Abstract
A raising number of surgeons have chosen laparoscopy-assisted gastrectomy (LAG) as an alternative to open gastrectomy (OG) with D2 lymph node dissection for treatment of advanced gastric cancer (ADG). But no meta-analysis has been performed to evaluate the value of LAG versus OG with regard to safety and efficacy for treatment of ADG. A comprehensive literature research was performed in PubMed, Web of Science and Embase to identify studies that compared LAG and OG with D2 lymph node dissection for treatment of ADG. Data of interest were checked and subjected to meta-analysis with RevMan 5.1 software. 11 studies with 1904 patients (982 in LAG and 922 in OG) were enrolled. Pooled risk ratios (RR) and weighted mean difference (WMD) with 95% confidence intervals (CI) were appropriately derived from random-effects models or fixed-effects models. Compared with OG, LAG was associated with less blood loss (WMD = -144.47; P < 0.05), shorter time of first flatus time (WMD = -0.91; P < 0.05) and postoperative hospital stay (WMD = -3.27; P < 0.05), and lower morbidity (RR = 0.70; P < 0.05), but longer operation time (WMD = 41.78; P < 0.05). No significant differences were noted in terms of harvested lymph nodes (WMD = 1.85; P = 0.09), pathological N stage (χ2 3.97; P = 0.26), tumor size (WMD = -0.05; P = 0.81), mortality (RR 0.82; P = 0.76), cancer recurrence rate (RR 0.77; P = 0.18) and 3-year overall survival rate (RR 1.09; P = 0.18). Compared with OG, LAG with D2 lymph node dissection for ADG had the advantages of minimal invasion, faster recovery, and fewer complications, and it could achieve the same degree of radicality, harvested lymph nodes, short-term and long-term prognosis as OG, though the operation time was slightly longer.
Keywords: Advanced gastric cancer, laparoscopy-assisted gastrectomy, open gastrectomy, D2 lymph node dissection, meta-analysis
Introduction
Although there have been great improvements in the diagnosis and treatment of gastric cancer, it remains a major health problem as the fourth most common cancer and the second leading cause of cancer death worldwide and China is classified as a high incidence area for gastric cancer [1,2]. Surgery is the cornerstone in the treatment of gastric cancer [3] which includes conventional open gastrectomy and laparoscopy-assisted gastrectomy.
Complete resection along with lymph node dissection has been accepted as the only possibly curative treatment for gastric cancer [4]. LAG for early gastric cancer (EGC) was first performed in 1991 [5], since then the use of this procedure has been rapidly increasing in EGC high prevalence countries in Asia. “Gastric Cancer Treatment Guidelines in Japan” were implemented in 2010 and recommend laparoscopy-assisted distal, or total gastrectomy with D2 dissection temporarily for clinical research [6]. In fact, the implementation of LAG with D2 dissection is already technically feasible for treating EGC [7-9].
However, many controversies remain on whether this technique could be applied in AGC. It remains to be confirmed whether laparoscopic surgery can still guarantee the advantage of minimal invasion, whether it increases perioperative complications and mortality, and wheth er it can achieve the same degree of radicality as open surgery. Regarding to the inclusion criteria used to select studies, the recent reports not only reported patients with AGC, but also included patients with EGC in a study [10-12]. There are few reports related to the efficacy of LAG with D2 lymph node dissection for AGC merely in a study.
Therefore, we performed a meta-analysis by comparing LAG with OG with D2 lymph node dissection for AGC with regard to their short- and long-term outcomes to elucidate the current status of LAG.
Materials and methods
Search strategy
The publications were identified by searching the major medical electronic databases such as PubMed, Web of Science and Embase, for relevant articles published between January 2000 and September 2013. Registry was performed, using the following Mesh search headings and text words: “laparoscopy-assisted gastrectomy”, “laparoscopic-assisted gastrectomy”, “open gastrectomy”, “conventional gastrectomy”, “gastric cancer”, “gastric carcinoma”, “D2 dissection”. Logical combinations of these and related terms (stomach, neoplasm) were used to maximize sensitivity, articles published in English as a limit. Title and abstracts of each identified publication were screened, and only publications that reported the clinical outcomes of this analysis were further retrieved. Each of these publications was independently and thoroughly reviewed by 3 reviewers.
Inclusion and exclusion criteria
All clinical studies should meet the following criteria for the meta-analysis: (1) study type included RCTs and NRCTs; (2) clinical studies compared LAG versus OG with D2 lymph node dissection for treatment of advanced gastric cancer; (3) outcome assessment of studies included short and long-term outcomes; (4) The manuscript was written in English. The papers containing any of the following criteria were excluded: (1) robot-assisted gastrectomy; (2) no OG as a control; (3) recurrent gastric cancer or palliative resection cases; (4) abstract only; (5) duplicate publication or the publication did not provide sufficient data.
