Abstract
We describe a case series of seven patients presenting to an emergency department with symptoms of paralytic shellfish poisoning. They developed varying degrees of nausea, vomiting, diarrhea, weakness, ataxia and paresthesias after eating mussels harvested from a beach near their resort. Four patients were admitted to the hospital, one due to increasing respiratory failure requiring endotracheal intubation and the remainder for respiratory monitoring. All patients made a full recovery, most within 24 hours. The ability to recognize and identify paralytic shellfish poisoning and manage its complications are important to providers of emergency medicine.
INTRODUCTION
Paralytic shellfish poisoning is a foodborne illness that typically develops after consumption of shellfish contaminated with saxitoxin. During blooms of toxic algae, especially dinoflagellates of the genera Alexandrium, feeding molluscan bivalves and other shellfish concentrate the toxin and are unsafe to consume.1 Within hours of eating shellfish contaminated with toxic levels of saxitoxin, victims develop gastrointestinal distress and neurological symptoms, ranging from benign circumoral paresthesias and tingling of the extremities to ataxia, dysphagia, and changes in mental status.2 Many patients describe a sensation of “floating” or dissociation. Hypertension and tachycardia have been reported.3 While most patients recover without treatment, weakness may rapidly progress to respiratory paralysis and asphyxiation. Currently there are no antidotes to saxitoxin and treatment is supportive.1
While much is known about saxitoxin and its relationship to blooms of toxic dinoflagellates, reports of paralytic shellfish poisoning and descriptions of findings in its victims are infrequent in the medical literature. We describe seven patients with presumed paralytic shellfish poisoning, including one requiring intubation and respiratory support. Patients with paralytic shellfish poisoning most often present in coastal locations, but the international distribution of shellfish as a food substance expands the risk of paralytic shellfish poisoning to anywhere in the world. Knowledge of all shellfish poisoning syndromes is important, but the ability of the emergency medicine provider to recognize paralytic shellfish poisoning facilitates the anticipation and management of potentially rapidly progressive muscle paralysis and respiratory arrest.
CASE SERIES
The poison center was contacted by emergency medicine staff at a community hospital regarding management of seven patients presenting with suspected paralytic shellfish poisoning. The patients were part of a group visiting the area and staying at a nearby resort. Around midnight, they harvested mussels on the beach near their hotel and prepared them in a soup which they ate between midnight and 2 a.m. Approximately 1–2 hours later, they began experiencing various symptoms: peripheral paresthesias (7/7), nausea (5/7), vomiting (4/7), diarrhea (3/7), ataxia (3/7), weakness (2/7), and shortness of breath (1/7). There were 4 males and 3 females, aged 19 to 67 years. The most affected was a 62 year old female who developed dysarthria and a floating sensation in addition to nausea and vomiting. She was transported by emergency medical services after she became ataxic, fell and could not stand up. At the hospital, her exam revealed pronounced dysarthria and diminished gag reflex in addition to her subjective dyspnea, oral paresthesia, and sensation of her throat closing up. Due to concerns of impending airway compromise, the patient was emergently intubated, placed on a ventilator, and transferred to the intensive care unit (ICU). Her laboratory results were remarkable only for hypokalemia (2.5 mmol/L) which improved with repletion. She was extubated after 24 hours and then discharged home in good condition the following day.
The etiology of her low potassium is uncertain and a literature search failed to find any known association with saxitoxin. Gastrointestinal loss from vomiting and diarrhea is unlikely considering the short duration of the symptoms. There was no documentation of beta agonists given in the Emergency Department; however emergency medical services records were not available and therefore unknown if any medications given en route. Since the patient was on an antihypertensive medication (patient did not know which one), she may have had underlying sub-acute or chronic hypokalemia. Basic electrolytes were checked on all of the patients and no one else exhibited hypokalemia, results ranged from 3.7 to 4.1 mmol/L.
After the initial patient was intubated, all remaining patients had serial peak flow measurements obtained (Table). The expected normal values based on heights and weights were not included in the records. Only patient “F” had height and weight recorded, allowing calculation of expected peak flow rate. His effort of 470 Liters per minute was 89% of expected per standard peak flow chart. Six of the patients were hypertensive, either at triage or during their hospital course. Tachycardia was present in four patients, three at triage and one later in the emergency department.
Table.
