Abstract
Introduction
Vertebral fracture (VF) incidence following glucocorticoid (GC) initiation has not been previously reported in pediatric nephrotic syndrome.
Methods
VF were assessed on radiographs (Genant method); lumbar spine bone mineral density (LS BMD) was evaluated by dual-energy x-ray absorptiometry.
Results
Sixty-five children were followed to 12 months post-GC initiation (median age: 5.4 years, range 2.3 to 17.9). Three of 54 children with radiographs (6%, 95% CI 2 to 15%) had incident VF at 1 year. The mean LS BMD Z-score was below the healthy average at baseline (mean ± SD −0.5 ± 1.1 p=0.001) and at 3 months (−0.6 ± 1.1 p<0.001), but not at 6 months (−0.3 ± 1.3, p=0.066) or 12 months (−0.3 ± 1.2, p=0.066). Mixed effect modeling showed a significant increase in LS BMD Z-scores between 3 and 12 months (0.22 SD, 95% CI 0.08 to 0.36, p=0.003). A sub-group (N=16; 25%) had LS BMD Z-scores that were ≤ −1.0 at 12 months. In these children, each additional 1000 mg/m2 of GC received in the first 3 months was associated with a decrease in LS BMD Z-score by 0.39 at 12 months (95% CI, −0.71 to −0.07; p=0.017).
Conclusions
The incidence of VF at 1 year was low and LS BMD Z-scores improved by 12 months in the majority. Twenty-five percent of children had LS BMD Z-scores ≤ −1.0 at 12 months. In these children, LS BMD Z-scores were inversely associated with early GC exposure, despite similar GC exposure compared to the rest of the cohort.
Keywords: Nephrotic syndrome, Children, Osteoporosis, Vertebral fracture, Bone mineral density
Introduction
Pediatric nephrotic syndrome (NS) is defined by nephrotic range proteinuria, generalized edema, and hypoalbuminuria with normal renal function. With the first episode of idiopathic NS, children are typically treated with high-dose glucocorticoid (GC) therapy for a total of 12 weeks, which will achieve remission in close to 90% [1]. In two-thirds of cases, there are episodic relapses requiring repeated courses of GC [2]. GC therapy is well-known for its deleterious effect on basic cellular mechanisms that are important in the development and maintenance of bone strength [3]. GC induce apoptosis of the cells which sense bone tissue strain (the osteocytes) and inhibit osteoblastogenesis and thereby bone formation [4]. In addition, GC initially cause prolonged survival of osteoclasts, giving rise to excessive skeletal resorption, followed by inhibition of osteoclastogenesis leading to a low bone turnover state [4]. A large pediatric study showed higher rates of extremity fractures among children who had received GC to treat a variety of underlying diseases [5], and the association between GC therapy and vertebral fractures (VF) has also been shown in studies of children with GC-treated rheumatic disorders [6–8]. In adults with systemic disorders, VF are frequent in the first year following GC therapy, an observation that has been attributed to rapid loss of bone mass that has been observed in the first few months of GC exposure [3].
Through prospective study, we previously described an inverse relationship between lumbar spine bone mineral density (LS BMD) Z-scores and short-term GC therapy in pediatric GC-treated NS [9]. After a median of 18 days following GC initiation, every 1 mg/m2 increase in GC therapy (prednisone equivalents) was associated with a decrease in spine BMD Z-score of almost 0.4 standard deviation (SD). This finding was consistent with a recent report in adult GC-treated NS in which significant declines in spine BMD Z-scores were evident after just 12 weeks of GC therapy [10]. Similarly, a large cross-sectional case-control study showed lower spine bone mineral content after 4 years of GC exposure in children with steroid-sensitive NS, suggesting the potential for residual bone mass deficits in the longer term [11].
To date, there have been no data describing the incidence of overt bone fragility among children with GC-treated NS. Feber et al. [9] found that 8% of children with GC-treated NS and short-term GC exposure (maximum 37 days) had asymptomatic prevalent VF. On the other hand, a recent report described painful moderate VF in a young boy with NS following 5 years of intermittent GC therapy [12]. In view of these findings, we sought to determine the following features of skeletal development among children with GC-treated NS: (1) the incidence of VF at 12 months following GC initiation, (2) the change in spine BMD Z-scores over the same time period, and (3) the relationship between key clinical variables and the observed skeletal phenotype.
Subjects and Methods
Patients were recruited through pediatric nephrology clinics in 10 children’s hospitals across Canada as part of the STeroid-associated Osteoporosis in the Pediatric Population (STOPP) research program. Children from one month to 17 years of age with NS were enrolled (N = 80) from 2005 to 2007, with the baseline bone health assessment initiated within 37 days of GC therapy [13].
Children were included in the study if they met the clinical criteria for NS as defined by the International Society of Kidney Disease in Children [14], including edema, proteinuria (>960 mg/m2/day) or urine protein/creatinine (>0.2 g/mmol), and serum albumin (<25 g/L). Idiopathic NS was diagnosed either clinically (following an appropriate response to GC treatment in the first month of therapy, presumed minimal change disease) or confirmed by renal biopsy. NS due to focal segmental glomerulosclerosis and membranoproliferative glomerulonephritis were also confirmed on renal biopsy. The diagnosis of Henoch-Schöenlein Purpura was made on clinical grounds.
Children were not eligible for the study if GC had previously been used at any time for treatment of the underlying disease. Patients were also excluded if they had received intravenous or oral GC for more than 14 consecutive days in the 12 months preceding study enrolment to treat any other medical condition (e.g. asthma), if they had received prior medication for osteoporosis, or if they had received calcium or vitamin D supplementation that exceeded the Dietary Reference Intake for age at the time that the study was conducted [15]. The study was approved by the Research Ethics Board in each participating institution and informed consent/assent was obtained prior to study enrolment, as appropriate.
Clinical data
All children were treated with GC therapy according to standard clinical care in each of the participating nephrology clinics, with a typical starting dose of 60 mg/m2/day. Children with idiopathic NS were further classified into one of three groups depending on their response to GC therapy: (1) steroid-sensitive NS, (2) steroid-dependent NS, or (3) steroid-resistant NS. Steroid-sensitive NS was further classified by: (a) an absence of relapses, (b) infrequent number of relapses (less than 2 relapses within a 6 month period or less than 4 in 12 months), or (c) frequent relapses (2 or more relapses within a 6 month period or 4 or more per year [16]. Glomerular filtration rate (GFR) was calculated using the updated Schwartz formula ( ) [17] at baseline and 3 months.