Data extraction and quality assessment
Data extraction and quality assessment were conducted independently by two authors (Yu-Ling Huang and Hai-Guan Lin). Relevant data included: author, year of publication, geographical region, sample size, study period, laparoscopic technique. The short-time outcomes included operation time, blood loss, flatus time, postoperative hospital stay, harvested lymph nodes, N stage, tumor size, morbidity (defined as the incidence of postoperative complications), mortality (defined as hospital mortality), recurrence rate and survival rate. Postoperative complications were classified as duodenal stump fistula, anastomotic leakage, anastomotic stenosis, intra-abdominal bleeding, ileus, pancreatitis, intra-abdominal abscess, wound infection and pulmonary infection. The long-time outcomes included cancer recurrence and survival rate. We used a star scoring system [13] based on criteria related to study design, comparability of patient groups, and outcome assessment to assess literature quality. The total score was 9 stars, and the quality of each study was graded as level 1 (0-5 stars) or level 2 (6-9 stars).
Statistical analysis
All statistical calculations were analyzed using WMDs for continuous variables and RRs for dichotomous variables. Data for continuous variables in the form of means and standard deviation allowed statistical analysis. A randomeffect model was used to avoid statistical heterogeneity between the studies owing to the high heterogeneity of the studies, otherwise, fixed-effects model was used [14]. Heterogeneity was assessed by using the χ2 test. The 95% confidence intervals (CIs) were established. P ≤ 0.05 was considered to indicate statistical significance. Statistical analysis were performed using the Review Manager Version 5.1 downloaded from Cochrane Library.
Results
According to the search strategy and inclusion criteria, a total of 11 studies that included 1904 (982 in LAG and 922 in OG) gastrectomies with D2 lymph node resection for AGC were considered eligible for our meta-analysis [15-25]. Ten studies were carried out by the scholars in Asia [15-18,20-25], and only one was done by the Western investigators [19]. The characteristics (Table 1) and quality assessment (Table 2) of the reported studies are shown, and each study had a score of ≥ 6 stars.
Table 1.
Characteristics of included study
| Reference | Country | Study period | Total | LAG | OG | Follow up | Laparoscopy technique | |
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| LAG | OG | |||||||
| HUR et al (2008) | Korea | Apr 2004-Mar 2007 | 51 | 26 | 25 | 6-47 m | 6-47 m | LADG |
| DU et al (2009) | China | Jun 2004-Dec 2008 | 168 | 78 | 90 | 4-58 m | 4-58 m | LADG |
| Huang et al (2010) | China | Jan 2007-Jun 2008 | 135 | 66 | 69 | 1-19 m | 1-19 m | LADG |
| Cai et al (2011) | China | Mar 2008-Dec 2009 | 96 | 49 | 47 | 4-36 m | 4-36 m | LAG |
| Scatizzi et al (2011) | Italy | Jan 2006-Jun 2009 | 60 | 30 | 30 | 2-37 m | 7-42 m | LADG |
| Shuang et al (2011) | China | Aug 2005-Dec 2007 | 70 | 35 | 35 | 23-50 m | 27-50 m | LADG |
| Chen et al (2012) | China | Jan 2008-Dec 2010 | 346 | 224 | 112 | 1-48 m | 1-48 m | LAG |
| Hamabe et al (2012) | Japan | Jan 2000-Dec 2009 | 167 | 66 | 101 | 5 y | 5 y | LAG |
| Kim et al (2013) | Korea | Jan 2011-Dec 2011 | 346 | 139 | 207 | - | - | LATG |
| Lin et al (2013) | China | Jan 2008-Dec 2010 | 166 | 83 | 83 | 12-50 m | 12-50 m | LAG |
| Shinohara et al (2013) | Japan | Oct 1997-Dec 2008 | 309 | 186 | 123 | 25-58.5 m | 25-58.5 m | LAG |
m: month; y: year; LADG: laparoscopy-assisted distal gastrectomy; LAG: laparoscopy-assisted gastrectomy; LATG: laparoscopy-assisted total gastrectomy; OG: open gastrectomy.
Table 2.
Quality Assessment Scoring of Studies
| Selection | Comparability of group | Outcome | ||||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| Reference | 1 | 2 | 3 | 4 | 5 | 6 | 7 | Total |
| HUR et al (2008) | * | * | * | * | ** | 6 | ||
| DU et al (2009) | * | * | * | * | ** | * | 7 | |
| Huang et al (2010) | * | * | * | * | * | * | 6 | |
| Cai et al (2011) | * | * | * | ** | ** | * | 8 | |
| Scatizzi et al (2011) | * | * | * | ** | ** | * | 8 | |
| Shuang et al (2011) | * | * | * | ** | * | * | 7 | |
| Chen et al (2012) | * | * | * | ** | ** | * | 8 | |
| Hamabe et al (2012) | * | * | * | * | ** | * | 7 | |
| Kim et al (2013) | * | * | * | ** | ** | 7 | ||
| Lin et al (2013) | * | * | * | ** | ** | * | 8 | |
| Shinohara et al (2013) | * | * | * | ** | * | * | 7 | |
Quality was assessed using a star scoring system. Selection for treatment: 1, inclusion criteria reported; 2, representability of patients undergoing LAG with D2 lymph node dissection to population undergoing surgery for AGC; 3, representability of patients undergoing OG with D2 lymph node dissection to population undergoing surgery for AGC. Comparability of groups (if yes to all, 2 stars; if one of these characteristics was not reported, 1 star; if the two groups differed, no star): 4, age, sex, and body mass index (BMI); 5, tumor site, tumor histological type, tumor size, and tumor stage. Outcome assessment: 8, > 8 outcomes clearly recorded, 1 star; 9, quality of follow-up, 1 star if > 90 patients were followed up for five years.
