Abstract
The majority of endometrioid endometrial carcinomas (EEC) is diagnosed at stage I. Among these, 30% present myometrial invasion (stage IB), which is associated with tumor spread and relapse after primary treatment. Although an increased expression of RUNX1/AML1 and ERM/ETV5 in EEC have been suggested to be associated with early events of myometrial infiltration, there is no data regarding its expression along the evolution of EEC and possible associations with other clinicopathological parameters. Therefore, ERM/ETV5 and RUNX1/AML1 protein and gene expression profiles were assessed in different EEC stages to evaluate their role in endometrial carcinogenesis. RUNX1/AML1 and ERM/ETV5 proteins were analyzed by immunohistochemistry in 219 formalin fixed paraffin embedded endometrioid tumors and in 12 normal atrophic and proliferative endometrium samples. RUNX1/AML1 and ERM/ETV5 genes expression were analyzed by RT-qPCR. RUNX1/AML1 and ERM/ETV5 expression were decreased with increasing EEC stage, with a positive correlation between protein and gene expression for ERM/ETV5, but not for RUNX1/AML1. Both proteins were present in the nucleus of the tumor cells, whereas RUNX1/AML1, but not ERM/ETV5, was expressed in 7 out of 12 normal endometrial samples, with its expression being restricted to the cytoplasm of the positive cells. We concluded that there is a higher expression of ERM/ETV5 in early stages of EEC, whereas there seems to be a RUNX1/AML1 translocation from cytoplasm to nucleus in EEC neoplastic transformation.
Keywords: endometrioid endometrial carcinoma, myometrial invasion, ERM/ETV5, RUNX1/AML1, differential gene expression
Introduction
Endometrial cancer is the most common gynecologic malignancy among women, with 189 000 new cases and 45 000 related deaths occurring each year.1 Obesity, persistent anovulatory cycles, nulliparity, and exogenous estrogen exposure are etiologically associated with the disease.2-4 Surgery is the main treatment for endometrial cancer, and adjuvant therapy can be applied according to specific tumor features, such as myometrial invasion.
Tumors confined in the corpus uteri (stage I) can be classified in stage IA and stage IB,5 according to myometrial invasion depth, with 30% of the cases possessing over 50% of myometrial invasion (stage IB).6,7 Myometrial invasion is considered an independent predictive outcome factor and deep invasion is frequently associated with poorly differentiated tumors, lymph node metastasis, high rates of recurrence, and decreasing overall survival.7,8
The vast majority (about 90%) of endometrial tumors are adenocarcinomas, stratified in type I or II based on histologic characteristics and clinical behavior.9 Endometrioid tumors (Type I) (EEC) represent 80% to 90% of cases, arise from previous hyperplasia, are well-differentiated, related to obesity and estrogen exposure, and have generally favorable prognosis.10 PTEN inactivation is the most frequent genetic alteration and mutations in KRAS (around 20%) and CTNNB1 (around 30%) are also frequently observed in EEC.11-14
ERM/ETV5 and RUNX1/AML1 expression have been suggested to be associated with deep myometrial invasion in EEC.15,16 ERM/ETV5 is a transcription factor that has a conserved aminoacid sequence responsible for a DNA-binding domain that regulates the expression of a variety of genes, such as stromelysin-1, vimentin, and ICAM-1.17,18RUNX1/AML1 is an oncogene related to acute myeloid leukemia that induces cell proliferation through increased transcription of genes involved in G1-S transition phase.19,20 Furthermore, RUNX1/AML1 expression has been associated with metastatic adenocarcinomas from several tissues, such as uterus, ovaries, and breast.21
Therefore, ERM/ETV5 and RUNX1/AML1 protein and gene expression profiles were analyzed in a large series of endometrioid tumors in order to evaluate their association with neoplastic progression, etiology, and prognosis.
Results
Clinicopathological features
Table 1 shows the clinicopathological characteristics of patients with EEC, with most of them presenting hypertension (69.9%), obesity or overweight (83.9%), and stage I (71.7%), particularly stage IA (51.6%) tumors. The mean follow up period was 55 mo and relapse was observed in 13.2% of cases. Cancer-related death happened in 55.1% of recurrent cases and the overall survival was 86.3%, confirming that EEC possesses a good prognosis. Association of overall survival and disease-free survival with all clinicopathological data and assessed markers were performed. The only variable associated with decreased overall survival (P = 0.01) and recurrence (P = 0.001) was tumor stage.
