For over 30 years the endothelium has assumed greater and greater importance in our understanding of the development of vascular pathology. This includes the discoveries that the endothelium releases powerful vasodilator and antiplatelet mediators, prostacyclin and nitric oxide, as well as its role in governing permeability, inflammation, and monocyte/macrophage infiltration of the blood vessel. In this issue of Circulation, Fan and colleagues1 show that boosting endothelial-derived oxidants in the mouse aorta by overexpression of the NADPH oxidase isoform, Nox2, during prolonged angiotensin II-induced hypertension, results in a high incidence of infra-renal aortic dissection.
Aortic dissections are often associated with aneurysms, which may affect both thoracic (TAA) and abdominal (AAA) regions, but can occur also in the absence of aneurysm. There are currently no approved drug treatments against these deadly aortopathies. Limited treatment options include blood pressure control, as a means of decreasing the risk of rupture, and endovascular or open surgical repair, a procedure with high risk of morbidity and mortality. Although the elucidation of the mechanisms responsible for early aortic pathological changes that precede overt dissection remains very challenging in humans, animal models suggest that medial degeneration, consisting of vascular smooth muscle cell apoptosis and elastin fragmentation, are early features. The work by Fan et al. identifies release from the endothelium of the pro-inflammatory cytokine cyclophillin A (cypA), upon angiotensin II administration, as a potential paracrine culprit mediating early degenerative events in vascular smooth muscle resulting in aortic dissection. Surprisingly, such a short-term dramatic effect was not a consequence of the hypertensive response to angiotensin II, as equal pressor doses of norepinephrine did not cause dissection. Instead, the authors present evidence that when Nox2 is over-expressed in the endothelium, angiotensin II causes excessive aortic inflammation and remodeling leading to dissection because the augmented production of oxidants promotes the secretion of cypA. The novel model they present, therefore, offers unique evidence of the powerful influence of the endothelium on arterial wall structure as well as insights into potential paracrine mediators within the arterial wall that may lead to aortic dissection. Despite the high rate of dissection and the dramatic MRI pictures of the extent of the dissection along the aorta presented by the authors, they did not report on any sudden deaths, the most dreaded consequence of aortic dissection in man. Hopefully, further understanding of the molecular mechanisms of dissection will provide therapeutic insights to prevent this cause of sudden death in man.
Aortic dilatations and dissections, primarily in the thoracic region, are often a co-morbidity of monogenic syndromes, such as Marfan’s, Loeys-Dietz’s and Ehlers Danlos’s syndromes, characterized by genetic alterations in extracellular matrix components, including fibrillin-1 and collagen. These gene mutations result in pathological aortic remodeling and enlargement, which can progressively worsen into a thoracic aortic aneurysm. Similarly, mutations in MYH11 and ACTA2, two major vascular smooth muscle structural proteins, result in aneurysm. In addition, genetic mutations in transforming growth factor β (TGFβ)2, a cytokine with fibrotic effects on vascular smooth muscle, or TGFβ receptors (TGFβR1 and TGFβR2)3, downstream effectors (SMADs)4 or inhibitors (SKI)5, have been implicated in the etiology of aortic dilatation and dissection6. Losartan, an angiotensin II receptor blocker commonly used as an anti-hypertensive medication, has recently entered clinical trials for the treatment of thoracic aortic aneurysms in Marfan’s and related syndromes7, for its ability to antagonize the downstream effector TGFβ8, as well as to decrease hemodynamic stress on the dilated aorta, by lowering blood pressure. In contrast to purported deleterious actions in the thoracic aorta, TGFβ was protective in angiotensin II-induced AAA in normocholesterolemic mice by inhibiting inflammatory cell infiltration and consequent vascular smooth muscle cell apoptosis and extracellular matrix degradation9. This underscores that molecular mechanisms may differ amongst the phenotypic spectrum of aortic aneurysms and dissections based on etiology and/or on location, making a “one-fits-all” therapeutic approach problematic. However, the fact that cypA mediates VSMC proliferation and recruitment of inflammatory cells in several arterial disease models including ligated carotid arteries, atherosclerosis and angiotensin II-induced aneurysms10 would suggest that cypA may be a common inflammatory mediator and a possible therapeutic target for several diseases of arterial remodeling characterized by oxidant overproduction and inflammatory cell infiltration.