Clinicopathological findings
To produce such kind of study, patients in OG group were mainly matched those of the LAG group with regard to age, sex, and BMI in enrolled studies. And harvested lymph node, pathological N stage and tumor size were retrospectively selected.
Similar number of harvested lymph nodes were found between LAG group and OG group (WMD = 1.85; 95% CI -0.32, 4.02; P = 0.09) with the random-effect model due to marked heterogeneity (I2 = 81%) (Figure 1).
Figure 1.
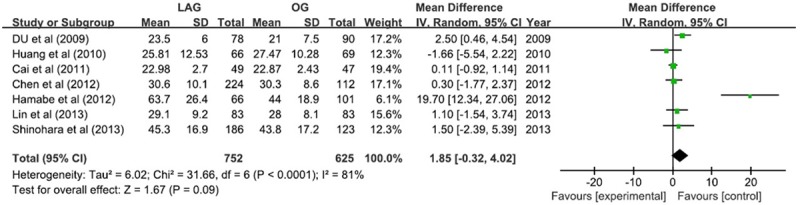
Harvested lymph nodes.
The overall pathological N stage showed no significant difference between LAG and OG (χ2 3.97; P = 0.26).
Similar size of tumor was found between LAG group and OG group (WMD = -0.05; 95% CI -0.47, 0.37; P=0.81) with the random-effect model due to moderate heterogeneity (I2 = 69%) (Figure 2).
Figure 2.
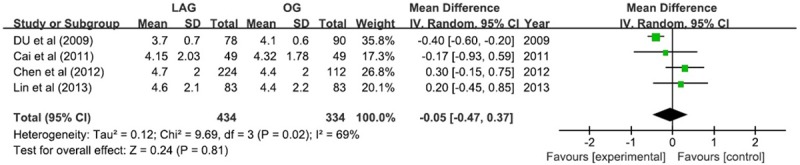
Tumor size.
Surgery-related findings
The duration of operation time in LAG group was 41.78 min longer than that in OG group (WMD 41.78; 95% CI 14.49, 69.08; P <; 0.05) with the random-effect model due to significant heterogeneity (I2 = 95%) (Figure 3).
Figure 3.
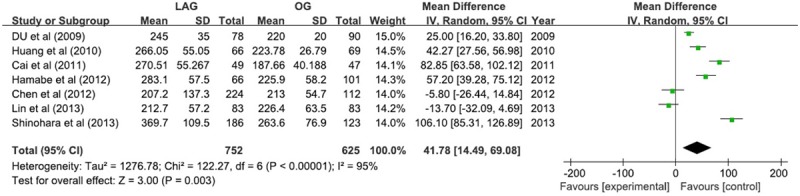
Operation time.
The results of blood loss was in favor of LAG group, with a reduction of 144.47 ml (WMD -144.47; 95% CI -194.01, -94.93; P <; 0.05). The level of heterogeneity was noteworthy (I2 = 89%), so the randomized effect model was used (Figure 4).
Figure 4.
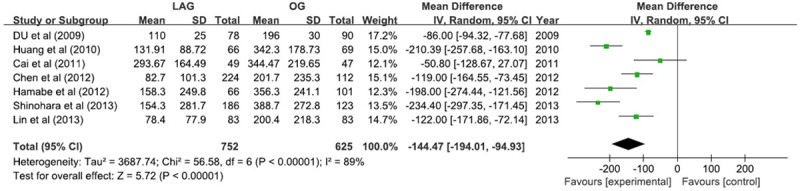
Blood loss.
Postoperative findings
The bowel function recovery could be assessed by first flatus time and there was 0.91 day earlier in LAG group (WMD -0.91; 95% CI -1.19, -0.62; P <; 0.05). A marked heterogeneity was observed (I2 = 78%) (Figure 5).
Figure 5.
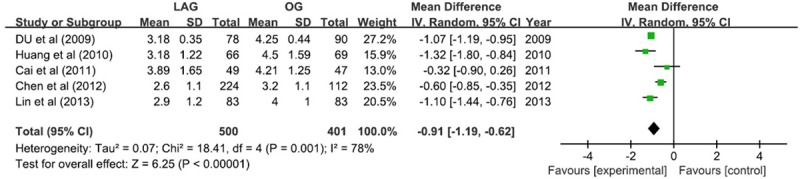
First flatus.
The postoperative hospital stay showed a favorable tendency for LAG group with 3.27 days shorter (WMD -2.69; 95% CI -4.96, -1.58; P <; 0.05). The result showed a remarkable heterogeneity (I2 = 92%) (Figure 6).
Figure 6.
