Abstract
Patterns of behavior within societies have long been visualized and interpreted using maps. Mapping the occurrence of sleep across individuals within a society could offer clues as to functional aspects of sleep. In spite of this, a detailed spatial analysis of sleep has never been conducted on an invertebrate society. We introduce the concept of mapping sleep across an insect society, and provide an empirical example, mapping sleep patterns within colonies of European honey bees (Apis mellifera L.). Honey bees face variables such as temperature and position of resources within their colony's nest that may impact their sleep. We mapped sleep behavior and temperature of worker bees and produced maps of their nest's comb contents as the colony grew and contents changed. By following marked bees, we discovered that individuals slept in many locations, but bees of different worker castes slept in different areas of the nest relative to position of the brood and surrounding temperature. Older worker bees generally slept outside cells, closer to the perimeter of the nest, in colder regions, and away from uncapped brood. Younger worker bees generally slept inside cells and closer to the center of the nest, and spent more time asleep than awake when surrounded by uncapped brood. The average surface temperature of sleeping foragers was lower than the surface temperature of their surroundings, offering a possible indicator of sleep for this caste. We propose mechanisms that could generate caste-dependent sleep patterns and discuss functional significance of these patterns.
Introduction
Maps help to integrate data in ways that clarify patterns or relationships in the lives of organisms. Mapping social phenomena can reveal the spread of disease [1], routes of migration [2], foraging paths [3], organization with respect to division of labor [4] or brood sorting [5], spatial segregation of individuals within a colony [6], or spatial dynamics of competing colonies [7]. Social insect colonies, and honey bee (Apis mellifera L.) colonies in particular, lend themselves well to mapping of behavior. Honey bee activity has been visualized outside the nest with respect to flight paths [8], [9], simulated flight paths relative to landmarks [10], and inside the nest for spatial organization of waggle dance information [11] and patterns generated by removal rates of comb contents [12]. Seeley [13] created maps depicting twelve of the most commonly performed tasks within a nest of honey bees. Conspicuously absent, however, are maps depicting where bees reside when not performing tasks. Sleep is a behavior that has never been mapped extensively across an invertebrate society, in spite of its potential ecological and evolutionary significance. Studies mapping sleep of invertebrates are limited to measurements of individual inactivity, usually within highly artificial settings (e.g., fruit fly stasis within test tubes, [14]). This is in contrast with the more extensive literature devoted to the study of vertebrate sleep sites, which has offered insight as to some functional implications of sleeping socially, especially with respect to vigilance and predator avoidance [15], [16], [17].
Sleep is defined behaviorally by a suite of characters [18], [19], [20] that have been identified in honey bees [21], [22] (Fig. 1; see Materials & Methods for operational definition). Honey bee workers typically progress through a chronological sequence of task-based castes, beginning adulthood as cell cleaners [23], [24], [25], later tending brood and queen as nurse bees, then receiving and storing nectar as food storers [26], and ultimately serving as the colony's foragers [13]. Because the different worker castes engage in tasks that have some spatial component, we hypothesized that bees belonging to different worker castes would sleep in different areas of the nest, depending in part on distance from the bustling brood comb. By sleeping while exposed to an incessantly working mass of siblings cleaning cells and tending the brood, bees could face frequent disturbance and sleep fragmentation. Following Kaiser's [21] observations of unknown caste members sleeping near the perimeter of the comb and Klein et al's [27] observations of a single forager over 24 h, we predicted that foragers sleep closer to the perimeter of the comb and away from uncapped brood cells (brood cells not yet capped by the juveniles' older sisters). In contrast, we predicted that younger castes sleep inside cells within the brood comb area.
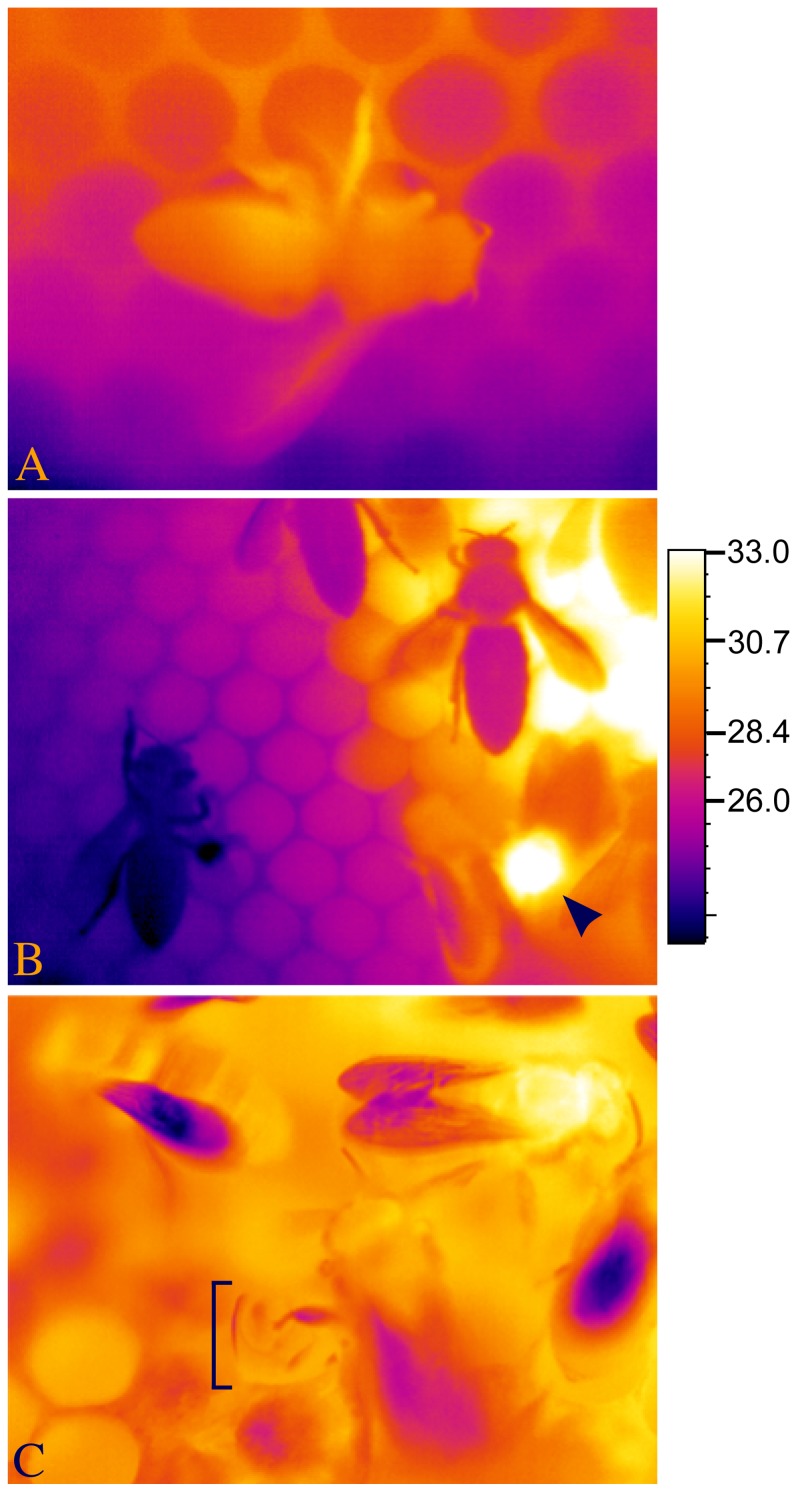
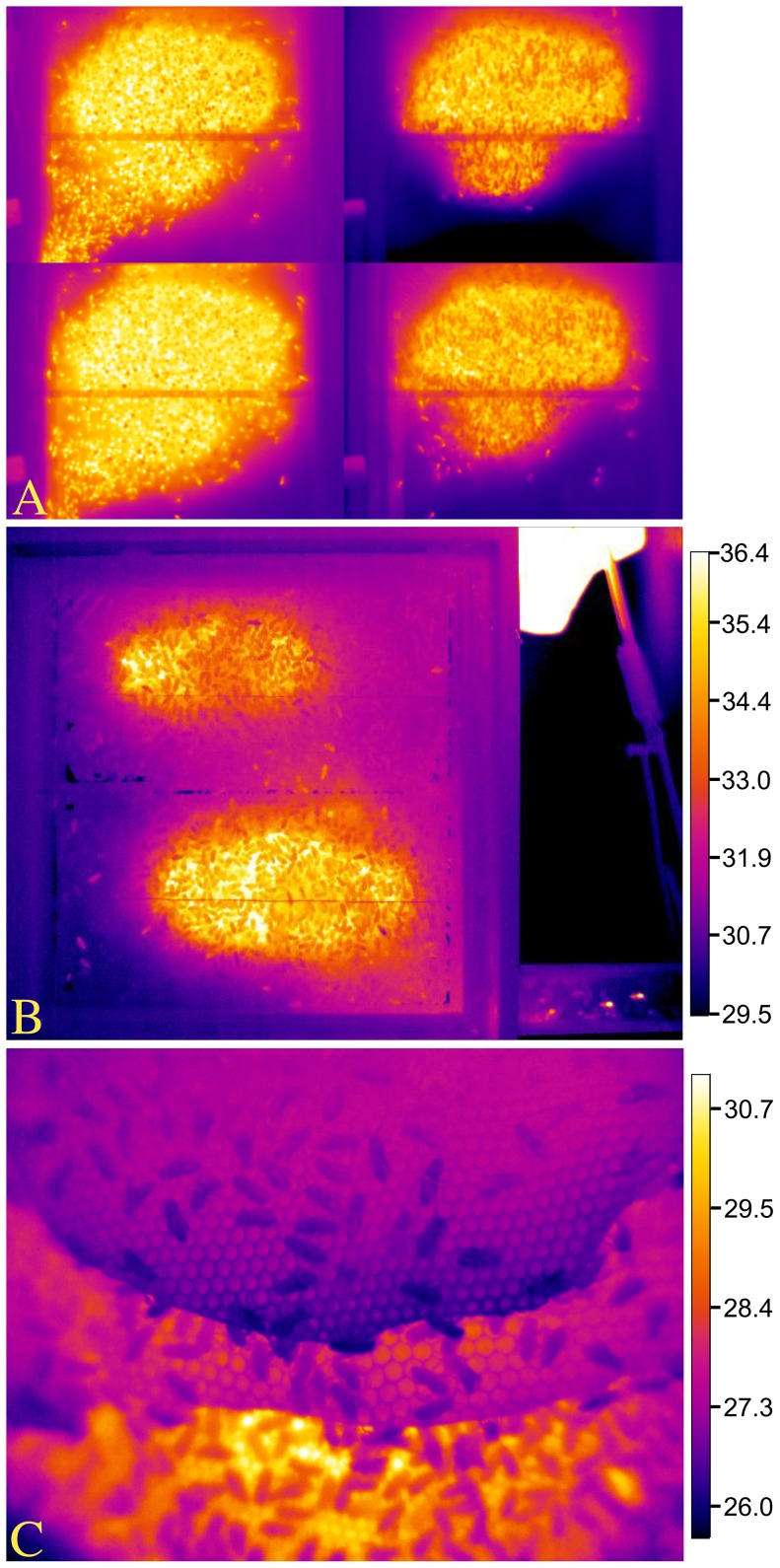
Figure 1. Infrared images of sleeping honey bees.