Additional clinical data were obtained at baseline and every 3 months for a total of 12 months. Height, weight and pubertal staging according to Marshall and Tanner [18, 19] were determined as previously described [13]. Height, weight, and body mass index (BMI; weight (kg) divided by height (meters)2) raw values were transformed into age- and gender-specific Z-scores according to the United States Centers for Disease Control National Center for Health Statistics normative database [20]; for children under 2 years of age, body mass index Z-scores were calculated according to the World Health Organization child growth standards [21]. The presence or absence of back pain reported by the participant was recorded at each study visit, and the spine was palpated for tenderness at baseline and at 12 months. Dietary calcium and vitamin D intake were assessed by a validated food frequency questionnaire [22]. Daily intake (diet plus supplement) was expressed as the percent of the Adequate Intake value based on Dietary Reference Intakes in place at the time the study was conducted [15]. The percentage of adequate intake scores was then classified as <50% of the age-related Dietary Reference Intake, ≥50 and <100%, or ≥100% of the Dietary Reference Intake. Physical activity was assessed through the Habitual Activity Estimation Scale [23, 24].
Quantification of glucocorticoid exposure
The dose of systemic GC therapy (oral and intravenous) received during the 12 month observation period was converted into prednisone equivalents with results expressed as [25–27]: 1) cumulative GC dose, the amount of GC in prednisone equivalents (mg/m2) received during the observation period; 2) average GC dose, the cumulative dose in prednisone equivalents (mg/m2) divided by the total number of days during the observation period; and 3) GC dose intensity, the cumulative dose in prednisone equivalents (mg/m2), divided by the number of days in receipt of steroids during the observation period.
Vertebral fracture assessment
Lateral thoracolumbar spine radiographs were obtained at baseline and at 12 months, with VF assessment based on the Genant semi-quantitative method from T4 to L4 [28]. Vertebral bodies were graded according to the extent of the difference in height ratios from 100% when the anterior vertebral height was compared to the posterior height, the middle height to the posterior height, and the posterior height to the posterior height of adjacent vertebral bodies. The scores corresponded to the following differences in height ratios: Grade 0: 20% or less (normal); Grade 1 fracture (mild): > 20 to 25%; Grade 2 fracture (moderate): > 25 to 40%; Grade 3 fracture (severe): > 40%. Minimal physiological rounding of vertebral bodies in the mid-thoracic region of the spine, as can be seen in normal children, was assigned Grade 0 [29, 30]. An incident VF was defined as an increase in the Genant grade by at least 1 compared to baseline.
Lumbar spine bone mineral density, bone age, and metacarpal morphometry
LS BMD was measured in the anterior-posterior direction from L1 to L4 by dual-energy x-ray absorptiometry (DXA) using either Hologic (QDR 4500, 4 centers; Discovery, 2 centers; Delphi, one center) or Lunar Prodigy (3 centers) systems at baseline, 3, 6 and 12 months. Machines were cross-calibrated as previously described [13]. Data were converted to Hologic units and areal BMD Z-scores were generated using the Hologic 12.4 normative database, a reference database provided by the manufacturer that spans the age ranges included in this study. Volumetric BMD (vBMD) Z-scores were generated according to a published Canadian reference database [31]. Radiographs of the left hand and wrist were obtained at baseline and 12 months to determine bone age and to calculate the percent cortical area at the second metacarpal bone, as previously reported [13].
Statistical analysis
Categorical variables were summarized using frequency and percentage. Continuous variables were summarized using mean and SD or median (25th percentile, 75th percentile; or minimum, maximum). The 95% confidence intervals (CI) for the proportions of patients with incident vertebral deformities were calculated using the Wilson score method [32]. At each time point, height, weight and LS BMD Z-score for the entire cohort was compared to the healthy average (i.e. Z-score = 0.0) using one sample Student’s t-tests.
Height, weight, and LS BMD Z-score evaluated at baseline, 3, 6, and 12 months were modeled separately using mixed-effects models. In the analyses, an unstructured covariance matrix was specified to take into account the correlation of the repeated measurements within the same subject. Additionally, a mixed effects regression model was fit to study the multivariate relationship of clinically relevant parameters with LS BMD Z-score over time. Specifically, this model tested whether the association between LS BMD Z-score and GC exposure differed according to time point (by including an interaction term between GC exposure and time point). Gender and physical maturity were important clinical variables included in this model. Age, bone age, and Tanner Staging (Stages 1, 2 versus 3 to 5) were evaluated individually and since age was most strongly associated with LS BMD Z-score, it was included in the analysis to represent physical maturity.
A multiple linear regression model with the LS BMD Z-score at 12 months as the dependent variable was fit to examine the relationship between GC exposure and other clinically relevant parameters for the entire cohort. GC exposure between each of the LS BMD time points in the 12 month period was included in the model (specifically, from GC initiation to the baseline LS BMD, from the baseline LS BMD to 3 months, and from 3 to 12 months) as well as age, gender and BMI.
Previously, it has been shown that the risk of vertebral and forearm fractures in children increases for every 1.0 SD below the healthy average in LS BMD Z-score [33, 34]. Given these observations, we sought to differentiate children with LS BMD Z-scores ≤ −1.0 at 12 months from those without. An exact 95% CI for the proportion of patients with LS BMD Z-score ≤ −1.0 was calculated and the estimate was compared to the expected value in a normal population using the binomial test for proportions. Characteristics of the two groups were compared using chi-square or Fisher’s exact test for categorical variables and Mann-Whitney for continuous variables. For LS BMD Z-score, the group comparisons were made with adjustment for BMI Z-score.
Multivariate linear regression modeling (adjusting for age, gender and BMI) was used to explore whether the effect of GC exposure differed among the two LS BMD-based groupings of children. This model included interaction terms between GC exposure at the various time points and the LS BMD group indicator variable. All regression models with LS BMD Z-score as the dependent variable included BMI Z-score. Analyses were conducted using IBM SPSS Statistics 19.0.0.1 (IBM Corporation New York, NY, USA) or SAS 9.2 (SAS Institute Inc., Cary, NC, USA).
Results
Clinical characteristics
A total of 128 children were approached for participation in the study; 44 declined, 3 were excluded because of failure to undergo a bone health evaluation within the baseline timeline and one was excluded for failure to meet inclusion criteria. Of the 80 children enrolled in the study, 65 completed the LS BMD at 12 months. The reasons for lack of follow-up on 15 children at 12 months are presented in Fig. 1. The clinical profile at baseline for the 15 children without data at 12 months did not differ significantly from those with complete follow-up (data not shown). The baseline LS BMD was determined at a median of 18 days from GC initiation (inter-quartile range (IQR): 9, 27) and the baseline spine radiograph was done at a median of 17 days (IQR: 8, 27). The number of children no longer receiving GC therapy increased progressively over time, with 14% having ceased therapy at 3 months, 29% at 6 months and 51% at 12 months. Furthermore, the cumulative amount of GC per month was progressively lower over the course of the observation period in the cohort, as well (data not shown).
Fig 1.