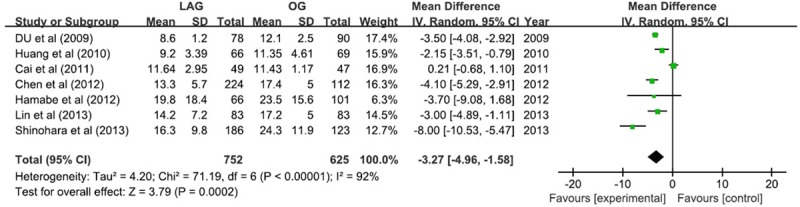
Postoperative hospital stay.
Postoperative complications and hospital mortality
The overall incidence of postoperative complications was less in LAG group (RR 0.70; 95% CI 0.57, 0.86; P <; 0.05) (Figure 7). But the subgroup analysis of postoperative complications showed that the patients had less wound infection (RR 0.26; 95% CI 0.13, 0.56; P <; 0.05) and intra-abdominal abscess (RR 0.49; 95% CI 0.25, 0.96; P <; 0.05) in LAG group, and there was no statistical difference with regard to duodenal stump fistula, anastomotic leakage, anastomotic stenosis, intra-abdominal bleeding, ileus, pancreatitis, and pulmonary infection (Table 3).
Figure 7.
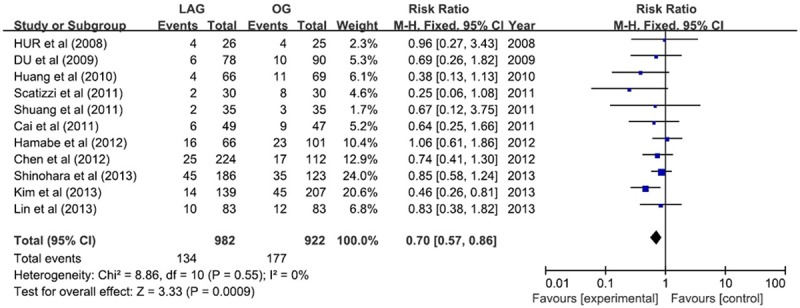
Postoperative complications.
Table 3.
Total complications and subgroup meta-analysis results
| Outcomes | No. of study | Sample size | Heterogeneity P, I2 | RR 95% CI | P | |
|---|---|---|---|---|---|---|
|
|
||||||
| LAG | OG | |||||
| Total complications | 11 | 982 | 922 | 0.55, 0% | 0.70, [0.57, 0.86] | 0.0009 |
| Duodenal stump fistula | 8 | 712 | 717 | 0.89, 0% | 0.94, [0.38, 2.31] | 0.89 |
| Anastomotic leakage | 8 | 855 | 793 | 0.67, 0% | 0.71, [0.34, 1.50] | 0.37 |
| Anastomotic stenosis | 6 | 776 | 716 | 0.74, 0% | 1.04, [0.49, 2.22] | 0.92 |
| Abdominal abscess | 5 | 698 | 626 | 0.51, 0% | 0.49, [0.25, 0.96] | 0.04 |
| Abdominal bleeding | 7 | 646 | 616 | 0.65, 0% | 0.94, [0.39, 2.29] | 0.89 |
| Postoperative ileus | 5 | 573 | 579 | 0.35, 10% | 0.90, [0.33, 2.46] | 0.84 |
| Pancreatitis | 5 | 594 | 454 | 0.94, 0% | 1.05, [0.51, 2.18] | 0.89 |
| Wound infection | 7 | 579 | 596 | 0.72, 0% | 0.26, [0.13, 0.56] | 0.0005 |
| Pulmonary infection | 5 | 508 | 465 | 0.07, 54% | 0.70, [0.36, 1.36] | 0.29 |
The hospital mortality rate was found to be no significant difference (RR 0.82; 95% CI 0.23, 2.88; P = 0.76) (Figure 8).
Figure 8.
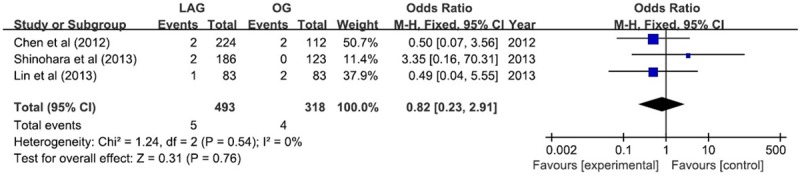
Hospital mortality.
Long-term outcomes
Tumor recurrence was reported in 4 studies, which verified no remarkable difference in LAG group compared with OG group (RR, 0.77; 95% CI, 0.52 1.13; P = 0.18), but the result was associated with low-grade heterogeneity between studies (P = 0.15, I2 = 41%) (Figure 9).
Figure 9.
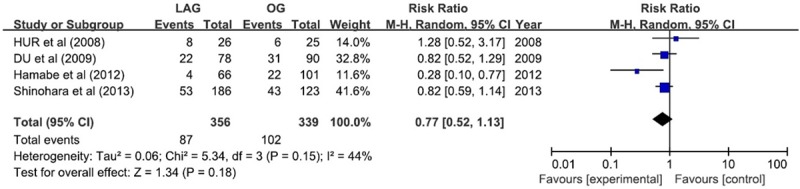
Recurrence rate.
