Abstract
BACKGROUND
With modernization, cardiometabolic disease risk has increased in low and middle-income countries. To better understand cardiometabolic disease etiology, we evaluated the patterning risk factors in a susceptible young adult population.
METHODS AND RESULTS
Participants included 1,621 individuals from the 2005 Cebu Longitudinal Health and Nutrition Survey. Using cluster analysis, we grouped individuals by the following biomarkers: triglycerides, HDL and LDL cholesterol, C-reactive protein, blood pressure, homeostasis model assessment of insulin resistance, and fasting glucose. Using multinomial logistic regression models we assessed how diet, adiposity, and environment predicted cardiometabolic clusters. We identified 5 distinct sex-specific clusters: 1) Healthy/High HDL cholesterol (with the addition of high LDL cholesterol in women); 2) Healthy/Low blood pressure; 3) High blood pressure; 4) Insulin resistant/High triglycerides; and 5) High C-reactive protein. Low HDL cholesterol was the most prevalent risk factor (63%). In men and women, a higher intake of saturated fat increased the likelihood of being in the healthy clusters. In men, poorer environmental hygiene increased the likelihood of being in the High C-reactive protein cluster, compared to the healthy clusters (OR 0.74 [95% CI 0.60–0.90] and 0.83 [0.70–0.99]). Adiposity most strongly associated with membership to the Insulin resistant/high triglyceride cluster.
CONCLUSIONS
Despite the population’s youth and leanness, cluster analysis found patterns of cardiometabolic risk. While adiposity measures predicted clustering, diet and environment also independently predicted clustering, emphasizing the importance of screening lean and overweight individuals for cardiometabolic risk. Finding predictors of risk in early adulthood could help inform prevention efforts for future disease.
Keywords: Young adult, Risk factors, Cluster analysis, Adiposity, Cardiovascular diseases
INTRODUCTION
Low and middle-income countries undergoing rapid nutrition and lifestyle changes display an increasing burden of obesity, visceral adiposity, and associated diseases.1–3 These concerns are heightened for Asians and young adults. The risk of cardiometabolic (CM) diseases has been shown to be elevated among Asians at lower levels of BMI, prompting the World Health Organization to recommend the use of a lower BMI cut-point to define overweight in this population.4 In addition, overweight young adults are likely to remain overweight throughout life and have increased risk of CM diseases, such as cardiovascular disease and type 2 diabetes.1, 5–7
Substantial literature links obesity to insulin resistance, dyslipidemia, hypertension, and inflammation, and consequently to elevated risk of CM diseases.8–11 These factors tend to co-occur, leading to the definition of the metabolic syndrome (MetS).12 However, using the MetS definition presents several problems. First, there is a lack of research demonstrating that MetS stems from a common underlying pathophysiology:13–15 treatment of MetS is no different than treating the specific CM factors present.16,17 In addition, objectively evaluating the clustering of CM risk factors, rather than the diagnosis of MetS, is more useful for predicting and preventing disease.18,19 Lastly, the inclusion/exclusion of specific CM risk factors in the MetS definition is unfounded. For example, in-flammation, as indicated commonly by elevated C-reactive protein (CRP), is often not included in the classic MetS definition, despite that it predicts CVD and type II diabetes independent of MetS status.11
Motivated by the downfalls of applying a uniform MetS definition, we used cluster analysis to identify groups of young adults, from the 2005 Cebu Longitudinal Health and Nutrition Survey (CLHNS), who share similar patterns of CM risk factors. Furthermore, differences in the prevalence and patterns of co-occurrence of these risk factors likely reflect variation in modifiable and non-modifiable characteristics. However, there is a lack of research relating such characteristics to the clustering of CM risk factors, particularly among young adults. Thus we sought to determine how diet, adiposity, environment, and sex related to the clustering of CM risk factors in Filipino young adults.
This study population is ideal for our research question because 1) the majority of participants did not have any clinical disease; 2) Cebu is undergoing a rapid nutrition and lifestyle transition; and 3) the CLHNS includes detailed diet, lifestyle, anthropometric, and biomarker data. By using an at-risk young adult population, we can gain a better understanding of how modifiable and non-modifiable characteristics relate to CM risk factors in young adulthood, which can help inform prevention strategies for future CM disease.
MATERIALS AND METHODS
Survey design
Data come from the CLHNS. This ongoing community-based survey follows a cohort of 3080 infants born in 1983 to 1984.20 Briefly, the CLHNS is a community-based cohort of women and their index children followed since 1983. The original participants included all pregnant women from 33 randomly selected communities of Metro Cebu, who gave birth between May 1, 1983, and April 30, 1984. Surveys took place immediately after birth, bimonthly for 2 years, in 1991, 1994–5, 1998–99, 2002, and 2005. In 2005, fasting blood was drawn for CVD biomarkers and genetics. Here we use data from the index children still participating in the 2005 CLHNS.
Blood samples were collected on 1790 individuals. Excluding women who were pregnant at the time of blood draw, we clustered 1621 (889 men and 732 women) individuals with complete fasting biomarker data and with CRP levels < 10 mg/L (a level representing current/recent illness rather than low-level basal inflammation).19 Of those clustered, 1,569 individuals with complete diet, socioeconomic, and anthropometric data were included in the multivariate analysis (871 men and 698 women). All data were collected with informed consent, using protocols approved by the institutional review board of the University of North Carolina, Chapel Hill.
Cardiometabolic biomarkers
Fasting plasma CM biomarkers included triglycerides (TG), HDL cholesterol (HDL-C), LDL cholesterol (LDL-C), glucose, insulin, and C-reactive protein (CRP). Other biomarkers included homeostatic model assessment insulin resistance (HOMA-IR) and systolic and diastolic blood pressure (BP). Details regarding obtainment of these biomarkers are described previously.21 We used cutpoints for these biomarkers based on recommendations from the International Diabetes Federation (IDF), the American Heart Association, and other previously recognized and accepted cutpoints (Table 1).8, 19, 22, 23
Table 1.
Criteria for defining elevated cardiometabolic risk
Anthropometry
Body weight, height, and waist circumference (WC) were measured using standard techniques24. BMI was calculated as the ratio of weight (kg) to height (m2). We used cutpoints for Asians to define overweight (OW) as a BMI ≥ 23 kg/m2.25 Cutpoints for Asians define central adiposity as WC ≥ 80 cm for women and WC ≥ 90 cm for men8; since less than 8% of individuals have WC above these cutpoints, we used median values (men = 71 cm and women = 66.5 cm) to define at-risk groups.