Disposition of patients
The mean height Z-scores were similar to the healthy average at all time points (baseline mean ± SD = 0.2 ± 1.0, p=0.059; 3 months 0.01 ± 0.9, p=0.947; 6 months 0.2 ± 0.9, p=0.068; 12 months 0.2 ± 1.1, p=0.118), while weight Z-scores were consistently above the healthy average during the 12 month period (baseline mean ± SD = 0.7 ± 1.1, p<0.001; 3 months 0.7 ± 1.0, p<0.001; 6 months 0.7 ± 1.1, p<0.001; 12 months 0.6 ± 1.1, p<0.001). Height, weight and BMI Z-scores did not change significantly from baseline to 12 months (height Z-score Δ mean ± SD = 0.0 ± 0.5, p=0.737; weight Z-score Δ mean ± SD = 0.0± 0.9, p=0.669; BMI Z-score Δ mean ± SD = −0.1 ± 1.2, p=0.541). Additional clinical features are shown in Table 1.
Table 1.
Clinical Characteristics
| Clinical Characteristics | N=65a |
|---|---|
| Demographic Data | |
| Male, n (%) | 36 (55) |
| Age at 12 months (years), median (min, max) | 5.4 (2.3, 17.9) |
| Bone age at 12 months (years), median (min, max) | 5.0 (2.0, 18.0) |
| Nephrotic Syndrome (NS), n (%) | |
| Idiopathic NS (n=58) | |
| Without renal biopsy (presumed minimal change disease) | 32 (49) |
| Confirmed on renal biopsy (minimal change disease) | 15 (23) |
| Focal segmental glomerulosclerosis | 11 (17) |
| Secondary NS (n=7) | |
| Henoch-Schöenlein purpura | 5 (8) |
| Other, membranoproliferative glomerulonephritis type 1 | 2 (3) |
| Idiopathic NS by Response to Glucocorticoid (GC) Therapy, n=58 | |
| GC-sensitive NS | 42 (72) |
| Absence of relapse | 22 (38) |
| Infrequent relapses | 12 (20) |
| Frequent relapses | 8 (14) |
| GC-dependent NS | 9 (16) |
| GC-resistant NS | 7 (12) |
| Renal Function | |
| GFR at study entry, mean (SD), n=56 | 127 (34) |
| GFR at 3 months, mean (SD), n=22 | 110 (35) |
| Anthropometry | |
| Height Z-score, mean (SD) | 0.2 (1.1) |
| Weight Z-score, mean (SD) | 0.6 (1.1)* |
| BMI Z-score, mean (SD) | 0.7 (1.1)* |
| Pubertal stage, N (%), n=61 | |
| Stage 1 | 51 (84) |
| Stage 2–5 | 10 (16) |
| LS BMD Z-Score at 12 Months, n (%) | |
| < − 2 | 6 (9) |
| ≥ −2 and < − 1 | 10 (15) |
| ≥ −1 and < 0 | 24 (37) |
| ≥ 0 | 25 (39) |
| Second Metacarpal Morphometry at 12 months, mean (SD) | |
| Metacarpal length Z-score | 0.7 (1.1)* |
| Combined cortical thickness Z-score | 0.4 (0.9)* |
| Percent cortical area Z-score | 0.2 (0.9) |
| Glucocorticoid (GC) Therapy to 12 months | |
| Number of days between GC initiation and 12 Month spine DXA, mean (SD) | 387 (21.2) |
| Number of days in receipt of GC, median (IQR) | 143 (104, 228) |
| Cumulative GC dose (mg/m2), mean (SD) | 6,266 (3,465) |
| Average GC dose (mg/m2/day), mean (SD) | 16.3 (9.3) |
| GC dose intensity (mg/m2/day), mean (SD) | 39.7 (11.0) |
| Vitamin D and Calcium Intake Over 12 Months, n (%) | |
| Average daily vitamin D intakeb, n=48 | |
| < 50 % | 9 (19) |
| 50 – <100 % | 17 (35) |
| ≥ 100% | 22 (46) |
| Vitamin D supplementation – Yes | 37 (57) |
| Average daily calcium intakeb, n=48 | |
| < 50 % | 1 (2) |
| 50 – <100 % | 8 (17) |
| ≥ 100% | 39 (81) |
| Calcium supplementation – Yes | 27 (42) |
Statistically significant compared to healthy population (one sample t-test)
For data expressed as percentages, the denominator is 65 children unless otherwise specified
Combined dietary plus supplemental intake, expressed as the % of the Dietary Reference Intake for age
Incident vertebral fractures
Of the 80 children enrolled in the study, 54 children had complete spine radiographs at 12 months. Fifteen of the 26 children excluded from the 12 month VF analysis were removed for the reasons outlined in Figure 1. An additional 11 children were excluded from the 12 month VF analysis for the following reasons: 1 child did not have a baseline spine radiograph, obviating the ability to determine VF incidence, 2 children had radiographs of insufficient quality for VF assessment, and 8 children missed the 12 month spine radiograph procedure. The clinical profile at baseline of the 26 children excluded from the 12 month VF analysis was no different compared to those with complete spine radiograph data to 12 months.
At 12 months, 3 of the 54 children (6%, 95% CI 2% to 15%; 2 girls) sustained single incident VF (all mild, anterior wedge fractures) in the mid-thoracic region (T4, T7, and T8); these three fractures were all new fractures in previously normal vertebral bodies. All 3 of these children (1 boy and 2 girls) had idiopathic NS and were pre-pubertal (Tanner Stage 1). None had back pain at baseline or during the 12 months follow-up.
Given the small number of children with incident VF, formal statistical comparison between those with (N=3) and without (N=51) VF was not undertaken. However, we noted that the 3 children with incident VF had a median (min, max) average GC exposure over 12 months of 9.4 (4.5, 17.7) mg/m2/day compared to 15.7 (4.2, 45.4) among children without incident fractures. In addition, renal function was normal in these 3 children. We also observed the following LS BMD Z-scores in the 3 children with incident VF: patient 1: −0.01 at baseline, 0.4 at 3 months, 0.6 at 6 months and 1.1 at 12 months; patient 2: −2.6, −1.7, −1.7 and −1.4 at baseline, 3, 6 and 12 months; patient 3: −1.4, −1.5, −0.9 and −0.6 at the same time points. As such, one of the 3 children had a LS BMD Z-score less than −1.0 at 12 months. In addition, 2 out of 3 children with incident VF were GC-sensitive without relapses and the remaining patient was GC-sensitive with infrequent relapses.
Bone mineral density
The mean LS BMD Z-score for all children was significantly lower than the healthy average at the baseline visit (mean ± SD, −0.5 ± 1.1, p<0.001) and at 3 months (−0.6 ± 1.1, p<0.001) but not at 6 months (−0.3 ± 1.3, p=0.066) or 12 months (−0.3 ± 1.2, p=0.066). Mixed effect modeling showed that significant increases in LS BMD Z-score (controlling for BMI Z-scores) occurred between baseline and 12 months (β 0.18, 95% CI 0.02 to 0.35, p=0.032) and also between 3 and 12 months (β 0.22, 95% CI 0.08 to 0.36, p=0.003), as shown in Fig. 2. Mixed effect modeling also revealed that the association between LS BMD Z-score and GC exposure differed by time point (p=0.003). At baseline (median 18 days after GC initiation), every unit increase in cumulative GC exposure (1000 mg/m2) was associated with a decrease in LS BMD Z-score of 0.27 (95% CI −0.41 to −0.14, p=0.0001), controlling for age, gender, and BMI Z-score. This relationship was not observed at the other time points (3 months β −0.06, 95% CI −0.14 to 0.02, p=0.172; 6 months β −0.05, 95% CI −0.10 to 0.01, p=0.082; 12 months β −0.02, 95% CI −0.06 to 0.03, p=0.455). We explored whether there was a relationship between the changes in LS BMD Z-scores over the 12 months and the changes in second metacarpal percent cortical area Z-score by visual inspection of the data. No evident relationship was detected.