Serial peak flow measurements obtained after initial patient was intubated.
| Patient age/gender | Symptoms | Triage vital signs | Peak blood pressure, mm Hg | Peak heartrate, bpm | Vital signs at time of disposition | Peak Flow Rates* | Admitted? |
|---|---|---|---|---|---|---|---|
| A | Dizzy, nausea | 118/75 | 165/117 | 100 | 109/78 | 325 | No |
| 19 | HR 95 | HR 74 | 370 | ||||
| Female | RR16 | RR12 | 380 | ||||
| 96% RA | 97% RA | ||||||
| B | Vomiting, diarrhea, facial numbness, ataxia, arm & leg weakness | 149/10 | 151/87 | 93 | 99/58 | 290 | ICU |
| 43 | HR 92 | HR 67 | 280 | ||||
| Female | RR16 | RR16 | 320 | ||||
| 99% RA | 99% RA | 330 | |||||
| C | Vomiting, paresthesia | 192/119 | 192/119 | 110 | 156/106 | 310 | ICU |
| 47 | HR 110 | HR 101 | 300 | ||||
| Male | RR14 | RR18 | 275 | ||||
| 99% RA | 96% RA | 275 | |||||
| D | Facial & fingertip paresthesia | 149/100 | 158/95 | 89 | 158/95 | 450 | No |
| 67 | HR 86 RR12 | HR 87 | 400 | ||||
| Male | 97% RA | 98% RA | 500 | ||||
| E | Floating sensation, dizzy, vomiting, diarrhea, facial & fingertip paresthesias | 153/113 | 163/107 | 96 | 159/92 | 400 | ICU |
| 49 | HR 96 | HR 95 | 450 | ||||
| Male | RR12 | RR15 | |||||
| 96% RA | 99% RA | ||||||
| F | Facial & fingertip paresthesia, dizzy | 129/87 | 129/87 | 112 | 118/62 | 420 | No |
| 52 | HR 112 | HR 86 | 470 | ||||
| Male | RR16 | RR14 | |||||
| 97% RA | 96% RA | ||||||
| G | Floating sensation, dyspnea, vomiting, diarrhea | 175/84 | 226/128 | 123 | 114/72 | none | ICU (Intubated) |
| 62 | HR 97 | HR 96 | |||||
| Female | RR16 | RR20 | |||||
| 100% RA | 100% (vent) |
HR, heart rate; RR, respiration rate; bpm, beats per minute; ICU, intensive care unit; RA, room air
Three patients were admitted to the ICU for monitoring of respiratory status and were subsequently discharged within 24 hours. The remaining patients were evaluated in the emergency department, monitored, and discharged with resolved or resolving symptoms. No urine or serum saxitoxin levels were obtained from the patients. The beach where the family had harvested their mussels was under restriction for collecting shellfish due to elevated saxitoxin levels; however the patients failed to see the Department of Health notice due to the darkness. On 24 September 2012, the day after the patients’ presentation, saxitoxin levels obtained from shellfish from the beach were found to average 6250 mcg/100 gm of shellfish meat (Figure).
Figure.
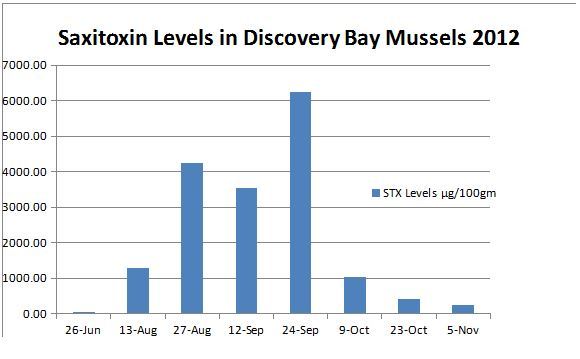
Saxitoxin levels found in shellfish meat.