Elastase infusions or calcium chloride instillation induce elastin fragmentation and mimic the structural defects of human aneurysmal aortas, and are also features of mice bearing mutation for genes required for structural integrity of the aortic wall, such as fibrillin-1, fibulin-4 and TGFβ receptors. In addition, apolipoprotein E or low density lipoprotein receptor null mice administered angiotensin II have been extensively used as experimental models of aortic aneurysm, in part because of the reproducibility of the outcome, but despite the fact that hypercholesterolemia is not an independent risk factor for aneurysms in man. In the absence of the apolipoprotein E null genetic background, elevated doses of angiotensin II (commonly 3.2mg/kg/d) are required to induce aneurysms in mice and occur with a lower incidence than in hypercholesterolemic mice, although they cause similar tissue pathology. The relevance of angiotensin II-infusion models to the human pathology is still debated11, largely because direct comparisons between animal and human specimens at early stages of the disease are not feasible. The striking finding by Fan et al. is that angiotensin II infusion, even at the modest dose of 1mg/kg/d in endothelial Nox-2 transgenic mice, was sufficient to cause dissections in 45% of their normolipidemic mice.
Angiotensin II infusion in rodents has been employed as a model of renin-dependent hypertension for more than 50 years. Despite the fact that the rapidity of development and severity of hypertension is not often reproduced in spontaneously hypertensive patients, the model continues to provide unique insights into the pathogenesis of hypertension and the mechanisms of its clinical sequelae. Two of the most exciting recent mechanistic observations provided by the model indicate the importance of NADPH oxidase in brain nuclei that control sympathetic nerve traffic12, and the involvement of T-cells in mediating the hypertensive response to angiotensin II13. These two new directions stem from the original fundamental observations that angiotensin II stimulates NADPH oxidase-derived oxidants and contributes to vasoconstriction14, hypertrophy and remodeling of the vascular wall15, and atherosclerosis16. The key NADPH oxidase involved contains the heme-binding subunit Nox2, or gp91phox, the isoform that accounts for superoxide anion production by neutrophils, macrophages, and other myeloid cells. A Nox2 deficient knockout mouse had diminished pressor and hypertrophic response to angiotensin II17, but that study left open the question addressed in Fan et al. of whether Nox2 expression in different cell types was important. Interestingly, Nox2, even in the normal aortic wall, is concentrated in the endothelium as well as in adventitial fibroblasts18, and these two sites also are where leukocytes increase during angiotensin II infusion. This inflammatory cell influx caused by angiotensin II is key as demonstrated by the fact that leukocyte infiltration as well as the pressor and hypertrophic response is diminished in a chemokine receptor knockout mouse19. Fan et al. show that the increase in reactive oxygen species (ROS) that they have induced in the endothelium, leads to increased adhesion molecule VCAM1 expression throughout the aortic wall, providing evidence that the greater inflammatory response due to a paracrine mediator is at the root of the increased incidence of aortic dissection.
Fan et al. also provide insights into the paracrine relationships within the vascular wall that mediate the response to angiotensin II. Earlier elegant studies showed that cypA in smooth muscle cells promotes inflammation and activation of proteolytic enzymes, and that mice doubly deficient in cypA and apolipoprotein E were prevented from developing aortic aneurysms during angiotensin II infusion20. In a clever series of studies using conditioned medium of cultured endothelial cells and aorta from endothelial Nox2 transgenic mice, Fan et al. show that endothelial oxidants promote cypA production which in turn “primes” smooth muscle cells through Erk phosphorylation and increased oxidants. This, in turn, is responsible for the activation of proteolytic enzymes that destroy elastin and lead to dissection. The authors leave unaddressed the question of whether cell specific genetic deletion of Nox2 in aortic endothelium might prevent much of the aortic pathology in response to angiotensin II, which would further highlight the importance of paracrine mediators.
