Abstract
p16 is a cell cycle inhibitor that is frequently inactivated in various types of tumours. In spite of p16 importance and its association with clinical behaviour in the genesis of Oral Squamous Cell Carcinoma (OSCC) is not understood fully. The aim of study was to examine the impact of p16 and CK19 in the development and progression of tumour with other clinical behaviours. In the present study, expression profiles of p16 and Cytokeratin19 (CK19) protein were analysed through immunohistochemistry using anti-p16 and anti-CK19 antibody. Expressions pattern of p16 and CK19 were noticed 40% and 58% in the OSCC respectively. Whereas expressions pattern were different in control cases for both markers as p16 (70%) and CK19 (20%). There was progressive loss of p16 expression from oral inflammatory lesion to OSCC and this differences were statically significant (p<0.05). The positivity of p16 were observed in gender where its expression pattern did not reach statistical significance (p>0.05). Expression pattern of CK19 were observed and its expression increased according to the grade and stage of cancer. Furthermore, CK19 expression was seen to be significantly higher in male in the age group ≥50 years (p<0.05). This finding shows that p16 and CK19 may have potential as a prognostic marker in human OSCC and important molecular event in pathogenesis of oral carcinoma.
Keywords: Oral squamous cell carcinoma, p16INK4a, CK-19 and IHC
Introduction
Oral cancer is multi-factorial diseases including genetic and epigenetic alterations and also a major global health problem worldwide [1-5]. It is the sixth most common cancer worldwide accounting for 4% of all malignancies in male and 2% in women [6]. Several factors such as cigarette smoking, gutkha/nut chewing and alcohol are responsible for the alteration in DNA and gene function. In the long term, polycyclic hydrocarbon and nicotine of cigarette smoke causes cellular damage and play a vital role in the DNA adduct formation resulting in alterations in squamous cells. P16 tumour suppressor gene also known as p16INK4a is a member of the INK4 family of CDK inhibitors. By inhibiting phosphorylation of Rb, p16INK4A promotes the formation of an Rb-E2F repressive transcriptional complex, which also blocks cell cycle progression at the G1-S restriction point [7,8]. Altered or loss of p16 expression has been noticed in oral premalignant lesions and tumour oral cavity [9-12]. The exact reason of inactivation of p16 in the tumour progression is not known entirely but various types of mutation play a notable role in this vista.
Another protein such as Cytokeratin19 also involve in the development and progression of oral cancer. Cytokeratin, constitute the largest family of intermediate filament proteins, and are subdivided into two types; type I contains CK9-CK23 and other type II constitutes CK1-CK8 subclasses [13]. Earlier studies showed a range for the percentage of CK19 positive OSCC specimens from 29 to 100% [14-16]. A number of earlier investigator showed that either loss of p16 or overexpression of CK19 in oral squamous cell carcinoma. However, no study has examined expression of both proteins in the same tumour and their role in the development and progression of oral carcinoma. Therefore, we utilized immunohistochemistry to measure expression of p16 and cytokeratin in the same oral squamous cell carcinoma and their relation with age, gender and other clinical outcome.
Materials and methods
Subjects population
Formalin fixed oral tumour with OSSC were retrieved from the department of pathology for immunohistochemical staining by using an anti-p16INK4A and anti-CK19 mouse monoclonal antibody (ChemMate ™ EnVision, +/HRP/DAB, rabbit/ mouse two-step staining). Hematoxylin and Eosin staining was done on all cases to confirm the tumour characteristics (Figure 1). A total one hundred cases of OSCC (76 Male and 24 female) and 90 (75 male and 15 female) cases of inflammatory lesions of oral were taken as control. The median age was 62 years (range, 22-78). The tumour cases were 36 in the oral cavity, 31 the pharynx, and 33 the larynx. According to TNM Staging system, tumour were diagnosed as 13 in stage I, 25 stage II, 30 stage III and 32 cases stage IV. Histopathologically, the oral cancers were categorized as well differentiated 22 cases, moderately differentiated 34 cases and poorly differentiated 42 cases. The inflammatory lesions of oral mucosa were taken as control. Keeping the marker profile in view the cases were further divided according to age into two groups: Less than 50 years & ≥50 years.