DISCUSSION
Shellfish poisoning occurs after ingestion of organisms contaminated by infectious agents or concentrated toxins. Toxins concentrated in the flesh of shellfish can produce syndromes that include paralytic shellfish poisoning, amnesic shellfish poisoning, diarrheic shellfish poisoning, and neurologic shellfish poisoning.1 Shellfish poisoning syndromes provide a confusing array of overlapping signs and symptoms. Providers may fail to recognize these syndromes and may attribute findings to cerebrovascular events, intoxication, psychiatric illness, or other disorders.4
Paralytic shellfish poisoning is a growing problem worldwide and occurs seasonally in the coastal United States.5 Saxitoxin is produced by certain dinoflagellates and concentrated in the flesh of filter feeding mollusks, including clams, oysters, and mussels. These dinoflagellates are bloom-forming microalgae that thrive in calm, warm waters. They include about ten Alexandrium species in the waters surrounding the United States. Dinoflagellate blooms are commonly called “red tide,” but can occur with other color changes in water (green, brown, or yellow) and toxic levels of saxitoxin can occur in clear-appearing water. This has led to the recommendation that such events be called “harmful algal blooms” instead of “red tides.”6,7 First isolated from butter clams (Saxidomus gigantea), saxitoxin is a potent neurotoxin three orders of magnitude more toxic than sodium cyanide (Lethal Dose 50: saxitoxin 10mcg/kg, cyanide 10mg/kg).8,9 Saxitoxin has also been associated with pufferfish, cyanobacteria, and other marine animals.10
Consumption of saxitoxin from any source produces a syndrome through the blockade of voltage-gated fast sodium channels, inhibiting signal propagation in neural tissue.1 Symptoms commonly include paresthesias of the mouth, face, and lips; odd feelings of floating; tingling of the extremities; and rapidly-progressive weakness and paralysis. Gastrointestinal distress occurs in many victims and hypertension is commonly seen in paralytic shellfish poisoning patients.3 Most victims fully recover without medical care, but some need control of gastrointestinal distress, and some require airway control and mechanical ventilation.4 Predictors of the severity of neurological toxicity have not been identified. Symptom onset is rapid, usually within the first 1–2 hours, and recovery within 24 to 48 hours. Some patients describe fatigue for several days after paralytic shellfish poisoning.1
The diagnosis of paralytic shellfish poisoning is almost always made clinically, but can be confirmed by measurement of saxitoxin levels either in the shellfish meat or the patient’s urine or serum.11 In our case series, the diagnosis was based on clinical presentation, geographic location, and temporal association with eating bivalves from a beach with known toxic levels of saxitoxin. The Washington Department of Health (DOH) became involved at the onset of the case and tested shellfish from that beach for saxitoxin, domoic acid, & okadaic acid; only saxitoxin was above the safety threshold and measured 6025 mcg/100 gm (beaches are closed when levels are greater than 80 mcg/100 gm). During the course of its investigation, the DOH determined that paralytic shellfish poisoning was the causative agent. Although other toxins, especially tetrodotoxin, ciquatoxin, and brevetoxin can cause a similar constellation of symptoms, these are very unlikely since not endemic to this particular location and the onset occurred after eating mussels caught locally. In addition, the neurologic symptoms make a food borne illness such as Vibrio species unlikely. Chemical and nerve agents should also be considered, however, the short duration of symptoms and a known exposure to a biologic toxin, saxitoxin, makes this extremely unlikely as well.
Our cases of paralytic shellfish poisoning demonstrated the common findings of neurologic and gastrointestinal symptoms.3 Five of the seven patients were hypertensive at triage and the most symptomatic patient showed the highest blood pressure in the group. Only one patient did not develop hypertension. Four of the patients were monitored in an intensive care setting, one required airway control and mechanical ventilation. Two of the patients described the characteristic “floating sensation” frequently seen in paralytic shellfish poisoning. In addition, six patients had peak expiratory flow rates measured to evaluate and monitor ventilatory status. Of note, the peak expiratory flow rates of only two patients (A & B) progressively improved as symptoms resolved.
Three additional cases of suspected paralytic shellfish poisoning were identified by searching Washington Poison Center records from the 6-month period May to October 2012 for the key words “paralytic,” “shellfish,” “clams,” “mussels,” “oysters,” and “food poisoning.” The 10 cases of paralytic shellfish poisoning identified in Washington in 2012 represent a considerable increase over the average number reported in recent years (0–2 cases annually in Washington State since 1998). The actual number of cases of paralytic shellfish poisoning in Washington is likely underestimated, as many cases of paralytic shellfish poisoning go unreported.4
Saxitoxin levels are measured in shellfish collected from public beaches in Washington State every two weeks in the summer months.12 Symptoms usually develop in humans at saxitoxin levels significantly above 80 mcg per gram of shellfish meat.13 When saxitoxin levels exceed 80 mcg per 100 gram of shellfish meat, beaches are closed, marked with signs, and identified on the Washington State Shellfish Program website.12 Such closures occur annually on the coastal areas of Washington; however the beaches of southern Puget Sound usually remain open. The summer of 2012 was unique with many days of sun and light wind in the Puget Sound, and may have promoted the growth of Alexandrium, necessitating beach closure much further south than usual.
