Abstract
Type 1 diabetic osteoporosis results from impaired osteoblast activity and death. Therefore, anti-resorptive treatments may not effectively treat bone loss in this patient population. Intermittent parathyroid hormone (PTH) treatment stimulates bone remodeling and increases bone density in healthy subjects. However, PTH effects may be limited in patients with diseases that interfere with its signaling. Here, we examined the ability of 8 and 40 μg/kg intermittent PTH to counteract diabetic bone loss. PTH treatment reduced fat pad mass and blood glucose levels in non-diabetic PTH treated mice, consistent with PTH affecting glucose homeostasis. However, PTH treatment did not significantly affect general body parameters, including the blood glucose levels, of type 1 diabetic mice. We found that the high dose of PTH significantly increased tibial trabecular bone density parameters in control and diabetic mice, and the lower dose elevated trabecular bone parameters in diabetic mice. The increased bone density was due to increased mineral apposition and osteoblast surface, all of which are defective in type 1 diabetes. PTH treatment suppressed osteoblast apoptosis in diabetic bone, which could further contribute to the bone-enhancing effects. In addition, PTH treatment (40 μg/kg) reversed preexisting bone loss from diabetes. We conclude that intermittent PTH may increase type 1 diabetic trabecular bone volume through its anabolic effects on osteoblasts.
Keywords: diabetes, bone, PTH, osteoporosis, osteoblast, death, bone recovery
INTRODUCTION
Bone is a highly dynamic tissue that is constantly remodeling to maintain blood calcium homeostasis and to respond to altered demand for structural support. Osteoblasts (bone forming cells) and osteoclasts (bone resorbing cells) work simultaneously to repair microcracks and maintain bone density and strength. Certain diseases and conditions can alter the balance of bone formation and resorption and can often lead to bone loss in men and women, which heightens the risk of osteoporosis from aging and menopause (McCabe, 2007). One such disease is type 1 (T1-) diabetes, in which patients are hyperglycemic and hypoinsulinemic and display bone loss and increased fracture risk (Auwerx et al., 1988; Follak et al., 2004; Gandhi et al., 2005; Kemink et al., 2000; Levin et al., 1976). As with secondary osteoporosis resulting from any disease, understanding the mechanism of T1-diabetic bone loss is necessary for choosing the best therapies. Osteoporosis from T1-diabetes is marked by decreased bone formation (and unchanged or decreased bone resorption) in both humans and animals (Botolin et al., 2005; Bouillon et al., 1995; Danne et al., 1997; Fowlkes et al., 2008; Goodman and Hori, 1984; Holl et al., 1998; McCabe, 2007; Motyl et al., 2009). Reduced bone formation markers in diabetes include reduced serum osteocalcin levels as well as reduced mRNA levels of osteocalcin, runt-related transcription factor 2 (runx2), osterix and Dlx5 in bone. This suggests impaired differentiation and maturation of osteoblasts. It has been suggested that reduced marrow stromal cell differentiation to the osteoblast lineage could also conribute to suppressed bone formation and be related to the increased number of adipocytes in the bone marrow (both osteoblasts and adipocytes arise from mesenchymal stem cells (MSCs)) (Botolin et al., 2005; Botolin and McCabe, 2007; Fowlkes et al., 2008; Martin and McCabe, 2007; Motyl et al., 2009). However, recent studies have demonstrated a disconnect between osteoblast and adipocyte differentiation in T1-diabetes. For example, we demonstrated that inhibition of marrow adiposity with leptin or the PPARg inhibitor bisphenol-A-diglycidyl ether (BADGE) was unable to prevent bone loss from T1-diabetes (Botolin and McCabe, 2006b; Motyl and McCabe, 2009b). In addition to reduced maturation, we found that osteoblasts undergo apoptosis as early as 2 days after blood glucose levels become elevated in the streptozotocin mouse model of T1-diabetes and at comparably early time points in the spontaneously diabetic mice (Coe et al., 2009). Thus, a treatment that could target osteoblasts (by promoting differentiation and/or preventing apoptosis) may benefit the bone health of patients with diabetes.
Most treatments for osteoporosis are anti-resorptive, meaning they work by inhibiting osteoclast activity. Bisphosphonates (the most widely used antiresorptive therapy) have a similar structure to inorganic pyrophosphate and can stably incorporate into bone (Russell and Rogers, 1999; Tashjian and Gagel, 2006). When osteoclasts encounter bisphosphonates embedded in bone, resorption is halted, and osteoclasts often undergo apoptosis (Russell, 2006). This could present a problem for fracture repair and everyday remodeling of microcracks (Abrahamsen et al., 2009; Goh et al., 2007; Kwek et al., 2008; Lenart et al., 2008; Neviaser et al., 2008). In T1-diabetes, bone resorption is already suppressed (McCabe, 2007); therefore, further suppression of resorption with antiresorptive therapies may worsen impaired fracture healing in these patients. Compared to the total patient population receiving bisphosphonates for osteoporosis, diabetic patients were nearly 5 times more likely to be diagnosed with osteonecrosis of the jaw (Khamaisi et al., 2007). Other antiresorptive treatments for osteoporosis exist such as hormone replacement therapy, selective estrogen receptor modulators (SERMs), and calcitonin. Because bone loss from T1-diabetes is predominantly the result of reduced bone formation, not increased resorption, anabolic therapies that target bone formation directly may be the most appropriate treatment for T1-diabetic patients.
Currently, the only anabolic treatment approved for use in the US is the N-terminus of parathyroid hormone (PTH), also called teriparatide (Vahle et al., 2004; Vahle et al., 2002). The mechanism of PTH action in bone is complex and dependent on dosing. Chronic elevation of serum PTH levels causes net bone resorption and calcium release (Jilka et al.; Ma et al., 2001). Intermittent (daily subcutaneous injections) PTH treatment, on the other hand, causes a net increase in bone formation and reduction of fracture risk in humans and laboratory animals (Barnes et al., 2008; Burr et al., 2001; Dempster et al., 1993; Jilka, 2007; Neer et al., 2001). However, intermittent PTH treatment at high doses (much greater than the standard human dose of 20 μg/kg/day), while beneficial for bone density, have been associated with osteosarcoma in rats (Vahle et al., 2004; Vahle et al., 2002).
There are no reports examining the efficacy of PTH treatment for T1-diabetic bone loss in humans. In laboratory animals, intermittent PTH treatment has proven to be anabolic (Dempster et al., 1993), and is effective at increasing bone formation in models of unloading, ovariectomy, and alcohol consumption (Burr et al., 2001; Liu and Kalu, 1990; Liu et al., 1991; Sibonga et al., 2007; Tanaka et al., 2004; Turner et al., 1998). Additionally, 4-week intermittent PTH treatment of STZ-diabetic rats improves bone density parameters at 4, 6 and 8 weeks after diabetes induction (Tsuchida et al., 2000). Similarly, PTH related protein (PTHrP) treatment of streptozotocin-diabetic mice also enhances bone formation markers and bone density (Lozano et al., 2009). The effects of 8 compared to 40 μg/kg intermittent PTH treatment on diabetic bone loss has yet to be examined with regard to its impact on bone density loss and recovery and on molecular parameters of diabetic bone (such as adiposity and lineage markers).
Here we examined the efficacy of intermittent PTH therapy on several aspects of bone loss in T1-diabetic mice (bone formation, osteoblast apoptosis, and bone resorption). We used a lower dose (8 μg/kg body weight) as well as a standard dose use in the literature (40 μg/kg). Both doses were sufficient to restore trabecular bone density of T1-diabetic mice back to untreated control levels. We demonstrated that this effect was due to increased osteoblast maturity, viability and mineralization. We also found that PTH was capable of restoring diabetic bone density to normal levels even when initiated after bone loss had already occurred. This is crucial for PTH to be an effective clinical treatment of osteoporosis in already diagnosed type I diabetic patients. Taken together, our clinically relevant findings warrant further exploration of the use of PTH in treating bone loss human diabetic subjects.
MATERIALS AND METHODS
Diabetes induction
Male adult (15–16 week) BALB/c mice were obtained from Harlan Sprague Dawley (Indianapolis, IN). All mice were maintained on a 12-hour light, 12-hour dark cycle at 23 °C, were given standard lab chow, and had food and water ad libitum. At 14 weeks of age, when mice were in the plateau phase of growth, mice were given intraperitoneal injections of streptozotocin (50 mg/kg; to induce diabetes) or 0.1 M citrate buffer pH 4.5 vehicle (control) for five consecutive days. Diabetes was confirmed 12 days after the first injection with an AccuChek compact glucometer (Roche, Nutley, NJ) and a drop of blood collected from the saphenous vein. Blood glucose over 300 mg/dl was considered diabetic.