Different duration of follow-up among studies ranged from 1 to 60 months; The long-term survival rate of all available studies revealed no significant difference between the two groups. Data from 3 studies involving 315 participants were available to calculate 3-year overall survival rate for LAG compared with OG [15,16,18], the result was found no remarkable difference (RR 1.09; 95% CI 0.96 1.23; P = 0.18), and the result was not associated with significant heterogeneity (P = 0.85, I2 = 0%) (Figure 10).
Figure 10.
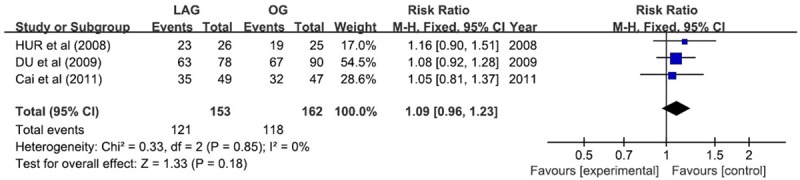
3-year overall survival rate.
Discussion
With known benefits to a minimally invasive approach, laparoscopic techniques for treating patients with early gastric cancer have substantially increased, which have shown oncologic and long-term survival equivalency to the open technique [26,27]. Meanwhile, an increasing number of surgeons have become concerned about laparoscopic surgery for AGC. In the world, sixty-eighty percent of patients diagnosed with gastric carcinoma are in advanced-stage [28]. It would seem more important to study laparoscopic techniques for AGC. D2 lymph node dissection showed the benefits for fit patients with early- and intermediate-stage disease [29,30], for the reason that D2 dissection was possible to remove more positive nodes than D1 dissection [31]. But D2 dissection was also thought to be a more appropriate treatment for patients with advanced disease [32], in centers that could demonstrate low operative mortality [33]. The Japanese gastric cancer treatment guidelines have adopted D2 lymph node dissection as the standard technique for AGC. Nevertheless, debate on oncological adequacy and postoperative outcomes makes the use of LAG with D2 lymph node dissection for AGC still controversial, it remains questionable on account of the technical difficulty of D2 lymph node dissection [34,35]. Therefore, we performed this meta-analysis to assess the value of LAG with D2 lymph node dissection for AGC.
For meta-analysis, RCT is the most ideal tools, but no RCTs were found. Eventually, 11 non-randomized comparative cohort studies were included. Based on the fact that curative resection of AGC involving extended lymphadenectomy was well accepted by Eastern Asian countries, such as Japan, Korea and China, and some specialized centers in Europe [32,36,37], 10 studies were carried out by the scholars in Asia, and only one was done by the Western investigators. No significant difference of characteristics such as age, sex, and BMI were found between the two groups, indicating the fact that the two groups were comparable.
To assess the quality of oncological adequacy and indicate the long-term outcome, the number of harvested lymph nodes is an important subject. Similar number of harvested lymph nodes (WMD = 1.85; 95% CI -0.32, 4.02; P = 0.09) was found between LAG and OG with D2 lymph nodes for ADG which matched with Ding’s and Wang’s meta-analysis [6,38]. Though some authors reported that more lymph nodes were harvested during OTG compared with LTG, although no statistical difference was found [39,40]. The pathological N stage and tumor size are also important to evaluate criterion that showed no significant difference between LAG and OG in our meta-analysis. Moreover, the comparability of the two groups was verified again.
In this meta-analysis, operation time was 41.78 min longer in LAG group than that in OG group. The familiarity with the laparoscopic system and the skill of the surgeons influence the length of the time [41]. It is believed that the time for LAG will decrease in the future. Less blood loss was found in LAG group and scholars have found that less blood loss can reduce the risk of acute or late adverse effects such as acute lung injury, volume overload, hypothermia, etc [42]. The advantages also reflected in faster bowel function recovery and shorter postoperative hospital stay which are similar with previous meta-analysis [6,38,43].
D2 dissection is a more technically demanding and time-consuming procedure and concern also exists that whether a more extensive lymphadenectomy could be associated with higher morbidity [44]. However, it is reported in previous meta-analysis about LAG with D2 lymph node dissection for gastric cancer (including EGC and AGC) compared with OG revealed a lower frequency of total postoperative complications [6,38,45]. We observed the same result when we pooled the data together, but differences were found in subgroup analysis, it showed that the patients had less wound infection and intra-abdominal abscess in LAG group, and there was no statistical difference with regard to duodenal stump fistula, anastomotic leakage, anastomotic stenosis, intraabdominal bleeding, ileus, pancreatitis and pulmonary infection. Meanwhile, hospital mortality rate was found to be no significant difference.
Cancer recurrence and long-term survival rate are two critical outcomes for evaluating surgical interventions in oncological therapy. Preceding scholars have affirmed that tumor recurrence was similar in the LAG group compared with the OG group [6,38], and we got the same result. The duration of follow-up among studies ranged from 1 to 60 months; the long-term survival rate of all available studies were summarized, which revealed that there was no significant difference between the two groups in all the studies. Data from 3 studies involving 315 participants were available to calculate 3-year overall survival rate for LAG compared with OG, and the result showed no remarkable difference, which is matched with previous analysis [43].