Fig 2.
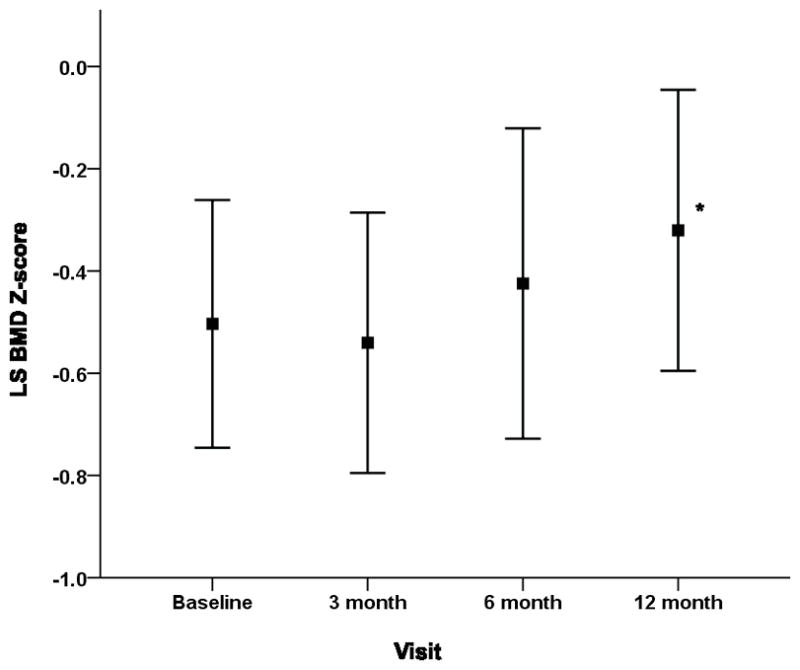
Mean lumbar spine bone mineral density Z-scores (adjusted for BMI Z-score) and 95% CI at baseline, 3, 6 and 12 months post-glucocorticoid initiation. * Significant differences in mean lumbar spine bone mineral density Z-scores at 12 months compared to baseline.
Given our interest in the skeletal status of these children at 1 year post-GC initiation, we evaluated the relationship between GC exposure and LS BMD Z-score at 12 months, controlling for age, gender, and BMI Z-score. No statistically significant relationships were observed between LS BMD Z-score at 12 months and cumulative GC dose (1000 mg/m2) from the time of GC initiation to the baseline bone health evaluation (p=0.928), from the baseline evaluation to 3 months (p=0.053), nor from 3 to 12 months (p=0.742).
A sub-group (N=16; 25%) was identified with LS BMD Z-scores that were ≤ −1 at 12 months. These children had lower height Z-scores at 12 months (mean ± SD = −0.3 ± 1.0 versus 0.4 ± 1.1, p=0.085) and weight Z-scores (0.1 ± 1.0 versus 0.8 ± 1.1, p = 0.048) compared to the remainder of the cohort. Following adjustment for BMI Z-scores, the LS BMD Z-scores in these 16 children were low at all time points relative to the rest of the cohort (baseline −1.7 ± 0.9 versus −0.1 ± 0.9, p<0.001, 3 months −1.6 ± 0.8 versus −0.2 ± 0.8, p<0.001, 6 months −2.0 ± 1.0 versus 0.1 ± 1.0, p<0.001, 12 months −1.8 ± 0.8 versus 0.2 ± 0.8, p<0.001). Linear regression modeling adjusting for change in BMI confirmed the lack of increase in spine BMD Z-score from baseline to 12 months in these children compared to the rest of cohort (BMD Z-score Δ mean ± SD = −0.2 ± 0.7, p=0.394 compared with 0.3 ± 0.7, p=0.0007).
Furthermore, the mean ± SD LS vBMD Z-score at 12 months was lower in these children (−0.7 ± 0.9 versus 1.3 ± 1.2, p<0.001). However, they were not significantly different from the remainder of the cohort in terms of age, bone age, gender, NS diagnosis, type of response to GC therapy, pubertal status at 12 months, second metacarpal percent cortical area Z-score, back pain, physical activity, GFR, or vitamin D and calcium intake (data not shown).
Over the 12 month period, the total quantity and duration of GC therapy for these 16 children were similar to the rest of the cohort. Specifically, over the 12 month period the median (min, max) cumulative GC dose (mg/m2) was 4,389 (2,296, 14,444) for these 16 children and 6,084 (1,568, 17,710) for the children with higher LS BMD Z-scores (p=0.35). Over the one-year period, the median (min, max) number of days in receipt of GC was 110 (44, 345) for those with persistently low spine BMD Z-scores compared to 148 (44, 377) for those without (p=0.33). Similarly, there were no significant differences between the two groups for the total quantity and duration of GC therapy in the first 3 months, as follows: for the 16 children with low spine BMD Z-scores at 12 months, the median cumulative GC dose (mg/m2) in the first 3 months was 3,706 (range 2,334 to 7,149) compared to 3,452 (1,655 to 7,400) for the remainder of the cohort (p=0.29). During the first three months, the median number of days in receipt of steroids was 70 (44 to 129) for those with low spine BMD Z-scores versus 74 (44 to 120) for those without (p=0.87).
On the other hand, within the sub-group of 16 children with lower BMD Z-scores at 12 months, each additional 1000 mg/m2 of cumulative GC received in the 3 months following the baseline evaluation was associated with a decrease in LS BMD Z-score of 0.39 at 12 months (95% CI, −0.71 to −0.07; p=0.017; controlling for age, gender, and BMI Z-scores; Fig. 3). For the other 49 patients there was no association between cumulative GC exposure from baseline to 3 months and LS BMD Z-score at 12 months (β +0.04, 95% CI, −0.16 to 0.24, p=0.692).
Fig 3.
Relationship between cumulative glucocorticoid dose from baseline to 3 months (mg/m2) for those with lumbar spine BMD Z-scores at 12 months ≤ −1.0 (N=16; circles and dashed regression line) compared to those with lumbar spine BMD Z-scores > −1.0 (N=49; triangles, solid regression line).