Figure 1.
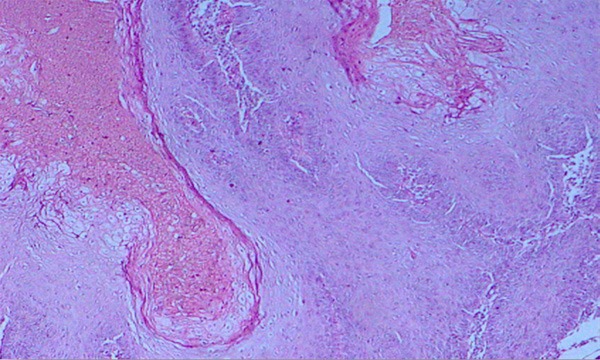
Well Differentiated Squamous Cell Carcinoma. H & E stain X400.
Expressional analysis of tumour markers
Formalin fixed paraffin-embedded tissue blocks were cut in 5 microns thick serial sections and expressional evaluation of P16 and CK were made as per previously described methods [17]. Finally chromogen Diaminobenzidine (DAB) was used and section were counterstain with hematoxylin.
Scoring method
Tumors were classified as p16 and CK negative (i.e. low expression) if less than 10% of cells displayed positivity. If equal to or greater than 10% of cells were positive for P16 and CK19 (i.e. high expression) were considered as positive. The percentage of p16 and CK positive cells was calculated independently by two pathologists. The correlations between p16 and CK expression and patient age, sex and histological grading and staging were studied.
Statistical analysis
Chi square (λ2) test was performed to find out the possible correlation among p16, CK19 and other clinical parameters in oral cancer and inflammatory lesions of oral tissue. Statistical significance was defined as P<0.05.
Results
In our study a total of 100 cases of Oral Squamous Cell Carcinoma (OSCC) and 90 cases of oral inflammatory lesions (as a control) were collected and analysed for pI6 and CK19 protein expression. The age of cancer patients were ranged from 20-76 years with a mean age of 56 years. However, for the control cases this figure was different and most of the patients were younger age (less than 38 years) with mean being 32±10 years. In our study, incidences of carcinoma were significantly higher in male as ratio 4:1 (male v/s females). The similar patterns were observed in control cases where mostly patients were male with ratio 4:1.
Expressional evaluation of p16 protein
The expression of p16 protein in inflammatory lesions of oral tissue and Squamous Cell Carcinoma was observed in cytoplasm. Expression of p16 was not detected in nucleus and other cell components (Figures 2 and 3). Expression of p16 was high in inflammatory lesions of oral tissue (70%) but low in cancer cases (40%). The result reveals the significant difference of positivity of p16 in the normal epithelium and cancerous tissue (p<0.05). Further, the expressions of p16 were different in different grade where it was 54% in well differentiation 47% in moderately differentiation and 25% poor differentiation. The results showed the progression in tumour grade where the rate of p16 expression significantly decreased (p<0.05). As per tumor characteristics parameter, p16 expression was associated with poor differentiation, lymph node involvement, distant metastasis and advance stages of OSCC (Table 1).
Figure 2.
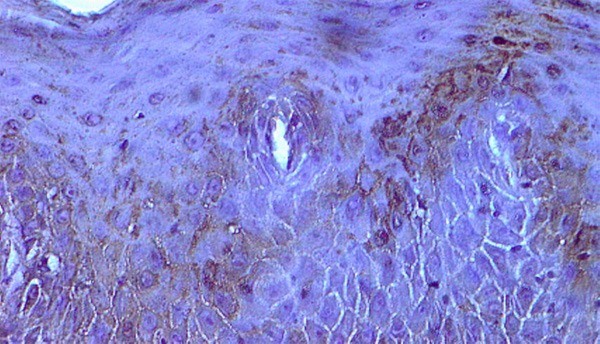
P16 expression in the cytoplasm of well differentiated squamous cell carcinoma X400.
Figure 3.