It is apparent that the paracrine relationships within the arterial wall induced by angiotensin II are multiple and complex, but that superoxide anion produced by NADPH oxidase, whether it be in endothelial cells, leukocytes, or adventitial fibroblasts is key. It is made clear by Fan et al. that endothelial oxidants can augment generation of cypA, but which oxidant species are involved and their cellular sites and enzymatic sources of origin is important to consider. In a supplemental figure, Fan et al. show that an inhibitor of nitric oxide synthase prevents much of the angiotensin II-induced ROS production by endothelial cells that overexpress Nox2, suggesting the possibility that uncoupled eNOS and the generation of the reaction product of superoxide anion and nitric oxide, peroxynitrite, is at work. Indeed, the footprint of peroxynitrite, nitrotyrosine, is abundantly localized in the aortic intima and adventitia, but not in the media, after angiotensin II18. In the media, evidence would suggest that hydrogen peroxide is the oxidant species at work, as angiotensin II-induced hypertrophy and aneurysm formation are prevented by smooth muscle specific overexpression of catalase21, 22. Either peroxynitrite or hydrogen peroxide can contribute, by transcriptional14 and post-transcriptional23 mechanisms, to the increase in matrix metalloproteinase expression and activation that was shown so well by Fan et al. to occur throughout the aortic wall and accounts for the elastin breaks and dissections they reported.
In considering multiple potential therapeutic targets to inhibit the molecular and cellular events leading to aortic dissection (Figure 1), it is important to realize that the same mechanisms that account for hypertension caused by angiotensin II, are clearly different from those which account for arterial hypertrophy and remodeling. For example, inhibiting the acute activation of NFκB by replacement of IκBα with IκBβ, nearly abolished the intense fibrotic response to angiotensin II in the aorta and heart without affecting the pressor response24.
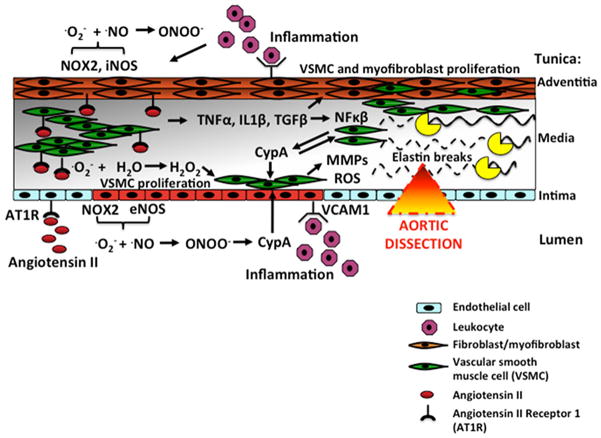
Figure 1.
Molecular mechanisms in the vascular wall leading to aortic dissection. In the presence of angiotensin II, endothelial Nox2-derived oxidants (·O2− and ONOO−) stimulate endothelial cyclophillin A (cypA) production, which acts as a paracrine factor to activate metalloproteinases (MMPs) and ROS production in VSMC. MMPs, in turn, degrade elastin, causing aortic dissection. Angiotensin II elicits an array of oxidants (·O−2, ONOO−, H2O2) and inflammatory responses (NFκβ) within the arterial wall via AT1 receptors (AT1R), which stimulate VSMC and fibroblast proliferation and inflammatory influx in the vascular wall, all contributing to remodeling and fibrosis. Angiotensin II also stimulates cypA production in VSMC, further contributing to oxidants and inflammation.