PTH Treatment
PTH (Bachem, Torrance, CA) was stored in glass vials topped with argon gas at −80 °C as a 10−4 M stock in 4 mM HCl supplemented with 0.1% bovine serum albumin. PTH did not go through more than one freeze/thaw cycle. Immediately before injection, stock PTH was diluted in ice cold 0.9% saline. Control and diabetic mice were subjected to daily subcutaneous injections of each PTH dosing regimen: 1) daily vehicle treatment from day 0 until harvest (day 40), 2) daily 8 μg/kg PTH from days 0–40, 3) daily 40 μg/kg PTH from day 0–40 or 4) daily vehicle treatment from days 0–19 followed by daily 40 μg/kg PTH from days 20–40. At the indicated time points, mice were harvested and serum was collected, tissues were weighed, and tissues and bones were either fixed in formalin or frozen in liquid nitrogen and stored at −80°C. All animal procedures were performed in accordance with Michigan State University Institutional Animal Care and Use Committee.
Micro-computed tomography (μCT) analyses
Fixed bones were scanned in a medium of 70% ethanol using a GE Explore Locus μCT system at a voxel resolution of 20 μm3 obtained from 720 views. Beam angle of increment was 0.5 and beam strength was set at 80 kVp and 450 mA. Each run included bones from each treatment group and a calibration phantom to standardize grayscale values and maintain consistency. Based on autothreshold and segmentation analyses of multiple bone samples, a fixed threshold (800) was used to separate bone from bone marrow. Accuracy was verified by comparison of the original and segmented image slices. Cortical bone analyses were made in a defined 2 mm3 cube in the mid-diaphysis immediately proximal to the distal tibial-fibular junction, with the exception of cortical bone mineral density (BMD), which were made in a 0.1 mm3 cube. Trabecular bone analyses were performed in a manually defined region of trabecular bone beginning at 0.17 mm (1% of the total length) distal to the growth plate of the proximal tibia and extending 2 mm toward the diaphysis, and excluding the outer cortical shell. Trabecular bone mineral content (BMC), BMD, bone volume fraction (BV/TV), thickness (Tb.Th), separation (Tb.Sp) and number (Tb.N) and cortical BMD, moment of inertia (MOI), thickness, inner and outer perimeter, and marrow, cortical and total area values were computed by a GE Healthcare MicroView software application for visualization and analysis of volumetric image data. Trabecular isosurface images were taken from a cylindrical region in the tibia immediately distal to the proximal growth plate measuring 0.8 mm in length and diameter.
Bone histology and histomorphometry
Fixed femur samples were dehydrated and infiltrated using a routine overnight processing schedule. Samples were then embedded in paraffin and 5 mm sections obtained. Slides were stained for tartrate-resistant acid phosphatase (TRAP) activity according to manufacturer protocol (387A-1KT, Sigma, St. Louis, MO). The identity of the sections was not revealed until all measures were obtained. Measurements were obtained from two separate people and averaged. Osteoclast surface area was measured and expressed as a percentage of total bone surface in the femur trabecular region ranging from the distal growth plate to 2 mm proximal. Osteoblasts were counted and expressed relative to total trabecular bone surface. Adipocytes, greater than 30 μm in diameter, were counted in the same area.
To detect cell death in vivo, the TACS·XL® Basic In Situ Apoptosis Detection Kit was used according to manufacturer protocol (Trevigen Inc., Gaithersburg, MD). Positive controls included slides incubated with nuclease. Five trabecular regions were examined for each section. Total osteoblast number counted ranged between 45 and 150 per mouse. Three people carried out the analyses and obtained similar results.
For mineral apposition rate (MAR), mice were injected intraperitoneally with 200 ml of 10 mg/ml calcein (Sigma, St. Louis, MO, USA) at 9 and 2 days before harvest. Sections were photographed under fluorescent light and the distance between lines of calcein was measured. All photomicrograph measurements were performed with Image Pro Plus software (Media Cybernetics, Inc., Bethesda, MD).
RNA analyses
Tibias were cleaned of muscle and connective tissue, snap frozen in liquid nitrogen and stored at −80°C. Frozen tibias were crushed under liquid nitrogen conditions with a Bessman Tissue Pulverizer (Spectrum Laboratories, Inc., Rancho Dominguez, CA). RNA was isolated with Tri Reagent (Molecular Research Center, Inc., Cincinnati, OH) and integrity was assessed by formaldehyde-agarose gel electrophoresis. cDNA was synthesized by reverse transcription with Superscript II Reverse Transcriptase Kit and oligo dT(12-18) primers (Invitrogen, Carlsbad, CA) and amplified by real-time PCR with iQ SYBR Green Supermix (Biorad, Hercules, CA) and gene-specific primers synthesized by Integrated DNA Technologies (Coralville, IA). Hypoxanthine guanine phosphoribosyl transferase (HPRT) mRNA levels do not fluctuate in diabetes or with PTH treatment and were used as an internal control. HPRT was amplified using 5′-AAG CCT AAG ATG AGC GCA AG-3′ and 5′-TTA CTA GGC AGA TGG CCA CA-3′ (Vengellur and LaPres, 2004). Osteocalcin was amplified using 5′-ACG GTA TCA CTA TTT AGG ACC TGT G-3′ and 5′-ACT TTA TTT TGG AGC TGC TGT GAC-3′ (Ontiveros and McCabe, 2003). TRAP5 was amplified using 5′-AAT GCC TCG ACC TGG GA-3′ and 5′-CGT AGT CCT CCT TGG CTG CT-3′ (Wiren et al., 2004). Receptor activator of nuclear factor kappa-B ligand (RANKL) was amplified using 5′-TTT GCA GGA CTC GAC TCT GGA G-3′ and 5′-TCC CTC CTT TCA TCA GGT TAT GAG-3′ (Zhao et al., 2002). OPG was amplified using 5′-GAA GAA GAT CAT CCA AGA CAT TGA C-3′ and 5′-TCC ATA AAC TGA GTA GCT TCA GGA G-3′ (Motyl and McCabe, 2009a). Real time PCR was carried out for 40 cycles using the iCycler (Bio-Rad) and data were evaluated using the iCycler software. Each cycle consisted of 95°C for 15 s, 60°C for 30 s (except for osteocalcin which had an annealing temperature of 65°C), and 72°C for 30 s. cDNA-free samples, a negative control, did not produce amplicons. Melting curve and gel analyses (sizing, isolation, and sequencing) were used to verify single products of the appropriate base pair size.
Statistical Analyses
All measurements are presented as mean ± standard error. Statistically significant (α = 0.05) main effects (of PTH dose or diabetes) as well as PTH x diabetes interaction (which would indicate diabetes altering PTH effects or visa versa) were determined using factorial analysis of variance (ANOVA) and one-way ANOVA with Tukey HSD post hoc test (where necessary) with SPSS statistical software (Chicago, IL). Student’s t-test was also used to determine significance where necessary.
RESULTS
Diabetes induction and body composition
Diabetes was induced in 15–16 week old adult male mice, at the plateau phase of their growth. At the same time, mice began a PTH treatment regimen. Control and diabetic mice were injected daily with either vehicle, low dose (8 μg/kg) PTH, or high dose (40 μg/kg) PTH. Mice were harvested 40 days after the first injection. Statistical analysis with ANOVA indicated a significant (p < 0.05) effect from diabetes on blood glucose, as expected. ANOVA did not detect a significant interaction between PTH treatment and diabetes or PTH treatment and blood glucose levels, indicating that PTH treatment did not interfere with diabetes induction (Table I). Similar to previous findings, diabetic mice weighed 9% less than controls at the end of the study (Motyl and McCabe, 2009b). Weight loss was due in part to muscle and fat loss: diabetic mice had 16% lower tibialis anterior mass, 41% lower femoral fat pad mass and 57% lower perirenal fat pad mass (Table I). When analyzed by factorial ANOVA, neither 8 nor 40 μg/kg PTH treatment regimens altered the diabetes-induced loss of total body, fat or muscle mass (Table I).
Table I.
Blood glucose, muscle and fat composition of control and diabetic, vehicle and PTH treated mice at 40 days.
| Vehicle
|
8 μg/kg PTH
|
40 μg/kg PTH
|
||||
|---|---|---|---|---|---|---|
| C (n = 19) | D (n = 17) | C (n = 13) | D (n = 12) | C (n = 14) | D (n = 15) | |
|
|
|
|
||||
| Non-fasting glucose (mg/dl) | 173 ± 8 | 491 ± 27* | 151 ± 9@ | 531 ± 14* | 164 ± 13 | 534 ± 19* |
| Body mass (g) | 27.9 ± 0.4 | 25.5 ± 0.5* | 28.6 ± 0.4 | 25.6 ± 0.6* | 29.0 ± 0.5 | 26.3 ± 0.5* |
| Tibialis anterior (mg) | 50 ± 1 | 42 ± 3* | 52 ± 1 | 43 ± 1* | 52 ± 1 | 43 ± 1* |
| Femoral fat pad (mg) | 133 ± 5 | 78 ± 7* | 112 ± 8^ | 76 ± 6* | 114 ± 9@ | 75 ± 6* |
| Perirenal fat pad (mg) | 37 ± 3 | 16 ± 2* | 31 ± 3@ | 16 ± 2* | 32 ± 4 | 14 ± 2* |
| Marrow adipocyte #/mm2 § | 7 ± 1 | 20 ± 3* | 12 ± 2^ | 23 ± 7* | 9 ± 3 | 17 ± 3* |
Abbreviations: C, control; D, diabetic; PTH, parathyroid hormone, no., number.