(A) A sleeping honey bee outside of a cell is relatively immobile, typically hangs in the direction of gravity (in this case she hung horizontally, facing right), and discontinuously ventilates, pumping her abdomen (metasoma) in the anterior-posterior direction. This bee was exhibiting deep sleep, with extended bouts of antennal immobility. (B) Sleeping bees are affected by surrounding temperature, as with the bee on the perimeter of the hot brood comb (upper right) and the inverted bee, hanging (lower left) on the plastic window a short distance from the brood comb. For comparison, note the mobile bee at lower right with the relatively hot thorax (arrowhead). (C) Bees also slept inside cells, primarily when they were young adults. Here, framed by the bracket, is the abdomen of a sleeping bee slightly protruding from a cell (leg of neighboring bee resting on the distal tip of her abdomen), distinguished as sleeping by her discontinuous ventilatory movements. Temperatures of bees sleeping inside cells were not recorded. B.A.K. took all images with FLIR thermal cameras on non-experiment days under different ambient temperature conditions; °C temperature scale only relevant for (B), and values were adjusted for thermal camera settings (see Materials and Methods).
Investigating the spatial patterns of sleep in colonies of honey bees could benefit from a visualization technique that distinguishes sleeping from non-sleeping individuals. Honey bees of unknown caste have been reported to sleep at ambient temperature at the perimeter of their nest [21] or when resting inside cells [28], and foragers have been reported to sleep within a certain range of ambient temperatures when isolated [21], [29], [30] or in unreported locations of the nest [30]. If honey bees of different worker castes sleep in different areas of the nest, is temperature, alone or in combination with nest landmarks, a good predictor for determining which honey bees are asleep? We hypothesized that ontogenetic changes and caste-dependent demands of aging honey bees would result in predictable thermal and sleep patterns across a colony's nest. We applied remote sensing thermography to map caste-dependent sleep by individually marking newly eclosed honey bees and recording their behaviors at different stages of their adult lives. Our aim was to uncover caste-dependent sleep patterns with respect to a bee's location in the nest. We identified spatial patterns among sleeping bees within their nest, and propose a possible role of thermography in further mapping sleep behavior in honey bees.
Materials & Methods
We studied the sleep behavior, location, and surface temperatures associated with Carniolan worker honey bees (A. mellifera carnica Pollman, 1879) shortly after eclosion and during subsequent periods of their adult lives as the bees changed castes. We observed two colonies in separate years; differences between the studies of Colony 1 (2006) and Colony 2 (2008) are noted throughout. First, we installed a two-frame observation hive [31] in a temperature-controlled room at the bee research facility of the University of Würzburg (Würzburg, Germany, 49°46′47″N, 9°58′31″E), and allowed the hive of bees unrestricted access to the outdoors, where bees freely foraged during the day. We introduced 49 recently eclosed, individually-marked worker bees to Colony 1 on 2 June 2006 and 49 to Colony 2 on 14 August 2008. The bees had been extracted within hours of eclosing from a brood comb placed in a 35°C incubator (Colony 1), or had been extracted directly from six different outdoor hives (Colony 2). We individually marked the dorsal mesosoma (referred elsewhere as thorax), and dorsal and ventral metasoma (referred elsewhere as abdomen) of the bees using either model paints (Games Workshop, Nottingham, UK; Colony 1) or oil-based markers (Sharpie, Oak Brook, IL, USA; Colony 2). Neither marking method notably affected surface temperature readings in preliminary tests. Further preparations for surface temperature recordings included replacing the observation hives' glass windows with transparent polypropylene giftwrap (pbs-factory, Artikel 00347, Rheinland-Pfalz, Germany) and adjusting thermal camera settings (emissivity of honey bees 0.97–1.0, transmissivity of polypropylene 0.89). The giftwrap produced a nonlinear error when recording temperature as temperature increased, so absolute temperature measurements reported in this study have been adjusted. We calculated error by recording thermal images of a bee corpse through giftwrap and again without giftwrap, incrementally adjusting the bee's temperature by inserting a carbon film resistor within the body and controlling voltage with a transformer.
String stretched across both sides of the hive created a grid that was visible relative to the nest when thermally photographed. We mounted a thermal camera (FLIR S40 for Colony 1, FLIR SC660 for Colony 2, FLIR Systems Inc., Boston, MA, USA; accuracy ± 1°C or 1% of reading) on an adjustable, rolling monopod. We moved the camera from section to section of the grid and recorded data for each marked bee. We lined the hive and feet of our observation chair with dense foam to reduce substrate-borne vibrations. We also eliminated all ambient light. The hives were perpetually lit on each side with a desk lamp (Colony 1: 25 W, 230 V; Colony 2: Megaman, Compact 2000HPF 30 W, 4000 K) covered with red acetate filters (Colony 2: #27 Medium Red, transparency = 4%, peak at 670 nm, Supergel by Rosco, Stamford, CT, USA). Closer examination of behaviors was facilitated with a headlamp, also covered with the same red filter, selected because honey bees are reported to be less sensitive to frequencies beyond 600 nm [32] or 650 nm [33]. Although a preliminary test showed that bees could detect the filtered light sources, their behavior did not noticeably change if the bees were exposed to gradual changes in light intensity.
Observations
Fifteen hours and 20 h after we collected bees for Colony 1 and 2 (3 h and 8.5 h after introduction into the hive, respectively), the bees were integrated into the colony, with no signs of aggression by other bees and no abnormal grooming. B.A.K. and M.S. systematically scanned for marked bees one section of the nest at a time, surveying bees every hour. As cell cleaners aged and became foragers, we recorded data for 24 consecutive hours during each of these two caste periods for Colony 1 (2 and 24 June 2006). As cell cleaners aged and became nurse bees, then food storers, we recorded data for 6 daytime hours and 6 nighttime hours during each of these three caste periods for Colony 2 (1000–1600 h and 2200–0400 h; 15, 18, and 26 August 2008). We began monitoring cell cleaners on the 1st day after they eclosed, nurse bees on the 4th day, food storers on the 12th day and foragers on the 23rd day (Fig. S1). We selected dates based on typical age-related caste determination in workers [34], [35].
We recorded thermal images of marked bees by pointing at each bee with soft forceps (marked with a pointer on one end to distinguish orientation of the head) as the thermal camera automatically recorded images every second. We verbally recorded each bee's behavior and identity (Olympus VN-4100PC Digital Voice Recorder, or audio track of Sony Handycam DCR-HC65, Tokyo, Japan), and confirmed identity of bees with a dim, handheld LED light. Due to temporary camera malfunction, we were unable to report data for nurse bees or food storers in Colony 1. To supplement the declining number of marked workers as Colony 1's bees became foragers, we captured and marked ten additional foragers of unknown age returning to the entrance of the hive.