Dietary data
Dietary data were derived from two 24-hour dietary recalls and the mean intake was used in the analyses. A nutritionist reviewed all dietary recalls immediately after collection. When implausible values were found, interviewers revisited respondents for verification. Energy and saturated fat intakes were calculated using the Philippines Food Composition Tables.26, 27
Sociodemographic and lifestyle characteristics
We included the following sociodemographic and lifestyle characteristics in our analysis: household assets, urbanicity, environmental hygiene, graduation status, smoking status, alcohol consumption, and level of physical activity.
The assets score, ranging from 0 to 10, measures household economic status. It reflects the type of lighting used, ownership of house, type of housing material, and ownership of selected assets: television, air conditioner, tape recorder, refrigerator, and motor vehicle. We dichotomized this variable at the median, ≤ 5 assets or > 5 assets. The urbanicity index is comprised of 7 components derived from CLHNS barangay-level survey data.28 A higher score designates a more urbanized barangay. We dichotomized this variable at the median, ≤ 43 or > 43. The hygiene score measures environmental cleanliness using data on the interviewer’s rating of cooking area, immediate area around the house, toilet type, and water source. The score ranges from 0 to 9 with larger values indicating greater cleanliness.29 High school (HS) graduation status was classified as yes or no. Smoking and alcohol consumption were assessed as yes or no. The majority of women did not smoke (> 93%) therefore we did not include this covariate in their analysis.
Physical activity was assessed by asking respondents to report time spent in all activities during a typical day. Each activity was assigned a metabolic equivalent (MET) value using the updated Compendium of Physical Activities. We identified minutes/week of moderate to vigorous physical activity (MVPA=METS >3) performed during occupation, leisure time, and household activities to approximate an overall minutes/week of MVPA. The majority of women did not participate in any MVPA (82%), thus MVPA was only included in the analysis of the men. We categorized physical activity: no MVPA, low to medium amounts of MVPA (<sex-specific median of 720 minutes/week), and high amounts of MVPA (≥720 minutes/week).
Statistical analysis
We performed a K-means cluster analysis to identify groups of young adults with similar CM risk factor patterns using SAS PROC FASTCLUS (SAS version 9.2, SAS Institute, Cary, NC).30 Since final cluster solutions are sensitive to initial seed values, we used a more objective approach to picking a cluster solution by creating an algorithm to maximize the ratio of between-cluster variance to within-cluster variance (largest R2).21
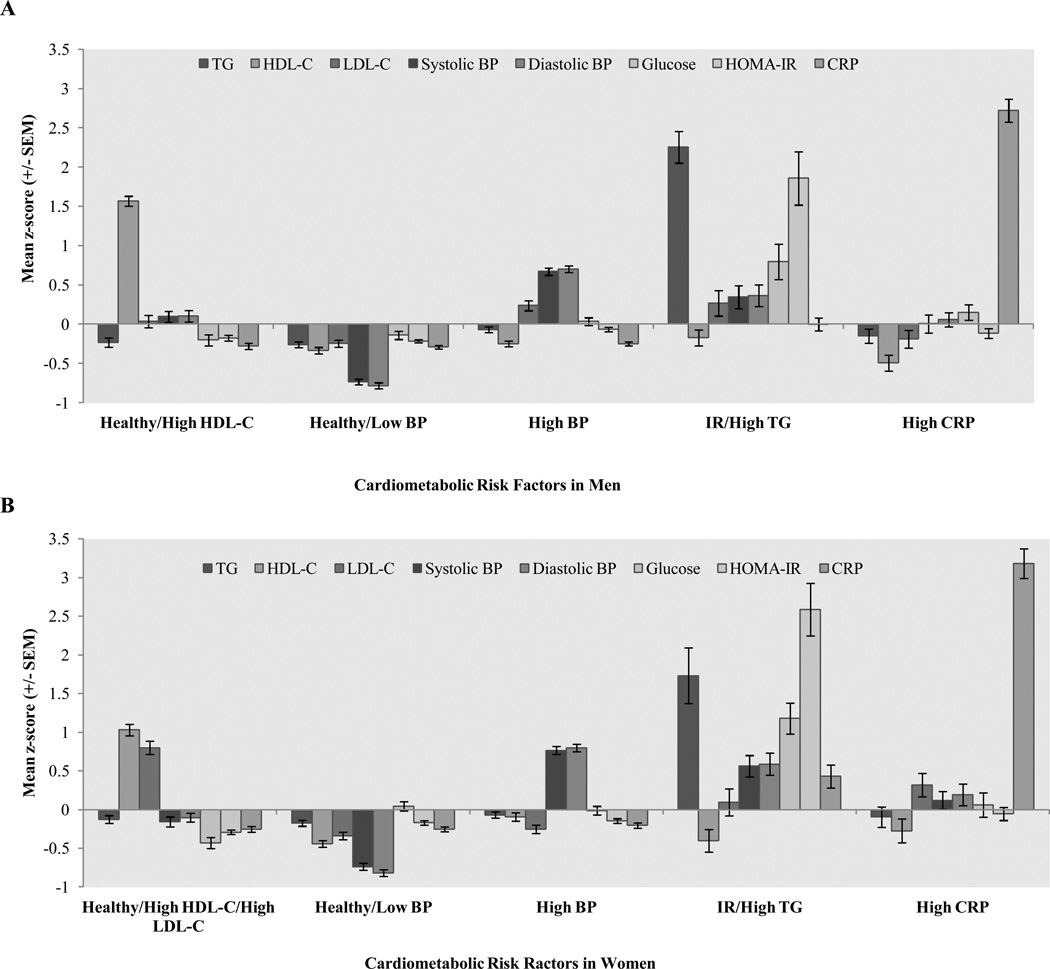
Cluster analysis was conducted separately in the women and men to account for differences in patterns of CM risk by sex. The variables entered into the cluster analysis were chosen to represent hypertension, inflammation, insulin resistance, and lipid abnormalities, and included sample and sex-specific standardized values (z-scores) of TG, HDL-C, LDL-C, systolic BP, diastolic BP, glucose, HOMA-IR, and CRP (Figure 1).
Figure 1. Mean z-scores of fasting biomarkers by cardiometabolic cluster.
Mean z-scores by cardiometabolic cluster for the eight fasting biomarkers used as input variables in the cluster analysis. A: Mean z-scores of biomarkers in young adult men. B: Mean z-scores of biomarkers in young adult women.
We used sex-specific multinomial logistic regression models in Stata version 12.0 (Stata Corporation, College Station, TX, 2006) to examine predictors of cluster membership in young adults. For men and women, the full models included the following covariates: high WC, OW status, % energy intake from saturated fat, energy intake, alcohol consumption, household assets, urbanicity, environmental hygiene, and education status; smoking status and level of physical activity were additionally included for men. We used the multivariate nutrient density method to control for confounding and to remove extraneous variation due to total energy intake.31 Multicollinearity between % of energy intake from saturated fat and total energy intake was not an issue (correlation coefficient<0.4).