In conclusion, LAG with D2 lymph nodes dissection is a feasible and safe procedure for AGC. It is superior to OG in minimal invasion, faster recovery, fewer complications and recurrences, and it can achieve the same degree of short- and long-term prognosis as OG. However, with the benefits of less blood loss, earlier postoperative recovery, reduced postoperative complications, and similar harvested lymph nodes, LAG with D2 lymph nodes dissection for the treatment of AGC can only be performed instead of OG by the experienced surgeons. Meanwhile, the well-designed RCTs are expected to be published to allow a more convincing evaluation.
Acknowledgements
We acknowledge all authors whose publications could be included in our meta-analysis.
Disclosure of conflict of interest
None.
References
- 1.Crew KD, Neugut AI. Epidemiology of gastric cancer. World J Gastroenterol. 2006;12:354–362. doi: 10.3748/wjg.v12.i3.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 3.Li QG, Li P, Tang D, Chen J, Wang DR. Impact of postoperative complications on long-term survival after radical resection for gastric cancer. World J Gastroenterol. 2013;19:4060–4065. doi: 10.3748/wjg.v19.i25.4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shin D, Park SS. Clinical importance and surgical decision-making regarding proximal resection margin for gastric cancer. World J Gastrointest Oncol. 2013;5:4–11. doi: 10.4251/wjgo.v5.i1.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kitano S, Iso Y, Moriyama M, Sugimachi K. Laparoscopy-assisted Billroth I gastrectomy. Surg Laparosc Endosc. 1994;4:146–148. [PubMed] [Google Scholar]
- 6.Ding J, Liao GQ, Liu HL, Liu S, Tang J. Meta-analysis of laparoscopy-assisted distal gastrectomy with D2 lymph node dissection for gastric cancer. J Surg Oncol. 2012;105:297–303. doi: 10.1002/jso.22098. [DOI] [PubMed] [Google Scholar]
- 7.Kim MG, Kim BS, Kim TH, Kim KC, Yook JH, Kim BS. The effects of laparoscopic assisted total gastrectomy on surgical outcomes in the treatment of gastric cancer. J Korean Surg Soc. 2011;80:245–250. doi: 10.4174/jkss.2011.80.4.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eom BW, Kim YW, Lee SE, Ryu KW, Lee JH, Yoon HM, Cho SJ, Kook MC, Kim SJ. Survival and surgical outcomes after laparoscopy-assisted total gastrectomy for gastric cancer: casecontrol study. Surg Endosc. 2012;26:3273–3281. doi: 10.1007/s00464-012-2338-9. [DOI] [PubMed] [Google Scholar]
- 9.Zeng YK, Yang ZL, Peng JS, Lin HS, Cai L. Laparoscopy-assisted versus open cancer: evidence from randomized and nonrandomized clinical trials. Ann Surg. 2012;256:39–52. doi: 10.1097/SLA.0b013e3182583e2e. [DOI] [PubMed] [Google Scholar]
- 10.Wang JB, Huang CM, Zheng CH, Li P, Xie JW, Lin BJ, Lu HS. Efficiency of laparoscopic D2 radical gastrectomy in gastric cancer: Experiences of 218 patients. Zhonghua Wai Ke Za Zhi. 2010;48:502–505. [PubMed] [Google Scholar]
- 11.Xu J, Dong YH, Zhao BY, Ding W, Chen Z, Wu SS. Clinical analysis of laparoscopic D2 lymphadenectomy for distal gastric cancer. Zhonghua Wei Chang Wai Ke Za Zhi. 2012;15:1269–1272. [PubMed] [Google Scholar]
- 12.Chen K, Xu X, Mou Y, Pan Y, Zhang R, Zhou Y, Wu D, Huang C. Totally laparoscopic distal gastrectomy with D2 lymphadenectomy and Billroth II gastrojejunostomy for gastric cancer: short- and medium-term results of 139 consecutive cases from a single institution. Int J Med Sci. 2013;10:1462–1470. doi: 10.7150/ijms.6632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Athanasiou T, Al-Ruzzeh S, Kumar P, Crossman MC, Amrani M, Pepper JR, Del Stanbridge R, Casula R, Glenville B. Off-pump myocardial revascularization is associated with less incidence of stroke in elderly patients. Ann Thorac Surg. 2004;77:745–753. doi: 10.1016/j.athoracsur.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 14.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 15.Hur H, Jeon HM, Kim W. Laparoscopy-assisted distal gastrectomy with D2 lymphadenectomy for T2b advanced gastric cancers: three years’ experience. J Surg Oncol. 2008;98:515–519. doi: 10.1002/jso.21155. [DOI] [PubMed] [Google Scholar]