We transcribed bee caste, individual identity, and behavior data from voice-recorded notes. Behavior included different sleep states, distinguished from wakeful activity by a bee's relaxed immobility and discontinuously ventilating abdomen. We examined each bee for 3–5 sec to determine her behavior. If she was potentially asleep inside or outside a cell we examined her for discontinuous ventilation, marked by a minimum of ten seconds without visible anterior-posterior abdominal pumping motions. Our ten-second pause between abdominal pumping bouts was based on measurements of honey bees inside cells made by Kleinhenz et al. [28], which appear to be shorter than the average respiratory pause measured by Kovac et al. [36] in isolated bees in metabolic chambers. If outside a cell, we reported antennae as immobile (deep sleep), or exhibiting swaying motions or minute twitches (light sleep) [27], [37], [38]. Reduced antennal mobility correlates with higher response thresholds, and antennal immobility for extended periods may be suggestive of a deeper sleep state [21].
We transcribed a bee's location, or calculated it from thermal images. Infrared images were relayed to a computer and analyzed using camera-specific software (FLIR Systems ThermaCAM Researcher Professional software version 2.9). We recorded the average surface temperature of a bee's thorax (Tth) and the average surface temperature of her surroundings (Tsurr), taken as the average temperature within a circle with the radius of one bee body length (Fig. 2). We report the difference of the two to indicate the temperature of the bee relative to the surface temperature of her surroundings (Tdiff). Tsurr included the surface temperature of wax comb with a range of contents, wood from the hive frames, or bees; when calculating Tsurr, we did not exclude the area in which the examined bee appears. We also mapped the contents of comb cells for Colony 1 within 24 h of each census (5 and 25 June 2006) so that we could analyze bee behavior and temperature with respect to placement in the nest, particularly with respect to uncapped brood. We manually labeled cell contents on hive windows, removed and scanned these windows, and colored the discreet comb contents with different colors in Adobe Photoshop v.7.0 (Adobe Systems, San Jose, CA, USA).
Figure 2. Collection of temperature data from a sequence of infrared images.
The forceps were held open to encompass the heat-generating thorax of the bee (top) and camera software computed the average temperature within the selected region, represented by a circle in the inset image. For recording the surface temperature surrounding a bee (Tsurr), forceps were removed (bottom) so as not to influence the measurement, taken as the average temperature within the larger circle (radius = bee body length). Tdiff is the difference of the average temperature within the large circle (Tsurr) from the average temperature in the smaller circle (Tth). Temperature scale values (°C) were adjusted for thermal camera settings.
Over the course of the study, Colony 1 grew from 1500 to 2800 individuals. Colony 2 housed about 2000 bees, and apparently lacked brood. Room air temperature varied moderately during study recording sessions (2006: 25.9°C, range = 24.7–26.6°C; 2008: 28.2°C, range = 24.6–28.4°C). The sun rose ca. 0510–0554 h and set ca. 2045–2130 h (CEST). For purposes of this study, daytime is defined as 0600–2200 h and nighttime as 2200–0600 h (CEST) to approximate ambient light (sunrise to sunset) conditions. No bees were observed to prematurely forage and by observing cell contents, we confirmed caste identity of several nurse bees and food storers.
Analysis
All analyses are based on a total of 84 h of audio data and 78 h of thermal data (cell cleaners: 32 h, nurse bees: 12 h, food storers: 10 h, foragers: 24 h). Data include bee identity, behavior, position relative to the nest's perimeter (x, y coordinates), cell contents below bee, Tth, and Tsurr (Raw Data S1). Analyses computing placement in the nest were conducted using Python (version 2.6, http://www.python.org). Results from tests using continuous response variables and behavior as a categorical, independent variable are products of linear mixed-effects models, programmed in R [39], with bee group (caste), Tsurr, Tdiff, and position in the nest as fixed effects, and individual bee identity as a random factor (i.e., observations were nested within bee; Models S1). Linear mixed-effects models were fit using the lmer function in the lme4 package [40]. We used the multcomp package to perform likelihood-ratio tests to distinguish between competing models [41]; because of the complicated and unbalanced nature of the data (e.g. missing data, correlated covariates), we could not run standard likelihood-ratio tests [42]. We performed binary logistic regression using R, with Tsurr as continuous predictor and behavior as the dependent variable. Means ± standard error means (s.e.m.) reported throughout the text and figure legends were calculated from averaged values, one value per bee. We set alpha at 0.05 and report two-tailed P-values for all tests.
Results
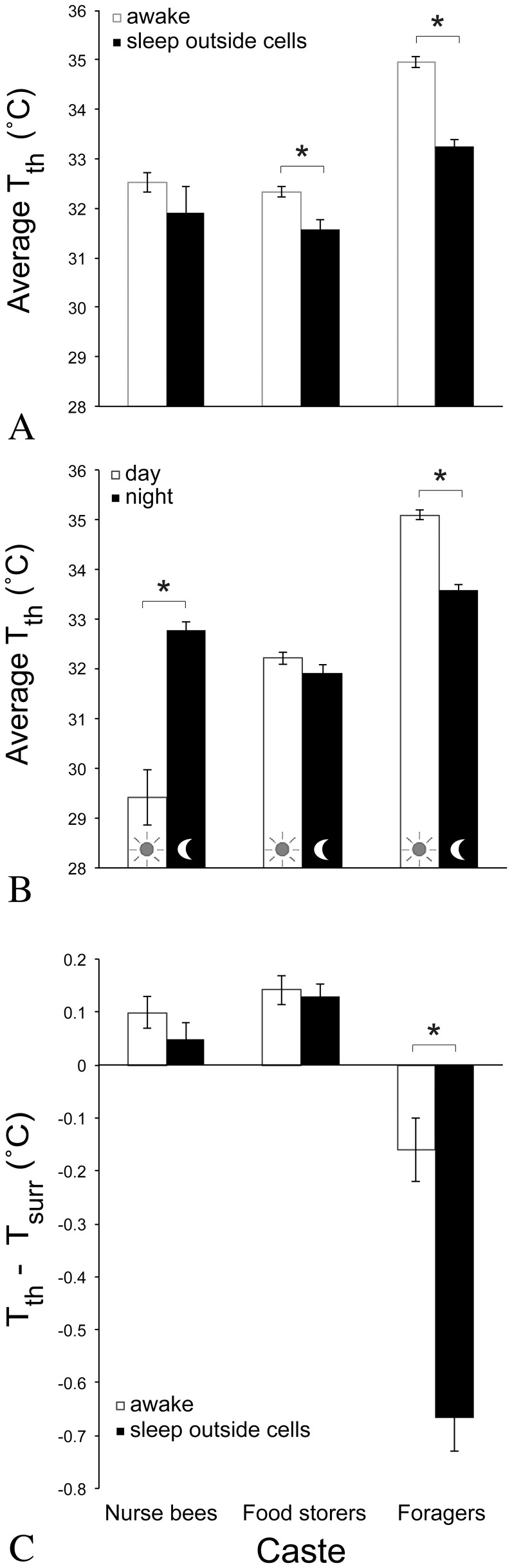
Honey bees belonging to all worker castes exhibited sleep (see Materials & Methods for operational definition). Cell cleaners slept inside cells, while each subsequent age caste slept less and less inside cells, with foragers having slept exclusively outside of cells (Table 1). Individual bees slept in many different areas over the course of a 24-h period (Fig. 3), but different worker castes consistently slept in different areas of the nest relative to position of brood comb and surrounding temperature, Tsurr (see below). The only caste to clearly exhibit day-night periodicity with regard to their sleep was the foraging caste, with more total sleep at night (z = −6.05, P<0.00001; Fig. 4) and more deep sleep at night than during the day (z = −2.32, P = 0.038; n = 199 observations, 32 bees).
Table 1. Percent and total number of observations honey bees of different worker castes engaged in wakefulness and sleep.
| Worker castes | ||||||||
| Behavior | Cell cleaners (n = 95 bees) | Nurse bees (n = 47 bees) | Food storers (n = 43 bees) | Foragers (n = 32 bees) | ||||
| Light sleep | 0.0% | 0 | 6.4% | 32 | 8.6% | 31 | 12.0% | 24 |
| Deep sleep | 0.1% | 1 | 6.8% | 34 | 23.9% | 86 | 14.6% | 29 |
| Sleep inside cells | 21.9% | 196 | 19.5% | 97 | 7.5% | 27 | 0.0% | 0 |
| Total sleep | 22.0% | 197 | 32.7% | 163 | 40.0% | 144 | 26.6% | 53 |
| Awake | 78.0% | 697 | 67.3% | 335 | 60.0% | 216 | 73.4% | 146 |
| Total | 100% | 894 | 100% | 498 | 100% | 360 | 100% | 199 |
Percent of sleep inside cells decreased with age and caste (99.5% of sleep for cell cleaners, 59.5% for nurse bees, 18.8% for food storers, and 0% for foragers; numbers in table represent percent of total observations, including when bees were awake). All observations for each bee are included.