We conducted manual backwards elimination (likelihood ratio test) to test whether each covariate improved model fit. If it did not improve model fit and also did not predict cluster membership the covariate was removed. Throughout our analysis we used α < 0.05 as the criterion for significance.
RESULTS
Prevalence of CM risk
Baseline characteristics are presented in Tables 2 and 3 for men and women respectively. Men had a high prevalence of low HDL-C (60%), while a low prevalence of elevated LDL-C (6%), elevated fasting glucose (3%), elevated HOMA-IR (3%), and elevated CRP (7%). Women had a high prevalence of low HDL-C (68%), while a low prevalence of elevated TG (9%), hypertension (2%), elevated fasting glucose (3%), elevated CRP (8%), and elevated HOMA-IR (4.5%). In comparison to women, men had a higher prevalence of elevated TG and hypertension. While in comparison to men, women had a higher prevalence of low HDL-C, elevated LDL-C, and elevated HOMA-IR.
Table 2.
Characteristics of young adult men in the 2005 CLHNS
| All Men (n= 871) |
Healthy/High HDL-C (n= 139) |
Healthy/Low BP (n= 312) |
High BP (n= 282) |
IR/High TG (n= 65) |
High CRP (n= 73) |
|
|---|---|---|---|---|---|---|
| Age, y | 21.0 ± 0.0 | 20.9 ± 0.0 | 20.9 ± 0.0 | 21.0 ± 0.0 | 20.9 ± 0.0 | 21.0 ± 0.0 |
| Cardiometabolic biomarkers† | ||||||
| Elevated triglycerides (%) | 19.7 ± 1.3 | 15.3 ± 3.0 | 11.1 ± 1.8 | 15.9 ± 2.1 | 87.7 ± 4.1 | 20.0 ± 4.6 |
| Low HDL cholesterol (%) | 59.6 ± 1.6 | 0.0 ± 0.0 | 72.7 ± 2.5 | 69.3 ± 2.7 | 67.7 ± 5.8 | 74.7 ± 5.1 |
| Elevated LDL cholesterol (%) | 5.7 ± 0.8 | 4.9 ± 1.8 | 2.9 ± 0.9 | 7.9 ± 1.6 | 10.8 ± 3.9 | 6.7 ± 2.9 |
| Hypertension (%) | 19.0 ± 1.3 | 20.8 ± 3.4 | 0.0 ± 0.0 | 37.6 ± 2.8 | 29.2 ± 5.7 | 14.7 ± 4.1 |
| Elevated fasting glucose (%) | 3.1 ± 0.6 | 1.4 ± 1.0 | 1.6 ± 0.7 | 2.1 ± 0.8 | 15.4 ± 4.5 | 6.7 ± 2.9 |
| Elevated HOMA-IR (%) | 2.5 ± 0.5 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.7 ± 0.5 | 29.2 ± 5.7 | 1.3 ± 1.3 |
| Elevated CRP (%) | 7.1 ± 0.9 | 0.7 ± 0.7 | 0.0 ± 0.0 | 0.0 ± 0.0 | 3.1 ± 2.2 | 80.0 ± 4.6 |
| Anthropometrics | ||||||
| Waist circumference (WC; cm) | 72.1 ± 0.2 | 71.2 ± 0.6 | 69.5 ± 0.3 | 73.9 ± 0.5 | 80.2 ± 1.3 | 71.3 ± 0.7 |
| High WC‡ (%) | 48.1 ± 1.7 | 45.3 ± 4.2 | 34.2 ± 2.7 | 60.1 ± 2.9 | 81.3 ± 4.9 | 37.0 ± 5.7 |
| BMI (kg/m2) | 21.0 ± 0.1 | 20.8 ± 0.2 | 20.0 ± 0.1 | 21.6 ± 0.2 | 24.2 ± 0.5 | 20.9 ± 0.3 |
| Overweight§ (%) | 19.4 ± 1.3 | 20.9 ± 3.5 | 6.4 ± 1.4 | 26.1 ± 2.6 | 50.0 ± 6.3 | 19.2 ± 4.6 |
| Dietary | ||||||
| Energy (kcal) | 2,221.8 ± 35.2 | 2,330.5 ± 87.4 | 2,154.0 ± 53.5 | 2,237.1 ± 66.0 | 2,376.1 ± 158.1 | 2,110.6 ± 107.2 |
| Saturated fat (%) | 7.8 ± 0.2 | 8.9 ± 0.5 | 7.7 ± 0.3 | 7.5 ± 0.3 | 8.6 ± 0.6 | 6.7 ± 0.4 |
| Cigarette smoking (%) | 49.3 ± 1.7 | 46.0 ± 4.2 | 54.0 ± 2.8 | 44.2 ± 3.0 | 60.9 ± 6.1 | 45.2 ± 5.9 |
| Alcohol drinking (%) | 85.2 ± 1.2 | 89.2 ± 2.6 | 81.5 ± 2.2 | 85.9 ± 2.1 | 85.9 ± 4.4 | 90.4 ± 3.5 |
| Socioeconomic | ||||||
| Number of assets | 5.2 ± 0.1 | 5.5 ± 0.2 | 4.9 ± 0.1 | 5.2 ± 0.1 | 6.1 ± 0.3 | 5.1 ± 0.2 |
| Hygiene score | 6.1 ± 0.1 | 6.5 ± 0.1 | 5.9 ± 0.1 | 6.2 ± 0.1 | 6.4 ± 0.2 | 5.7 ± 0.2 |
| Urbanicity score | 41.2 ± 0.5 | 43.7 ± 1.1 | 39.9 ± 0.8 | 41.0 ± 0.8 | 42.7 ± 1.6 | 41.2 ± 1.6 |
| Graduate d high school (%) | 60.2 ± 1.7 | 71.2 ± 3.9 | 53.8 ± 2.8 | 61.0 ± 2.9 | 68.8 ± 5.8 | 56.2 ± 5.8 |
Data are means ± SE or % ± SE.
Cutpoints are defined using Table 1.
High waist circumference defined as >71cm for men;
BMI ≥ 23kg/m2
Table 3.