In addition, other agonists, such as endothelin, thromboxane A2, or cytokines such as TNFβ can stimulate many of the pathways that angiotensin II does so potently and rapidly via the AT1 receptor, leaving the door open for potential trouble during the sole use of specific AT1 receptor antagonists. NFκB is clearly central to aortic pathologies, and yet its direct inhibition has been both elusive and considered unwise for the long term. Broad-spectrum agents to inhibit the inflammatory response, such as statins, are attractive though less specific possibilities25. Making a target of the oxidants or their enzymatic sources, which are so clearly inherent to the mechanisms presented by Fan et al. has been a failure, indicated by the lack of conclusive evidence that antioxidants benefit cardiovascular disease. Directly targeting Nox2 chronically has been deemed unwise because of its central role in leukocyte activation in infection. This potential side effect also has limited direct targeting of cypA which is implicated in a wide variety of pathologies in addition to aortic dissection, including cancer, viral infections, asthma, and rheumatoid arthritis10. The most specific agent, cyclosporine, which binds directly to cypA, exerts profound immunosuppressive effects via its intracellular binding partner, calcineurin. There are agents in development that specifically bind to extracellular cypA avoiding these immunosuppressive effects10. What would be useful are agents that effectively treat elevated blood pressure and, at the same time, prevent the long-term sequelae of vascular remodeling that accompany hypertension, so strikingly demonstrated in the study by Fan et al.
CypA might also represent a novel biomarker of intramural degeneration preceding overt aortic aneurysm or dissection. Elevated serum or plasma levels of cypA are found in patients with unstable angina or after acute myocardial infarction26 and with stable coronary artery disease27. Prospective studies on the temporal relationship between circulating cypA levels and the progression of aortic aneurysms or dissections are warranted to validate cypA as a diagnostic tool to identify patients more likely to benefit from long-term monitoring or immediate elective surgery.
Acknowledgments
Funding Sources: The authors are supported in part by research grants from the National Institutes of Health, R01 HL105287, R01 HL031607, R37 HL104017 as well as an NHLBI Contract No. HHSN268201000031C.
Footnotes
Conflict of Interest Disclosures: None.
References
- 1.Fan LM, Douglas G, Bendall JK, McNeill E, Crabtree mJ, Hale AB, Mai A, Li J, McAteer M, Schneider JE, Choudhury RP, Channon KM. Endothelial Cell-Specific ROS Production Increases Susceptibility to Aortic Dissection. Circulation. 2014;129:XX–XXX. doi: 10.1161/CIRCULATIONAHA.113.005062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lindsay ME, Schepers D, Bolar NA, Doyle JJ, Gallo E, Fert-Bober J, Kempers MJ, Fishman EK, Chen Y, Myers L, Bjeda D, Oswald G, Elias AF, Levy HP, Anderlid BM, Yang MH, Bongers EM, Timmermans J, Braverman AC, Canham N, Mortier GR, Brunner HG, Byers PH, Van Eyk J, Van Laer L, Dietz HC, Loeys BL. Loss-of-function mutations in TGFB2 cause a syndromic presentation of thoracic aortic aneurysm. Nat Genet. 2012;44:922–927. doi: 10.1038/ng.2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Loeys BL, Schwarze U, Holm T, Callewaert BL, Thomas GH, Pannu H, De Backer JF, Oswald GL, Symoens S, Manouvrier S, Roberts AE, Faravelli F, Greco MA, Pyeritz RE, Milewicz DM, Coucke PJ, Cameron DE, Braverman AC, Byers PH, De Paepe AM, Dietz HC. Aneurysm syndromes caused by mutations in the TGF-beta receptor. N Engl J Med. 2006;355:788–798. doi: 10.1056/NEJMoa055695. [DOI] [PubMed] [Google Scholar]