Paralytic shellfish poisoning is a preventable condition. Because commercially harvested shellfish are routinely tested for saxitoxin, victims are typically hobbyists, collecting clams or mussels from local beaches. While avoiding non-commercially harvested shellfish is the best way to prevent paralytic shellfish poisoning, shellfish harvesting is a common practice for some citizens of Washington State and a cultural tradition for many members of the Pacific Northwest native tribes. The patients identified in this series harvested mussels in darkness and did not see warning signs posted on the beach. None of the victims checked the Washington State Shellfish Program website – they reported a practice of collecting squid and shellfish annually from that site and never needing to check for beach closure in the past. The unusual closure of southern Puget Sound beaches in 2012, failure to review online information, and darkness obscuring posted warning signs contributed to this outbreak of paralytic shellfish poisoning.
Patients with shellfish poisoning most often present in coastal locations. Knowledge of these syndromes and the ability to recognize them is important to Emergency Medicine providers in coastal areas. However, the wide distribution of shellfish as a food product makes knowledge of shellfish poisoning syndromes, especially paralytic shellfish poisoning with its risk of rapidly progressive paralysis of respiratory muscles and need for ventilation, important to all Emergency Medicine providers regardless of their location. Prompt recognition of cases of paralytic shellfish poisoning can prevent complications and death in individual patients, and provide an opportunity to limit the impact of an outbreak by coordinating investigation and intervention with the local health department and poison control center.
Footnotes
Full text available through open access at http://escholarship.org/uc/uciem_westjem
Conflicts of Interest: By the WestJEM article submission agreement, all authors are required to disclose all affiliations, funding sources and financial or management relationships that could be perceived as potential sources of bias. The authors disclosed none. The paper is the opinions of the authors and by no means reflects either the opinions or interests of the United States Army, Madigan Army Medical Center, the Department of Defense, or the United States Government.
REFERENCES
- 1.Etheridge SM. Paralytic shellfish poisoning: seafood safety and human health perspectives. Toxicon. 2010;56(2):108–122. doi: 10.1016/j.toxicon.2009.12.013. [DOI] [PubMed] [Google Scholar]
- 2.Gessner BD, Middaugh JP. Paralytic shellfish poisoning in Alaska: a 20-year retrospective. Am J Epidemiol; Paralytic shellfish poisoning – southeast Alaska; May–June 2011; 1995. pp. 766–770. [DOI] [PubMed] [Google Scholar]
- 3.Gessner BD, Bell P, Doucette GJ, Moczydlowski E, et al. Hypertension and identification of toxin in human urine and serum following a cluster of mussel-associated paralytic shellfish poisoning outbreaks. Toxicon. 1997;35(5):711–722. doi: 10.1016/s0041-0101(96)00154-7. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention (CDC) MMWR Morb Mortal Wkly Rep. 2011 Nov 18;60(45):1554–1556. [PubMed] [Google Scholar]
- 5.Van Dolah FM. Marine algal toxins: origins, health effects, and their increased occurrences. Environ Health Perspect. 2000;108:133–141. doi: 10.1289/ehp.00108s1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.James KJ, Carey B, O’Halloran J, et al. Shellfish toxicity: human health implications of marine algal toxins. Epidemiol Infect. 2010;138(7):927–940. doi: 10.1017/S0950268810000853. [DOI] [PubMed] [Google Scholar]
- 7.Smayda TJ. Harmful algal blooms: their ecophysiology and general relevance to phytoplankton blooms in the sea. Limnol Oceanogr. 1997;42:1137–1153. [Google Scholar]
- 8.Schantz EJ, Ghazarossian VE, Schnoes HK, et al. The structure of saxitoxin (letter) J Am Chem Soc. 1975;97(5):1238. doi: 10.1021/ja00838a045. [DOI] [PubMed] [Google Scholar]
- 9.Narahashi T. Mechanism of tetrodotoxin and saxitoxin action. Tu AT, editor. Marine Toxins and Venoms. 1998;3:185–210. [Google Scholar]
- 10.Deeds JR, Landsberg JH, Etheridge SM, et al. Non-traditional vectors for paralytic shellfish poisoning. Mar Drugs. 2008;6:308–348. doi: 10.3390/md20080015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rodrigues SM, de Carvalho M, Mestre T, et al. Paralytic shellfish poisoning due to ingestion of Gymnodinium catenatum contaminated cockles – application f the AOAC HPLC official method. Toxicon. 2012;59(5):558–566. doi: 10.1016/j.toxicon.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 12.Biotoxin web page, Washington State Department of Health. Available at: http://www.doh.wa.gov/ehp/sf/BiotoxinProgram.htm.
- 13.Wekell JC, Hurst J, Lefebvre KA. The origin of the regulatory limits for PSP and ASP toxins in shellfish. J Shellfish Res. 2004;23(3):927–930. [Google Scholar]