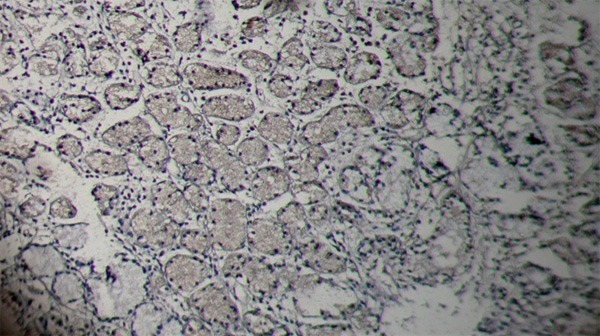
Salivary gland-pleomorphic adenoma without p16 expression X400.
Table 1.
Correlation between p16, CK and associated clinical outcome
| Factors | Total no. | p16+ve (%) | P-value | CK19+ve | P-value |
|---|---|---|---|---|---|
| Age | |||||
| ≤50 | 36 | 11 (30.55) | p>0.05 | 13 (36) | p>0.05 |
| >50 | 64 | 29 (45.3) | 45 (70) | ||
| Male | 76 | 27 (59.62) | p>0.05 | 48 (63) | p<0.05 |
| Female | 24 | 13 (54.16) | 10 (41) | ||
| Well differentiated Carcinoma | 22 | 12 (54) | p<0.05 | 10 (45) | p<0.05 |
| Moderately differentiated | 34 | 16 (47) | 16 (47) | ||
| Poorly differentiated Carcinoma | 44 | 12 (25) | 30 (68) | ||
| Stage | |||||
| I | 13 | 7 (53) | P<0.05 | 2 (15) | p<0.05 |
| II | 25 | 12 (48) | 14 (56) | ||
| III | 30 | 13 (43) | 17 (56) | ||
| IV | 32 | 8 (25) | 20 (62) | ||
| Lymph node involvement | |||||
| No | 45 | 16 (35.5) | P<0.05 | 21 (46) | p<0.05 |
| N1+N2 | 55 | 24 (43.63) | 27 (49) |
Keeping the p16 marker profile in view; cases were further divided according to age into two groups: Less than 50 years & ≥50 years. However, the expressions pattern of P16 was not significant with age and gender (p>0.05) (Table 1).
Clinico-pathological significance of CK19
Cytoplasmic immunoreaction for CK19 was considered positive and found in 58/100 cases (58%) of Oral Squamous Cell carcinoma (Figure 4). The intensity of CK19 expressions were further analyzed and found that 17 cases were positive (10-25% cells), 20 cases were (26-50%) and 21 cases were more than 50 percentage; while inflammatory lesions of oral tissue has shown less positivity. Expressions of CK19 result reveal the significant difference between the control cases (20%) and cancerous tissue (58%) (p<0.05) (Figure 5). Negativity of CK19 was observed in tumour cases. Regarding the expression profile in different grade, we have noticed that, CK19 was expressed in the majority of tumour cells of poorly differentiated OSCC whereas it was low in well differentiated OSCC (Table 1). The expression pattern between the grades was statistically significant as CK19 expression was high in high grade tumour. When CK19 positivity pattern were analysed, CK19 expression was associated with stage of the tumour and CK19 expression was increasing according to stage (Table 1). This difference in expression pattern of CK19 according to stage was statistically significant. CK19 expression on the basis of age and sex was high in male patients (66%) ≥50 years and insignificant in less than 50 age group (47%) (p>0.05) (Table 2).
Figure 4.
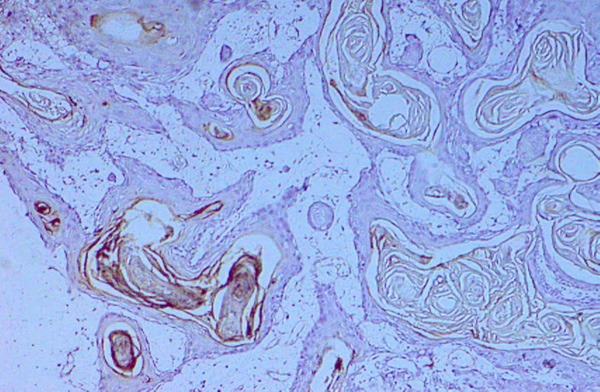
Well differentiated squamous cell carcinoma with cytoplasmic expression of CK19 X400.