- 4.van de Laar IM, Oldenburg RA, Pals G, Roos-Hesselink JW, de Graaf BM, Verhagen JM, Hoedemaekers YM, Willemsen R, Severijnen LA, Venselaar H, Vriend G, Pattynama PM, Collee M, Majoor-Krakauer D, Poldermans D, Frohn-Mulder IM, Micha D, Timmermans J, Hilhorst-Hofstee Y, Bierma-Zeinstra SM, Willems PJ, Kros JM, Oei EH, Oostra BA, Wessels MW, Bertoli-Avella AM. Mutations in SMAD3 cause a syndromic form of aortic aneurysms and dissections with early-onset osteoarthritis. Nat Genet. 2011;43:121–126. doi: 10.1038/ng.744. [DOI] [PubMed] [Google Scholar]
- 5.Doyle AJ, Doyle JJ, Bessling SL, Maragh S, Lindsay ME, Schepers D, Gillis E, Mortier G, Homfray T, Sauls K, Norris RA, Huso ND, Leahy D, Mohr DW, Caulfield MJ, Scott AF, Destree A, Hennekam RC, Arn PH, Curry CJ, Van Laer L, McCallion AS, Loeys BL, Dietz HC. Mutations in the TGF-beta repressor SKI cause Shprintzen-Goldberg syndrome with aortic aneurysm. Nat Genet. 2012;44:1249–1254. doi: 10.1038/ng.2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gomez D, Al Haj Zen A, Borges LF, Philippe M, Gutierrez PS, Jondeau G, Michel JB, Vranckx R. Syndromic and non-syndromic aneurysms of the human ascending aorta share activation of the Smad2 pathway. J Pathol. 2009;218:131–142. doi: 10.1002/path.2516. [DOI] [PubMed] [Google Scholar]
- 7.Brooke BS, Habashi JP, Judge DP, Patel N, Loeys B, Dietz HC., 3rd Angiotensin II blockade and aortic-root dilation in Marfan’s syndrome. N Engl J Med. 2008;358:2787–2795. doi: 10.1056/NEJMoa0706585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Habashi JP, Judge DP, Holm TM, Cohn RD, Loeys BL, Cooper TK, Myers L, Klein EC, Liu G, Calvi C, Podowski M, Neptune ER, Halushka MK, Bedja D, Gabrielson K, Rifkin DB, Carta L, Ramirez F, Huso DL, Dietz HC. Losartan, an AT1 antagonist, prevents aortic aneurysm in a mouse model of Marfan syndrome. Science. 2006;312:117–121. doi: 10.1126/science.1124287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Y, Ait-Oufella H, Herbin O, Bonnin P, Ramkhelawon B, Taleb S, Huang J, Offenstadt G, Combadiere C, Renia L, Johnson JL, Tharaux PL, Tedgui A, Mallat Z. TGF-beta activity protects against inflammatory aortic aneurysm progression and complications in angiotensin II-infused mice. J Clin Invest. 2010;120:422–432. doi: 10.1172/JCI38136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nigro P, Pompilio G, Capogrossi MC. Cyclophilin A: a key player for human disease. Cell Death Dis. 2013;4:e888. doi: 10.1038/cddis.2013.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bruemmer D, Daugherty A, Lu H, Rateri DL. Relevance of angiotensin II-induced aortic pathologies in mice to human aortic aneurysms. Ann N Y Acad Sci. 2011;1245:7–10. doi: 10.1111/j.1749-6632.2011.06332.x. [DOI] [PubMed] [Google Scholar]
- 12.Lob HE, Schultz D, Marvar PJ, Davisson RL, Harrison DG. Role of the NADPH oxidases in the subfornical organ in angiotensin II-induced hypertension. Hypertension. 2013;61:382–387. doi: 10.1161/HYPERTENSIONAHA.111.00546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Trott DW, Harrison DG. The immune system in hypertension. Adv Physiol Educ. 2014;38:20–24. doi: 10.1152/advan.00063.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rajagopalan S, Kurz S, Munzel T, Tarpey M, Freeman BA, Griendling KK, Harrison DG. Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation. Contribution to alterations of vasomotor tone. J Clin Invest. 1996;97:1916–1923. doi: 10.1172/JCI118623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zafari AM, Ushio-Fukai M, Akers M, Yin Q, Shah A, Harrison DG, Taylor WR, Griendling KK. Role of NADH/NADPH oxidase-derived H2O2 in angiotensin II-induced vascular hypertrophy. Hypertension. 1998;32:488–495. doi: 10.1161/01.hyp.32.3.488. [DOI] [PubMed] [Google Scholar]