Characteristics of young adult women in the 2005 CLHNS
| All Women (n= 698) |
Healthy/High HDL-C /High LDL-C (n= 138) |
Healthy/Low BP (n= 248) |
High BP (n= 228) |
IR/High TG (n= 46) |
High CRP (n= 38) |
|
|---|---|---|---|---|---|---|
| Age, y | 20.9 ± 0.0 | 21.0 ± 0.0 | 20.9 ± 0.0 | 20.9 ± 0.0 | 20.9 ± 0.1 | 20.9 ± 0.1 |
| Cardiometabolic biomarkers† | ||||||
| Elevated triglycerides (%) | 8.6 ± 1.0 | 3.8 ± 1.5 | 4.4 ± 1.3 | 7.7 ± 1.8 | 50.0 ± 7.3 | 9.8 ± 4.7 |
| Low HDL cholesterol (%) | 67.8 ± 1.7 | 27.2 ± 3.6 | 85.7 ± 2.2 | 72.5 ± 2.9 | 79.2 ± 5.9 | 73.2 ± 7.0 |
| Elevated LDL cholesterol (%) | 12.3 ± 1.2 | 32.3 ± 3.7 | 3.6 ± 1.2 | 4.7 ± 1.4 | 20.8 ± 5.9 | 22.0 ± 6.5 |
| Hypertension (%) | 2.0 ± 0.5 | 0.0 ± 0.0 | 0.0 ± 0.0 | 5.6 ± 1.5 | 4.2 ± 2.9 | 0.0 ± 0.0 |
| Elevated fasting glucose (%) | 3.0 ± 0.6 | 0.0 ± 0.0 | 1.6 ± 0.8 | 1.7 ± 0.9 | 27.1 ± 6.5 | 2.4 ± 2.4 |
| Elevated HOMA-IR (%) | 4.5 ± 0.8 | 0.0 ± 0.0 | 0.8 ± 0.6 | 0.0 ± 0.0 | 62.5 ± 7.1 | 2.4 ± 2.4 |
| Elevated CRP (%) | 7.5 ± 1.0 | 1.3 ± 0.9 | 1.6 ± 0.8 | 1.3 ± 0.7 | 14.6 ± 5.1 | 95.1 ± 3.4 |
| Anthropometrics | ||||||
| Waist circumference (WC; cm) | 67.9 ± 0.3 | 66.7 ± 0.6 | 65.6 ± 0.3 | 69.0 ± 0.5 | 76.8 ± 1.7 | 70.4 ± 1.4 |
| High WC‡ (%) | 48.1 ± 1.9 | 43.5 ± 4.2 | 36.0 ± 3.1 | 56.8 ± 3.3 | 78.3 ± 6.1 | 55.3 ± 8.2 |
| BMI (kg/m2) | 20.3 ± 0.1 | 19.9 ± 0.2 | 19.3 ± 0.1 | 20.7 ± 0.2 | 24.2 ± 0.8 | 21.0 ± 0.6 |
| Overweight§ (%) | 15.2 ± 1.4 | 12.3 ± 2.8 | 6.1 ± 1.5 | 18.1 ± 2.6 | 50.0 ± 7.5 | 26.3 ± 7.2 |
| Dietary | ||||||
| Energy (kcal) | 1,605.6 ± 33.1 | 1,588.7 ± 72.1 | 1,601.8 ± 61.1 | 1,629.8 ± 57.3 | 1,493.8 ± 88.4 | 1,683.7 ± 120.4 |
| Saturated fat (%) | 8.5 ± 0.2 | 9.5 ± 0.4 | 8.6 ± 0.3 | 8.0 ± 0.3 | 8.5 ± 0.7 | 7.5 ± 0.6 |
| Cigarette smoking (%) | 6.8 ± 1.0 | 6.6 ± 2.1 | 6.5 ± 1.6 | 6.2 ± 1.6 | 10.9 ± 4.6 | 7.9 ± 4.4 |
| Alcohol drinking (%) | 55.0 ± 1.9 | 57.4 ± 4.3 | 52.2 ± 3.2 | 56.4 ± 3.3 | 56.5 ± 7.4 | 55.3 ± 8.2 |
| Socioeconomic | ||||||
| Number of assets | 5.3 ± 0.1 | 5.5 ± 0.2 | 5.2 ± 0.1 | 5.3 ± 0.1 | 5.3 ± 0.2 | 5.1 ± 0.3 |
| Hygiene score | 6.2 ± 0.1 | 6.4 ± 0.1 | 6.1 ± 0.1 | 6.0 ± 0.1 | 6.3 ± 0.2 | 6.2 ± 0.3 |
| Urbanicity score | 41.4 ± 0.5 | 40.7 ± 1.2 | 41.6 ± 0.8 | 40.8 ± 0.9 | 42.3 ± 2.0 | 44.9 ± 2.1 |
| Graduated high school (%) | 78.3 ± 1.6 | 86.2 ± 2.9 | 79.4 ± 2.6 | 72.7 ± 3.0 | 76.1 ± 6.4 | 78.9 ± 6.7 |
Data are means ± SE or % ± SE.
Cutpoints are defined using Table 1.
High waist circumference defined as >66.5cm for women;
BMI ≥ 23kg/m2
Cluster analysis
We conducted a series of cluster analyses with 3 to 6 clusters specified, and chose the 5-cluster solution for both men and women because it yielded distinct CM risk factor patterns and each cluster contained approximately ≥ 5% of the sample.32 The 5-cluster solutions had R2= 0.35 and R2 = 0.36 in men and women respectively, indicating slightly more than 1/3rd of the variance in CM biomarkers was explained by the clusters. For men we identified the five clusters as: (1) Healthy/High HDL-C, (2) Healthy/Low BP, (3) High BP, (4) Insulin resistant (IR)/High TG, and (5) High CRP. For the women we identified the same five clusters except the first cluster also included LDL-C: (1) Healthy/High HDL-C/High LDL-C. We named the clusters according to what risk factor(s) had the highest/lowest mean relative to other clusters; the term “healthy” represents low z-scores for the majority of CM biomarkers (except HDL-C). We ordered these clusters such that clusters 1–5 in men and women represented similar CM patterns.
Cardiometabolic patterns in young adult men
Mean z-scores of the CM biomarkers varied markedly by cluster (Figure 1), as did the prevalence of risk factors defined by cutpoints to represent “high risk” (Tables 2 and 3). Men in the Healthy/High HDL-C cluster (n=144, 16%) had the zero prevalence of low HDL-C. Men in the Healthy/Low BP cluster (n=315, 35%) had the lowest prevalence of hypertension (0%) and a high prevalence of low HDL-C (73%). Men in the High BP cluster (n=290, 33%) had a relatively high prevalence of hypertension (38%) and low HDL-C (69%). Men in the IR/High TG cluster (n=65, 7%) had highest prevalence of elevated TG (88%), elevated fasting glucose (15%), and elevated HOMA-IR (29%); in addition, these men had a high prevalence of low HDL-C (68%). Lastly, men in the High CRP cluster (n=75, 8%) had the highest prevalence of elevated CRP (80%), and a high prevalence of low HDL-C (75%).