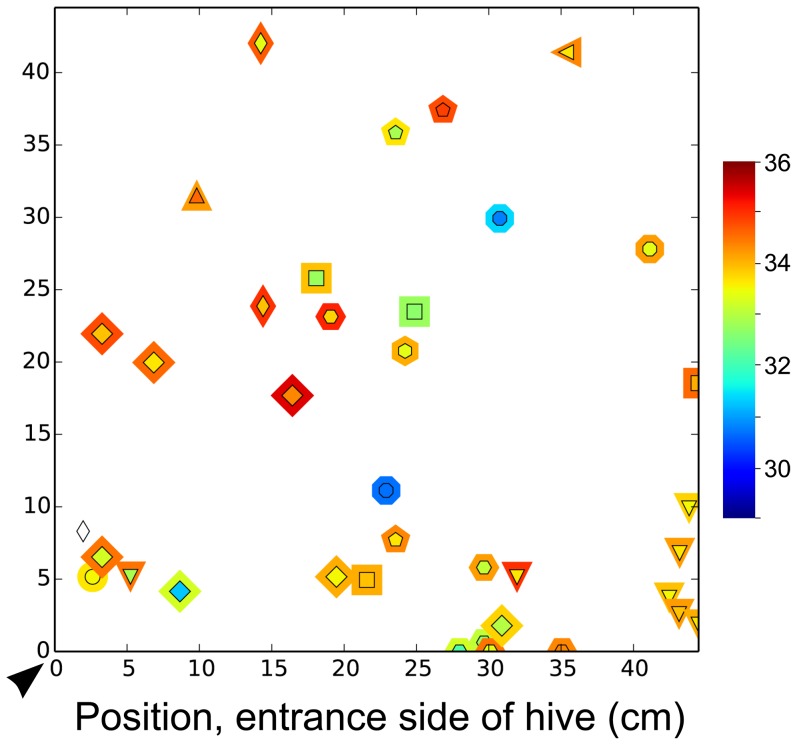
Figure 3. Sleep positions of individual foragers with respect to temperatures Tth and Tsurr.
Unique shapes (including uniquely oriented shapes) distinguish different foragers (e.g., every triangle facing down represents data from one individual at different times, while every triangle facing up represents data from a different individual, squares = a third individual, etc.). Shapes represent Tth (inner shape) and Tsurr (outer shape) for each observation of a marked, sleeping forager over 24 h on the entrance side of the hive (she may have also slept on the reverse side of the hive, as pictured in Fig. S3). Temperatures (°C) correspond with the color scale (white = no data, represented by diamond at lower left). Hive entrance/exit is indicated by arrowhead, and was restricted to one side of the hive. All relevant forager data are included in graph (n = 11 bees), but we treated forager as a random factor in mixed effects analyses to statistically cope with repeated measures of individuals. See Figs. S2 and S3 for differences across behaviors and worker castes.
Figure 4. Proportion of observations bees of different worker castes were asleep during the day and night.
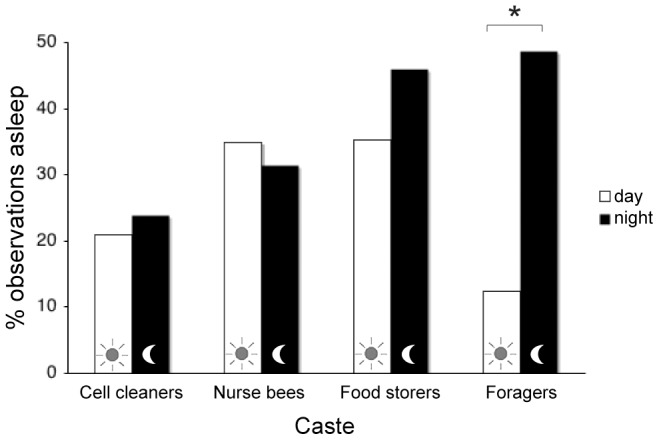
Younger castes slept with no distinction between day and night. The clearest statistical distinction (signified with an asterisk, applying a linear mixed-effects model) appeared in foragers, with more time spent asleep during the night.
Sleep and proximity to the perimeter of nest
Our first objective was to calculate a bee's position relative to the perimeter of the comb and correlate this distance to a bee's behavior. Worker bees could position themselves anywhere between the perimeter of the comb (0 cm) and the center of the comb (22.25 cm from the nearest edge), the center being where brood are typically tended.
When worker bees slept inside cells, they tended to be at the same distance from the perimeter as when awake (e.g., Colony 2 cell cleaners: z = 1.41, P = 0.20, n = 158 observations of 44 bees), although cell cleaners in Colony 1 may have been slightly closer to the perimeter when asleep than when awake (z = 2.10, P = 0.050, n = 413 observations, 47 bees) (Fig. 5, S2).
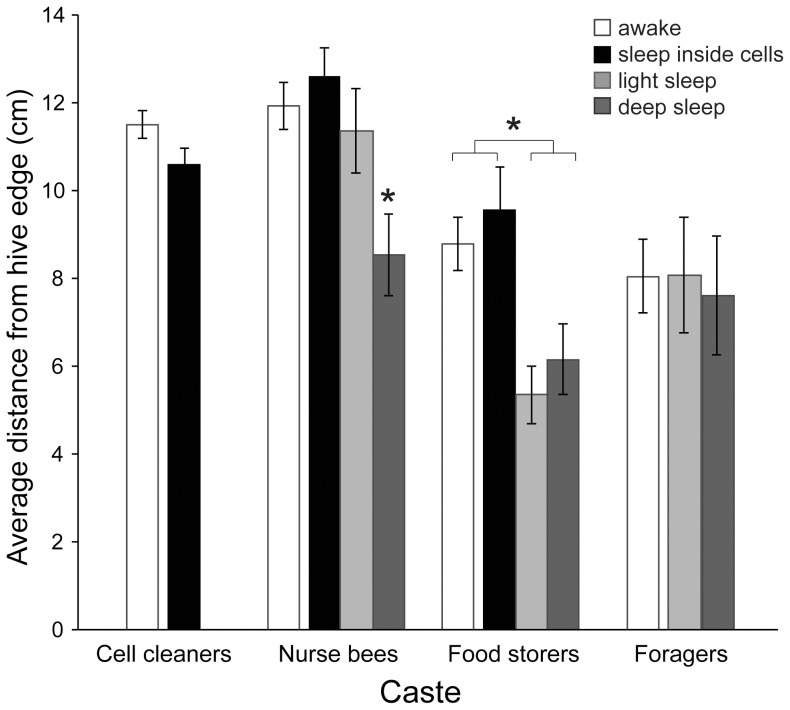
Figure 5. Distance from the nest's perimeter with respect to behavior and caste.
Younger castes (cell cleaners and nurse bees) slept closer to the center of the nest (i.e., farther from the perimeter) than older castes (food storers and foragers). Bees slept outside cells closer to the perimeter of the nest than when they slept inside cells or were awake, except in the case of foragers, who were active during the day near the nest entrance. Cell cleaners did not sleep outside cells and foragers did not sleep inside cells, hence the absence of relevant bars. These data represent averages for castes calculated from average values per bee (± s.e.m.). Asterisks signify statistically significant differences within castes.
When worker bees slept outside of comb cells, they tended to do so closer to the perimeter of the nest than when awake or sleeping inside cells (Fig. 5). Nurse bees exhibited light sleep at the same distance from the perimeter of the nest as when they were awake or sleeping inside cells, but they exhibited deep sleep when they were closer to the perimeter of the nest (z = 3.94, P = 0.0001; n = 469 observations, 47 bees) (Fig. 5, S2), and when nurse bees became food storers, both light and deep sleep were exhibited closer to the perimeter than when they were awake or sleeping inside cells (z = 4.43, P<0.00001; n = 332 observations, 43 bees) (Fig. 5, S3). Unexpectedly, foragers were not closer to the nest's perimeter when asleep than when awake. See possible explanations for this in the Discussion. Food storers and foragers spent more time both asleep and awake closer to the perimeter of the nest than younger castes (asleep: F 3,158 = 27.87, P<0.0001; awake: F 3,204 = 19.77, P<0.0001, ANOVA of pooled data), but no caste spent time closer to the perimeter with respect to day vs. night.
Sleep position relative to surrounding temperature
Temperatures, particularly away from brood comb, fluctuate in regions of the nest, so we investigated the thermal position of bees with respect to day vs. night, as well as with regard to behavior. Our objective was to determine if differing thermal environments within the nest could serve as a predictor of a bee's behavior. We performed a binary logistic regression analysis to test the probability of predicting a bee's behavior by her Tsurr.
When cell cleaners and nurse bees were asleep inside cells, they were in warmer regions of the nest than when they were awake (cell cleaners: 34.9±0.1°C vs. 34.3±0.1°C, n = 68 & 189, respectively; z = −2.89, P = 0.004). Nurse bees occasionally slept outside cells, and did so in regions not significantly thermally different from the regions they were in when awake (inside: 33.4±0.4°C, outside: 32.2±0.5°C vs. awake: 32.5±0.1°C, n = 51 & 115). The regions in which food storers slept were colder than the regions in which they were awake (asleep 31.6±0.2°C vs. awake 32.2±0.1°C, n = 31 & 63; z = 2.82, P = 0.005), but only when sleeping outside cells (31.5±0.2°C), not when sleeping inside cells (31.8±0.3°C). Likewise, the regions in which foragers slept (always outside cells) were colder than the regions in which foragers were awake (asleep 33.9±0.2°C vs. awake 35.1±0.1°C, n = 53 & 145; z = 5.03, P<0.00001) (Fig. 6).
