Abstract
Glutathione S-transferase T1 (GSTT1) null genotype has been indicated to be correlated with preterm delivery (PTD) susceptibility, but study results were still debatable. Thus, a meta-analysis was conducted. PubMed, EMBASE, and CNKI were searched. Odds ratios (ORs) with 95% confidence intervals (CIs) were used to calculate the strength of association in the random-effects model or fixed-effects model. Nine case-control studies with a total of 2526 cases and 4565 controls were eligible. The null genotype of GSTT1 was associated with a significantly increased risk of PTD when compared with present genotype (OR = 1.18; 95% CI 1.05-1.33; I2 = 33). In the subgroup analysis according to ethnicity, significantly increased PTD risk was observed in Asians (OR = 1.20; 95% CI 1.01-1.33; I2 = 0%) but not in Caucasians (OR = 1.32; 95% CI 0.89-1.97; I2 = 77). This meta-analysis suggested that GSTT1 null genotype may be associated with the risk of PTD.
Keywords: Preterm delivery, GSTT1, meta-analysis, polymorphism
Introduction
Preterm delivery (PTD), defined by the World Health Organization (WHO) as birth occurring before 37 weeks of gestation, is considered a major global health problem and is strongly associated with neonatal mortality as well as short- and long-term morbidity [1]. There is a common view that PTD is a multifactorial disease caused by complex interactions between a variety of genetic and environmental factors.
Glutathione S-transferases (GSTs) are well known for removing environmental pollutants and endogenous toxic compounds as part of the phase II detoxification process through glutathionylation of diverse electrophilic substrates. Glutathione S-transferase T1 (GSTT1) belongs to GSTT (θ), which has been identified in human liver. It is located on 22q11.23, and encodes a protein consisting of 240 amino acids. The length of GSTT1 gene is 8092 bp with 5 exons and 4 introns. The genotype of GSTT1 allele homozygous deletion is GSTT1 null. Recently, the association between the GSTT1 null genotype and susceptibility of PTD has been investigated extensively [2-10]. However, the the results were conflicted and inconclusive. Therefore, we performed this meta-analysis to precisely estimate the association between the GSTT1 null genotype and risk of PTD.
Materials and methods
Search for publications
Online electronic databases (PubMed, EMBASE and CNKI) was searched using the search terms: (Glutathione S-transferase T1 or GSTT1) and (polymorphism or variant or variation) and (“preterm delivery” or “preterm birth”). Additional studies were identified by a hand search from reference of original studies or review articles on this topic. There was no language restriction.
Inclusion and exclusion criteria
The major inclusion criteria were: (1) case-control studies or cohort studies; (2) investigating the association between GSTT1 null genotype and PTD risk; (3) available genotype distribution information in cases and controls or odds ratio (OR) with its 95% confidence intervals (CIs). The major reasons for exclusion of studies were: (1) reviews and repeated literatures; (2) case-only studies; (3) studies without detail genotype frequencies.
Data extraction
The following data were recorded from each article: first author, year of publication, ethnicity of participants, numbers of cases and controls, and genotype number in cases and controls. The data were extracted by two of the authors independently. Discrepancies between these two authors were resolved by discussion.
Statistical analysis
The strength of association between the GSTT1 null genotype and risk of PTD was assessed by calculating OR with 95% CI. A statistical test for heterogeneity was performed based on the Q statistic. The P > 0.10 of the Q-test indicated a lack of heterogeneity among studies. If heterogeneity was observed among the studies, the random-effects model was used to estimate the pooled OR (the DerSimonian and Laird method). Otherwise, the fixed-effects model was adopted (the Mantel-Haenszel method). Stratified analysis was performed by ethnicity. Cumulative meta-analysis was also performed. Sensitivity analysis was conducted through sequentially excluded individual studies to assess the stability of the results. Potential publication bias was examined visually in a funnel plot and Egger’s test [11]. All statistical tests were performed with the software STATA version 11.0 (Stata Corporation, College station, TX, USA). A P value < 0.05 was considered statistically significant.
Results
Characteristics of studies
A total of nine case-control studies with 2526 cases and 4565 controls on the association between GSTT1 null genotype and risk of PTD were included for this meta-analysis [2-10]. There were five studies of Asian population and three studies of Caucasian population. One study was performed in African population. The characteristics of each case-control study and the genotype in each study are presented in Table 1.