Cardiometabolic patterns in young adult women
Mean z-scores of the CM biomarkers varied markedly by cluster (Figure 1), as did the prevalence of risk factors defined by cutpoints to represent “high risk” (Tables 2 and 3). Women in the Healthy/High HDL-C/High LDL-C cluster (n=158, 22%) had the lowest prevalence of low HDL-C (27%) and a relatively high prevalence of LDL-C (32%); none of these women had hypertension. Women in the Healthy/Low BP cluster (n=252, 34%) had no hypertension and a high prevalence of low HDL-C (86%). Women in the High BP cluster (n=233, 32%) had a relatively high prevalence of hypertension (6%), and low HDL-C (73%). Women in the IR/High TG cluster (n=48, 7%) had highest prevalence of elevated TG (50%), elevated fasting glucose (27%), and elevated HOMA-IR (63%); in addition, these women had a high prevalence of low HDL-C (79%). Lastly, women in the High CRP cluster (n=41, 6%) had the highest prevalence of elevated CRP (95%) and a high prevalence of low HDL-C (73%); none of these women had hypertension.
Multivariable analysis in young adult men
The final multivariate model in the men included the following covariates: high WC, OW status, % of energy intake from saturated fat, energy intake, household assets, smoking status, alcohol consumption, and environmental hygiene (Table 4).
Table 4.
Predictors of cluster membership
| Predicted male cluster | ||||
| Referent male cluster | Healthy/Low BP | High BP | IR/High TG | High CRP |
| − OW† [0.32 (0.16,0.64)] | + High WC‡ [1.87 (1.15,3.04)] | + High WC‡ [3.68 (1.62,8.36)] | − Satfat§ [0.43 (0.22,0.86)] | |
| − Alcohol [0.51 (0.27,0.96)] | + OW† [2.17 (1.02,4.64)] | − Hygiene [0.74 (0.60,0.90)] | ||
| Healthy/High HDL-C | + Assets [2.14 (1.06,4.32)] | |||
| + Smoking [2.04 (1.06,3.90)] | ||||
| + High WC‡ [1.92 (1.32,2.78)] | + High WC‡ [3.78 (1.77,8.06)] | + OW† [5.12 (2.13,12.33)] | ||
| + OW† [3.46 (1.95,6.16)] | + OW† [6.80 (3.21,14.42)] | − Satfat§ [0.51 (0.27,0.98)] | ||
| − Smoking [0.63 (0.44,0.89)] | + Assets [2.72 (1.42,5.24)] | + Assets [1.94 (1.10,3.42)] | ||
| Healthy/Low BP | − Smoking [0.56 (0.33,0.97)] | |||
| + Alcohol [2.83 (1.19,6.72)] | ||||
| − Hygiene [0.83 (0.70,0.99)] | ||||
| + OW† [1.96 (1.02,3.77)] | − High WC† [0.34 (0.18,0.64)] | |||
| High BP | + Assets [2.42 (1.27,4.60)] | − Hygiene [0.82 (0.69,0.98)] | ||
| + Smoking [2.28 (1.25,4.14)] | ||||
| IR/High TG | − High WC‡ [0.17 (0.07,0.43)] | |||
| − Smoking [0.39 (0.19,0.82)] | ||||
| Predicted female cluster | ||||
| Referent female cluster | Healthy/Low BP | High BP | IR/High TG | High CRP |
| − Satfat§ [0.46 (0.28,0.78)] | + OW† [4.57 (1.90,10.95)] | − Satfat§ [0.22 (0.08,0.61)] | ||
| Healthy/High HDL-C/High LDL-C | + Energy¶ [1.40 (1.00,1.96)] | + Energy¶ [1.73 (1.02,2.91)] | ||
| − HS Grad [0.51 (0.29,0.92)] | + Urban [2.88 (1.30,6.39)] | |||
| + High WC‡ [1.86 (1.24,2.77)] | + High WC‡ [2.94 (1.24,6.95)] | + OW† [4.12 (1.49,11.40)] | ||
| Healthy/Low BP | + OW† [2.24 (1.17,4.29)] | + OW† [8.26 (3.50,19.50)] | − Satfat§ [0.35 (0.13,0.92)] | |
| + Urban [2.81 (1.31,6.04)] | ||||
| High BP | + OW† [3.69 (1.72,7.92)] | + Urban [2.82 (1.32,6.04)] | ||
| IR/High TG | ||||
Cells display +/− association of predictors with cluster membership. Data are OR (95% CI).
Overweight;
Waist Circumference;
Percentage of total energy intake from saturated fat; scaled (divided by 10) when imputed in the multinomial logistic regression to ease interpretation;
Energy intake was also scaled; units were kJ/1000.
Compared to the Healthy/High HDL-C cluster: being normal weight and not consuming alcohol increased the likelihood of being in the Healthy/Low BP cluster; higher WC increased the likelihood of being in the High BP cluster; higher WC, being OW, having more assets, and smoking increased the likelihood of being in the IR/High TG cluster; decreased % of energy intake from saturated fat and lower environmental hygiene increased the likelihood of being in the High CRP cluster.
Compared to the Healthy/Low BP cluster: higher WC, being OW, and not smoking increased the likelihood of being in the High BP cluster; higher WC, being OW, and having more assets increased the likelihood of being in the IR/High TG cluster; being OW, decreased % of energy intake from saturated fat, having more assets, not smoking, alcohol consumption, and decreased environmental hygiene increased the likelihood of being in the High CRP cluster.
Compared to the High BP cluster: being OW, having more assets, and smoking increased the likelihood of being in the IR/High TG cluster; lower WC and decreased environmental hygiene increased the likelihood of being in the High CRP cluster.
Compared to the IR/High TG cluster, lower WC and not smoking increased the likelihood of being in the High CRP cluster.
Multivariable analysis in young adult women
The final multivariate model in the women included the following covariates: high WC, OW status, % of energy intake from saturated fat, energy intake, urbanicity, and HS graduation status (Table 4).
Compared to the Healthy/High HDL-C/High LDL-C cluster: no covariates increased the likelihood of being in the Healthy/Low BP cluster; decreased % of energy intake from saturated fat, increased energy intake, and not graduating from HS increased the likelihood of being in the High BP cluster; being OW increased the likelihood of being in the IR/High TG cluster; decreased % of energy intake from saturated fat, increased energy intake, and lower urbanicity increased the likelihood of being in the High CRP cluster.