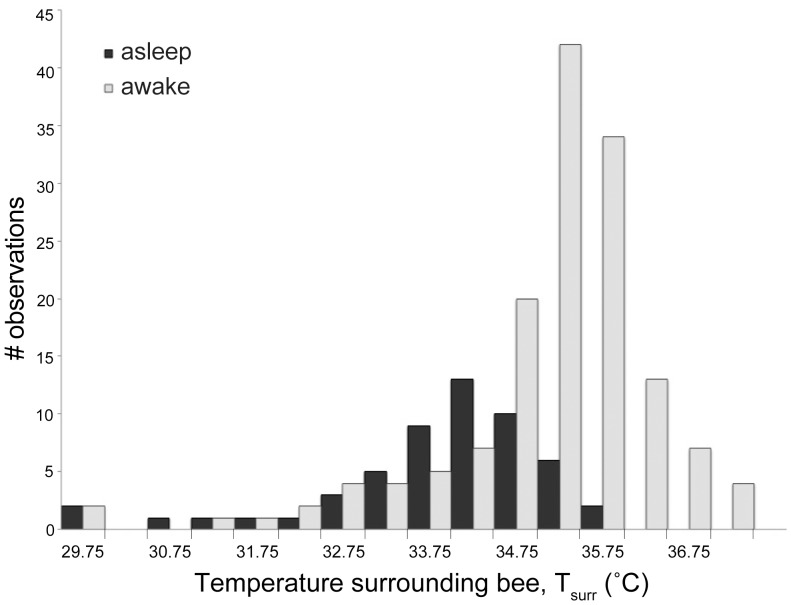
Figure 6. Surface temperature surrounding foragers as a continuous predictor of foragers' behavior.
When Tsurr was lower, foragers tended to be asleep; when Tsurr was higher, foragers tended to be awake. Temperatures represent bins of +/−0.25°C (e.g., 29.75°C = 29.50–29.99°C; the unlabeled 30.25°C = 30.00–30.49°C). All data were collected from foragers outside cells. Food storers also exhibited lower Tsurr when asleep than when awake.
Applying the same binary logistic regression analysis, we examined Tsurr's ability to predict if a bee was observed during the day or night. Cell cleaners and nurse bees spent more time with warmer surroundings during the night than during the day (cell cleaners: 34.8±0.1°C vs. day 33.9±0.2°C, z = 4.47, P<0.00001; nurse bees: 33.0±0.2°C vs. day 29.4±0.5°C, z = 4.80, P<0.00001). Food storers, however, did not spend more time in colder or warmer regions of the nest (31.9±0.2°C vs. day 32.1±0.1°C, z = −0.95, P = 0.34) and foragers spent more time in colder regions of the nest at night than during the day (34.0±0.1°C vs. 35.3±0.1°C, n = 77 & 121; z = −5.72, P<0.00001).
Sleep temperature
We recorded the average temperature of the dorsal surface of a bee's thorax (Tth) and took the difference of Tsurr from Tth as a measure of a bee's temperature relative to her surroundings (Tdiff) to test if variation in Tth or Tdiff is explained by a bee's behavior. All data were extracted from bees when they were exposed (i.e., not inside cells).
Food storers were colder when in deep sleep than when awake (31.6±0.2°C vs. 32.3±0.1°C; deep: z = −3.22, P = 0.004; light: z = −2.25, P = 0.070; n = 83 observations, 36 bees). Likewise, foragers were colder when in either light sleep (33.0±0.28°C, z = −6.55, P<0.00001) or deep sleep than when awake (33.4±0.1°C vs. 35.0±0.1°C, z = −5.55, P<0.00001, n = 199 observations, 32 bees). But in nurse bees, Tth did not differ between light and deep sleep than when awake (z = −0.73 & 0.30, P = 0.84 & 0.99, light & deep sleep, respectively; n = 133 observations, 42 bees) (Fig. 7A).
Figure 7. Average thoracic surface temperature of worker honey bees (Tth) with regard to caste, behavior, day vs. night, or Tsurr.
All measurements were taken from worker bees outside of cells; cell cleaners slept exclusively inside cells, so are excluded. Data represent averages for castes (± s.e.m.). Asterisks signify statistically significant differences within castes. (A) Average thoracic surface temperature of bees (Tth) awake vs. asleep, (B) day vs. night, and (C) awake vs. asleep relative to surrounding surface temperatures (Tdiff).
Day and night had an effect on some of the bees' Tth. Nurse bees were warmer at night than during the day (32.8±0.2°C vs. 29.7±0.5°C, z = 5.89, P<0.00001; n = 133 obs., 42 bees). Food storers' Tth did not differ between day and night (32.2±0.1°C vs. 31.9±0.2°C, z = −1.36, P = 0.29; n = 83 obs., 36 bees) (Fig. 7B). Foragers were colder at night than during the day (33.6±0.1°C vs. 35.1±0.1°C, z = −8.08, P<0.00001; n = 199 obs., 32 bees). Day or night, foragers were colder (i.e., Tth was lower) when asleep than when awake (day: z = 4.87, P<0.00001, n = 121 obs., 27 bees; night: z = 3.54, P = 0.0008, n = 78 obs., 24 bees), and this colder sleeping temperature did not significantly differ between day and night (z = −1.58, P = 0.19; n = 53 obs., 16 bees). Sleeping food storers also showed no difference between day and night (z = 0.50, P = 0.81; n = 22 obs., 18 bees). In contrast, sleeping nurse bees were colder during the day than at night (z = 2.39, P = 0.023; n = 22 obs., 9 bees) (Fig. 8).
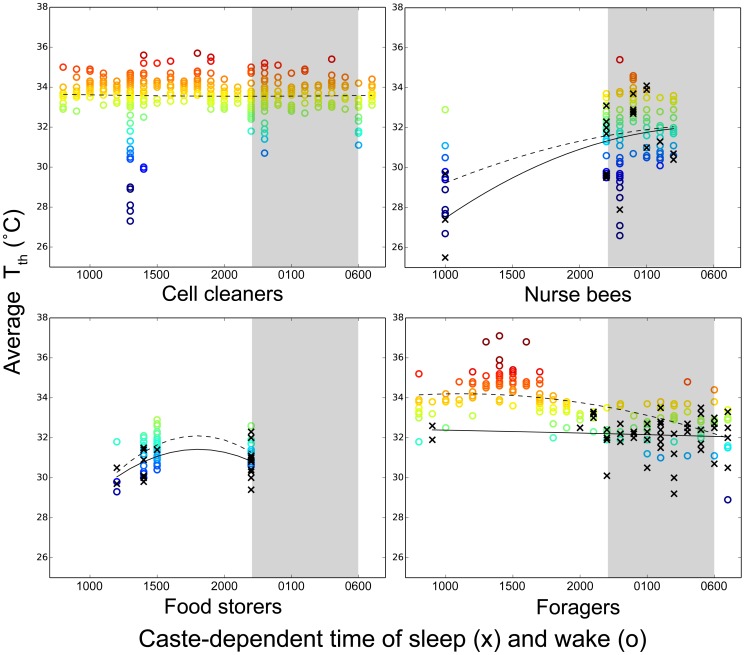
Figure 8. Average thoracic surface temperature of honey bee workers (Tth) over the course of 24 h with respect to caste and behavior.
An observation of an awake bee is represented by o with dashed lines fitting the data; an observation of a sleeping bee is represented by x with solid lines fitting the data. Gray backdrop represents nighttime and colors correspond with the temperature scale on y-axes and with Figs. 3, S2, and S3. Average Tth is reported per bee per census period and, although all bee data are included in these graphs, we treated bee as a random factor in mixed effects analyses. All measures were taken from bees outside of cells; no data exist for cell cleaners sleeping outside cells, hence the absence of x in the cell cleaner graph.
The only caste for which Tdiff differed between sleeping and awake bees was the foragers. A sleeping forager's Tdiff was more pronounced than an awake forager's (−0.66±0.06°C vs. −0.16±0.06°C, z = −4.08, P<0.001; n = 198 obs., 32 bees), particularly during deep sleep (−0.74±0.07°C) (Fig. 7C). Examples of Tdiff in which Tth < Tsurr are visible in Figs. 3 and S3.
All bees outside of cells were colder when situated closer to the perimeter of the nest, although foragers were colder specifically at night when closer to the perimeter of the nest (T∼day/night*proximity to perimeter; z = 2.79, P = 0.017; n = 199 observations, 32 bees). We could not examine sleep by cell cleaners with respect to their Tth or Tdiff because cell cleaners slept inside cells, but awake cell cleaners' Tth and Tdiff did not differ day vs. night (Fig. 8).