Figure 5.
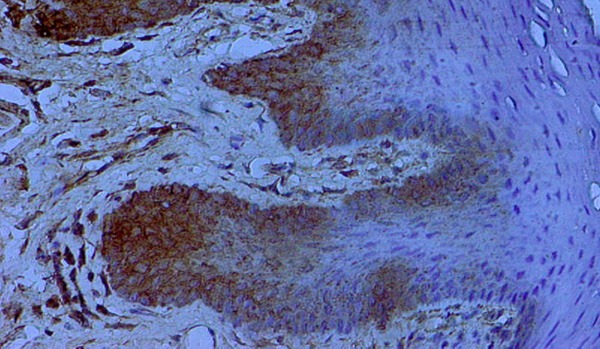
Lingual fibro-epithelial polyp with CK expression in the inflammatory lesions of oral mucosa X400.
Table 2.
Relationship between CK expression, sex and age
| Sex | Age | CK Positivity | |||
|---|---|---|---|---|---|
|
| |||||
| Total cases | Positive cases | Percentage | P-value | ||
| Male | <50 | 28 | 16 | 57% | P<0.05 |
| ≥50 | 48 | 32 | 66% | ||
| Female | <50 | 8 | 04 | 50% | P>0.05 |
| ≥50 | 16 | 06 | 37% | ||
| Total | 100 | 58 | 58% | ||
Correlation between p16 and CK19 expressions in control and oral squamous cell carcinoma cases
In the current study, a significant negative correlation (P<0.05) was observed between CK19 overexpression and loss of P16 expression in OSCC. Furthermore, loss of expression of p16 marker was observed according to tumour stage and grade whereas CK19 expression pattern was directly correlated to the grade and stage of OSCC. Therefore, we speculate that tumor prognostic features correlated with CK19 and p16 expression in OSCC. Another important negative correlation found in both markers in cancer and control cases, where p16 expression was high in control cases but low in cancer cases while CK19 expression profile was high in OSSC cases.
Discussion
Oral cancer is one of the major culprits in health problem and epidemiological studies have shown that several factors are responsible for the development and progression of the oral cancer. The major etiologic factors in the genesis of oral carcinoma include tobacco chewing/smoking, alcohol consumption and HPV [18-21]. Another forms of tobacco use such as nass (an aqueous or oily mixture of tobacco, ash and lime), smoking of bedi (cheap cigarettes in which tobacco is rolled in a temburni leaf) and reverse smoking in which the lighted end of a cigarette is held within the mouth [22] shows a vital effect in oral cancer development. Still the exact cause of the development and progression of oral cancer is not fully known but it is thought that genetic and metabolic pathways alteration is a one of major reason. Earlier investigators has shown that, the loss of PTEN, p16, overexpression of hormonal receptors and VEGF play a role in the development and progression of oral carcinoma [5,17,23,24].
Incidence of oral cancer was high in older age group as compared to younger worldwide [23-26] and also more frequent in male patients as compared to female [23-26]. Our finding also supporting the previous statement as the peak incidence of oral cancer was seen in the older age group patients. The exact reason for that is not well known but it might be due to the cumulative effects of long time exposures to various types of carcinogens. The incidence of oral cancer is high in male worldwide but exact reason of difference in incidence is not understood fully; it is thought that habit of smoking are higher in men than women; it is also recognized that men starts these habits prior than women [23-26].
Various molecular diagnostic markers are used to diagnose the cancer but still constructive diagnostic marker is needed to help in the early diagnosis of cancer. In our study, first time reporting that CK19 and p16 in the same Squamous Cell Carcinomas on the basis of age, gender and other clinical aspect. In current study, expression of p16 was low in OSCC cases (40) as compared of control cases (70%). This finding is in accordance with other earlier report as loss of p16 was observed in tumour cases. Numerous report showed that, p16 is absent or rarely expressed in head and neck SCC [27-29] and OSCC [27]. An important finding also showed that, expression of p16 was found only in three cases (18.75%) of OSCC [30]. This study also reported that p16 expression was significantly low in high grade and stage of the tumour. Our finding supports the recent study on PTEN protein expression profile that showed decreased PTEN expression according to the grade [23]. Several other studies have reported that, loss of p16 expressions were observed in high grade tumour. A report in the support of p16 showed loss of p16INK4a expression with tumour stage and progression [31]. The present study reveals that progressive loss of P16 expression was seen in invasions and metastatic tumour. Keeping the marker profile in view, the expression pattern were analysed according to age, gender and histological grade where p16 expression did not show any significant data in gender and age (p≥0.05) but was low in higher grade tumour.