Table 1. Clinicopathological characteristics of 219 patients diagnosed with endometrioid endometrial adenocarcinoma (EEC).
| Characteristics | Patients (%) |
|---|---|
| Median age (years) | 63 |
| Variation | 42–89 |
| Hypertension | |
| Yes | 153 (69.9) |
| No | 66 (30.1) |
| Diabetes | |
| Yes | 55 (25.1) |
| No | 164 (74.9) |
| Heart diseases | |
| Yes | 17 (7.8) |
| No | 202 (92.2) |
| Obesity | |
| Yes | 111 (50.7) |
| No | 106 (48.4) |
| Overweight | |
| Yes | 71 (32.4) |
| No | 146 (66.7) |
| Nulliparity | |
| Yes | 29 (13.2) |
| No | 185 (84.5) |
| Contraceptive use | |
| Yes | 79 (36.1) |
| No | 113 (51.6) |
| Exogenous estrogen therapy | |
| Yes | 15 (6.8) |
| No | 175 (79.9) |
| FIGO stage | |
| IA | 113 (51.6) |
| IB | 44 (20.1) |
| II | 27 (12.3) |
| III | 29 (13.2) |
| IV | 6 (2.7) |
| Histological grade | |
| Well differentiated (G1) | 67 (30.6) |
| Moderate differentiated (G2) | 91 (41.6) |
| Poorly differentiated (G3) | 61 (27.9) |
| Recurrence | |
| Yes | 29 (13.2) |
| No | 190 (86.8) |
ETV5 detection profile
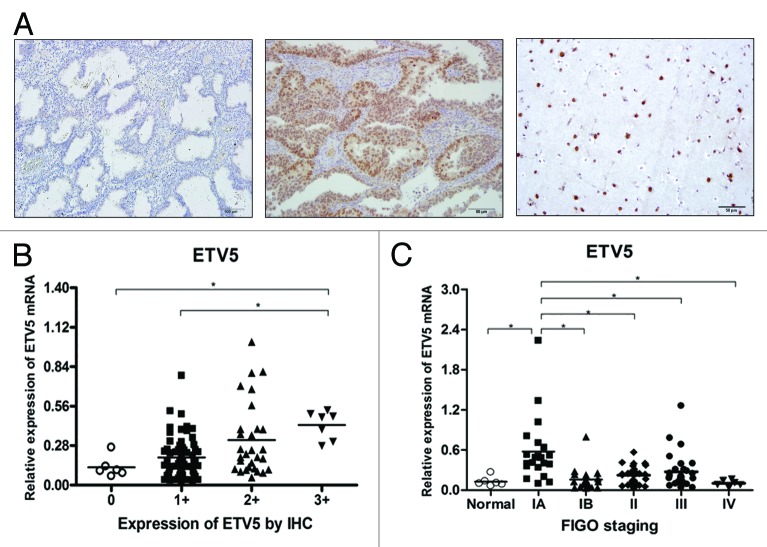
ERM/ETV5 protein profile was evaluated by immunohistochemistry (IHC) in normal endometrium and EECs, and was detected only in tumors. Figure 1A shows that the immunostaining was restricted to glandular epithelial tumor cells, observed predominantly in nucleus. ERM/ETV5 protein was not detected in the stromal compartment. The staining score evaluation revealed that most tumors expressed low levels of ERM/ETV5, with 53.4% of tumors (117 cases) considered as score 1+; 33.8% (74 cases) as score 2+, and only 12.8% (28 cases) as score 3+. However, among the latter, 22 cases (79%) were stage IA tumors, resulting in a positive association between high expression of ERM/ETV5 and IA stage tumors (Table 2). There was no association between ERM/ETV5 protein expression and any other clinicopathological characteristic.