Sleep relative to position of brood comb
Cell cleaners were more likely to be asleep than awake as the proportion of cells in their vicinity increasingly consisted of uncapped brood (surrounded by 32.27±0.03% vs. 22.56±0.03% uncapped brood, t 83 = 2.14, P = 0.036; with trend in mixed effects model: z = −1.79, P = 0.074; n = 413 observations, 47 bees from Colony 1). After cell cleaners eventually developed into foragers, they were more likely to be awake than engaged in deep sleep as the proportion of cells in their vicinity increasingly consisted of uncapped brood (surrounded by 43.11±0.05% vs. 23.96±0.08% uncapped brood, z = 2.45, P = 0.027; n = 199 observations, 32 bees) (Fig. 9). We did not record cell contents for nurse bee and food storer stages.
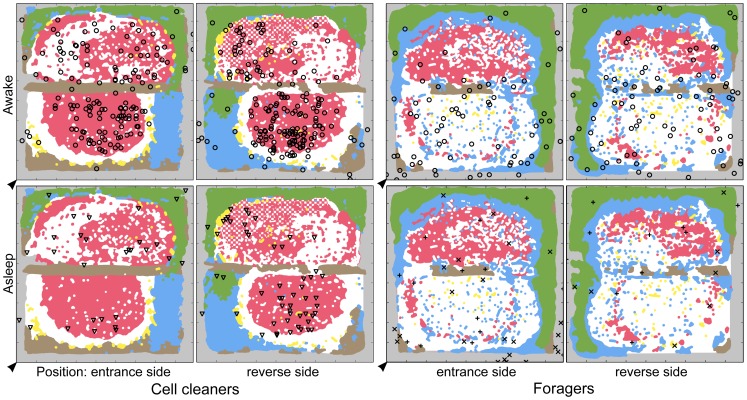
Figure 9. Caste-dependent maps of behavior with regard to comb cell contents.
Cell cleaners (Colony 1 pictured) were awake (o) and asleep (inside cells; triangles) primarily in the brood comb area, spending more time asleep than awake with uncapped brood in their midst. Foragers, on the other hand, spent more time awake (o) than asleep (x = deep sleep, + = light sleep) near uncapped brood comb. White = uncapped brood, red = capped brood, yellow = pollen cells, blue = uncapped nectar, green = capped honey, brown = empty comb, gray = no comb. Nest entrance/exit is indicated by an arrowhead, and was restricted to one side of the nest.
Discussion
Our objective was to describe caste-dependent sleep patterns in a honey bee colony, to produce “sleep maps” that reveal these patterns, and to discuss the patterns' functional significance. Sleep-site fidelity did not occur in individuals, contrary to what Clark and Gillingham [43] reported in anole lizards. However, spatial sleep patterns did occur among worker castes. The youngest workers (cell cleaners) were more often asleep than awake when surrounded by uncapped brood, while the opposite was true in the eldest workers (foragers) (Fig. 9). Workers slept less and less inside cells as they aged. Bees asleep inside cells were at the same distance from the perimeter of the nest as bees that were awake, while bees that slept outside cells did so closer to the perimeter of the nest than bees awake or asleep inside cells. Foragers appeared to sleep, and especially to exhibit deep sleep, on the periphery of the nest (Fig. S3), but two behaviors confounded this result: activity near the hive entrance (causing many awake bees to be near the perimeter), and sleep on wood frames running through the center of the nest. A two-frame observation hive is composed of one frame sitting above a second frame, creating an artificial break in the comb running through the center of the nest. Some bees were found sleeping on these centrally-located wood frames. Were proximity to the nest perimeter to be used to predict worker sleep in the future, forager activity near the hive entrance should be accounted for and all nest areas without comb (i.e., frames) should be treated as perimeters.
Why do honey bees sleep inside cells, why does this differ temporally among castes, and why is there a spatial distinction between sleep inside and outside cells within the same caste of bees? Sleep outside cells exposes bees to the arousing interactions of wakeful, mobile siblings ([27], supplementary video) and the greater density of bees found in the brood comb area could mean more frequent arousals and more fragmented sleep. By sleeping closer to the perimeter of the nest, exposed bees may increase their sleep. Consistent with this hypothesis, Klein et al. [27] reported increasing durations of uninterrupted sleep outside cells as bees aged/changed castes. Sleep inside cells may constitute an adaptive response to avoiding sleep fragmentation within the busy brood comb area. Younger bees slept more frequently inside cells than older bees and this could be the result of differential cell vacancy rates, with empty cells less frequently available in the brood comb, and more readily inhabited by bees already working in the brood comb area and within actual comb cells. Older bees may sleep less (food storers) or not at all (foragers) inside cells closer to the perimeter of the nest because they do not face the same degree of disturbance as they would if exposed in the brood comb. Sleep away from the brood comb may be a result of either learned or instinctual avoidance, or simple displacement due to repeated disturbance in the brood comb.
The temperature of a bee, of her surroundings, and the difference between the two appear to correlate with sleep behavior, offering specific opportunities for using temperature when identifying sleep across a colony of bees. We tested the probability of predicting a worker bee's behavior by her Tsurr and found that sleep inside cells occurred either in areas as warm as when the bees were awake (nurse bees and food storers) or even warmer areas than when awake (cell cleaners). Sleep outside of cells occurred in colder regions than when awake (food storers and foragers), except in our small sample involving nurse bees. A bee can impact Tsurr, as best demonstrated by the actions of heater bees [28], [44]. A resting bee can be weakly endothermic [36], although when not heating, the effect of a bee's body on Tsurr is less dramatic, or insignificant (Fig. 1A–C). It is worth noting that surrounding surface temperature can differ from ambient temperature experienced by a bee. This may account for the difference between our measure of Tsurr encircling the average sleeping forager (33.8±0.2°C) and the preferred ambient temperature of sleep, as reported by Kaiser et al. [29] in isolated foragers (23–26°C, with extremes of 21 and 29°C) and Schmolz et al. [30] in isolated foragers (28°C, range: 26–29°C) and foragers within a nest (27.9°C, range: 23.8–30.8°C), or of worker Bombus atratus bumblebees [45]. On the other hand, our average measure of a forager's Tth during deep sleep (33.4±0.2°C) was congruent with measurements of resting bees inside cells (32.7±0.1–33.4±0.3°C) [28], and our lowest Tsurr recordings are commonplace inside nests away from the brood area [21], [46].
We tested if Tth or Tdiff is explained by a bee's behavior. Tth is lower when food storers and foragers are asleep than when they are awake and this lower Tth is statistically indistinguishable between day and night (Fig. 8). A relatively static lower sleeping temperature for the older castes is a consequence of sleeping closer to the colder periphery of the nest, although this correlation was confounded in foragers by their activity near the nest entrance. Tth of sleeping nurse bees did not differ from their wakeful Tth due either to insufficient sample size of nurse bees sleeping outside cells, or because sleep bouts are shorter in nurse bees than in older workers [27], not allowing for Tth to significantly decrease. Cell cleaners' Tth did not vary significantly when awake, likely due to the consistent warmth of the brood comb area within which cell cleaners in Colony 1 worked.
Brood comb is warmer and the bees are typically more active than in other regions of the nest, setting the stage for brood comb location to impact sleep positioning. We found the proportion of uncapped brood comb in the vicinity of cell cleaners and foragers correlated with behavior: cell cleaners in Colony 1 (Colony 2 had no brood) tended to be asleep more often and foragers less often when surrounded by uncapped brood. Brood comb is often centrally located, but can vary across observation hives (Fig. 10A,B), and the organization of brood comb and of thermal microclimates in an observation hive will differ from that of the three-dimensional hive box [47], [48] or architecture of a feral colony's nest. Observation hives are typically two-sided, but they are not as three-dimensional as natural nests, which consist of a series of parallel combs (Fig. 10C). Recording undisturbed behavior between the parallel combs of a more naturalistic hive (e.g., www.hobos.de), would be necessary to establish any similarities or differences between what we observed in observation hives and what may be occurring in more natural, three-dimensional nests.
Figure 10. Infrared images revealing thermal activity across beehives.
(A) Sequence of colony-scale changes across the entrance side of Colony 1. In clockwise order from the upper left corner, 1700, 0400, 0900 and 1500 h, respectively. Entrance/exit is in the lower left corner of the hive, leading out tube at left of each image. Brood comb is most easily seen as the glowing warm area at 0400 h. (B) Observation hive containing Colony 2, with filter-covered lamp at upper right, and bees visibly exiting hive tunnel at lower right. (C) Exposed nest composed of parallel sheets of comb, set up by Dirk Ahrens-Lagast to induce bees to construct a more natural nest architecture; not used in study. B.A.K. took all images with FLIR thermal cameras on non-experiment days under different ambient temperature conditions. Temperature scale values (°C) were adjusted for thermal camera settings (see Materials and Methods).