In this study, we reported that the expression profile of CK19 was high in cancer cases (58%) as compared to the control. The exact reason of the expression difference of CK19 in control and cancer cases is not known fully but it might be due to the alteration in p16 gene due to smoking/carcinogens that damage the DNA and alter the squamous cell. As per CK19 expression pattern is concerned, CK19 expression profile were different from p16 and showed that CK19 expressions were increasing according to grade and also expression was high in cancer cases. The increased expression of keratin has been associated with higher tumour proliferation rate and poorer prognosis [32-34].This finding was in accordance to other protein expression such as VEGF and bcl2 that was increasing according to grade of the tumour [23,24]. In our study, we noted that in males with the age ≥50 years, the expression of CK19 was significantly higher (86%) as compared to the <50 years of age group (50%) (p<0.05). The finding also showed strong negative association of loss P16 expression and CK19 positive expression (p<0.05) in oral carcinoma. When we analysed the expressional profile of both markers; we found that p16 and CK were negatively correlated according to grade as according to the grade of the tumour when.
Conclusions
Expressional profile of both markers (p16 and CK19) was different in same tumour. CK19 positivity was associated with age whereas p16 showed insignificant expression. The expression of P16 decreased while CK19 increased with the tumour grade.
This finding also shows that progressive loss of p16 and high expression of CK19 according to tumour metastatic; when p16 and CK19 evaluated together may have more accurate prediction of clinical outcome/prognostic marker in human OSCC and important molecular event in pathogenesis of oral carcinoma.
Disclosure of conflict of interest
None.
References
- 1.Mehdi SJ, Ali A, Rizvi MA. Parkin Gene Alterations in Ovarian Carcinoma from Northern Indian population. Pathol Oncol Res. 2010;17:579–586. doi: 10.1007/s12253-010-9351-x. [DOI] [PubMed] [Google Scholar]
- 2.Ahmed HG, Abusail SM, Eltom FM, Babiker AY. Frequency and genotype of human papillomavirus among Sudanese patients with head and neck tumors. Ecancermedicalscience. 2012;6:282. doi: 10.3332/ecancer.2012.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aldebasi YH, Rahmani AH, Khan AA, Aly SM. The effect of vascular endothelial growth factor in the progression of bladder cancer and diabetic retinopathy. Int J Clin Exp Med. 2013;6:239–51. [PMC free article] [PubMed] [Google Scholar]
- 4.Babiker AY, Eltom FM, Abdalaziz MS, Rahmani A, Abusail S, Ahmed HG. Screening for high risk human papilloma virus (HR-HPV) subtypes, among Sudanese patients with oral lesions. Int J Clin Exp Med. 2013;6:275–281. [PMC free article] [PubMed] [Google Scholar]
- 5.Rahmani AH, Alzohairy M, Babiker AY, Khan AA, Aly SM, Rizvi MA. Implication of androgen receptor in urinary bladder cancer: a critical mini review. Int J Mol Epidemiol Genet. 2013;4:150–155. [PMC free article] [PubMed] [Google Scholar]
- 6.Boyle P, Macfarlane GJ, Maisonneuve P, Zheng T, Scully C, Tedesco B. Epidemiology of mouth cancer in 1989: a review. J R Soc Med. 1990;83:724–730. doi: 10.1177/014107689008301116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang HS, Postigo AA, Dean DC. Active transcriptional repression by the Rb-E2F complex mediates G1 arrest triggered by p16INK4a, TGFbeta, and contact inhibition. Cell. 1999;97:53–61. doi: 10.1016/s0092-8674(00)80714-x. [DOI] [PubMed] [Google Scholar]