Table 1.
Characteristics of the studies included in this meta-analysis
| First author/Year | No. of | No. of | Case | Control | |||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| Ethnicity | Case | Control | Present | Null | Present | Null | |
| Nukui/2004 | Caucasian | 51 | 904 | 28 | 23 | 690 | 214 |
| Suh 1/2008 | Asian | 117 | 118 | 60 | 57 | 66 | 52 |
| Suh 2/2008 | Asian | 145 | 120 | 78 | 67 | 64 | 56 |
| Tsai/2008 | Caucasian | 571 | 1178 | 131 | 440 | 283 | 895 |
| Zhang/2008 | Asian | 91 | 177 | 47 | 44 | 99 | 78 |
| Tsai/2011 | African | 518 | 512 | 128 | 390 | 152 | 360 |
| Lou/2012 | Asian | 198 | 524 | 103 | 95 | 283 | 241 |
| Mustafa/2013 | Asian | 156 | 151 | 92 | 64 | 106 | 45 |
| Zheng/2013 | Caucasian | 679 | 881 | 568 | 111 | 745 | 136 |
Results of meta-analysis
The null genotype of GSTT1 was associated with a significantly increased risk of PTD when compared with present genotype (OR = 1.18; 95% CI 1.05-1.33; I 2 = 33; Figure 1). In the subgroup analysis according to ethnicity, significantly increased PTD risk was observed in Asians (OR = 1.20; 95% CI 1.01-1.33; I 2 = 0%) but not in Caucasians (OR = 1.32; 95% CI 0.89-1.97; I 2 = 77). As shown in Figure 2, significant associations were evident with each addition of more data over time. The results showed that the pooled ORs tended to be stable. A single study involved in the meta-analysis was deleted each time to reflect the influence of the individual data set to the pooled ORs, and the corresponding pooled ORs were not materially altered (Figure 3). Funnel plot and Egger’s test were performed to assess the publication bias of literatures. Figure 4 shows the funnel plot for the assessment of publication bias. The shape of the funnel plot did not reveal any evidence of obvious asymmetry (Figure 4). Egger’s test did not find the evidence of publication bias (P = 0.13).
Figure 1.
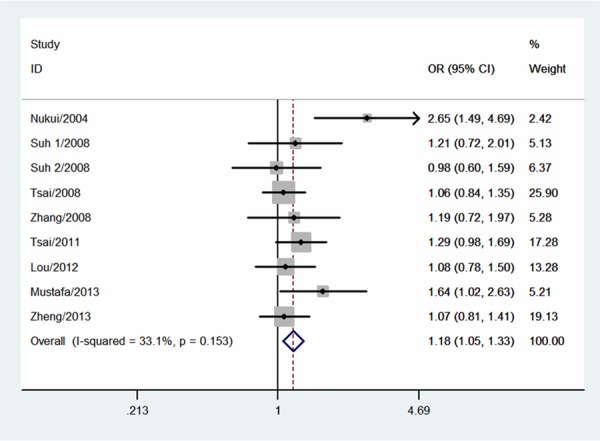
Meta-analysis for the association between GSTT1 null genotype and risk of PTD.
Figure 2.
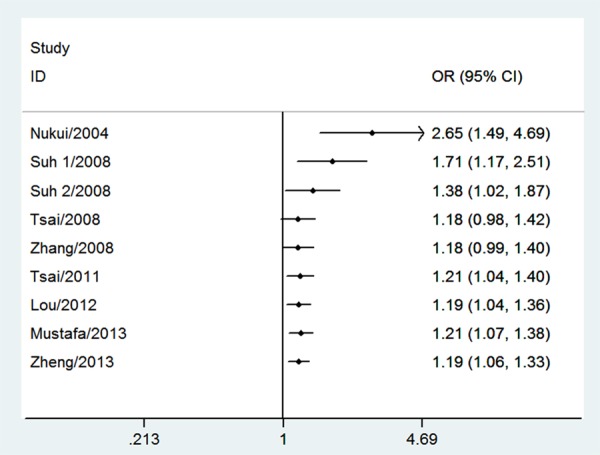
Cumulative meta-analysis of association between GSTT1 null genotype and risk of PTD.
Figure 3.