Caste-dependent sleep patterns may be the consequence of selection pressures for sleeping in warmer or cooler areas. For example, honey bees may experience a trade-off between the benefits of warmth and obtaining unfragmented sleep, with more heat lost when alone [49]. By sleeping in colder areas, food storers and foragers may conserve energy, and by sleeping in warmer areas, cell cleaners may increase neural development or facilitate consolidation of memories. Schmolz et al. [30] reported that foragers sleep ectothermically and hypothesized that foragers select cool, but not maximally cool regions to sleep for the purpose of conserving energy while still promoting regenerative processes during sleep. Stabentheiner et al. [50] reported that ectothermy is most common in the youngest bees (0 to ∼2 d) and proposed that visitation to warm cells within the brood comb serves to increase flight muscle development. Additional explanations could include reduction of pathogen spread by segregation of sleeping castes, or an increased protection of younger, less expendable bees at the center of the nest. Alternatively, an awake bee may simply fall asleep without changing her location, or change location due to non-sleep-related reasons.
The observed caste-dependent patterns of sleep were consistent with previous studies of honey bees, including sleep inside and outside of cells [27], day-night periodicity of sleep in foragers [21], [27], [51] and the absence of periodicity in the younger castes (cell cleaners and nurse bees) [27], [52], [53], [54]. Food storers did not exhibit day-night periodicity in this study, but have previously been reported to exhibit either circadian sleep [27] or, in the case of a single subject, ultradian periodicity (12 h sleep-wake cycles) [52]. Future investigations, including testing our predictions to map spatial and temporal sleep behavior in colonies of honey bees, may add insight about functional attributes of sleep in a social setting.
Supporting Information
Timeline of data collection (black bars on timelines) for both Colony 1 and Colony 2. We scheduled census times to fall within periods distinguishing the age-based worker castes. The beginning of the timeline represents eclosion, or the start of adulthood.
(TIF)
Position of cell cleaners and nurse bees with respect to behavior and temperatures Tth and Tsurr. Concentric circles represent Tth (inner circle) and Tsurr (outer halo) for each honey bee observation. Temperatures (°C) correspond with the color scale at lower right (white = no data). Hive entrance/exit is indicated by an arrowhead, and was restricted to one side of the hive. All bee data are included in these graphs, but we treated bee as a random factor in mixed effects analyses to statistically cope with repeated measures of individual bees. Note that cell cleaners slept exclusively inside cells, so no Tth data were available for sleeping bees.
(TIF)
Position of food storers and foragers with respect to behavior and temperatures Tth and Tsurr. Concentric circles represent Tth (inner circle) and Tsurr (outer halo) for each honey bee observation. Temperatures (°C) correspond with the color scale at lower left (white = no data). Hive entrance/exit is indicated by an arrowhead, and was restricted to one side of the hive. All bee data are included in these graphs, but we treated bee as a random factor in mixed effects analyses to statistically cope with repeated measures of individual bees. Note that foragers exhibited wakeful activity near hive entrance, which eliminated average wake-sleep differences in distance from the nest perimeter. For a more focused look at the changing sleep sites of foragers, see Fig. 3.
(TIF)
Raw data, used for analyses in R. Caste: c = cell cleaner, n = nurse bee, fs = food storer, f = forager. Newforager: yes = forager added to supplement dwindling sample of foragers in Colony 1; this category was not used in analyses. d_n: d = day, n = night. Behav = a more specific set of behavioral categories than Beh. cm_edge = position of bee, in cm from edge of hive. UnderColor = the color of the hive map on which the bee is positioned (e.g., white signifies the region of the comb in which cells contain uncapped brood).
(CSV)
Models used in statistical analyses. Models are written for analysis in R.
(R)
Acknowledgments
We thank the University of Würzburg and all members of the BEEgroup for facilitating our research. Dirk Ahrens-Lagast was instrumental as our skilled and creative beekeeper. Hartmut Vierle, Sven Mayer, Rebecca Basile, Marco Kleinhenz, and Christian Lutsch offered assistance and guidance, and Walter Kaiser paid a special visit to discuss our research. Uma Bhat, Kathryn Busby, Clairissa Dewstow and Laura Still helped transcribe data. Melissa Bingham of the Statistical Consulting Center at UW–La Crosse, Samuel Scarpino, and Sabrina Amador-Vargas assisted with statistical advice, however any errors of fact or interpretation remain the sole responsibility of the authors. B.A.K. also thanks the Section of Integrative Biology at the University of Texas at Austin, Jon und Hanna Ahrens-Lagast for translation assistance, Anja Weidenmüller, Christoph Kleineidam, and the honey bees. Thomas Seeley, Walter Kaiser, Rebecca Basile, the Mueller lab, and four anonymous reviewers kindly critiqued earlier versions of this paper.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.
Funding Statement
BAK received the following funding: Doctoral Dissertation Improvement Grant #0710142, National Science Foundation (http://www.nsf.gov). Deutscher Akademischer Austausch Dienst #A0670415 (https://www.daad.org). Environmental Science Institute, The University of Texas at Austin (no number; http://www.esi.utexas.edu). Bienenforschung Würzburg e.V. (no number; http://www.bienenforschung.biozentrum.uni-wuerzburg.de/bienenforschung_wuerzburg_ev/). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Hay SI, Guerra CA, Gething PW, Patil AP, Tatem AJ, et al. (2009) A world malaria map: Plasmodium falciparum endemicity in 2007. PLoS Medicine 6: 286–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Witteveen BH, Worthy GAJ, Roth JD (2009) Tracing migratory movements of breeding North Pacific humpback whales using stable isotope analysis. Marine Ecology-Progress Series 393: 173–183. [Google Scholar]
- 3. Noser R, Byrne RW (2010) How do wild baboons (Papio ursinus) plan their routes? Travel among multiple high-quality food sources with inter-group competition. Anim Cogn 13: 145–155. [DOI] [PubMed] [Google Scholar]
- 4. Jandt JM, Dornhaus A (2009) Spatial organization and division of labour in the bumblebee Bombus impatiens . Anim Behav 77: 641–651. [Google Scholar]
- 5. Franks NR, Sendova-Franks AB (1992) Brood sorting by ants: distributing the workload over the work-surface. Behav Ecol Sociobiol 30: 109–123. [Google Scholar]
- 6. Baracchi D, Zaccaroni M, Cervo R, Turillazzi S (2010) Home range analysis in the study of spatial organization on the comb in the paper wasp Polistes dominulus . Ethology 116: 579–587. [Google Scholar]
- 7. Adams ES, Tschinkel WR (1995) Spatial dynamics of colony interactions in young populations of the fire ant Solenopsis invicta . Oecologia 102: 156–163. [DOI] [PubMed] [Google Scholar]
- 8. Menzel R, Greggers U, Smith A, Berger S, Brandt R, et al. (2005) Honey bees navigate according to a map-like spatial memory. P Natl Acad Sci USA 102: 3040–3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Riley JR, Greggers U, Smith AD, Reynolds DR, Menzel R (2005) The flight paths of honeybees recruited by the waggle dance. Nature 435: 205–207. [DOI] [PubMed] [Google Scholar]
- 10. Cartwright BA, Collett TS (1987) Landmark maps for honeybees. Biol Cybern 57: 85–93. [Google Scholar]
- 11.von Frisch K (1967) The Dance Language and Orientation of Bees. Cambridge: The Belknap Press. [Google Scholar]
- 12. Camazine S (1991) Self-Organizing Pattern Formation on the Combs of Honey Bee Colonies. Behav Ecol Sociobiol 28: 61–76. [Google Scholar]
- 13. Seeley TD (1982) Adaptive significance of the age polyethism schedule in honeybee colonies. Behav Ecol Sociobiol 11: 287–293. [Google Scholar]
- 14. Hendricks JC, Finn SM, Panckeri KA, Chavkin J, Williams JA, et al. (2000) Rest in Drosophila is a sleep-like state. Neuron 25: 129–138. [DOI] [PubMed] [Google Scholar]
- 15. Anderson JR (1998) Sleep, sleeping sites, and sleep-related activities: awakening to their significance. Am J Primatol 46: 63–75. [DOI] [PubMed] [Google Scholar]
- 16. Rattenborg NC, Lima SL, Amlaner CJ (1999) Half-awake to the risk of predation. Nature 397: 397–398. [DOI] [PubMed] [Google Scholar]
- 17. Anderson JR (2000) Sleep-related behavioural adaptations in free-ranging anthropoid primates. Sleep Med Rev 4: 355–373. [DOI] [PubMed] [Google Scholar]
- 18.Piéron H (1913) Le Problème Physiologique du Sommeil. Paris: Masson. [Google Scholar]
- 19. Flanigan WF, Wilcox RH, Rechtschaffen A (1973) The EEG and behavioral continuum of the crocodilian, Caimen sclerops . Electroencephalogr Clin Neurophysiol 34: 521–538. [DOI] [PubMed] [Google Scholar]
- 20.Tobler I (1985) Deprivation of sleep and rest in vertebrates and invertebrates. In: Inoue S, Borbely AA, editors. Endogenous Sleep Substances and Sleep Regulation (Taniguchi Symposia, series no. 8). Utrecht: VNU Science Press. pp. 57–66. [Google Scholar]
- 21. Kaiser W (1988) Busy bees need rest, too: Behavioural and electromyographical sleep signs in honeybees. J Comp Physiol A 163: 565–584. [Google Scholar]
- 22. Sauer S, Herrmann E, Kaiser W (2004) Sleep deprivation in honey bees. J Sleep Res 13: 145–152. [DOI] [PubMed] [Google Scholar]
- 23. Seeley TD, Kolmes SA (1991) Age polyethism for hive duties in honey bees–illusion or reality? Ethology 87: 284–297. [Google Scholar]
- 24. Moore D, Angel JE, Cheeseman IM, Fahrbach SE, Robinson GE (1998) Timekeeping in the honey bee colony: integration of circadian rhythms and division of labor. Behav Ecol Sociobiol 43: 147–160. [Google Scholar]
- 25. Moore D (2001) Honey bee circadian clocks: behavioral control from individual workers to whole-colony rhythms. J Insect Physiol 47: 843–857. [Google Scholar]
- 26. Johnson BR (2008) With-in nest temporal polyethism in the honey bee. Behav Ecol Sociobiol 62: 777–784. [Google Scholar]
- 27. Klein BA, Olzsowy KM, Klein A, Saunders KM, Seeley TD (2008) Caste-dependent sleep of worker honey bees. J Exper Biol 211: 3028–3040 10.1242/jeb.017426 [DOI] [PubMed] [Google Scholar]
- 28. Kleinhenz M, Bujok B, Fuchs S, Tautz J (2003) Hot bees in empty broodnest cells: heating from within. J Exp Biol 206: 4217–4231. [DOI] [PubMed] [Google Scholar]
- 29. Kaiser W, Faltin T, Bayer G (2002) Sleep in a temperature gradient–behavioural recordings from forager honey bees. J Sleep Res 11 suppl.: 115–116. [Google Scholar]
- 30. Schmolz E, Hoffmeister D, Lamprecht I (2002) Calorimetric investigations on metabolic rates and thermoregulation of sleeping honeybees (Apis mellifera carnica). Thermochim Acta 383: 221–227. [Google Scholar]
- 31.Seeley TD (1995) The Wisdom of the Hive: The Social Physiology of Honey Bee Colonies. Cambridge: Harvard University Press. [Google Scholar]
- 32.von Frisch K, Lindauer M, Inst Wiss Film (1977) Nachweis des Farbensehens bei der Honigbiene. Film C 1263 des IWF, Göttingen. Publication of Lindauer M, Publ Wiss Film, Sekt Biol, Ser. 14: Nr. 23/C 1263 (1981), 8 S.