Compared to the Healthy/Low BP cluster: higher WC and being OW increased the likelihood of being in the High BP cluster; higher WC and being OW increased the likelihood of being in the IR/High TG cluster; being OW, decreased % of energy intake from saturated fat, and lower urbanicity increased the likelihood of being in the High CRP cluster.
Compared to the High BP cluster: being OW increased the likelihood of being in the IR/High TG cluster; decreased urbanicity increased the likelihood of being in the High CRP cluster.
No covariates distinguished the IR/High TG cluster from the High CRP cluster.
DISCUSSION
Cluster analysis is a useful tool for identifying groups of individuals who share similar CM risk factor patterns. In contrast with the MetS definition, cluster analysis allows for flexibility. For example, we included a measure of inflammation in the cluster analysis, a risk factor not commonly included in MetS definitions, which allowed us to identify a distinct group characterized primarily by elevated CRP levels. In addition, we did not include WC as a criterion for the clustering algorithm, unlike the IDF, which requires elevated WC in the definition.8 This enabled us to distinguish for which clusters elevated WC (a modifiable risk factor) predicted cluster membership.
By using cluster analysis, we were able to capture the heterogeneity in patterns of CM risk factor clustering. Research has demonstrated that mortality risk is dependent on the actual combinations of CM risk factors, highlighting the importance of understanding these sex differences in the clustering of CM risk factors.33 While our analysis found relatively similar CM risk clusters among men and women, the predictors of these clusters varied by sex. Perhaps as these young adults age more distinct CM patterns between men and women will emerge.
A high prevalence of low HDL-C, a risk factor for heart disease, has been reported in the Philippines and other Asian populations.34–36 This was reflected in the cluster analysis results: over 65% of men and 70% of women, not in the Healthy/High HDL-C cluster, had low HDL-C levels.
Previous work among the mothers in Cebu suggested that saturated fat intake, perhaps from coconut oil, could be protective against low HDL-C levels.21, 37 However in young adults, we saw saturated fat intake had varying relationships with different CM risk factors. In both men and women, decreased % energy intake from saturated fat predicted membership in the High CRP group when compared to the two Healthy clusters. In addition, a decrease in % saturated fat intake predicted membership in the High BP group in women, compared to the Healthy/High HDL-C/High LDL-C group.
The association of saturated fat intake with healthy CM profiles could reflect the types of saturated fats consumed in this population. Coconut oil, the most common and traditional cooking oil in Cebu, is rich in lauric acid.38 Lauric acid improves the total cholesterol to HDL-C ratio, more than any other saturated or unsaturated fatty acid, primarily by increasing HDL-C levels.39 In addition, a replacement of carbohydrates with lauric acid produces a decrease in this ratio.39 This proves especially relevant in our study population since over half of energy intake comes from carbohydrates, the majority of which are refined rice products. Other studies have found diets rich in coconut oil or in saturated fat do not alter markers of inflammation, fasting glucose, fasting insulin, HOMA-IR, or incident diabetes.40, 41
Men with poorer environmental hygiene (increased pathogenicity) were more likely to be in the High CRP cluster compared to the two Healthy clusters. These results support previous research conducted in the CLHNS and reinforce the involvement of pathogen exposure in activating pro-inflammatory pathways.29, 42, 43 But why do we fail to observe this hygiene effect in women? Adiposity relates more strongly with inflammation in women than in men, thus it is possible the effects of adiposity overwhelmed the effects of the hygiene score in women.15, 44
As expected, WC and OW status were the strongest predictors of membership in the IR/High TG cluster, underscoring the adverse health effects of excess visceral adipose tissue, for which WC serves as a proxy.45 WC is among the best-established predictors of CM risk and past work in the CLHNS and other populations support this notion.29, 42, 46, 47 Research has also demonstrated that increased WC predicts CM abnormalities in both normal weight and OW individuals, highlighting the potential for visceral fat to influence the development of CM risk factors, independent of BMI.48
This population has a low prevalence of overweight (18%). However, among normal weight individuals, CM risk factors were already present: 63% of the sample with BMI<23 kg/m2 had low HDL-C. Despite leanness, cluster analysis found patterns of CM risk. While measures of adiposity predicted some of these patterns, modifiable factors such as dietary intake and pathogen exposure also independently predicted cluster membership. This emphasizes the importance monitor and screen lean individuals for CM risk and future CM diseases, especially in Asian populations where the risk of CM diseases is elevated at a lower BMI (likely due to increased visceral fat at lower BMIs).4
Several limitations warrant mention. A limitation of cluster analysis is that not all individuals within a certain cluster necessarily share all characteristics. For example, in our “Healthy” clusters we found the average z-scores for CM risk biomarkers were relatively low (except HDL-C), but we cannot ascribe these low values to each individual in the cluster.
Attrition and selection bias are also concerns. Migration of the more educated, urban segment of the original cohort has left us with a sample that is no longer representative of the population from which it was drawn.20 The sample was further reduced due to selection criteria. From the full sample of 1,888 young adults in 2005, the multivariate analysis included those that were fasting and not pregnant with complete biomarker, anthropometric, and socioeconomic data, resulting in an analytic sample of 1,621. Comparing baseline socioeconomic characteristics, we found a lower percentage of HS graduates among women excluded vs. those included in the analysis (68% vs. 78% respectively, ANOVA p < 0.05).
In sum, despite the population’s young age, lack of clinical disease, and relative leanness, cluster analysis identified distinct patterns of CM risk factors. By using cluster analysis we made fewer assumptions regarding the underlying etiology and allowed relationships among CM risk factors to emerge from the data themselves. We found sex-specific clustering of CM risk factors and were able to evaluate how diet, adiposity, and environmental factors influenced these patterns. As expected, measures of adiposity predicted specific CM risk patterns. However, diet and environmental factors also independently predicted risk factor clustering. This emphasizes the importance of screening both lean and OW individuals for CM risk, especially in Asian populations where the risk of CM diseases is elevated at lower BMI.4 Future studies examining how CM risk patterns change longitudinally could provide insight to how CM risk evolves across the life course. Finding modifiable and non-modifiable predictors of CM risk in early adulthood could help inform targeted prevention efforts for future CM disease.
ACKNOWLEDGEMENTS
We thank the Office of Population Studies Foundation research and data collection teams and the study participants who generously provided their time for this study.
FUNDING SOURCES
This research was supported by National Institutes of Health grants R01-HL085144-03, R01-HD054501, and R01-TW05596.