Significance:
p < 0.05 compared treatment-matched control.
p < 0.05 compared to vehicle treated control.
p < 0.1 compared to vehicle treated control, by t-test.
n = 4–12 per group.
In light of recent findings suggesting a role for uncarboxylated osteocalcin (released during resorption) in regulating glucose homeostasis (Ferron 2010 page 296), we performed post-hoc analysis of non-diabetic blood glucose and body composition of vehicle and PTH treated mice. Blood glucose was slightly lower (p=0.08) in 8 μg/kg PTH treated mice, but not different with 40 μg/kg treatment (Table I). Interestingly, 8 μg/kg PTH treatment alone significantly reduced femoral fat pad mass and tended to reduce (p=0.07) perirenal fat pad mass in non-diabetic mice. The effect from higher dose PTH was not as strong: 40 μg/kg PTH tended to reduce femoral fat pad mass but did not significantly alter perirenal fat.
As we and others have previously demonstrated, loss of peripheral and visceral fat depots in diabetes was accompanied by increased bone marrow adiposity (Table I). Diabetes induced a 2.8-fold increase in marrow adipocyte number in vehicle treated mice. At the 8 or 40 μg/kg PTH treatment doses, marrow adiposity also increased by nearly 2-fold. The level of marrow adiposity did not significantly differ between diabetic mice of each group.
PTH Counteracted Diabetic Bone Loss
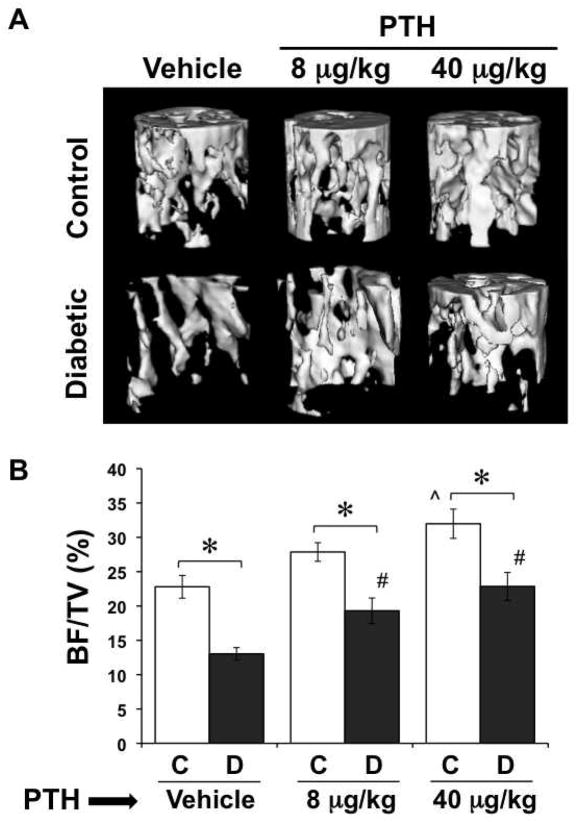
When analyzed by ANOVA, diabetes significantly (p < 0.05) impacted trabecular bone parameters (Figure 1, Table II). As we have demonstrated in the past, diabetes reduced tibia trabecular BMC, BMD, BV/TV, trabecular thickness, and trabecular number (Figure 1, Table II) (Botolin et al., 2005). Significant PTH effects were also found with all of the above parameters in both euglycemic and diabetic mice. Specifically, the high dose PTH (40 μg/kg), but not the low dose (8 μg/kg), treatment significantly increased trabecular parameters in euglycemic mice (Figure 1, Table II). Although PTH treated diabetic mice had a reduction in trabecular parameters compared to PTH treated euglycemic mice, bone density did not decline to the level of vehicle treated diabetics. Furthermore, the bone parameters of the high dose (40 μg/kg) PTH treated diabetic group were more closely aligned with those of healthy untreated controls and were significantly elevated (with the exception of trabecular spacing, which was significantly decreased) compared to the vehicle treated diabetic mice. The increase in bone density in diabetic PTH treated mice (compare to vehicle treated diabetics) was higher with the 40 μg/kg dose of PTH than with the 8 μg/kg dose, but 8 μg/kg PTH still produced a significant increase in BV/TV and trabecular number. For example, trabecular number increased 25% in 8 μg/kg PTH treated diabetics and 39% in 40 μg/kg PTH treated diabetics. Thus, the PTH treatment was successful in raising “basal” bone parameters such that a diabetes induced decrease in bone density was still within the healthy control mouse range.
Figure 1. PTH treatment counteracted trabecular bone loss from T1-diabetes.
Diabetes was induced with STZ at 14 weeks of age. At the same time, control (citrate buffer only) and diabetic mice were started on a daily regimen of subcutaneous injections of PTH (8 or 40 μg/kg) or saline vehicle. PTH treatment was continued for the remainder of the study. Mice were harvested at 40 days after the first streptozotocin injection and tibias were analyzed by μCT. (A) Representative three-dimensional isosurface images of the trabecular bone of the proximal tibia of control and diabetic, vehicle and PTH treated mice. (B) BV/TV of control (white bars) and diabetic (gray bars), vehicle and PTH treated mouse tibias. Bars represent mean ± standard error. n ≥ 12 per group. Significance between groups was determined with a post-hoc test (only after factorial ANOVA determined significance). *p < 0.05 compared to treatment (vehicle, 8 or 40 μg/kg PTH) matched control. ^p < 0.05 compared to vehicle-treated control. #p < 0.05 compared to vehicle-treated diabetic.
Table II.
μCT analysis of control (C) and diabetic (D), vehicle and PTH treated mice at 40 dpi.
| Vehicle
|
8 mg/kg PTH
|
40 mg/kg PTH
|
||||
|---|---|---|---|---|---|---|
| C (n = 15) | D (n = 17) | C (n = 13) | D (n = 12) | C (n = 13) | D (n = 15) | |
|
|
|
|
||||
| TRABECULAR | ||||||
| BMC (mg) | 0.78 ± 0.03 | 0.62 ± 0.02* | 0.84 ± 0.07 | 0.70 ± 0.04* | 0.92 ± 0.06^ | 0.79 ± 0.05*# |
| BMD (mg/cm3) | 262 ± 9 | 208 ± 6* | 283 ± 12 | 237 ± 13* | 307 ± 17^ | 262 ± 14*# |
| BV/TV (%) | 22.8 ± 1.7 | 13.0 ± 0.9* | 27.9 ± 1.4 | 19.3 ± 1.2*# | 32.0 ± 2.1^ | 22.9 ± 2.0*# |
| Tb.Th (μm) | 48 ± 2 | 36 ± 1* | 54 ± 2 | 42 ± 2* | 61 ± 4^ | 44 ± 2*# |
| Tb.Sp (μm) | 260 ± 19 | 277 ± 13 | 221 ± 23 | 247 ± 16 | 216 ± 18 | 227 ± 20# |
| Tb.N (mm−1) | 4.8 ± 0.3 | 3.6 ± 0.2* | 5.4 ± 0.2 | 4.5 ± 0.2*# | 5.6 ± 0.1^ | 5.0 ± 0.3*# |
| CORTICAL | ||||||
| Ct.Th (μm) | 328 ± 6 | 319 ± 7 | 344 ± 4 | 330 ± 7 | 360 ± 6^ | 329 ± 4* |
| MOI (mm4) | 0.091 ± 0.004 | 0.090 ± 0.004 | 0.087 ± 0.004 | 0.080 ± 0.005 | 0.102 ± 0.005 | 0.090 ± 0.006 |
| Ec.Pm (mm) | 1.52 ± 0.03 | 1.55 ± 0.04 | 1.53 ± 0.04 | 1.48 ± 0.04 | 1.56 ± 0.04 | 1.61 ± 0.05 |
| Ps.Pm (mm) | 3.72 ± 0.07 | 3.66 ± 0.05 | 3.82 ± 0.07 | 3.67 ± 0.07 | 3.95 ± 0.06^ | 3.86 ± 0.08 |
| Ma.Ar (mm2) | 0.15 ± 0.01 | 0.16 ± 0.01 | 0.16 ± 0.01 | 0.15 ± 0.01 | 0.16 ± 0.01 | 0.17 ± 0.01 |
| Ct. Ar (mm2) | 0.80 ± 0.02 | 0.77 ± 0.02 | 0.86 ± 0.02 | 0.79 ± 0.3 | 0.92 ± 0.02^ | 0.83 ± 0.03* |
| Tt.Ar (mm2) | 0.95 ± 0.03 | 0.93 ± 0.02 | 1.02 ± 0.03 | 0.93 ± 0.04 | 1.08 ± 0.03^ | 1.00 ± 0.04 |
| BMD (mg/cm3) | 1071 ± 24 | 1042 ± 16 | 1086 ± 16 | 1071 ± 18 | 1065 ± 25 | 1060 ± 13 |
Abbreviations: A, area; BMC, bone mineral content; BMD, bone mineral density, BV/TV, bone volume fraction; Tb.Th, trabecular thickness; Tb.Sp, trabecular separation; Tb.N, trabecular number; Ct.Th, cortical thickness; MOI, moment of inertia; Ec.Pm, endocortical perimeter; Ps.Pm periosteal perimeter; Ma.Ar, marrow area; Ct.Ar, cortical bone area; Tt.Ar total cross sectional area.