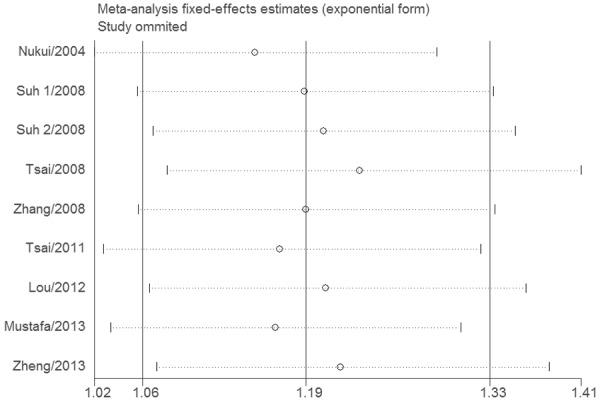
Sensitivity analysis of association between GSTT1 null genotype and risk of PTD.
Figure 4.
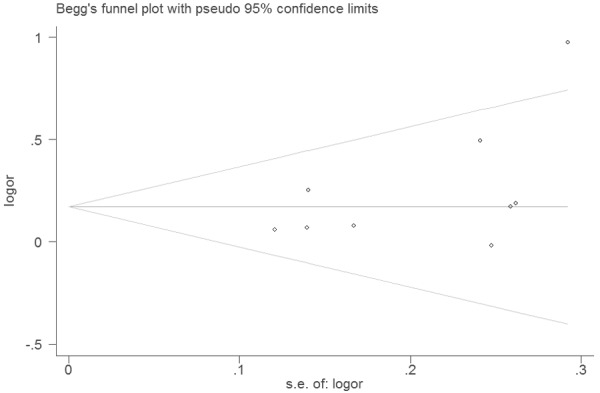
Funnel plot of associations between GSTT1 null genotype and risk of PTD.
Discussion
We performed a systematic search of the literature and combined the available results in this meta-analysis. GSTT1 null genotype has been studied extensively about the relationship with PTD [2-10]. Previous results of the studies on the relationship between GSTT1 null genotype and PTD risk were contradictory. These inconsistent results were possibly because of the low statistical powers of individual studies. Consequently, the meta-analysis was needed to provide a quantitative approach for combining the results of various studies with the same topic. To our knowledge, this was the first meta-analysis which investigated the association between the GSTT1 null genotype and risk of PTD.
We found that GSTT1 null genotype was a risk factor for developing PTD. The results suggested that carriers of GSTT1 null genotype had an 18% increased PTD risk. In the subgroup analysis by ethnicity, we noted that Asians carrying GSTT1 null genotype had an increased PTD risk, while Caucasians carrying GSTT1 null genotype did not have an increased PTD risk. On the one hand, it was possible that this difference might be affected by exposure to various environmental factors. However, no reported article was performed to assess the effect of GSTT1-environment interactions in different ethnicities. In the future, more studies should be designed to analyze these associations. On the other hand, it was possible that considerable heterogeneity (I 2 = 77%) may have distorted the result. More studies with Caucasians are still needed to evaluate the effect on PTD risk through the GSTT1 null genotype.
Oxidative stress has been related to PTD. Weber et al. found that the lowest quintile of retinol in cord blood was associated with an increased risk for PTD [12]. Pathak and colleagues showed that higher levels of some of the organochlorine pesticides may be associated with increased oxidative stress and PTD. Oxidative stress may causes oxidative DNA damage in fetal tissues and can lead to PTD by causing a reduction in placental blood flow. Previous studies have shown that individuals with GSTT1 null genotype have an impaired detoxification. Thus, impaired GSTT1 function may lead to serious DNA damage. It is biologically plausible that the GSTT1 null genotype may increase risk of PTD.
Heterogeneity is a potential problem that may affect the interpretation of the results. However, no significant heterogeneity existed in this meta-analysis. In addition, funnel plots and Egger’s tests did not find potential publication bias. Results from one-way sensitivity analysis and cumulative meta-analysis suggested high stability and reliability of our results. All together, these results suggested that results of this meta-analysis were reliable.
Some limitations should be addressed. First, there was only one case-control study investigated the association of GSTT1 null genotype and risk of PTD in Africans. Therefore, more studies with large sample sizes are needed to further identify the association among African. Second, because small negative studies are less likely to published, the possibility of publication bias cannot be ruled out completely, even though the Egger’s test and funnel plots did not provide the evidence of publication bias in this meta-analysis. Third, a lack of original data from the eligible studies limited evaluation of the effects of the gene-gene and gene-environment interactions during PTD development.