Footnotes
CONTRIBUTION: Zubair and L.S. Adair had full access to study data and take full responsibility for the integrity of the data and accuracy of the analysis. L.S. Adair is the Principal Investigator for which the study was based; N. Zubair and L.S. Adair designed research; C.W. Kuzawa, and T.W. McDade led the laboratory data analysis; N. Zubair and L.S. Adair performed the statistical analysis; N. Zubair wrote the initial draft of the manuscript; N. Zubair, L. S. Adair, C.W. Kuzawa, N. R. Lee, and T.W. McDade reviewed and revised the drafts. All authors read and approved the final manuscript.
DISCLOSURES
None.
REFERENCES
- 1.Yoon KH, Lee JH, Kim JW, Cho JH, Choi YH, Ko SH, Zimmet P, Son HY. Epidemic obesity and type 2 diabetes in Asia. Lancet. 2006;368:1681–1688. doi: 10.1016/S0140-6736(06)69703-1. [DOI] [PubMed] [Google Scholar]
- 2.Popkin BM, Adair LS, Ng SW. Global nutrition transition and the pandemic of obesity in developing countries. Nutr Rev. 2012;70:3–21. doi: 10.1111/j.1753-4887.2011.00456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Popkin BM. The Nutrition Transition and Obesity in the Developing World. J Nutr. 2001;131:871S–873S. doi: 10.1093/jn/131.3.871S. [DOI] [PubMed] [Google Scholar]
- 4.Choo V. WHO reassesses appropriate body-mass index for Asian populations. Lancet. 2002;360:235. doi: 10.1016/S0140-6736(02)09512-0. [DOI] [PubMed] [Google Scholar]
- 5.Singh A, Mulder C, Twisk JWR, Van Mechelen W, Chinapaw MJM. Tracking of childhood overweight into adulthood: a systematic review of the literature. Obes Rev. 2008;9:474–488. doi: 10.1111/j.1467-789X.2008.00475.x. [DOI] [PubMed] [Google Scholar]
- 6.Guo SS, Huang C, Maynard LM, Demerath E, Towne B, Chumlea WC, Siervogel RM. Body mass index during childhood, adolescence and young adulthood in relation to adult overweight and adiposity: the Fels Longitudinal Study. Int J Obes Relat Metab Disord. 2000;24:1628–1635. doi: 10.1038/sj.ijo.0801461. [DOI] [PubMed] [Google Scholar]
- 7.Zimmet P, Alberti G, Kaufman F, Tajima N, Silink M, Arslanian S, Wong G, Bennett P, Shaw J, Caprio S. The metabolic syndrome in children and adolescents. Lancet. 2007;369:2059. doi: 10.1016/S0140-6736(07)60958-1. [DOI] [PubMed] [Google Scholar]
- 8.Alberti KG, Zimmet P, Shaw J IDF Epidemiology Task Force Consensus Group. The metabolic syndrome--a new worldwide definition. Lancet. 2005;366:1059–1062. doi: 10.1016/S0140-6736(05)67402-8. [DOI] [PubMed] [Google Scholar]
- 9.Bray GA, Bellanger T. Epidemiology, trends, and morbidities of obesity and the metabolic syndrome. Endocrine. 2006;29:109–117. doi: 10.1385/ENDO:29:1:109. [DOI] [PubMed] [Google Scholar]
- 10.Must A, Spadano J, Coakley EH, Field AE, Colditz G, Dietz WH. The disease burden associated with overweight and obesity. JAMA. 1999;282:1523. doi: 10.1001/jama.282.16.1523. [DOI] [PubMed] [Google Scholar]
- 11.Ridker PM, Wilson PW, Grundy SM. Should C-reactive protein be added to metabolic syndrome and to assessment of global cardiovascular risk? Circulation. 2004;109:2818–2825. doi: 10.1161/01.CIR.0000132467.45278.59. [DOI] [PubMed] [Google Scholar]
- 12.Reaven GM. Role of Insulin Resistance in Human Disease (Syndrome X): An Expanded Definition. Annual Review of Medicine. 1993;44:121–131. doi: 10.1146/annurev.me.44.020193.001005. [DOI] [PubMed] [Google Scholar]
- 13.Lakka HM, Laaksonen DE, Lakka TA, Niskanen LK, Kumpusalo E, Tuomilehto J, Salonen JT. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA. 2002;288:2709. doi: 10.1001/jama.288.21.2709. [DOI] [PubMed] [Google Scholar]
- 14.Lehto S, Laakso M. Cardiovascular risk factors clustering with endogenous hyperinsulinaemia predict death from coronary heart disease in patients with type II diabetes. Diabetologia. 2000;43:148–155. doi: 10.1007/s001250050023. [DOI] [PubMed] [Google Scholar]
- 15.de Roos NM, Bots ML, Katan MB. Replacement of dietary saturated fatty acids by trans fatty acids lowers serum HDL cholesterol and impairs endothelial function in healthy men and women. Arterioscler Thromb Vasc Biol. 2001;21:1233–1237. doi: 10.1161/hq0701.092161. [DOI] [PubMed] [Google Scholar]
- 16.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jones DW, Materson BJ, Oparil S, Wright JT. Seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension. 2003;42:1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 17.Smith SC, Jackson R, Pearson TA, Fuster V, Yusuf S, Faergeman O, Wood DA, Alderman M, Horgan J, Home P. Principles for national and regional guidelines on cardiovascular disease prevention. Circulation. 2004;109:3112–3121. doi: 10.1161/01.CIR.0000133427.35111.67. [DOI] [PubMed] [Google Scholar]
- 18.Kahn R, Buse J, Ferrannini E, Stern M. American Diabetes Association, European Association for the Study of Diabetes. The metabolic syndrome: time for a critical appraisal: joint statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2005;28:2289–2304. doi: 10.2337/diacare.28.9.2289. [DOI] [PubMed] [Google Scholar]
- 19.Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO, Criqui M, et al. Markers of Inflammation and Cardiovascular Disease: Application to Clinical and Public Health Practice: A Statement for Healthcare Professionals From the Centers for Disease Control and Prevention and the American Heart Association Circulation. 2003;107:499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- 20.Adair LS, Popkin BM, Akin JS, Guilkey DK, Gultiano S, Borja J, Perez L, Kuzawa CW, McDade T, Hindin MJ. Cohort profile: the Cebu longitudinal health and nutrition survey. Int J Epidemiol. 2011;40:619–625. doi: 10.1093/ije/dyq085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zubair N, Kuzawa CW, McDade TW, Adair LS. Cluster analysis reveals important determinants of cardiometabolic risk patterns in Filipino women. Asia Pac J Clin Nutr. 2012;21:271–281. [PMC free article] [PubMed] [Google Scholar]