Figure 1. (A) ERM/ETV5 protein expression by IHC. Left, negative sample of normal endometrial epithelium. Center, EEC-positive sample. Right, brain tissue (positive control). (B) ERM/ETV5 mRNA expression by RT-qPCR, normalized to β-actin, in EEC and normal endometrial samples, according to protein expression score by IHC. ERM/ETV5 mRNA expression is higher in scored 3+ cases in comparison with normal endometrial epithelium and with 1+ scored samples (P = 0.0007). (C) ERM/ETV5 mRNA expression by RT-qPCR, normalized to β-actin, in EEC samples of patients with different tumor stages and in normal endometrial samples. ERM/ETV5 mRNA expression is higher in IA stage EEC in comparison with other EEC stage or normal endometrial epithelium samples (P < 0.0001).
Table 2. Frequency of different IHC staining scores of ERM/ETV5 expression in stage IA compared with other EEC stages.
| Stage (FIGO) | IHC staining score for ERM/ETV5 expression | P value | ||
|---|---|---|---|---|
| Score 1+ n (%) | Score 2+ n (%) | Score 3+ n (%) | ||
| IA | 49 (43.4) | 42 (37.2) | 22 (19.5) | |
| IB + II | 45 (63.4) | 22 (31.0) | 4 (5.6) | 0.007 |
| III + IV | 23 (65.7) | 10 (28.6) | 2 (5.7) | 0.040 |
ERM/ETV5 gene expression was positive in all 105 EECs and in 6 normal endometrial samples analyzed (5 samples of proliferative and 1 of atrophic endometrium). There was a positive association between ERM/ETV5 gene and protein expression, as shown in Figure 1B. Furthermore, there was a statistically significant higher expression of ERM/ETV5 in IA stage tumors (P < 0.0001) when compared with other tumor stages and normal endometrial samples (Fig. 1C).
RUNX1 detection profile
RUNX1/AML1 protein profile was also evaluated and 58% of normal endometrial (7 out of 12 samples, with 3 proliferative and 4 atrophic endometrium) and 100% of tumor samples expressed this protein. Figure 2A shows that RUNX1/AML1 detection was restricted to glandular epithelial cells, with expression confined to the nucleus of tumor and to the cytoplasm of normal endometrial cells. The staining score evaluation of EECs showed an equal distribution, with 36.1% (79) of tumors classified as score 1+; 25.1% (55) as score 2+; and 38.8% (85) as score 3+. The score evaluation of the 7 normal endometrial samples showed that 2 were considered as score 1+, 2 as score 2+, and 3 as score 3+. There was no association between RUNX1/AML1 expression level and endometrial morphology.
Figure 2. (A) RUNX1/AML1 protein expression by IHC. From left to right, negative sample of normal endometrial epithelium, positive sample of normal endometrial epithelium (cytoplasmic staining), EEC-positive sample (nuclear staining), and placenta tissue (positive control). (B) RUNX1/AML1 mRNA expression by RT-qPCR, normalized to β-actin, in EEC and normal endometrial samples, according to protein expression score by IHC (concerning nuclear staining only). There was no significant difference of RUNX1/AML1 mRNA expression among stratified groups (P = 0.4589). (C) RUNX1/AML1 mRNA expression by RT-qPCR, normalized to β-actin, in EEC samples of patients with different EEC stages and in normal endometrial epithelium. RUNX1/AML1 mRNA expression is higher in IA stage EEC in comparison with other stage samples (P = 0.0002).
Differently from ERM/ETV5 protein expression, there was no association between RUNX1/AML1 protein expression and tumor stages, as shown in Table 3. Similarly, there was no association between RUNX1/AML1 expression and any other clinicopathological parameter evaluated in this study.
Table 3. Frequency of different IHC staining scores of RUNX1/AML1 expression in stage IA compared with other EEC stages.
| Stage (FIGO) | IHC staining score for RUNX1/AML1 expression | P value | ||
|---|---|---|---|---|
| Score 1+ n (%) | Score 2+ n (%) | Score 3+ n (%) | ||
| IA | 47 (41.6) | 23 (20.4) | 43 (38.1) | |
| IB + II | 23 (32.4) | 19 (26.8) | 29 (40.8) | 0.398 |
| III + IV | 9 (25.7) | 13 (37.1) | 13 (37.1) | 0.087 |
RUNX1/AML1 gene expression was positive in all cases of EEC and in 6 samples of normal endometrial epithelium evaluated (5 cases of proliferative endometrium and 1 of atrophic endometrium). Figure 2B shows that there was no association (P = 0.4589) between RUNX1/AML1 gene and its nuclear protein expression (samples of normal epithelium were considered negative since RUNX1/AML1 was expressed only in cytoplasm of normal endometrial cells). However, Figure 2C shows that, differently from its protein expression, there was a significantly higher expression of RUNX1/AML1 in IA stage tumors (P = 0.0002) when compared with other tumor stages or normal endometrial samples.