Signficance:
p < 0.05 compared to treatment matched control;
p < 0.05 compared to vehicle treated control;
p < 0.05 compared to vehicle treated diabetic.
Cortical bone thickness was also affected by diabetes and PTH treatment, according to ANOVA, but there was no significant interaction between diabetes and PTH. Thickness was increased in 40 μg/kg PTH treated euglycemic controls (compared to vehicle treated controls), but not in 8 μg/kg treated controls (Table II). The former can be attributed to an increase outer perimeter in the 40 μg/kg group, which consequently increased both cortical and total area. In contrast to our previous studies (Motyl and McCabe, 2009b), diabetes did not induce any significant changes in cortical bone parameters in vehicle treated mice. However, diabetes did reduce cortical thickness and cortical area in 40 μg/kg PTH treated mice compared to 40 μg/kg PTH treated controls, but these parameters were not significantly different from healthy untreated controls. No significant changes were found in cortical BMD, MOI, inner perimeter or marrow area.
PTH increased osteoblast parameters
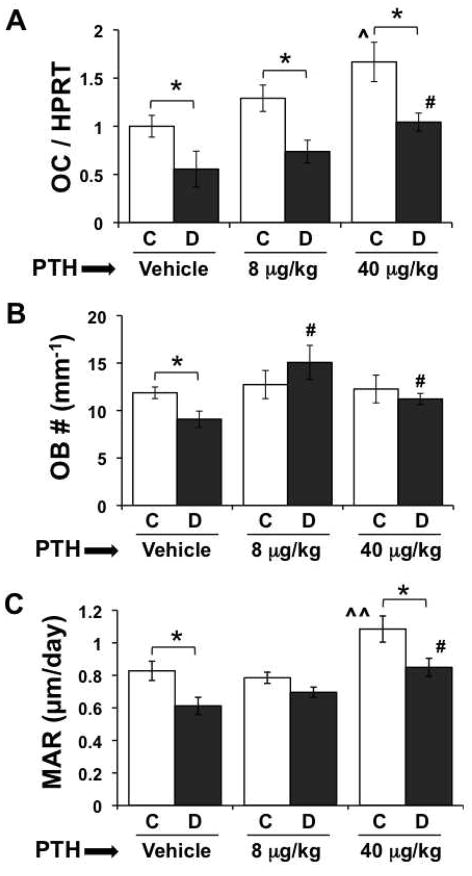
To examine the influence of PTH on osteoblast activity we measured osteocalcin gene expression in tibia, osteoblast number/bone surface and mineral apposition rate. PTH treatment of euglycemic control mice significantly (by factorial ANOVA) affected osteocalcin, such that there was a stepwise increase in mRNA levels that reached statistical significance in the 40 μg/kg PTH treated group (Figure 2A). Diabetes also significantly reduced bone OC mRNA levels in untreated mice, consistent with previous findings; this reduction remained evident in both PTH treated diabetic groups compared to PTH treated controls. However, as with the controls, PTH treatment increased osteocalcin in the diabetic mice compared to untreated diabetics, and this was significant with the 40 μg/kg dose. Furthermore, OC mRNA levels were not different in the diabetic 40 μg/kg mice compared to untreated controls, consistent with bone density being similar between these two groups. Examination of osteoblast number (per mm tibia trabecular bone surface) indicated a decrease in untreated diabetic compared to control mice, consistent with previous reports (Botolin et al., 2005). However, no differences were seen between 8 or 40 μg/kg PTH treated control groups compared to untreated controls (Figure 2B); this may be a result of less sensitivity in the 2D-histomorphometry compared to whole bone imaging and RNA analyses or may result from time dependent responses. Despite this, it was still possible to detect that PTH treatment prevented the reduction in osteoblast number in diabetics compared to control mice and significantly increased osteoblast numbers in treated compared to untreated diabetic mice (Figure 2B). Similarly, mineral apposition rate (MAR) was significantly decreased in untreated diabetic compared to control mice. In contrast, MAR was increased in the 40 μg/kg PTH control group compared to untreated and 8 μg/kg PTH treated controls (Figure 2C). Even though the MAR was significantly reduced in diabetic 40 μg/kg PTH treated mice compared to treatment-matched controls, the value of MAR in 40 μg/kg PTH treated mice was not different from untreated control mice and was significantly higher than untreated diabetic mice. Taken together, high dose PTH appears capable of promoting bone anabolic processes in diabetic mice by preventing diabetes-induced reduction of osteoblasts and by increasing MAR. Low dose PTH treatment affected some parameters albeit at a less robust level.
Figure 2. PTH promotes bone formation in diabetes.
(A) mRNA from whole frozen tibiae was converted to cDNA and amplified with primers specific for osteocalcin (OC) and the housekeeping gene HPRT. (B) Osteoblasts lining the surface of trabeculi in the distal femur were identified in hematoxylin stained slides based on morphology, counted, and expressed per mm bone surface. (C) The distance between calcein double labels was measured in undecalcified, unstained L5 vertebrae sections and expressed per day. Bars represent mean ± standard error of control (C, white bars) and diabetic (D, gray bars), vehicle, 8 μg/kg and 40 μg/kg PTH treated mice at 40 days after the first injection. PTH treatment was daily from 0 to 40 days. N = 4–8 per group. Significance between groups was determined with a post-hoc test (only after factorial ANOVA determined significance). *p < 0.05 compared to treatment (vehicle, 8 or 40 μg/kg PTH) matched control. ^p < 0.05 compared to vehicle treated control. ^^p < 0.05 compared to vehicle treated control and 8 μg/kg PTH treated control. #p < 0.05 compared to vehicle treated diabetic.
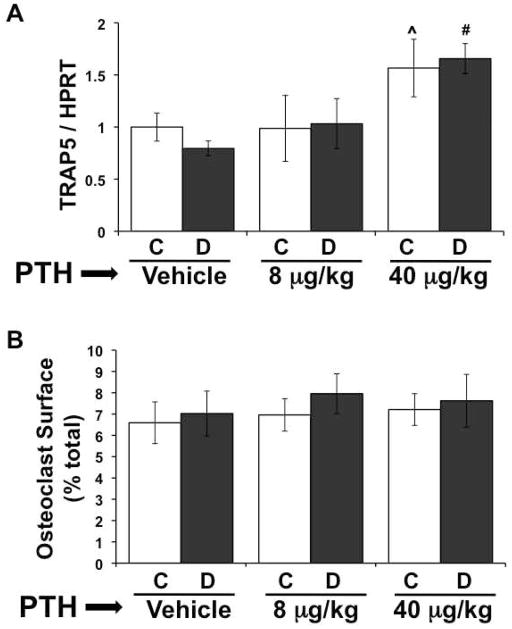
Effect of PTH on Bone Resorption
Levels of TRAP5 mRNA, a marker of active osteoclasts, were significantly elevated in the non-diabetic 40 μg/kg PTH treated group, but not in the 8 μg/kg group, compared to untreated controls (Figure 3A). This finding, in combination with increased bone formation, is indicative of increased remodeling overall in the 40 μg/kg group. As we demonstrated previously, diabetes did not alter TRAP5 expression in untreated mice, and this effect was consistent in the PTH treated groups. In agreement with overall increases in remodeling, the 40 μg/kg PTH treated diabetic mice had elevated TRAP5 expression compared to vehicle treated diabetic mice. We also examined osteoclast surface and did not find any significant diabetes or PTH effects, despite the increase in TRAP5 expression with the 40 μg/kg dose (Figure 3B). It is possible that there is more total resorption in these mice (since they have more bone surface), but when expressed as a function of total surface, it is unchanged. The fact that there is no change in osteoclast surface in diabetic vehicle treated mice compared to controls is consistent with our previous findings (Botolin et al., 2005).
Figure 3. High dose PTH promotes bone resorption in diabetic mice.
A) mRNA from frozen tibiae was converted to cDNA and amplified with primers specific for tartrate resistant acid phosphatase (TRAP5) and the housekeeping gene HPRT. (B) Osteoclasts were identified with TRAP5 staining in the distal femur. Length of trabecular bone surface covered by osteoclasts was measured and expressed as a percent of the total bone surface. Bars represent mean ± standard error of control (C, white bars) and diabetic (D, gray bars), vehicle, 8 μg/kg and 40 μg/kg PTH treated mice at 40 days. PTH treatment was daily from 0 to 40 days. N = 4–8 per group. Significance between groups was determined with a post-hoc test (only after factorial ANOVA determined significance). *p < 0.05 compared to treatment (vehicle, 8 or 40 μg/kg PTH) matched control. ^p < 0.05 compared to vehicle-treated control. #p < 0.05 compared to vehicle-treated diabetic.