- 8.Serrano M, Hannon GJ, Beach D. A new regulatory motif in cell-cycle control causing specific inhibition of cyclin D/CDK4. Nature. 1993;366:704–707. doi: 10.1038/366704a0. [DOI] [PubMed] [Google Scholar]
- 9.Papadimitrakopoulou V, Izzo J, Lippman SM, Lee JS, Fan YH, Clayman G, Ro JY, Hittelaman WN, Lotan R, Hong WK, Mao L. Frequent inactivation of p16INK4a in oral premalignant lesions. Oncogene. 1997;14:1799–1803. doi: 10.1038/sj.onc.1201010. [DOI] [PubMed] [Google Scholar]
- 10.Kresty LA, Mallery SR, Knobloch TJ, Song H, Lloyd M, Casto BC, Weghorst CM. Alterations of p16INK4a and p14ARF in patients with severe oral epithelial dysplasia. Cancer Res. 2002;62:5295–5300. [PubMed] [Google Scholar]
- 11.Riese U, Dahse R, Fiedler W, Theuer C, Koscielny S, Ernst G, Beleites E, Claussen U, von Eggeling F. Tumour suppressor gene p16 (CDKN2A) mutation status and promoter inactivation in head and neck cancer. Int J Mol Med. 1999;4:61–65. doi: 10.3892/ijmm.4.1.61. [DOI] [PubMed] [Google Scholar]
- 12.Nakahara Y, Shintani S, Mihara M, Kiyota A, Ueyama Y, Matsumura T. Alterations of Rb, p16INK4A and cyclin D1 in the tumourigenesis of oral squamous cell carcinomas. Cancer Lett. 2000;160:3–8. doi: 10.1016/s0304-3835(00)00546-2. [DOI] [PubMed] [Google Scholar]
- 13.Kanaji N, Bandoh S, Fujita J, Ishii T, Ishida T, Kubo A. Compensation of type I and type II cytokeratin pools in lung cancer. Lung Cancer. 2007;3:295–302. doi: 10.1016/j.lungcan.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 14.Nie M, Zhong L, Zeng G, Li B. The changes of cytokeratin 19 during oral carcinogenesis. Zhonghua Kou Qiang Yi Xue Za Zhi. 2002;37:187–90. [PubMed] [Google Scholar]
- 15.Zhong LP, Chen WT, Zhang CP. Increased CK19 expression correlated with pathologic differentiation grade and prognosis in oral squamous cell carcinoma patients. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;104:377–384. doi: 10.1016/j.tripleo.2006.07.019. [DOI] [PubMed] [Google Scholar]
- 16.Su L, Morgan PR, Thomas JA. Expression of keratin 14 and 19 mRNA and protein in normal oral epithelia, hairy leukoplakia, tongue biting and white sponge nevus. J Oral Pathol Med. 1993;22:183–9. doi: 10.1111/j.1600-0714.1993.tb01054.x. [DOI] [PubMed] [Google Scholar]
- 17.Rahmani A, Alzohairy M, Mandal AK, Rizvi MA. Expressional Evaluation of Androgen Receptor in Transitional Cell Carcinoma of Urinary Bladder Patients. British Journal of Medicine and Medical Research. 2011;1:233–238. [Google Scholar]
- 18.Kreimer AR, Clifford GM, Boyle P, Franceschi S. Human papillomavirus types in head and neck squamous cell carcinomas worldwide: a systematic review. Cancer Epidemiol Biomarkers Prev. 2005;14:467–475. doi: 10.1158/1055-9965.EPI-04-0551. [DOI] [PubMed] [Google Scholar]
- 19.Warnakulasuriya S, Sutherland G, Scully C. Tobacco, oral cancer, and treatment of dependence. Oral Oncol. 2005;41:244–260. doi: 10.1016/j.oraloncology.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 20.Blot WJ, McLaughlin JK, Winn DM. Smoking and drinking in relation to oral and pharyngeal cancer. Cancer Res. 1998;48:3282–7. [PubMed] [Google Scholar]