Discussion
A key finding in this study was that the proportion of children with incident VF at 12 months was relatively low (6%, 95% CI 2 to 15%). In addition, the clinical fracture burden was minimal, since each child with incident VF had mild asymptomatic vertebral deformities. This is in contrast to a paper by Rodd et al. [6] which showed a similar proportion of children with GC-treated rheumatic conditions had incident VF up to 12 months following GC initiation (6%, 95% CI 3 to 12%), but half of these children had moderate fractures and a third were symptomatic. This was despite doses of GC in the first year that were similar to those in our cohort (mean (± SD) cumulative GC dose in prednisone equivalents = 6,369 mg/m2 ± 5,146 among children with rheumatic conditions [6] versus 6,266 mg/m2 ± 3,465 among those in our NS cohort). These contrasting results highlight the importance of disease-specific studies to describe skeletal health in children with GC-treated disorders. Unlike in childhood rheumatic diseases, often characterized by persistent elevations in osteotoxic inflammatory cytokines despite treatment of the underlying disease [35], childhood NS usually responds rapidly to GC therapy, entering into complete quiescence while on GC therapy. While there are transient increases in inflammatory cytokines during the active phase of pediatric NS, there is little evidence to suggest independent, deleterious effects of the underlying disease on skeletal development in steroid-sensitive patients. As such, pediatric steroid-sensitive NS is considered an in vivo model of the impact of GC therapy on developing bone. We hypothesize that the underlying disease may partly explain the differences in the fracture severity and symptomatology in our largely steroid-sensitive NS cohort compared to children with rheumatic conditions and similar GC exposure [6].
What is the clinical significance of mild, asymptomatic VF such as observed in our cohort? Using the Genant method for VF characterization, the loss in vertebral height ratio was at a 20% threshold for defining fracture [28]. In pediatric leukemia, we have shown that Grade 1 prevalent VF diagnosed around the time of leukemia presentation were independently associated with increased odds of incident (i.e. new) VF at 12 months post-chemotherapy initiation [34]. Furthermore, Gaca et al. [30] showed that 95% of healthy children had an anterior:posterior vertebral height ratio loss of 11% or less, with no child exceeding a 14% loss in height ratio. Collectively, these data support the clinical significance of Grade 1 incident VF such as those observed in this report.
Another key finding in our study was that on average, LS BMD Z-scores were initially lower than expected (at a median of 18 days from the time of GC initiation) but then increased in the majority of children by 12 months following initiation of GC therapy. A number of studies have shown a significant decrease in LS BMD Z-scores in the first few weeks of GC therapy, both in children [36] and in adults [10] with NS. These observations are attributable to the acute effects of GC on bone, as demonstrated in animal models [37], and on trans-iliac bone specimens from adults [38]. On the other hand, the longer-term effects of GC therapy in pediatric NS are less consistent across studies. Gulati et al. [39] showed in a cohort of 88 children with idiopathic NS that spine BMD Z-scores were on average −1.6 SD below the healthy average at enrolment (at a mean of 3.2 ± 2.7 years following diagnosis) and remained low in the next 12 months. Hegarty et al. [40] showed that LS BMD age- and gender-matched Z-scores were normal in adults who had received GC therapy for NS during the pediatric years, while LS BMD T-scores were reduced. The most striking finding in that report [40] was a persistently low distal radius trabecular volumetric BMD (by peripheral quantitative computed tomography (pQCT)) – on average almost a full standard deviation below the healthy average decades following the treatment of pediatric NS.
pQCT has emerged as an effective skeletal imaging tool to more precisely define the changes that occur in cortical and trabecular compartments during growth. This is important, since DXA does not differentiate between these two structures, yet studies have shown divergent effects of GC on the two compartments [41]. Tsampalieros et al. [42] recently studied a longitudinal cohort of pediatric NS treated with a similar initial GC regime as in our study, and found that tibia trabecular volumetric BMD Z-scores were lower and cortical density and area Z-scores higher compared to controls around the time of diagnosis. Over the one-year follow-up period, trabecular and cortical BMD Z-scores did not change significantly; however, there was a decrease in cortical area Z-score that was hypothesized to result from a dampening effect of GC therapy on cortical modeling (i.e. on periosteal apposition). The higher cortical density was associated with higher GC doses, attenuated growth in tibial length and declines in cortical area Z-scores. Higher cortical density in GC-treated disorders has been observed in other cohorts [27, 41] and is attributed to low bone turnover giving rise to prolongation of the secondary mineralization phase. With improvement in bone turnover, cortical density appears to normalize [27], suggesting the abnormally high cortical density may not necessarily confer increases in bone strength.
These pQCT data are important, since the divergent effects of GC therapy on skeletal compartments have implications for our DXA-based observations about BMD development. Given the high cortical density and low trabecular Z-scores observed at diagnosis and after 12 months of GC therapy in the study by Tsampelieros et al. [42], when these two compartments are combined in the anterior-posterior DXA measurement, any net changes in the sum of the compartments must be considered in view of the more limited DXA technique. As such, longitudinal observations that show increases in BMD Z-scores by DXA over time (such as in our report) need to consider that such increases could arise from an increase in cortical density (which may not be beneficial to bone strength), or improvement in previously low trabecular density. In our overall cohort, which showed increases in spine BMD Z-scores by 12 months, we recognize that the precise structural basis for these changes remains unclear.
What is clear from our study is that there was an inverse relationship between spine BMD Z-score and GC exposure in the first few weeks of GC therapy (maximum 37 days). In addition, a sub-set of children appeared to be particularly sensitive to the BMD-attenuating effects of even short-term GC treatment, given that 25% of the cohort had low LS BMD Z-scores at 12 months that were inversely associated with the GC exposure in the first 3 months. These children showed lack of overall improvement in spine BMD Z-score between baseline and 12 months, in comparison to the rest of the cohort. Furthermore, our analyses showed no evidence of clinically distinguishing features in these children apart from the inverse association between GC exposure and LS BMD Z-score in the first 3 months. Since these patients did not receive more GC than the remainder of the cohort at any point during the observation period, our findings point to the possibility that some patients may have enhanced sensitivity to the early osteotoxic effects of GC.
There are limitations to our study which merit consideration. First, our population included children with NS of varying etiologies. Since the goal of the study was to capture data that comprehensively reflects the clinical setting, we sought to evaluate early changes secondary to GC therapy in an inception cohort of pediatric NS regardless of the specific etiology or the eventual responsiveness to GC therapy. As such, children with non-idiopathic NS (6 out 65) and an additional 8/65 patients with idiopathic GC-resistant NS were included in this cohort. The non-idiopathic forms of NS and the GC-resistant cases can be considered systemic diseases that may be associated with inflammation [43], which in turn has the potential to independently affect BMD when not in remission. On the other hand, the children with lower spine BMD Z-scores at 12 months did not receive more GC therapy at any time point over the observation period (considered a proxy measurement of disease severity), nor did they manifest lower GFR values compared to the rest of the cohort. In addition, only 3 of the 16 children with low spine BMD Z-scores at 12 months had a diagnosis other than steroid-sensitive idiopathic NS; when these children were removed from our analysis, the inverse relationship between spine BMD Z-score at 12 months and GC exposure in the first 3 months persisted (data not shown). A second limitation of this study is that, given the number of patients available for longitudinal study, we were unable to include more than a few variables in linear regression modeling on spine BMD and we were limited to only a description of the VF. Given the limitation of post-hoc sub-group analyses, our results from this approach are most useful as a starting point for future hypotheses as opposed to being considered definitive results.