- 16.Du XH, Li R, Chen L, Shen D, Li SY, Guo Q. Laparoscopy-assisted D2 radical distal gastrectomy for advanced gastric cancer: Initial experience. Chin Med J (Engl) 2009;122:1404–1407. [PubMed] [Google Scholar]
- 17.Huang JL, Wei HB, Zheng ZH, Wei B, Chen TF, Huang Y, Guo WP, Hu B. Laparoscopy-assisted D2 radical distal gastrectomy for advanced gastric cancer. Dig Surg. 2010;27:291–296. doi: 10.1159/000281818. [DOI] [PubMed] [Google Scholar]
- 18.Cai J, Wei D, Gao CF, Zhang CS, Zhang H, Zhao T. A prospective randomized study comparing open versus laparoscopy-assisted D2 radical gastrectomy in advanced gastric cancer. Dig Surg. 2011;28:331–337. doi: 10.1159/000330782. [DOI] [PubMed] [Google Scholar]
- 19.Scatizzi M, Kröning KC, Lenzi E, Moraldi L, Cantafio S, Feroci F. Laparoscopic versus open distal gastrectomy for locally advanced gastric cancer: a case-control study. Updates Surg. 2011;63:17–23. doi: 10.1007/s13304-011-0043-1. [DOI] [PubMed] [Google Scholar]
- 20.Shuang J, Qi S, Zheng J, Zhao Q, Li J, Kang Z, Hua J, Du J. A case-control study of laparoscopy-assisted and open distal gastrectomy for advanced gastric cancer. J Gastrointest Surg. 2011;15:57–62. doi: 10.1007/s11605-010-1361-1. [DOI] [PubMed] [Google Scholar]
- 21.Chen QY, Huang CM, Lin JX, Zheng CH, Li P, Xie JW, Wang JB, Lu J. Laparoscopy-assisted versus open D2 radical gastrectomy for advanced gastric cancer without serosal invasion: a case control study. World J Surg Oncol. 2012;10:248. doi: 10.1186/1477-7819-10-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hamabe A, Omori T, Tanaka K, Nishida T. Comparison of long-term results between laparoscopy-assisted gastrectomy and open gastrectomy with D2 lymph node dissection for advanced gastric cancer. Surg Endosc. 2012;26:1702–1709. doi: 10.1007/s00464-011-2096-0. [DOI] [PubMed] [Google Scholar]
- 23.Kim HS, Kim BS, Lee IS, Lee S, Yook JH, Kim BS. Comparison of totally laparoscopic total gastrectomy and open total gastrectomy for gastric cancer. J Laparoendosc Adv Surg Tech A. 2013;23:323–331. doi: 10.1089/lap.2012.0389. [DOI] [PubMed] [Google Scholar]
- 24.Lin JX, Huang CM, Zheng CH, Li P, Xie JW, Wang JB, Lu J. Laparoscopy-assisted gastrectomy with D2 lymph node dissection for advanced gastric cancer without serosa invasion: a matched cohort study from South China. World J Surg Oncol. 2013;11:4. doi: 10.1186/1477-7819-11-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shinohara T, Satoh S, Kanaya S, Ishida Y, Taniguchi K, Isogaki J, Inaba K, Yanaga K, Uyama I. Laparoscopic versus open D2 gastrectomy for advanced gastric cancer: a retrospective cohort study. Surg Endosc. 2013;27:286–294. doi: 10.1007/s00464-012-2442-x. [DOI] [PubMed] [Google Scholar]
- 26.Mochiki E, Kamiyama Y, Aihara R, Nakabayashi T, Asao T, Kuwano H. Laparoscopic assisted distal gastrectomy for early gastric cancer: five years’ experience. Surgery. 2005;137:317–322. doi: 10.1016/j.surg.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 27.Yakoub D, Athanasiou T, Tekkis P, Hanna GB. Laparoscopic assisted distal gastrectomy for early gastric cancer: is it an alternative to the open approach? Surg Oncol. 2009;18:322–333. doi: 10.1016/j.suronc.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 28.Parkin DM. Intemational variation. Oncogene. 2004;23:6329–6340. doi: 10.1038/sj.onc.1207726. [DOI] [PubMed] [Google Scholar]
- 29.Pugliese R, Maggioni D, Sansonna F, Costanzi A, Ferrari GC, Di Lernia S, Magistro C, De Martini P, Pugliese F. Subtotal gastrectomy with D2 dissection by minimally invasive surgery for distal adenocarcinoma of the stomach: results and 5-year survival. Surg Endosc. 2010;24:2594–2602. doi: 10.1007/s00464-010-1014-1. [DOI] [PubMed] [Google Scholar]
- 30.Zilberstein B, Mucerino DR, Yagi OK, Ribeiro-Junior U, Lopasso FP, Bresciani C, Jacob CE, Coimbra BG, Cecconello I. Results of D2 gastrectomy for gastric cancer: lymph node chain dissection or multiple node resection? Arq Bras Cir Dig. 2012;25:161–164. doi: 10.1590/s0102-67202012000300005. [DOI] [PubMed] [Google Scholar]
