neurologically impaired people. Physiotherapy. 2002;88:386–97. [Google Scholar]
- 24.Ono K, Ebara S, Fuji T, et al. Myelopathy hand. New clinical signs of cervical cord damage. J Bone Joint Surg Br. 1987;69:215–9. doi: 10.1302/0301-620X.69B2.3818752. [DOI] [PubMed] [Google Scholar]
- 25.Kadanka Z, Mares M, Bednarik J, et al. Predictive factors for spondylotic cervical myelopathy treated conservatively or surgically. Eur J Neurol. 2005;12:55–63. doi: 10.1111/j.1468-1331.2004.00896.x. [DOI] [PubMed] [Google Scholar]
- 26.Travlos A, Pant B, Eisen A. Transcranial magnetic stimulation for detection of preclinical cervical spondylotic myelopathy. Arch Phys Med Rehabil. 1992;73:442–6. [PubMed] [Google Scholar]
- 27.Kaneko K, Kawai S, Taguchi T, et al. Coexisting peripheral nerve and cervical cord compression. Spine (Phila Pa 1976) 1997;22:636–40. doi: 10.1097/00007632-199703150-00012. [DOI] [PubMed] [Google Scholar]
- 28.Bertalanffy H, Eggert HR. Clinical long-term results of anterior discectomy without fusion for treatment of cervical radiculopathy and myelopathy. A follow-up of 164 cases. Acta Neurochir (Wien) 1988;90:127–35. doi: 10.1007/BF01560567. [DOI] [PubMed] [Google Scholar]
- 29.Tetreault LA, Nouri A, Singh A, et al. Predictors of Outcome in Patients with Cervical Spondylotic Myelopathy undergoing Surgical Treatment: A Survey of Members from AOSpine International. World Neurosurg. 2013 doi: 10.1016/j.wneu.2013.09.023. in press. [DOI] [PubMed] [Google Scholar]
- 30.Chiles BW, 3rd, Leonard MA, Choudhri HF, et al. Cervical spondylotic myelopathy: patterns of neurological deficit and recovery after anterior cervical decompression. Neurosurgery. 1999;44:762–9. doi: 10.1097/00006123-199904000-00041. discussion 9–70. [DOI] [PubMed] [Google Scholar]
- 31.Yukawa Y, Kato F, Ito K, et al. “Ten second step test” as a new quantifiable parameter of cervical myelopathy. Spine (Phila Pa 1976) 2009;34:82–6. doi: 10.1097/BRS.0b013e31818e2b19. [DOI] [PubMed] [Google Scholar]
- 32.Holly LT, Matz PG, Anderson PA, et al. Functional outcomes assessment for cervical degenerative disease. J Neurosurg Spine. 2009;11:238–44. doi: 10.3171/2009.2.SPINE08715. [DOI] [PubMed] [Google Scholar]
- 33.Karpova A, Arun R, Davis AM, et al. Predictors of surgical outcome in cervical spondylotic myelopathy. Spine (Phila Pa 1976) 2013;38:392–400. doi: 10.1097/BRS.0b013e3182715bc3. [DOI] [PubMed] [Google Scholar]
- 34.Lesoin F, Bouasakao N, Clarisse J, et al. Results of surgical treatment of radiculomyelopathy caused by cervical arthrosis based on 1000 operations. Surg Neurol. 1985;23:350–5. doi: 10.1016/0090-3019(85)90205-8. [DOI] [PubMed] [Google Scholar]
- 35.Lyu RK, Tang LM, Chen CJ, et al. The use of evoked potentials for clinical correlation and surgical outcome in cervical spondylotic myelopathy with intramedullary high signal intensity on MRI. J Neurol Neurosurg Psychiatry. 2004;75:256–61. [PMC free article] [PubMed] [Google Scholar]
- 36.Holly LT, Matz PG, Anderson PA, et al. Clinical prognostic indicators of surgical outcome in cervical spondylotic myelopathy. J Neurosurg Spine. 2009;11:112–8. doi: 10.3171/2009.1.SPINE08718. [DOI] [PubMed] [Google Scholar]
- 37.Morishita Y, Hida S, Naito M, et al. Evaluation of cervical spondylotic myelopathy using somatosensory-evoked potentials. Int Orthop. 2005;29:343–6. doi: 10.1007/s00264-005-0002-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wasserman E, Epstein C, Ziemann U. The Oxford Handbook of Transcranial Stimulation. New York: Oxford University Press; 2008. [Google Scholar]
- 39.Nikolaidis I, Fouyas IP, Sandercock PA, et al. Surgery for cervical radiculopathy or myelopathy. Cochrane Database Syst Rev. 2010:Cd001466. doi: 10.1002/14651858.CD001466.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]