In conclusion, LS BMD Z-scores increased by 12 months post-GC initiation in the majority of patients with GS treated NS, and the incidence of VF at 1 year was low. Twenty-five percent of children had LS BMD Z-scores ≤ −1 at 12 months; in these children, LS BMD Z-scores at 12 months were inversely associated with early GC exposure despite an absence of greater GC exposure. The ongoing incidence of VF and changes in BMD development will be a focus of future monitoring in this cohort.
Acknowledgments
Funding: Primary Funding Source: The Canadian Institutes of Health Research Operating Grants Program (FRN 64285). Additional Funding Sources: The Canadian Child Health Clinician Scientist Program; The Canadian Institutes for Health Research New Investigator Program; The Children’s Hospital of Eastern Ontario Research Institute, University of Ottawa; The Women and Children’s Health Research Institute, University of Alberta
This study was primarily funded by an operating grant from the Canadian Institutes for Health Research (FRN 64285). Additional funding for this work has been provided to Dr. Leanne Ward by the Canadian Institutes for Health Research New Investigator Program, the Canadian Child Health Clinician Scientist Career Enhancement Program, a University of Ottawa Research Chair Award and the CHEO Departments of Pediatrics and Surgery. This work was also supported by the Children’s Hospital of Eastern Ontario Research Institute and the University of Alberta Women and Children’s Health Research Institute.
The Canadian STOPP Consortium would like to thank the following individuals:
The children and their families who participated in the study and without whom the STOPP study would not have been possible.
Research Associates who managed the study at the co-ordinating center (the Children’s Hospital of Eastern Ontario Ottawa, Ontario): Elizabeth Sykes (STOPP Project Manager), Maya Scharke (STOPP Data Analyst and Database Manager), Monica Tomiak (Statistical Analyses), Victor Konji (STOPP Publications and Presentations Committee Liaison and hand morphometry measurements), Steve Anderson (Children’s Hospital of Eastern Ontario Pediatric Bone Health Program Research Manager), Catherine Riddell (STOPP National Study Monitor); Research Associates who took care of the patients from the following institutions: Alberta Children’s Hospital, Calgary, Alberta: Eileen Pyra; British Columbia Children’s Hospital, Vancouver British Columbia: Terry Viczko, Sandy Hwang, Angelyne Sarmiento; Children’s Hospital of Eastern Ontario, Ottawa, Ontario: Heather Cosgrove, Josie MacLennan, Catherine Riddell; Children’s Hospital, London Health Sciences Centre, London, Ontario: Leila MacBean, Mala Ramu; McMaster Children’s Hospital, Hamilton, Ontario: Susan Docherty-Skippen; IWK Health Center, Halifax, Nova Scotia: Aleasha Warner; Montréal Children’s Hospital, Montréal, Québec: Diane Laforte, Maritza Laprise; Ste. Justine Hospital, Montréal, Québec: Claude Belleville, Natacha Gaulin Marion; Stollery Children’s Hospital, Edmonton, Alberta: Ronda Blasco, Amanda Mullins; Toronto Hospital for Sick Children, Toronto, Ontario: Michele Petrovic; Winnipeg Children’s Hospital, Winnipeg, Manitoba: Dan Catte, Erika Bloomfield. The Research Nurses, Support Staff and all the STOPP collaborators from the various Divisions of Nephrology, Oncology, Rheumatology and Radiology who have contributed to the care of the children enrolled in the study.
Abbreviations
- BMD
Bone mineral density
- CI
Confidence interval
- GFR
Glomerular filtration rate
- GC
Glucocorticoid
- IQR
Inter-quartile range
- LS
Lumbar spine
- NS
Nephrotic syndrome
- SD
Standard deviation
- VF
Vertebral fracture
The Canadian STeroid-associated Osteoporosis in the Pediatric Population (STOPP) Consortium (a pan-Canadian, pediatric bone health working group)
Co-ordinating Center
Children’s Hospital of Eastern Ontario, Ottawa, Ontario: Leanne M. Ward#,*,§ (Study Principal Investigator), Janusz Feber*,§ (Nephrology), Jacqueline Halton*,§ (Oncology), Roman Jurencak (Rheumatology), MaryAnn Matzinger (Radiology, Central Radiograph Analyses), Johannes Roth (Rheumatology), Nazih Shenouda§ (Radiology, Central Radiograph Analyses)
Ottawa Hospital Research Institute, Ottawa Methods Centre Ottawa, Ontario: David Moher*,§ (Research Methods), Tim Ramsay (Statistical Analyses)
Participating Centers
Alberta Children’s Hospital, Calgary, Alberta: David Stephure (Site Principal Investigator), Reinhard Kloiber (Radiology), Victor Lewis (Oncology), Julian Midgley (Nephrology), Paivi Miettunen (Rheumatology)
British Columbia Children’s Hospital, Vancouver, British Columbia: David Cabral* (Site Principal Investigator), David B. Dix (Oncology), Kristin Houghton (Rheumatology), Helen R. Nadel (Radiology)
British Columbia Women’s and Children’s Hospital and Health Sciences Center, Vancouver, British Columbia: Brian C. Lentle§ (Radiology)
Brock University, Faculty of Applied Health Sciences, St. Catharines, Ontario: John Hay§ (Physical Activity Measurements)
Children’s Hospital, London Health Sciences Centre, University of Western Ontario, London, Ontario: Robert Stein (Site Principal Investigator), Elizabeth Cairney (Oncology), Cheril Clarson (Bone Health), Guido Filler (Nephrology) §, Joanne Grimmer (Nephrology), Keith Sparrow (Radiology)
IWK Health Center, Halifax, Nova Scotia: Elizabeth Cummings (Site Principal Investigator), Conrad Fernandez (Oncology), Adam M. Huber§ (Rheumatology), Bianca Lang*,§ (Rheumatology), Kathy O’Brien (Radiology)
McMaster Children’s Hospital, Hamilton, Ontario: Stephanie Atkinson*,§ (Site Principal Investigator), Steve Arora (Nephrology), Ronald Barr§ (Oncology), Craig Coblentz (Radiology), Peter B. Dent (Rheumatology), Maggie Larche (Rheumatology), Colin Webber* (Radiology),
Montréal Children’s Hospital, Montréal, Québec: Celia Rodd§ (Site Principal Investigator), Sharon Abish (Oncology), Lorraine Bell (Nephrology), Rosie Scuccimarri (Rheumatology)
Shriners Hospital for Children, Montréal, Québec: Frank Rauch*,§ (Co-Chair, Publications and Presentations Committee and Ancillary Studies Committee), Francis Glorieux*
Ste. Justine Hospital, Montréal, Québec: Nathalie Alos* (Site Principal Investigator), Josée Dubois (Radiology), Caroline Laverdière (Oncology), Véronique Phan (Nephrology), Claire Saint-Cyr (Rheumatology)
Stollery Children’s Hospital, Edmonton, Alberta: Robert Couch* (Site Principal Investigator), Janet Ellsworth (Rheumatology), Claire LeBlanc (Rheumatology), Maury Pinsk (Nephrology), Kerry Siminoski§ (Radiology), Beverly Wilson (Oncology)
Toronto Hospital for Sick Children, Toronto, Ontario: Ronald Grant* (Site Principal Investigator), Martin Charron (Radiology), Diane Hebert (Nephrology)
Université de Sherbrooke, Department of family medicine, Sherbrooke, Québec: Isabelle Gaboury*,§ (Biostatistics)
Winnipeg Children’s Hospital, Winnipeg, Manitoba: Shayne Taback§ (Site Principal Investigator), Tom Blydt-Hansen (Nephrology), Sara Israels (Oncology), Kiem Oen (Rheumatology), Martin Reed (Radiology)
Footnotes
Principal Investigator;
Executive Committee Member;
Publications and Presentations Committee Member
Conflicts of Interest:
Dr. Leanne M. Ward has been a consultant to Novartis Pharmaceuticals Corporation, Merck Sharpe & Dohme Corp. and Amgen Inc. in the last 2 years. All other authors have no conflict of interest to declare.