- 21.Warnakulasuriya S, Trivedy C, Peters TJ. Areca nut use: an independent risk factor for oral cancer. BMJ. 2002;324:799–800. doi: 10.1136/bmj.324.7341.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.World Health Organization (WHO) Control of oral cancer in developing countries. Bull. 1984;62:817–830. [PMC free article] [PubMed] [Google Scholar]
- 23.Rahmani A, Alzohairy M, Babiker AY, Rizvi M, Ahmad GH. “Clinicopathological significance of PTEN and bcl2 expressions in oral squamous cell carcinoma”. Int J Clin Exp Pathol. 2012;5:965–971. [PMC free article] [PubMed] [Google Scholar]
- 24.Rahmani A, Alzohairy M, Habeeb K, Shish AK, Rizvi MA. Expressional evaluation of Vascular Endothelial Growth Factor (VEGF) protein in urinary bladder carcinoma patients exposed to cigarette smoke. Int J Clin Exp Pathol. 2012;5:195–202. [PMC free article] [PubMed] [Google Scholar]
- 25.Ahmed HG, Mahgoob RM. Impact of Toombak dipping in the etiology of oral cancer: Gender-exclusive hazard in the Sudan. J Cancer Res Ther. 2007;3:127–130. doi: 10.4103/0973-1482.34696. [DOI] [PubMed] [Google Scholar]
- 26.Ginawi IM, Mahgoub EA, Ahmed HG. Immunophenotyping of HPV types 16 and 18 among Sudanese patients with oral lesions. Oman Med J. 2012;27:201–206. doi: 10.5001/omj.2012.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Natarajan E, Saeb M, Crum CP, Woo SB, Mckee PH, Rheinwald JG. Co-expression of p16 (INK4A) and laminin 5 gamma2 by microinvasive and superficial squamous cell carcinomas in vivo and by migrating wound and senescent keratinocytes in culture. Am J Pathol. 2003;163:477–91. doi: 10.1016/s0002-9440(10)63677-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rocco JW, Sidransky D. p16(MTS-1/CDKN2/INK4a) in cancer progression. Exp Cell Res. 2001;264:42–55. doi: 10.1006/excr.2000.5149. [DOI] [PubMed] [Google Scholar]
- 29.Poi MJ, Yen T, Li J. Somatic INK4a-ARF locus mutations: a significant mechanism of gene inactivation in squamous cell carcinomas of the head and neck. Mol Carcinog. 2001;30:26–36. doi: 10.1002/1098-2744(200101)30:1<26::aid-mc1010>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 30.Buajeeb W, Poomsawat S, Punyasingh J, Sanguansin S. Expression of p16 in oral cancer and premalignant lesions. J Oral Pathol Med. 2009;38:104–108. doi: 10.1111/j.1600-0714.2008.00710.x. [DOI] [PubMed] [Google Scholar]
- 31.Pande P, Mathur M, Shukla NK, Ralhan R. PRb and p16 protein alterations in human oral tumorigenesis. Oral Oncol. 1998;34:396–403. doi: 10.1016/s1368-8375(98)00024-4. [DOI] [PubMed] [Google Scholar]
- 32.Zhong LP, Chen WT, Zhang CP. Increased CK19 expression correlated with pathologic differentiation grade and prognosis in oral squamous cell carcinoma patients. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;104:377–84. doi: 10.1016/j.tripleo.2006.07.019. [DOI] [PubMed] [Google Scholar]
- 33.Toyoshima T, Vairaktaris E, Nkenke E, Schlegel KA, Neukam FW, Ries J. Cytokeratin 17 mRNA expression has potential for diagnostic marker of oral squamous cell carcinoma. J Cancer Res Clin Oncol. 2008;134:515–21. doi: 10.1007/s00432-007-0308-8. [DOI] [PubMed] [Google Scholar]
- 34.Fillies T, Werkmeister R, Packeisen J, Brandt B, Morin P, Weingart D, Joos U, Buerger H. Cytokeratin 8/18 expression indicates a poor prognosis in squamous cell carcinomas of the oral cavity. BMC Cancer. 2006;6:10. doi: 10.1186/1471-2407-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]