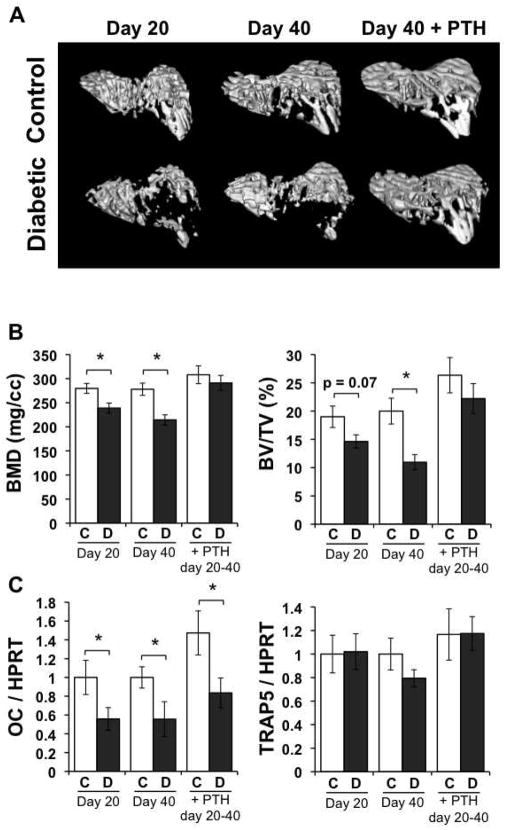
Diabetic bone loss can be reversed by PTH
Although PTH treatment was able to counteract bone loss when initiated simultaneously with diabetes induction (Figure 1 and Table II), we recognize that diagnosis of type 1 diabetes does not occur until after hyperglycemia is evident, and based on our findings, after early bone changes are initiated (Coe et al., 2009; Motyl et al., 2009). Therefore, we wanted to address whether PTH could improve bone density even after bone loss had already occurred. Diabetes was induced with streptozotocin as noted before. At 20 days after the start of the experiment, we harvested half of the control and diabetic mice to assess their bone parameters (and confirm bone loss) and began treatment of the other half with vehicle or PTH (40 μg/kg). As expected, diabetic mice at 20 days post-induction had significantly lower BMD and trended (p = 0.07) to have lower BV/TV (Figure 5A and 5B). Similarly, untreated diabetic mice at 40 days after the start of the experiment had lower BMD and BV/TV compared to untreated controls. Treating non-diabetic mice with 40 μg/kg PTH from days 20–40 was not enough to induce a significant increase in BMD or BV/TV compared to untreated controls. Conversely, treating the diabetic mice with PTH from days 20–40 increased BMD and BV/TV to levels not different from PTH-treated euglycemic controls, indicating short-term PTH imparts a stronger affect in diabetic mice than in controls.
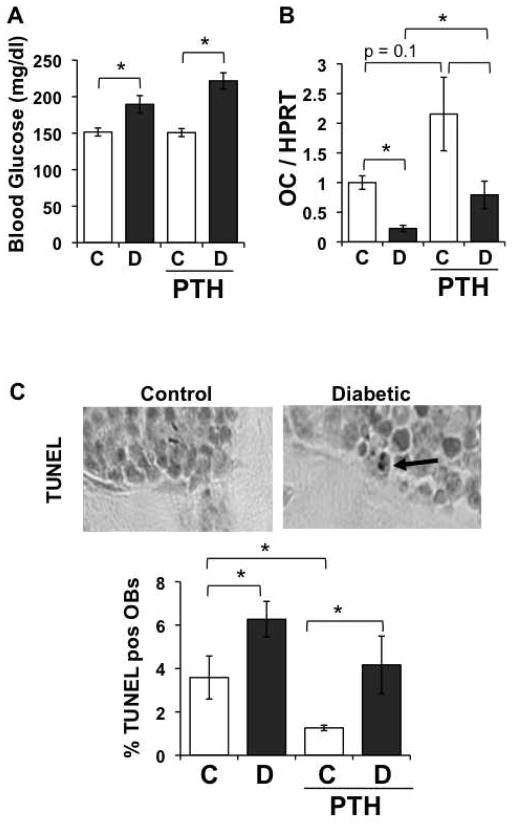
Figure 5. PTH ameliorates diabetes-induced osteoblast death.
Mice were injected with either citrate buffer (control) or STZ to induce hyperglycemia. At the same time, control and diabetic mice were started on a daily regimen of subcutaneous injections of PTH (40 μg/kg) or saline vehicle. PTH treatment was continued until harvest at 5 days. (A) Blood glucose was measured at the time of harvest and was not significantly altered by PTH-treatment. mRNA from frozen tibiae was converted to cDNA and amplified with primers specific for OC and the housekeeping gene HPRT (B). TUNEL positive osteoblasts were counted in trabecular bone in the distal femur and expressed relative to total osteoblasts (C). (D) Representative TUNEL stained femur sections. Dark brown staining is positive for DNA fragmentation. Bars represent mean ± standard error of control (C, white bars) and diabetic (D, gray bars), vehicle and 40 μg/kg PTH treated mice at 5 days. PTH treatment was daily from 0 to 5 days. N = 5–6 per group. Significance determined with student’s t-test. *p < 0.05 between bracketed bars.
To address osteoblast/osteoclast status in these mice we measured representative osteoblast and osteoclast markers, osteocalcin and TRAP5 mRNA levels (Figure 4C). Osteocalcin levels at 20 and 40 days were significantly decreased in untreated diabetic compared to control mouse bones. PTH treatment from days 20–40 promoted osteocalcin expression in both control and diabetic mice, although the increase was not significant. However, osteocalcin levels in PTH-treated diabetic mice were not different from untreated controls. Similar to our previous findings, the resorption marker TRAP5 was unchanged or tended to decrease in diabetic mice at days 20 and 40, respectively. PTH treatment for the last 20 days of the experiment was not enough to significantly increase TRAP5 levels in euglycemic or diabetic mice (although it trended to increase).
Figure 4. PTH promotes bone formation in diabetic mice when initiated after diabetic bone loss is detectable.
Mice were treated with streptozotocin to induce diabetes or citrate buffer (control). At 20 days, mice were either harvested, or treated with vehicle or 40 μg/kg PTH daily until harvest at 40 days. (A) Representative μCT isosurface images of trabecular bone immediately distal to the proximal growth plate. (B) Tibia bone mineral density (BMD) and bone volume fraction (BV/TV) from control (C, white bars) and diabetic (D, dark gray bars) untreated and PTH treated mice. (C) mRNA from frozen tibiae was converted to cDNA and amplified with primers specific for the bone formation marker osteocalcin (OC) or resorption marker TRAP5 and expressed relative to the housekeeping gene, HPRT. Bars represent mean ± standard error. N = 6 per group. Significance determined with student’s t-test. *p < 0.05 between bracketed bars.
Osteoblast viability is improved by PTH treatment
We previously demonstrated an increase in osteoblast death in diabetic mice (Coe et al., 2009) can contribute to the reduced osteoblast surface and MAR. Therefore, we examined the impact of PTH treatment on diabetes-induced osteoblast death. Diabetes was induced as before, control and diabetic mice were treated with daily injections of 40 μg/kg PTH, and mice harvested at 5 days after the first injections, a time point where osteoblast death has previously been identified (Coe et al., 2009)). Blood glucose levels were significantly elevated at this early time point (even though mice are not technically considered diabetic, defined as >300 mg/dl, at this time). PTH did not alter the blood glucose levels (Figure 5A). To address osteoblast status, we measured tibia mRNA levels of osteocalcin (Figure 5B). As we demonstrated in the past, osteocalcin mRNA levels were reduced nearly 5-fold in untreated diabetic mice compared to untreated controls (Motyl et al., 2009). PTH treatment tended to increase osteocalcin expression in non-diabetic mice (p = 0.1) and significantly increased osteocalcin expression in treated compared to untreated diabetic mice, similar to what we observed at 40 days (Figure 2). In contrast to osteocalcin expression, TUNEL positive osteoblasts increased in diabetic compared to control mice (Figure 5C). PTH-treatment was able to significantly lower baseline TUNEL staining. Although diabetes still induced osteoblast death in PTH-treated mice, the level of TUNEL staining was not different from untreated controls and trended to be lower than that in untreated diabetics (Figure 5C). These results suggest that the PTH reduction in basal osteoblast apoptosis could contribute to its bone health benefits in type I diabetic mice.
DISCUSSION
Osteoporosis is a severe complication of T1-diabetes that results from reduced bone formation, and unchanged or reduced resorption. Therefore, we determined whether anabolic PTH therapy was capable of enhancing and reversing T1-diabetic bone pathology. PTH counteracted trabecular bone changes when diabetic mice were treated daily for 40 days. PTH at a 40 μg/kg dose promoted overall bone remodeling by increasing osteocalcin expression, MAR, osteoblast number and TRAP5 expression in diabetic mice. In addition, PTH treatment lowered basal osteoblast apoptosis, such that diabetes-induced osteoblast apoptosis levels were not different from apoptosis levels in control, untreated mice. Finally, we also determined that PTH treatment was capable of reversing diabetic bone loss after significant trabecular bone changes had already occurred.