References
- 1.Hodson EM, Knight JF, Willis NS, Craig JC. Corticosteroid therapy for nephrotic syndrome in children. Cochrane Database Syst Rev. 2001;2 doi: 10.1002/14651858.CD001533. [DOI] [PubMed] [Google Scholar]
- 2.Tarshish P, Tobin JN, Bernstein J, Edelmann CM., Jr Prognostic significance of the early course of minimal change nephrotic syndrome: report of the International Study of Kidney Disease in Children. J Am Soc Nephrol. 1997;8:769–776. doi: 10.1681/ASN.V85769. [DOI] [PubMed] [Google Scholar]
- 3.Canalis E, Mazziotti G, Giustina A, Bilezikian JP. Glucocorticoid-induced osteoporosis: pathophysiology and therapy. Osteoporos Int. 2007;18:1319–1328. doi: 10.1007/s00198-007-0394-0. [DOI] [PubMed] [Google Scholar]
- 4.Weinstein RS. Glucocorticoid-induced osteoporosis and osteonecrosis. Endocrinol Metab Clin North Am. 2012;41:595–611. doi: 10.1016/j.ecl.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Staa TP, Cooper C, Leufkens HG, Bishop N. Children and the risk of fractures caused by oral corticosteroids. J Bone Miner Res. 2003;18:913–918. doi: 10.1359/jbmr.2003.18.5.913. [DOI] [PubMed] [Google Scholar]
- 6.Rodd C, Lang B, Ramsay T, et al. Incident vertebral fractures among children with rheumatic disorders 12 months after glucocorticoid initiation: a national observational study. Arthritis Care Res (Hoboken) 2012;64:122–131. doi: 10.1002/acr.20589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nakhla M, Scuccimarri R, Duffy KN, Chedeville G, Campillo S, Duffy CM, Azouz EM, Shenouda N, Sharma AK, Rodd C. Prevalence of Vertebral Fractures in Children with Chronic Rheumatic Diseases at Risk for Osteopenia. J Pediatr. 2009;154:438–443. doi: 10.1016/j.jpeds.2008.09.023. [DOI] [PubMed] [Google Scholar]
- 8.Varonos S, Ansell BM, Reeve J. Vertebral collapse in juvenile chronic arthritis: its relationship with glucocorticoid therapy. Calcif Tissue Int. 1987;41:75–78. doi: 10.1007/BF02555248. [DOI] [PubMed] [Google Scholar]
- 9.Feber J, Gaboury I, Ni A, et al. Skeletal findings in children recently initiating glucocorticoids for the treatment of nephrotic syndrome. Osteoporos Int. 2012;23:751–760. doi: 10.1007/s00198-011-1621-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fujita T, Satomura A, Hidaka M, Ohsawa I, Endo M, Ohi H. Acute alteration in bone mineral density and biochemical markers for bone metabolism in nephrotic patients receiving high-dose glucocorticoid and one-cycle etidronate therapy. Calcif Tissue Int. 2000;66:195–199. doi: 10.1007/s002230010039. [DOI] [PubMed] [Google Scholar]
- 11.Leonard MB, Feldman HI, Shults J, Zemel BS, Foster BJ, Stallings VA. Long-term, high-dose glucocorticoids and bone mineral content in childhood glucocorticoid-sensitive nephrotic syndrome. N Engl J Med. 2004;351:868–875. doi: 10.1056/NEJMoa040367. [DOI] [PubMed] [Google Scholar]
- 12.Sbrocchi AM, Rauch F, Matzinger M, Feber J, Ward LM. Vertebral fractures despite normal spine bone mineral density in a boy with nephrotic syndrome. Pediatr Nephrol. 2011;26:139–142. doi: 10.1007/s00467-010-1652-5. [DOI] [PubMed] [Google Scholar]
- 13.Halton J, Gaboury I, Grant R, et al. Advanced vertebral fracture among newly diagnosed children with acute lymphoblastic leukemia: results of the Canadian Steroid-Associated Osteoporosis in the Pediatric Population (STOPP) research program. J Bone Miner Res. 2009;24:1326–1334. doi: 10.1359/jbmr.090202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.International Study of kidney Disease in Children. The primary nephrotic syndrome in children. Identification of patients with minimal change nephrotic syndrome from initial response to prednisone. A report of the International Study of Kidney Disease in Children. J Pediatr. 1981;98:561–564. doi: 10.1016/s0022-3476(81)80760-3. [DOI] [PubMed] [Google Scholar]
- 15.Institute of Medicine. Dietary reference intakes for calcium, phosphorus, magnesium, Vitamin D, fluoride. Washington, DC: National Academy Press, Washington; 1997. [PubMed] [Google Scholar]
- 16.Gbadegesin R, Smoyer WE. Nephrotic syndrome. In: Geary DF, Schaefer F, editors. Comprehensive Pediatric Nephrology. Moseby Elsevier; 2008. [Google Scholar]
- 17.Schwartz GJ, Munoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, Furth SL. New equations to estimate GFR in children with CKD. J Am Soc Nephrol. 2009;20:629–637. doi: 10.1681/ASN.2008030287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Arch Dis Child. 1969;44:291–303. doi: 10.1136/adc.44.235.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marshall WA, Tanner JM. Variations in the pattern of pubertal changes in boys. Arch Dis Child. 1970;45:13–23. doi: 10.1136/adc.45.239.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ogden CL, Kuczmarski RJ, Flegal KM, Mei Z, Guo S, Wei R, Grummer-Strawn LM, Curtin LR, Roche AF, Johnson CL. Centers for Disease Control and Prevention 2000 growth charts for the United States: improvements to the 1977 National Center for Health Statistics version. Pediatrics. 2002;109:45–60. doi: 10.1542/peds.109.1.45. [DOI] [PubMed] [Google Scholar]
- 21.World Health Organization Multicentre Growth Reference Study Group. WHO Child Growth Standards: Length/height-for-age, weight-for-age, weight-for-length, weight-for-height and body mass index-for-age: Methods and development. World Health Organization; Geneva: 2006. pp. 229–300. [Google Scholar]
- 22.Musgrave KO, Giambalvo L, Leclerc HL, Cook RA, Rosen CJ. Validation of a quantitative food frequency questionnaire for rapid assessment of dietary calcium intake. J Am Diet Assoc. 1989;89:1484–1488. [PubMed] [Google Scholar]