- 22.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 23.Stern SE, Williams K, Ferrannini E, DeFronzo RA, Bogardus C, Stern MP. Identification of individuals with insulin resistance using routine clinical measurements. Diabetes. 2005;54:333–339. doi: 10.2337/diabetes.54.2.333. [DOI] [PubMed] [Google Scholar]
- 24.Lohman TG, Roche AF, Martorell R. Anthropometric Standardization Reference Manual: Human Kinetics Books Champaign. 1988 [Google Scholar]
- 25.WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157–163. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- 26.FNRI. Food Composition Tables Recommended for Use in the Philippines. Manila, Philippines: Food and Nutrition Research Institute; 1997. [Google Scholar]
- 27.Philippine Nutrition Facts & Figures. Manila, Philippines: Food and Nutrition Research Institute; 2001. [Google Scholar]
- 28.Dahly DL, Adair LS. Quantifying the urban environment: a scale measure of urbanicity outperforms the urban-rural dichotomy. Soc Sci Med. 2007;64:1407–1419. doi: 10.1016/j.socscimed.2006.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McDade TW, Rutherford JN, Adair L, Kuzawa C. Adiposity and pathogen exposure predict C-reactive protein in Filipino women. J Nutr. 2008;138:2442–2447. doi: 10.3945/jn.108.092700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aldenderfer M, Blashfield R. Cluster Analysis. Newbury Park, CA: SAGE Publications, Inc.; 1984. [Google Scholar]
- 31.Willett W. Nutritional Epidemiology. USA: Oxford University Press; 1998. [Google Scholar]
- 32.Boone-Heinonen J, Gordon-Larsen P, Adair LS. Obesogenic clusters: multidimensional adolescent obesity-related behaviors in the U.S. Ann Behav Med. 2008;36:217–230. doi: 10.1007/s12160-008-9074-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuk JL, Ardern CI. Age and sex differences in the clustering of metabolic syndrome factors: association with mortality risk. Diabetes Care. 2010;33:2457–2461. doi: 10.2337/dc10-0942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morales DD, Punzalan FE, Paz-Pacheco E, Sy RG, Duante CA. Metabolic syndrome in the Philippine general population: prevalence and risk for atherosclerotic cardiovascular disease and diabetes mellitus. Diab Vasc Dis Res. 2008;5:36–43. doi: 10.3132/dvdr.2008.007. [DOI] [PubMed] [Google Scholar]
- 35.Rutherford JN, McDade TW, Feranil AB, Adair LS, Kuzawa CW. High prevalence of low HDL-c in the Philippines compared to the US: population differences in associations with diet and BMI. Asia Pac J Clin Nutr. 2010;19:57–67. [PMC free article] [PubMed] [Google Scholar]
- 36.Huxley RR, Barzi F, Lam TH, Czernichow S, Fang X, Welborn T, et al. Isolated low levels of high-density lipoprotein cholesterol are associated with an increased risk of coronary heart disease: an individual participant data meta-analysis of 23 studies in the Asia-Pacific region. Circulation. 2011;124:2056–2064. doi: 10.1161/CIRCULATIONAHA.111.028373. [DOI] [PubMed] [Google Scholar]
- 37.Feranil AB, Duazo PL, Kuzawa CW, Adair LS. Coconut oil is associated with a beneficial lipid profile in pre-menopausal women in the Philippines. Asia Pac J Clin Nutr. 2011;20:190–195. [PMC free article] [PubMed] [Google Scholar]
- 38.Kuzawa CW, Adair LS, Avila JL, Cadungog JH, Le NA. Atherogenic lipid profiles in Filipino adolescents with low body mass index and low dietary fat intake. Am J Hum Biol. 2003;15:688–696. doi: 10.1002/ajhb.10200. [DOI] [PubMed] [Google Scholar]
- 39.Mensink RP, Zock PL, Kester AD, Katan MB. Effects of dietary fatty acids and carbohydrates on the ratio of serum total to HDL cholesterol and on serum lipids and apolipoproteins: a meta-analysis of 60 controlled trials. Am J Clin Nutr. 2003;77:1146–1155. doi: 10.1093/ajcn/77.5.1146. [DOI] [PubMed] [Google Scholar]
- 40.Voon PT, Ng TK, Lee VK, Nesaretnam K. Diets high in palmitic acid (16:0), lauric and myristic acids (12:0 +14:0), or oleic acid (18:1) do not alter postprandial or fasting plasma homocysteine and inflammatory markers in healthy Malaysian adults. Am J Clin Nutr. 2011;94:1451–1457. doi: 10.3945/ajcn.111.020107. [DOI] [PubMed] [Google Scholar]
- 41.Tinker LF, Bonds DE, Margolis KL, Manson JE, Howard BV, Larson J, et al. Low-fat dietary pattern and risk of treated diabetes mellitus in postmenopausal women: the Women's Health Initiative randomized controlled dietary modification trial. Arch Intern Med. 2008;168:1500–1511. doi: 10.1001/archinte.168.14.1500. [DOI] [PubMed] [Google Scholar]
- 42.Rexrode KM, Pradhan A, Manson JE, Buring JE, Ridker PM. Relationship of total and abdominal adiposity with CRP and IL-6 in women. Ann Epidemiol. 2003;13:674–682. doi: 10.1016/s1047-2797(03)00053-x. [DOI] [PubMed] [Google Scholar]
- 43.Jones KE, Patel NG, Levy MA, Storeygard A, Balk D, Gittleman JL, Daszak P. Global trends in emerging infectious diseases. Nature. 2008;451:990–993. doi: 10.1038/nature06536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Micha R, Mozaffarian D. Saturated fat and cardiometabolic risk factors, coronary heart disease, stroke, and diabetes: a fresh look at the evidence. Lipids. 2010;45:893–905. doi: 10.1007/s11745-010-3393-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Olefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Annu Rev Physiol. 2010;72:219–246. doi: 10.1146/annurev-physiol-021909-135846. [DOI] [PubMed] [Google Scholar]
- 46.Rutherford JN, McDade TW, Lee NR, Adair LS, Kuzawa C. Change in waist circumference over 11 years and current waist circumference independently predict elevated CRP in Filipino women. Am J Hum Biol. 2010;22:310–315. doi: 10.1002/ajhb.20986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ramachandran A, Snehalatha C, Yamuna A, Murugesan N, Narayan KM. Insulin resistance and clustering of cardiometabolic risk factors in urban teenagers in southern India. Diabetes Care. 2007;30:1828–1833. doi: 10.2337/dc06-2097. [DOI] [PubMed] [Google Scholar]
- 48.Araneta MRG, Barrett-Connor E. Ethnic Differences in Visceral Adipose Tissue and Type 2 Diabetes: Filipino, African-American, and White Women. Obesity. 2005;13:1458–1465. doi: 10.1038/oby.2005.176. [DOI] [PubMed] [Google Scholar]