Although diabetes significantly reduced osteoblast parameters, PTH treatment (40 μg/kg) increased baseline levels of bone anabolic markers such that the values in diabetic PTH-treated mice did not differ from those of untreated controls. This is consistent with PTH regulating osteoblast proliferation and differentiation (Krishnan et al., 2003; Pettway et al., 2008). Similarly, tail-suspended mice treated with PTH (40 μg/kg, 5 days/wk) had increased bone formation rates, albeit to a lesser extent than control mice (Tanaka et al., 2004). Our finding that bone parameters do not differ between diabetic PTH treated and untreated controls suggests that PTH may promote bone formation and overall remodeling in T1-diabetes. However, it should be noted that a recent study examining bone healing found that PTH treatment enhanced healing in control but not T1-diabetic rats (Kuchler et al., 2011). This study suggests that metabolic control may be necessary to obtain PTH anabolic benefits in T1-diabetes conditions. Differences between our study and this study are likely due to species differences, dosing (60 μg/kg compared to 8 or 40 μg/kg in our study) and the area of focus: bone remodeling compared to bone healing, the latter process requires vascular responsiveness and cartilage formation. Both areas need to be investigated further in clinical studies.
Interestingly, while we did not observe marked histological changes or bone density changes in control mice treated with low dose PTH (8 μg/kg), this dose successfully increased BV/TV, trabecular number and osteoblast number in diabetic compared to untreated diabetic mice. Consistent with an anabolic effect with low dose treatment, young C57BL/6 mice treated with 10 μg/kg PTH display an increase in trabecular bone density parameters (Niziolek et al., 2009). It should be noted that these doses, when compared to the human dose of 20 μg/kg, are still somewhat higher (5-fold) when taking into account equivalent surface area. Even lower doses, 1 μg/kg PTH, are still capable of increasing bone density in hind-limb unloaded rats (Turner et al., 2007), although it is unclear if they would have had an effect in healthy, load bearing rats in this study. Similarly, rats had increased BMC, BMD, and fracture healing when treated with 5–30 μg/kg PTH for more than 4 weeks (Alkhiary et al., 2005; Nakajima et al., 2002). Thus, it is possible that if our experiment had extended longer, we would have seen a significant effect with the 8 μg/kg dose in the control mice, and a stronger effect in the diabetic mice. Pettway, et al. demonstrated that the greatest increases in osteoblast proliferation occur after the first week of treatment (Pettway et al., 2008). This idea, in combination with the fact that lower dose (8 μg/kg) PTH is capable of improving bone density, suggests that an ideal treatment regimen might consist of an initial time period of high dose PTH to stimulate bone formation, followed by lower dose PTH to maintain bone density at a healthy level.
We further determined that PTH could restore bone density to normal levels even after bone loss had already occurred, consistent with other bone loss conditions, but has not been demonstrated with diabetes. Sibonga, et al. demonstrated that 80 μg/kg PTH could restore preexisting bone density in rats fed alcohol, which, like diabetes reduces bone formation (Sibonga et al., 2007). Additionally, the same dose of PTH reverses bone loss from ovariectomy in rats (Liu et al., 1991). Lozano, et al. demonstrated increased bone formation in diabetic mice treated with PTHrP, which signals through the shared PTH/PTHrP receptor, two weeks after diabetes was confirmed, a time point when bone loss should have been detectable (Botolin and McCabe, 2006b; Lozano et al., 2009; Motyl and McCabe, 2009a). It was important to address this issue because pathways responsible for bone loss from T1-diabetes (which are not completely understood) could overlap with the pathways for PTH induced bone formation, and if this were the case, then PTH might not have been an effective therapeutic.
Additionally, we determined that PTH-treatment of diabetic mice during the 20-day period had a greater effect than PTH-treatment of control mice for 20 days. It is possible that the diabetes-induced suppression of resorption actually helped bone density increase faster in the diabetic group, although we did not detect a significant suppression of TRAP5 expression from diabetes (p = 0.2) in this case. Along the same lines, PTH did not increase osteoblast number per bone surface (Figure 2B) in control mice, but it did prevent a decrease after diabetes induction, suggesting a stronger response of osteoblasts to PTH in a diabetic environment. In addition to PTH-induced osteoblast activity, there is ever-increasing evidence that PTH prevents osteoblast apoptosis. Bellido, et al. demonstrated a significant reduction of osteoblast apoptosis with 10–300 μg/kg PTH treatment for 28 days in female Swiss-Webster mice (Bellido et al., 2003). Here, we demonstrated that 40 μg/kg PTH reduced basal osteoblast apoptosis and reduced diabetes-induced increases in osteoblast apoptosis to a level comparable to that of untreated control mice (Figure 4). Recent evidence indicates that PTH promotes repair of DNA damage by increasing PCNA and Foxo3a (Schnoke et al., 2009).
Although it appeared that trabecular effects of PTH were stronger in diabetes, we have some evidence that cortical effects of PTH were weakened by diabetes. We found that 40 μg/kg PTH treatment increased cortical bone thickness and outer perimeter in euglycemic mice. This is consistent with other studies in ovariectomized mice and rats (Fox et al., 2006; Pierroz et al., 2006), however in our case, PTH could not increase cortical thickness in diabetic mice compared to diabetic controls (Table II). This suggests the possibility that cortical bone accrual in PTH treated mice is affected by diabetes whereas the mechanism of trabecular bone accrual is not. Calvi, et al. demonstrated a similar phenomenon: when the PTH receptor was constitutively active, trabecular bone density increased while periosteal MAR and cortical thickness decreased (Calvi et al., 2001), suggesting the mechanism of PTH action is location-dependent. Recently, Jilka, et al. found that although PTH has a profound anti-apoptotic effect in trabecular bone, cortical bone accrual from PTH is more likely due to pro-differentiation effects on preosteoblasts because of the comparatively low levels of osteoblast apoptosis in the periosteum, coupled with fast (2 day) increases in periosteal osteoblast number after PTH treatment (Jilka et al., 2009; Ogita et al., 2008). Levels of hyperglycemia equivalent to those in diabetes are known to have anti-differentiation as well as pro-apoptotic effects on osteoblasts (Botolin and McCabe, 2006a; Coe et al., 2009) and it is likely that both differentiation and apoptosis play a role in the diabetic osteoblast phenotype.
In summary, our data indicate that PTH reduces T1-diabetic bone loss in mice by promoting remodeling and reducing diabetes-induced osteoblast apoptosis. Because of its ability to restore bone density to normal levels, even after bone loss has already occurred, intermittent PTH therapy might be an option to promote bone formation and resorption, which are both depressed in diabetic patients. The long-term safety and mechanisms of PTH action on diseased bone and its repair is a future question that needs to be addressed in clinical studies.
Acknowledgments
Grant support: National Institutes of Health (RO1DK061184 and DK53904) and the American Diabetes Association (7-07-RA-105).
The authors thank Regina Irwin and Lindsay Coe for technical assistance and critical review of the manuscript, and the MSU Investigative Histology Laboratory for technical assistance. This work was funded by grants from the National Institutes of Health (RO1DK061184 and DK53904) and the American Diabetes Association (7-07-RA-105) to LRM.
Footnotes
Disclosure Statement: The authors have nothing to disclose.
The authors have no financial conflicts.