- 23.Hay J. Development and validation of the Habitual Activity Estimation Scale (HAES) Children and Exercise XIX. 1997;II:125–129. [Google Scholar]
- 24.Wells GD, Wilkes DL, Schneiderman-Walker J, Elmi M, Tullis E, Lands LC, Ratjen F, Coates AL. Reliability and validity of the habitual activity estimation scale (HAES) in patients with cystic fibrosis. Pediatr Pulmonol. 2008;43:345–353. doi: 10.1002/ppul.20737. [DOI] [PubMed] [Google Scholar]
- 25.van Staa TP, Leufkens HG, Cooper C. The epidemiology of corticosteroid-induced osteoporosis: a meta-analysis. Osteoporos Int. 2002;13:777–787. doi: 10.1007/s001980200108. [DOI] [PubMed] [Google Scholar]
- 26.Curtis JR, Westfall AO, Allison J, Bijlsma JW, Freeman A, George V, Kovac SH, Spettell CM, Saag KG. Population-based assessment of adverse events associated with long-term glucocorticoid use. Arthritis Rheum. 2006;55:420–426. doi: 10.1002/art.21984. [DOI] [PubMed] [Google Scholar]
- 27.Dubner SE, Shults J, Baldassano RN, Zemel BS, Thayu M, Burnham JM, Herskovitz RM, Howard KM, Leonard MB. Longitudinal assessment of bone density and structure in an incident cohort of children with Crohn’s disease. Gastroenterology. 2009;136:123–130. doi: 10.1053/j.gastro.2008.09.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Genant HK, Wu CY, van Kuijk C, Nevitt MC. Vertebral fracture assessment using a semiquantitative technique. J Bone Miner Res. 1993;8:1137–1148. doi: 10.1002/jbmr.5650080915. [DOI] [PubMed] [Google Scholar]
- 29.Keats TE, Smith TH. Year Book of Medical Publishers. 2. Chicago: 1977. An Atlas of Normal Developmental Anatomy. [Google Scholar]
- 30.Gaca AM, Barnhart HX, Bisset GS., 3rd Evaluation of wedging of lower thoracic and upper lumbar vertebral bodies in the pediatric population. AJR Am J Roentgenol. 2010;194:516–520. doi: 10.2214/AJR.09.3065. [DOI] [PubMed] [Google Scholar]
- 31.Webber CE, Beaumont LF, Morrison J, Sala A, Barr RD. Age-predicted values for lumbar spine, proximal femur, and whole-body bone mineral density: results from a population of normal children aged 3 to 18 years. Can Assoc Radiol J. 2007;58:37–45. [PubMed] [Google Scholar]
- 32.Newcombe RG. Two-sided confidence intervals for the single proportion: comparison of seven methods. Stat Med. 1998;17:857–872. doi: 10.1002/(sici)1097-0258(19980430)17:8<857::aid-sim777>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 33.Goulding A, Cannan R, Williams SM, Gold EJ, Taylor RW, Lewis-Barned NJ. Bone mineral density in girls with forearm fractures. J Bone Miner Res. 1998;13:143–148. doi: 10.1359/jbmr.1998.13.1.143. [DOI] [PubMed] [Google Scholar]
- 34.Alos N, Grant RM, Ramsay T, et al. High Incidence of Vertebral Fractures in Children With Acute Lymphoblastic Leukemia 12 Months After the Initiation of Therapy. J Clin Oncol. 2012;30:2760–2767. doi: 10.1200/JCO.2011.40.4830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Burnham JM. Inflammatory diseases and bone health in children. Curr Opin Rheumatol. 2012;24:548–553. doi: 10.1097/BOR.0b013e328356b0c2. [DOI] [PubMed] [Google Scholar]
- 36.Bak M, Serdaroglu E, Guclu R. Prophylactic calcium and vitamin D treatments in steroid-treated children with nephrotic syndrome. Pediatr Nephrol. 2006;21:350–354. doi: 10.1007/s00467-005-2118-z. [DOI] [PubMed] [Google Scholar]
- 37.Jia D, O’Brien CA, Stewart SA, Manolagas SC, Weinstein RS. Glucocorticoids act directly on osteoclasts to increase their life span and reduce bone density. Endocrinology. 2006;147:5592–5599. doi: 10.1210/en.2006-0459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dalle Carbonare L, Bertoldo F, Valenti MT, Zenari S, Zanatta M, Sella S, Giannini S, Cascio VL. Histomorphometric analysis of glucocorticoid-induced osteoporosis. Micron. 2005;36:645–652. doi: 10.1016/j.micron.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 39.Gulati S, Sharma RK, Gulati K, Singh U, Srivastava A. Longitudinal follow-up of bone mineral density in children with nephrotic syndrome and the role of calcium and vitamin D supplements. Nephrol Dial Transplant. 2005;20:1598–1603. doi: 10.1093/ndt/gfh809. [DOI] [PubMed] [Google Scholar]
- 40.Hegarty J, Mughal MZ, Adams J, Webb NJ. Reduced bone mineral density in adults treated with high-dose corticosteroids for childhood nephrotic syndrome. Kidney Int. 2005;68:2304–2309. doi: 10.1111/j.1523-1755.2005.00690.x. [DOI] [PubMed] [Google Scholar]
- 41.Wetzsteon RJ, Shults J, Zemel BS, Gupta PU, Burnham JM, Herskovitz RM, Howard KM, Leonard MB. Divergent effects of glucocorticoids on cortical and trabecular compartment BMD in childhood nephrotic syndrome. J Bone Miner Res. 2009;24:503–513. doi: 10.1359/JBMR.081101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tsampalieros A, Gupta P, Denburg MR, Shults J, Zemel BS, Mostoufi-Moab S, Wetzsteon RJ, Herskovitz RM, Whitehead KM, Leonard MB. Glucocorticoid effects on changes in bone mineral density and cortical structure in childhood nephrotic syndrome. J Bone Miner Res. 2013;28:480–488. doi: 10.1002/jbmr.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ostalska-Nowicka D, Zachwieja J, Nowicki M, Kaczmarek E, Siwinska A, Witt M. Vascular endothelial growth factor (VEGF-C1)-dependent inflammatory response of podocytes in nephrotic syndrome glomerulopathies in children: an immunohistochemical approach. Histopathology. 2005;46:176–183. doi: 10.1111/j.1365-2559.2005.02076.x. [DOI] [PubMed] [Google Scholar]