- 16.Tummala PE, Chen XL, Sundell CL, Laursen JB, Hammes CP, Alexander RW, Harrison DG, Medford RM. Angiotensin II induces vascular cell adhesion molecule-1 expression in rat vasculature: A potential link between the renin-angiotensin system and atherosclerosis. Circulation. 1999;100:1223–1229. doi: 10.1161/01.cir.100.11.1223. [DOI] [PubMed] [Google Scholar]
- 17.Murdoch CE, Alom-Ruiz SP, Wang M, Zhang M, Walker S, Yu B, Brewer A, Shah AM. Role of endothelial Nox2 NADPH oxidase in angiotensin II-induced hypertension and vasomotor dysfunction. Basic Res Cardiol. 2011;106:527–538. doi: 10.1007/s00395-011-0179-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang HD, Xu S, Johns DG, Du Y, Quinn MT, Cayatte AJ, Cohen RA. Role of NADPH oxidase in the vascular hypertrophic and oxidative stress response to angiotensin II in mice. Circulation Research. 2001;88:947–953. doi: 10.1161/hh0901.089987. [DOI] [PubMed] [Google Scholar]
- 19.Bush E, Maeda N, Kuziel WA, Dawson TC, Wilcox JN, DeLeon H, Taylor WR. CC chemokine receptor 2 is required for macrophage infiltration and vascular hypertrophy in angiotensin II-induced hypertension. Hypertension. 2000;36:360–363. doi: 10.1161/01.hyp.36.3.360. [DOI] [PubMed] [Google Scholar]
- 20.Satoh K, Nigro P, Matoba T, O’Dell MR, Cui Z, Shi X, Mohan A, Yan C, Abe J, Illig KA, Berk BC. Cyclophilin A enhances vascular oxidative stress and the development of angiotensin II-induced aortic aneurysms. Nat Med. 2009;15:649–656. doi: 10.1038/nm.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parastatidis I, Weiss D, Joseph G, Taylor WR. Overexpression of catalase in vascular smooth muscle cells prevents the formation of abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol. 2013;33:2389–2396. doi: 10.1161/ATVBAHA.113.302175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Y, Griendling KK, Dikalova A, Owens GK, Taylor WR. Vascular hypertrophy in angiotensin II-induced hypertension is mediated by vascular smooth muscle cell-derived H2O2. Hypertension. 2005;46:732–737. doi: 10.1161/01.HYP.0000182660.74266.6d. [DOI] [PubMed] [Google Scholar]
- 23.Jacob-Ferreira AL, Kondo MY, Baral PK, James MN, Holt A, Fan X, Schulz R. Phosphorylation status of 72 kDa MMP-2 determines its structure and activity in response to peroxynitrite. PLoS One. 2013;8:e71794. doi: 10.1371/journal.pone.0071794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu S, Zhi H, Hou X, Cohen RA, Jiang B. IkappaBbeta attenuates angiotensin II-induced cardiovascular inflammation and fibrosis in mice. Hypertension. 2011;58:310–316. doi: 10.1161/HYPERTENSIONAHA.111.172031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Attenhofer Jost CH, Greutmann M, Connolly HM, Weber R, Rohrbach M, Oxenius A, Kretschmar O, Luscher TF, Matyas G. Medical Treatment of Aortic Aneurysms in Marfan Syndrome and Other Heritable Conditions. Curr Cardiol Rev. 2014 Feb 14; doi: 10.2174/1573403X1002140506124902. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yan J, Zang X, Chen R, Yuan W, Gong J, Wang C, Li Y. The clinical implications of increased cyclophilin A levels in patients with acute coronary syndromes. Clin Chim Acta. 2012;413:691–695. doi: 10.1016/j.cca.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 27.Satoh K, Fukumoto Y, Sugimura K, Miura Y, Aoki T, Nochioka K, Tatebe S, Miyamichi-Yamamoto S, Shimizu T, Osaki S, Takagi Y, Tsuburaya R, Ito Y, Matsumoto Y, Nakayama M, Takeda M, Takahashi J, Ito K, Yasuda S, Shimokawa H. Plasma cyclophilin A is a novel biomarker for coronary artery disease. Circ J. 2013;77:447–455. doi: 10.1253/circj.cj-12-0805. [DOI] [PubMed] [Google Scholar]