- 33.Dustmann JH, Geffcken H (2000) Bienen können Farben unterscheiden. Nieders. Landesinstitut für Bienenkunde (Verleger), Celle.
- 34. Sakagami SF (1953) Untersuchungen über die Arbeitsteilung in einem Zwergvolk der Honigbiene. Beiträge zur Biologie des Bienenvolkes, Apis mellifera L. I. Jpn J Zool 11: 117–185. [Google Scholar]
- 35.Seeley TD (1985) Honeybee Ecology: A Study of Adaptation in Social Life. Princeton: Princeton University Press. [Google Scholar]
- 36. Kovac H, Stabentheiner A, Hetz SK, Petz M, Crailsheim K (2007) Respiration of resting honeybees. J Insect Physiol 53: 1250–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Klein BA, Klein A, Wray MK, Mueller UG, Seeley TD (2010) Sleep Deprivation impairs precision of waggle dance signaling in honey bees. P Natl Acad Sci USA 107: 22705–22709 10.1073/pnas.1009439108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Klein BA, Seeley TD (2011) Work or sleep? Honeybee foragers opportunistically nap during the day when forage is not available. Anim Behav 82: 77–83 10.1016/j.anbehav.2011.03.026 [DOI] [Google Scholar]
- 39.R Development Core Team (2005) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. isbn 3-900051-07-0, online.
- 40.Bates D, Sarkar D (2006) The lme4 Package, http://cran.r-project.org/src/contrib/Descriptions/lme4.html.
- 41. Hothorn T, Bretz F, Westfall P (2008) Simultaneous inference in general parametric models. Biometrical J 50: 346–363. [DOI] [PubMed] [Google Scholar]
- 42.Pinheiro J, Bates D (2002) Mixed-Effects Models in S and S-PLUS. New York: Springer. pp. 83–92.
- 43. Clark DL, Gillingham JC (1990) Sleep-site fidelity in two Puerto Rican lizards. Anim Behav 39: 1138–1148. [Google Scholar]
- 44. Bujok B, Kleinhenz M, Fuchs S, Tautz J (2002) Hot spots in the bee hive. Naturwissenschaften 89: 299–301. [DOI] [PubMed] [Google Scholar]
- 45. Vega L, Torres A, Hoffmann W, Lamprecht I (2011) Thermal investigations associated with the behaviour patterns of resting workers of Bombus atratus (Hymenoptera: Apidae). J Therm Anal Calorim 104: 233–237. [Google Scholar]
- 46. Hess WR (1926) Die Temperaturregulierung im Bienenvolk. Z Vergl Physiol 4: 465–487. [Google Scholar]
- 47. Szabo TI (1985) The thermology of wintering honeybee colonies in 4-colony packs as affected by various hive entrances. J Apicultural Res 24: 27–37. [Google Scholar]
- 48. Humphrey JAC, Dykes ES (2008) Thermal energy conduction in a honey bee comb due to cell-heating bees. J Theor Biol 250: 194–208. [DOI] [PubMed] [Google Scholar]
- 49. Fahrenholz L, Lamprecht I, Schricker B (1989) Thermal investigations of a honey bee colony: thermoregulation of the hive during summer and winter and heat production of members of different bee castes. J Comp Physiol B 159: 551–560. [Google Scholar]
- 50. Stabentheiner A, Kovac H, Brodschneider R (2010) Honeybee colony thermoregulation – regulatory mechanisms and contribution of individuals in dependence on age, location and thermal stress. PLoS One 5: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sauer S, Kinkelin M, Herrmann E, Kaiser W (2003) The dynamics of sleep-like behaviour in honey bees. J Comp Physiol A 189: 599–607. [DOI] [PubMed] [Google Scholar]
- 52. Sauer S, Menna-Barreto L, Kaiser W (1998) The temporal organization of rest and activity in newly emerged honey bees kept in isolation – initial results. Apidologie 29: 445–447. [Google Scholar]
- 53.Sauer S, Happel U, Neubecker R, Menna-Barreto L, Herrmann E, Kaiser W (1999) Ontogeny of the circadian rest-activity cycle in honey bees kept in isolation. In: Elsner N, Eysel U. Proceedings of the 1st Göttingen Conference of the German Neuroscience Society, vol. II: 27th Göttingen Neurobiology Conference. Stuttgart: Georg Thieme Verlag. p. 241. [Google Scholar]
- 54. Eban-Rothschild AD, Bloch G (2008) Differences in the sleep architecture of forager and young honeybees (Apis mellifera). J Exper Biol 211: 2408–2416. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Timeline of data collection (black bars on timelines) for both Colony 1 and Colony 2. We scheduled census times to fall within periods distinguishing the age-based worker castes. The beginning of the timeline represents eclosion, or the start of adulthood.
(TIF)
Position of cell cleaners and nurse bees with respect to behavior and temperatures Tth and Tsurr. Concentric circles represent Tth (inner circle) and Tsurr (outer halo) for each honey bee observation. Temperatures (°C) correspond with the color scale at lower right (white = no data). Hive entrance/exit is indicated by an arrowhead, and was restricted to one side of the hive. All bee data are included in these graphs, but we treated bee as a random factor in mixed effects analyses to statistically cope with repeated measures of individual bees. Note that cell cleaners slept exclusively inside cells, so no Tth data were available for sleeping bees.
(TIF)
Position of food storers and foragers with respect to behavior and temperatures Tth and Tsurr. Concentric circles represent Tth (inner circle) and Tsurr (outer halo) for each honey bee observation. Temperatures (°C) correspond with the color scale at lower left (white = no data). Hive entrance/exit is indicated by an arrowhead, and was restricted to one side of the hive. All bee data are included in these graphs, but we treated bee as a random factor in mixed effects analyses to statistically cope with repeated measures of individual bees. Note that foragers exhibited wakeful activity near hive entrance, which eliminated average wake-sleep differences in distance from the nest perimeter. For a more focused look at the changing sleep sites of foragers, see Fig. 3.
(TIF)
Raw data, used for analyses in R. Caste: c = cell cleaner, n = nurse bee, fs = food storer, f = forager. Newforager: yes = forager added to supplement dwindling sample of foragers in Colony 1; this category was not used in analyses. d_n: d = day, n = night. Behav = a more specific set of behavioral categories than Beh. cm_edge = position of bee, in cm from edge of hive. UnderColor = the color of the hive map on which the bee is positioned (e.g., white signifies the region of the comb in which cells contain uncapped brood).
(CSV)
Models used in statistical analyses. Models are written for analysis in R.
(R)
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.