- 31.de Manzoni G, Verlato G, Roviello F, Morgagni P, Di Leo A, Saragoni L, Marrelli D, Kurihara H, Pasini F. The new TNM classification of lymph node metastasis minimises stage migration problems in gastric cancer patients. Br J Cancer. 2002;87:171–174. doi: 10.1038/sj.bjc.6600432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Songun I, Putter H, Kranenbarg EM, Sasako M, van de Velde CJ. Surgical treatment of gastric cancer: 15-year follow-up results of the randomised nationwide Dutch D1D2 trial. Lancet Oncol. 2012;11:439–449. doi: 10.1016/S1470-2045(10)70070-X. [DOI] [PubMed] [Google Scholar]
- 33.Di Martino N, Izzo G, Cosenza A, Vicenzo L, Monaco L, Torelli F, Basciotti A, Brillantino A, Marra A. Total gastrectomy for gastric cancer: can the type of lymphadenectomy condition the long-term results? Suppl Tumori. 2005;4:S84–S85. [PubMed] [Google Scholar]
- 34.Miura S, Kodera Y, Fujiwara M, Ito S, Mochizuki Y, Yamamura Y, Hibi K, Ito K, Akiyama S, Nakao A. Paroscopy-assisted distal gastrectomy with systemic lymph node dissection: a critical reappraisal from the viewpoint of lymph node retrieval. J Am Coll Surg. 2004;198:933–938. doi: 10.1016/j.jamcollsurg.2004.01.021. [DOI] [PubMed] [Google Scholar]
- 35.Liang Y, Li G, Chen P, Yu J, Zhang C. Laparoscopic versus open gastrectomy for early distal gastric cancer: a meta-analysis. ANZ J Surg. 2011;81:673–680. doi: 10.1111/j.1445-2197.2010.05599.x. [DOI] [PubMed] [Google Scholar]
- 36.Dicken BJ, Bigam DL, Cass C, Mackey JR, Joy AA, Hamilton SM. Gastric adenocarcinoma: review and considerations for future directions. Ann Surg. 2005;241:27–39. doi: 10.1097/01.sla.0000149300.28588.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sasako M, Sano T, Yamamoto S, Kurokawa Y, Nashimoto A, Kurita A, Hiratsuka M, Tsujinaka T, Kinoshita T, Arai K, Yamamura Y, Okajima K Japan Clinical Oncology Group. D2 lymphadenectomy alone or with para-aortic nodal dissection for gastric cancer. N Engl J Med. 2008;359:453–462. doi: 10.1056/NEJMoa0707035. [DOI] [PubMed] [Google Scholar]
- 38.Wang W, Li Z, Tang J, Wang M, Wang B, Xu Z. Laparoscopic versus open total gastrectomy with D2 dissection for gastric cancer: a meta-analysis. J Cancer Res Clin Oncol. 2013;139:1721–1734. doi: 10.1007/s00432-013-1462-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sakuramoto S, Kikuchi S, Futawatari N, Katada N, Moriya H, Hirai K, Yamashita K, Watanabe M. Laparoscopy-assisted pancreas and spleen-preserving total gastrectomy for gastric cancer as compared with open total gastrectomy. Surg Endosc. 2009;23:2416–2423. doi: 10.1007/s00464-009-0371-0. [DOI] [PubMed] [Google Scholar]
- 40.Siani LM, Ferranti F, Benedetti M, De Carlo A, Quintiliani A. Laparoscopic versus open total mesorectal excision for stage I-III mid and low rectal cancer: a retrospective 5 years analysis. G Chir. 2012;33:404–408. [PubMed] [Google Scholar]
- 41.Kim MC, Jung GJ, Kim HH. Learning curve of laparoscopy-assisted distal gastrectomy with systemic lymphadenectomy for early gastric cancer. World J Gastroenterol. 2005;11:7508–7511. doi: 10.3748/wjg.v11.i47.7508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu WC, Smith TS, Henderson WG, Eaton CB, Poses RM, Uttley G, Mor V, Sharma SC, Vezeridis M, Khuri SF, Friedmann PD. Operative blood loss, blood transfusion, and 30-day mortality in older patients after major noncardiac surgery. Ann Surg. 2010;252:11–17. doi: 10.1097/SLA.0b013e3181e3e43f. [DOI] [PubMed] [Google Scholar]
- 43.Qiu J, Pankaj P, Jiang H, Zeng Y, Wu H. Laparoscopy versus open distal gastrectomy for advanced gastric cancer: a systematic review and meta-Analysis. Surg Laparosc Endosc Percutan Tech. 2013;23:1–7. doi: 10.1097/SLE.0b013e3182747af7. [DOI] [PubMed] [Google Scholar]
- 44.Ryu KW, Kim YW, Lee JH, Nam BH, Kook MC, Choi IJ, Bae JM. Surgical complications and the risk factors of laparoscopy-assisted distal gastrectomy in early gastric cancer. Ann Surg Oncol. 2008;15:1625–1631. doi: 10.1245/s10434-008-9845-x. [DOI] [PubMed] [Google Scholar]
- 45.Wei HB, Wei B, Qi CL, Chen TF, Huang Y, Zheng ZH, Huang JL, Fang JF. Laparoscopic versus open gastrectomy with D2 lymph node dissection for gastric cancer: a meta-analysis. Surg Laparosc Endosc Percutan Tech. 2011;21:383–390. doi: 10.1097/SLE.0b013e31822d02dc. [DOI] [PubMed] [Google Scholar]