Contributor Information
Katherine J. Motyl, Michigan State University, Departments of Physiology and Radiology, Biomedical Imaging Research Center, East Lansing, MI 48824
Laurie K. McCauley, University of Michigan, Department of Periodontics and Oral Medicine, Department of Pathology
Laura R. McCabe, Michigan State University, Departments of Physiology and Radiology, Biomedical Imaging Research Center, East Lansing, MI 48824
References
- Abrahamsen B, Eiken P, Eastell R. Subtrochanteric and diaphyseal femur fractures in patients treated with alendronate: a register-based national cohort study. J Bone Miner Res. 2009;24(6):1095–1102. doi: 10.1359/jbmr.081247. [DOI] [PubMed] [Google Scholar]
- Alkhiary YM, Gerstenfeld LC, Krall E, Westmore M, Sato M, Mitlak BH, Einhorn TA. Enhancement of experimental fracture-healing by systemic administration of recombinant human parathyroid hormone (PTH 1-34) J Bone Joint Surg Am. 2005;87(4):731–741. doi: 10.2106/JBJS.D.02115. [DOI] [PubMed] [Google Scholar]
- Auwerx J, Dequeker J, Bouillon R, Geusens P, Nijs J. Mineral metabolism and bone mass at peripheral and axial skeleton in diabetes mellitus. Diabetes. 1988;37(1):8–12. doi: 10.2337/diab.37.1.8. [DOI] [PubMed] [Google Scholar]
- Barnes GL, Kakar S, Vora S, Morgan EF, Gerstenfeld LC, Einhorn TA. Stimulation of fracture-healing with systemic intermittent parathyroid hormone treatment. J Bone Joint Surg Am. 2008;90(Suppl 1):120–127. doi: 10.2106/JBJS.G.01443. [DOI] [PubMed] [Google Scholar]
- Bellido T, Ali AA, Plotkin LI, Fu Q, Gubrij I, Roberson PK, Weinstein RS, O’Brien CA, Manolagas SC, Jilka RL. Proteasomal degradation of Runx2 shortens parathyroid hormone-induced anti-apoptotic signaling in osteoblasts. A putative explanation for why intermittent administration is needed for bone anabolism. J Biol Chem. 2003;278(50):50259–50272. doi: 10.1074/jbc.M307444200. [DOI] [PubMed] [Google Scholar]
- Botolin S, Faugere MC, Malluche H, Orth M, Meyer R, McCabe LR. Increased bone adiposity and peroxisomal proliferator-activated receptor-gamma2 expression in type I diabetic mice. Endocrinology. 2005;146(8):3622–3631. doi: 10.1210/en.2004-1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botolin S, McCabe LR. Chronic hyperglycemia modulates osteoblast gene expression through osmotic and non-osmotic pathways. J Cell Biochem. 2006a;99(2):411–424. doi: 10.1002/jcb.20842. [DOI] [PubMed] [Google Scholar]
- Botolin S, McCabe LR. Inhibition of PPARgamma prevents type I diabetic bone marrow adiposity but not bone loss. J Cell Physiol. 2006b;209(3):967–976. doi: 10.1002/jcp.20804. [DOI] [PubMed] [Google Scholar]
- Botolin S, McCabe LR. Bone loss and increased bone adiposity in spontaneous and pharmacologically induced diabetic mice. Endocrinology. 2007;148(1):198–205. doi: 10.1210/en.2006-1006. [DOI] [PubMed] [Google Scholar]
- Bouillon R, Bex M, Van Herck E, Laureys J, Dooms L, Lesaffre E, Ravussin E. Influence of age, sex, and insulin on osteoblast function: osteoblast dysfunction in diabetes mellitus. J Clin Endocrinol Metab. 1995;80(4):1194–1202. doi: 10.1210/jcem.80.4.7714089. [DOI] [PubMed] [Google Scholar]
- Burr DB, Hirano T, Turner CH, Hotchkiss C, Brommage R, Hock JM. Intermittently administered human parathyroid hormone(1-34) treatment increases intracortical bone turnover and porosity without reducing bone strength in the humerus of ovariectomized cynomolgus monkeys. J Bone Miner Res. 2001;16(1):157–165. doi: 10.1359/jbmr.2001.16.1.157. [DOI] [PubMed] [Google Scholar]
- Calvi LM, Sims NA, Hunzelman JL, Knight MC, Giovannetti A, Saxton JM, Kronenberg HM, Baron R, Schipani E. Activated parathyroid hormone/parathyroid hormone-related protein receptor in osteoblastic cells differentially affects cortical and trabecular bone. J Clin Invest. 2001;107(3):277–286. doi: 10.1172/JCI11296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coe LM, Irwin R, Lippner D, McCabe LR. The bone marrow microenvironment contributes to type I diabetes induced osteoblast death. J Cell Physiol. 2009 doi: 10.1002/jcp.22357. [DOI] [PubMed] [Google Scholar]
- Danne T, Kordonouri O, Enders I, Weber B. Factors influencing height and weight development in children with diabetes. Results of the Berlin Retinopathy Study. Diabetes Care. 1997;20(3):281–285. doi: 10.2337/diacare.20.3.281. [DOI] [PubMed] [Google Scholar]
- Dempster DW, Cosman F, Parisien M, Shen V, Lindsay R. Anabolic actions of parathyroid hormone on bone. Endocr Rev. 1993;14(6):690–709. doi: 10.1210/edrv-14-6-690. [DOI] [PubMed] [Google Scholar]
- Follak N, Kloting L, Wolf E, Merk H. Delayed remodeling in the early period of fracture healing in spontaneously diabetic BB/OK rats depending on the diabetic metabolic state. Histol Histopathol. 2004;19(2):473–486. doi: 10.14670/HH-19.473. [DOI] [PubMed] [Google Scholar]
- Fowlkes JL, Bunn RC, Liu L, Wahl EC, Coleman HN, Cockrell GE, Perrien DS, Lumpkin CK, Jr, Thrailkill KM. Runt-related transcription factor 2 (RUNX2) and RUNX2-related osteogenic genes are down-regulated throughout osteogenesis in type 1 diabetes mellitus. Endocrinology. 2008;149(4):1697–1704. doi: 10.1210/en.2007-1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox J, Miller MA, Newman MK, Metcalfe AF, Turner CH, Recker RR, Smith SY. Daily treatment of aged ovariectomized rats with human parathyroid hormone (1-84) for 12 months reverses bone loss and enhances trabecular and cortical bone strength. Calcif Tissue Int. 2006;79(4):262–272. doi: 10.1007/s00223-006-0108-1. [DOI] [PubMed] [Google Scholar]
- Gandhi A, Beam HA, O’Connor JP, Parsons JR, Lin SS. The effects of local insulin delivery on diabetic fracture healing. Bone. 2005;37(4):482–490. doi: 10.1016/j.bone.2005.04.039. [DOI] [PubMed] [Google Scholar]
- Goh SK, Yang KY, Koh JS, Wong MK, Chua SY, Chua DT, Howe TS. Subtrochanteric insufficiency fractures in patients on alendronate therapy: a caution. J Bone Joint Surg Br. 2007;89(3):349–353. doi: 10.1302/0301-620X.89B3.18146. [DOI] [PubMed] [Google Scholar]
- Goodman WG, Hori MT. Diminished bone formation in experimental diabetes. Relationship to osteoid maturation and mineralization. Diabetes. 1984;33(9):825–831. doi: 10.2337/diab.33.9.825. [DOI] [PubMed] [Google Scholar]
- Holl RW, Grabert M, Heinze E, Sorgo W, Debatin KM. Age at onset and long-term metabolic control affect height in type-1 diabetes mellitus. Eur J Pediatr. 1998;157(12):972–977. doi: 10.1007/s004310050980. [DOI] [PubMed] [Google Scholar]
- Jilka RL. Molecular and cellular mechanisms of the anabolic effect of intermittent PTH. Bone. 2007;40(6):1434–1446. doi: 10.1016/j.bone.2007.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jilka RL, O’Brien CA, Ali AA, Roberson PK, Weinstein RS, Manolagas SC. Intermittent PTH stimulates periosteal bone formation by actions on post-mitotic preosteoblasts. Bone. 2009;44(2):275–286. doi: 10.1016/j.bone.2008.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jilka RL, O’Brien CA, Bartell SM, Weinstein RS, Manolagas SC. Continuous elevation of PTH increases the number of osteoblasts via both osteoclast-dependent and -independent mechanisms. J Bone Miner Res. doi: 10.1002/jbmr.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemink SA, Hermus AR, Swinkels LM, Lutterman JA, Smals AG. Osteopenia in insulin-dependent diabetes mellitus; prevalence and aspects of pathophysiology. J Endocrinol Invest. 2000;23(5):295–303. doi: 10.1007/BF03343726. [DOI] [PubMed] [Google Scholar]
- Khamaisi M, Regev E, Yarom N, Avni B, Leitersdorf E, Raz I, Elad S. Possible association between diabetes and bisphosphonate-related jaw osteonecrosis. J Clin Endocrinol Metab. 2007;92(3):1172–1175. doi: 10.1210/jc.2006-2036. [DOI] [PubMed] [Google Scholar]
- Krishnan V, Moore TL, Ma YL, Helvering LM, Frolik CA, Valasek KM, Ducy P, Geiser AG. Parathyroid hormone bone anabolic action requires Cbfa1/Runx2-dependent signaling. Mol Endocrinol. 2003;17(3):423–435. doi: 10.1210/me.2002-0225. [DOI] [PubMed] [Google Scholar]
- Kuchler U, Spilka T, Baron K, Tangl S, Watzek G, Gruber R. Intermittent parathyroid hormone fails to stimulate osseointegration in diabetic rats. Clin Oral Implants Res. 2011 doi: 10.1111/j.1600-0501.2010.02047.x. [DOI] [PubMed] [Google Scholar]
- Kwek EB, Goh SK, Koh JS, Png MA, Howe TS. An emerging pattern of subtrochanteric stress fractures: a long-term complication of alendronate therapy? Injury. 2008;39(2):224–231. doi: 10.1016/j.injury.2007.08.036. [DOI] [PubMed] [Google Scholar]