Conclusion
In conclusion, this meta-analysis suggested that GSTT1 null genotype was associated with increased PTD risk. Further studies with large sample size were needed to confirm our findings.
Disclosure of conflict of interest
None.
References
- 1.Moster D, Lie RT, Markestad T. Long-term medical and social consequences of preterm birth. N Engl J Med. 2008;359:262–273. doi: 10.1056/NEJMoa0706475. [DOI] [PubMed] [Google Scholar]
- 2.Nukui T, Day RD, Sims CS, Ness RB, Romkes M. Maternal/newborn GSTT1 null genotype contributes to risk of preterm, low birthweight infants. Pharmacogenetics. 2004;14:569–576. doi: 10.1097/00008571-200409000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Suh YJ, Ha EH, Park H, Kim YJ, Kim H, Hong YC. GSTM1 polymorphism along with PM10 exposure contributes to the risk of preterm delivery. Mutat Res. 2008;656:62–67. doi: 10.1016/j.mrgentox.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 4.Suh YJ, Kim YJ, Park H, Park EA, Ha EH. Oxidative stress-related gene interactions with preterm delivery in Korean women. Am J Obstet Gynecol. 2008;198:541, e1–7. doi: 10.1016/j.ajog.2007.11.013. [DOI] [PubMed] [Google Scholar]
- 5.Tsai HJ, Liu X, Mestan K, Yu Y, Zhang S, Fang Y, Pearson C, Ortiz K, Zuckerman B, Bauchner H, Cerda S, Stubblefield PG, Xu X, Wang X. Maternal cigarette smoking, metabolic gene polymorphisms, and preterm delivery: new insights on GxE interactions and pathogenic pathways. Hum Genet. 2008;123:359–369. doi: 10.1007/s00439-008-0485-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang M, Chen Y, Dong S, Liu Y, Jin Y. Polymorphisms of GSTM1, GSTT1 and CYP1A1 genes in mothers and neonates related to susceptibility to preterm delivery. Wei Sheng Yan Jiu. 2008;37:155–158. [PubMed] [Google Scholar]
- 7.Tsai HJ, Hong X, Chen J, Liu X, Pearson C, Ortiz K, Hirsch E, Heffner L, Weeks DE, Zuckerman B, Wang X. Role of African ancestry and gene-environment interactions in predicting preterm birth. Obstet Gynecol. 2011;118:1081–1089. doi: 10.1097/AOG.0b013e31823389bb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luo YJ, Wen XZ, Ding P, He YH, Xie CB, Liu T, Lin JM, Yuan SX, Guo XL, Jia DQ, Chen LH, Huang BZ, Chen WQ. Interaction between maternal passive smoking during pregnancy and CYP1A1 and GSTs polymorphisms on spontaneous preterm delivery. PLoS One. 2012;7:e49155. doi: 10.1371/journal.pone.0049155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mustafa MD, Banerjee BD, Ahmed RS, Tripathi AK, Guleria K. Gene-environment interaction in preterm delivery with special reference to organochlorine pesticides. Mol Hum Reprod. 2013;19:35–42. doi: 10.1093/molehr/gas039. [DOI] [PubMed] [Google Scholar]
- 10.Zheng X, Feingold E, Ryckman KK, Shaffer JR, Boyd HA, Feenstra B, Melbye M, Marazita ML, Murray JC, Cuenco KT. Association of maternal CNVs in GSTT1/GSTT2 with smoking, preterm delivery, and low birth weight. Front Genet. 2013;4:196. doi: 10.3389/fgene.2013.00196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weber D, Stuetz W, Bernhard W, Franz A, Raith M, Grune T, Breusing N. Oxidative stress markers and micronutrients in maternal and cord blood in relation to neonatal outcome. Eur J Clin Nutr. 2014;68:215–222. doi: 10.1038/ejcn.2013.263. [DOI] [PubMed] [Google Scholar]
- 13.Pathak R, Suke SG, Ahmed T, Ahmed RS, Tripathi AK, Guleria K, Sharma CS, Makhijani SD, Banerjee BD. Organochlorine pesticide residue levels and oxidative stress in preterm delivery cases. Hum Exp Toxicol. 2010;29:351–358. doi: 10.1177/0748233710363334. [DOI] [PubMed] [Google Scholar]