- Lenart BA, Lorich DG, Lane JM. Atypical fractures of the femoral diaphysis in postmenopausal women taking alendronate. N Engl J Med. 2008;358(12):1304–1306. doi: 10.1056/NEJMc0707493. [DOI] [PubMed] [Google Scholar]
- Levin ME, Boisseau VC, Avioli LV. Effects of diabetes mellitus on bone mass in juvenile and adult-onset diabetes. N Engl J Med. 1976;294(5):241–245. doi: 10.1056/NEJM197601292940502. [DOI] [PubMed] [Google Scholar]
- Liu CC, Kalu DN. Human parathyroid hormone-(1-34) prevents bone loss and augments bone formation in sexually mature ovariectomized rats. J Bone Miner Res. 1990;5(9):973–982. doi: 10.1002/jbmr.5650050911. [DOI] [PubMed] [Google Scholar]
- Liu CC, Kalu DN, Salerno E, Echon R, Hollis BW, Ray M. Preexisting bone loss associated with ovariectomy in rats is reversed by parathyroid hormone. J Bone Miner Res. 1991;6(10):1071–1080. doi: 10.1002/jbmr.5650061008. [DOI] [PubMed] [Google Scholar]
- Lozano D, de Castro LF, Dapia S, Andrade-Zapata I, Manzarbeitia F, Alvarez-Arroyo MV, Gomez-Barrena E, Esbrit P. Role of parathyroid hormone-related protein in the decreased osteoblast function in diabetes-related osteopenia. Endocrinology. 2009;150(5):2027–2035. doi: 10.1210/en.2008-1108. [DOI] [PubMed] [Google Scholar]
- Ma YL, Cain RL, Halladay DL, Yang X, Zeng Q, Miles RR, Chandrasekhar S, Martin TJ, Onyia JE. Catabolic effects of continuous human PTH (1--38) in vivo is associated with sustained stimulation of RANKL and inhibition of osteoprotegerin and gene-associated bone formation. Endocrinology. 2001;142(9):4047–4054. doi: 10.1210/endo.142.9.8356. [DOI] [PubMed] [Google Scholar]
- Martin LM, McCabe LR. Type I diabetic bone phenotype is location but not gender dependent. Histochem Cell Biol. 2007;128(2):125–133. doi: 10.1007/s00418-007-0308-4. [DOI] [PubMed] [Google Scholar]
- McCabe LR. Understanding the pathology and mechanisms of type I diabetic bone loss. J Cell Biochem. 2007;102(6):1343–1357. doi: 10.1002/jcb.21573. [DOI] [PubMed] [Google Scholar]
- Motyl K, McCabe LR. Streptozotocin, Type I Diabetes Severity and Bone. Biol Proced Online. 2009a doi: 10.1007/s12575-009-9000-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motyl KJ, Botolin S, Irwin R, Appledorn DM, Kadakia T, Amalfitano A, Schwartz RC, McCabe LR. Bone inflammation and altered gene expression with type I diabetes early onset. J Cell Physiol. 2009;218(3):575–583. doi: 10.1002/jcp.21626. [DOI] [PubMed] [Google Scholar]
- Motyl KJ, McCabe LR. Leptin treatment prevents type I diabetic marrow adiposity but not bone loss in mice. J Cell Physiol. 2009b;218(2):376–384. doi: 10.1002/jcp.21608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima A, Shimoji N, Shiomi K, Shimizu S, Moriya H, Einhorn TA, Yamazaki M. Mechanisms for the enhancement of fracture healing in rats treated with intermittent low-dose human parathyroid hormone (1-34) J Bone Miner Res. 2002;17(11):2038–2047. doi: 10.1359/jbmr.2002.17.11.2038. [DOI] [PubMed] [Google Scholar]
- Neer RM, Arnaud CD, Zanchetta JR, Prince R, Gaich GA, Reginster JY, Hodsman AB, Eriksen EF, Ish-Shalom S, Genant HK, Wang O, Mitlak BH. Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med. 2001;344(19):1434–1441. doi: 10.1056/NEJM200105103441904. [DOI] [PubMed] [Google Scholar]
- Neviaser AS, Lane JM, Lenart BA, Edobor-Osula F, Lorich DG. Low-energy femoral shaft fractures associated with alendronate use. J Orthop Trauma. 2008;22(5):346–350. doi: 10.1097/BOT.0b013e318172841c. [DOI] [PubMed] [Google Scholar]
- Niziolek PJ, Murthy S, Ellis SN, Sukhija KB, Hornberger TA, Turner CH, Robling AG. Rapamycin impairs trabecular bone acquisition from high-dose but not low-dose intermittent parathyroid hormone treatment. J Cell Physiol. 2009;221(3):579–585. doi: 10.1002/jcp.21887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogita M, Rached MT, Dworakowski E, Bilezikian JP, Kousteni S. Differentiation and proliferation of periosteal osteoblast progenitors are differentially regulated by estrogens and intermittent parathyroid hormone administration. Endocrinology. 2008;149(11):5713–5723. doi: 10.1210/en.2008-0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ontiveros C, McCabe LR. Simulated microgravity suppresses osteoblast phenotype, Runx2 levels and AP-1 transactivation. J Cell Biochem. 2003;88(3):427–437. doi: 10.1002/jcb.10410. [DOI] [PubMed] [Google Scholar]
- Pettway GJ, Meganck JA, Koh AJ, Keller ET, Goldstein SA, McCauley LK. Parathyroid hormone mediates bone growth through the regulation of osteoblast proliferation and differentiation. Bone. 2008;42(4):806–818. doi: 10.1016/j.bone.2007.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierroz DD, Bouxsein ML, Rizzoli R, Ferrari SL. Combined treatment with a beta-blocker and intermittent PTH improves bone mass and microarchitecture in ovariectomized mice. Bone. 2006;39(2):260–267. doi: 10.1016/j.bone.2006.01.145. [DOI] [PubMed] [Google Scholar]
- Russell RG. Bisphosphonates: from bench to bedside. Ann N Y Acad Sci. 2006;1068:367–401. doi: 10.1196/annals.1346.041. [DOI] [PubMed] [Google Scholar]
- Russell RG, Rogers MJ. Bisphosphonates: from the laboratory to the clinic and back again. Bone. 1999;25(1):97–106. doi: 10.1016/s8756-3282(99)00116-7. [DOI] [PubMed] [Google Scholar]
- Schnoke M, Midura SB, Midura RJ. Parathyroid hormone suppresses osteoblast apoptosis by augmenting DNA repair. Bone. 2009;45(3):590–602. doi: 10.1016/j.bone.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibonga JD, Iwaniec UT, Shogren KL, Rosen CJ, Turner RT. Effects of parathyroid hormone (1-34) on tibia in an adult rat model for chronic alcohol abuse. Bone. 2007;40(4):1013–1020. doi: 10.1016/j.bone.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Tanaka S, Sakai A, Tanaka M, Otomo H, Okimoto N, Sakata T, Nakamura T. Skeletal unloading alleviates the anabolic action of intermittent PTH(1-34) in mouse tibia in association with inhibition of PTH-induced increase in c-fos mRNA in bone marrow cells. J Bone Miner Res. 2004;19(11):1813–1820. doi: 10.1359/JBMR.040808. [DOI] [PubMed] [Google Scholar]
- Tashjian AH, Jr, Gagel RF. Teriparatide [human PTH(1-34)]: 2.5 years of experience on the use and safety of the drug for the treatment of osteoporosis. J Bone Miner Res. 2006;21(3):354–365. doi: 10.1359/JBMR.051023. [DOI] [PubMed] [Google Scholar]
- Tsuchida T, Sato K, Miyakoshi N, Abe T, Kudo T, Tamura Y, Kasukawa Y, Suzuki K. Histomorphometric evaluation of the recovering effect of human parathyroid hormone (1-34) on bone structure and turnover in streptozotocin-induced diabetic rats. Calcif Tissue Int. 2000;66(3):229–233. doi: 10.1007/pl00005838. [DOI] [PubMed] [Google Scholar]
- Turner RT, Evans GL, Cavolina JM, Halloran B, Morey-Holton E. Programmed administration of parathyroid hormone increases bone formation and reduces bone loss in hindlimb-unloaded ovariectomized rats. Endocrinology. 1998;139(10):4086–4091. doi: 10.1210/endo.139.10.6227. [DOI] [PubMed] [Google Scholar]
- Turner RT, Evans GL, Lotinun S, Lapke PD, Iwaniec UT, Morey-Holton E. Dose-response effects of intermittent PTH on cancellous bone in hindlimb unloaded rats. J Bone Miner Res. 2007;22(1):64–71. doi: 10.1359/jbmr.061006. [DOI] [PubMed] [Google Scholar]
- Vahle JL, Long GG, Sandusky G, Westmore M, Ma YL, Sato M. Bone neoplasms in F344 rats given teriparatide [rhPTH(1-34)] are dependent on duration of treatment and dose. Toxicol Pathol. 2004;32(4):426–438. doi: 10.1080/01926230490462138. [DOI] [PubMed] [Google Scholar]
- Vahle JL, Sato M, Long GG, Young JK, Francis PC, Engelhardt JA, Westmore MS, Linda Y, Nold JB. Skeletal changes in rats given daily subcutaneous injections of recombinant human parathyroid hormone (1-34) for 2 years and relevance to human safety. Toxicol Pathol. 2002;30(3):312–321. doi: 10.1080/01926230252929882. [DOI] [PubMed] [Google Scholar]
- Vengellur A, LaPres JJ. The role of hypoxia inducible factor 1alpha in cobalt chloride induced cell death in mouse embryonic fibroblasts. Toxicol Sci. 2004;82(2):638–646. doi: 10.1093/toxsci/kfh278. [DOI] [PubMed] [Google Scholar]
- Wiren KM, Zhang XW, Toombs AR, Kasparcova V, Gentile MA, Harada S, Jepsen KJ. Targeted overexpression of androgen receptor in osteoblasts: unexpected complex bone phenotype in growing animals. Endocrinology. 2004;145(7):3507–3522. doi: 10.1210/en.2003-1016. [DOI] [PubMed] [Google Scholar]
- Zhao S, Zhang YK, Harris S, Ahuja SS, Bonewald LF. MLO-Y4 osteocyte-like cells support osteoclast formation and activation. J Bone Miner Res. 2002;17(11):2068–2079. doi: 10.1359/jbmr.2002.17.11.2068. [DOI] [PubMed] [Google Scholar]