Discussion
The present study demonstrated that ERM/ETV5 is expressed in EEC, but not in normal endometrium cells, whereas RUNX1/AML1 is expressed in both tumor and normal cells. Both genes are highly expressed in the early development of EEC, but were not associated with other clinicopathological parameters. Recurrence and decreased overall survival were associated with EEC advancing stages only.
We observed a positive association between ERM/ETV5 expression and IA stage EEC (just before myometrial invasion). In contrast, Planaguma et al. previously suggested that the highest expression of ERM/ETV5 was present in IC stage tumors (classified as IB in FIGO 2009 staging criteria, adopted in this study).15 ERM/ETV5 can act as nuclear effector of RAS-MAPK signaling pathway and regulates oncogenic target gene transcription.14,22,23 Furthermore, RAS signaling pathway alterations were also observed in premalignant lesions and are considered early events in endometrial carcinogenesis,24 supporting the role of ERM/ETV5 in the early stages of EEC. In fact, ERM/ETV5 seems to play a central role in inducing the epithelial-mesenchymal transition.20,25,26
However, different from Planaguma et al.,16 we did not detect ERM/ETV5 expression in atrophic endometrium. The previous study reported that ERM/ETV5 expression occurred preferentially in cytoplasm, with occasional expression in the nucleus. In our study, ETV5 expression was observed predominantly in the nucleus of glandular epithelial cells. This difference may be explained by the use of an antigen retrieval buffer with alkaline pH. During the process of antigen recovery, heat promotes denaturation of linked proteins and the different types of buffer sustain their conformation. These data show that ETV5 denatured protein conformation, once located in the nucleus, is more stable in an alkaline pH environment, what allows a proper reaction with the antibody. Once ERM/ETV5 is a transcription factor, the nuclear expression pattern seems to be more representative of the role of the protein.
Curiously, ERM/ETV5 has also been shown to be upregulated in early stages of breast tumors,21,27 suggesting that tumors which are etiologically associated with estrogen may require ERM/ETV5 expression during early neoplastic transformation. Estrogens may act through diverse mechanisms and c-Jun can regulate estrogen-dependent growth and differentiation.28 Nakae et al. demonstrated that c-Jun binds to a proline enriched region at the carboxi-terminal activation site of ERM/ETV5. This event increases its transcriptional activity by a feasible neutralization of ERM/ETV5 negative controlling domain in its transactivation domain.29 Moreover, Fugimoto et al. demonstrated that c-Jun mRNA expression is higher in endometrial tumors when compared with normal endometrial epithelium.30 So, the increased ERM/ETV5 protein profile described in our study may be associated with c-Jun expression. Nevertheless, we did not test this hypothesis, but we did not detect an association between exogenous estrogen exposure and a higher expression of ERM/ETV5.
The present study also showed that RUNX1/AML1 protein was detected in the nucleus of all tumors and in the cytoplasm of 58.3% of normal endometrial epithelium samples, suggesting that RUNX1/AML1 upregulation in the nuclear compartment can trigger endometrial carcinogenesis. RUNX1/AML1 had been previously detected in the perinuclear region of normal endometrial cells and mostly in the cytoplasm of EEC cells.15 Again this difference may be explained by the use of an antigen retrieval buffer with alkaline pH. RUNX1/AML1 protein location has been previously demonstrated to occur in the nucleus of leukemic cells,31 supporting our observation that RUNX1/AML1 nuclear translocation occurs in endometrial carcinogenesis.
RUNX1/AML1 mRNA was induced in IA stage tumors, confirming previous results from Planaguma et al. that RUNX1/AML1 gene overexpression is an early event of endometrial carcinogenesis.15 However, whereas we demonstrated that the highest expression occurred in IA stage tumors (just before deep myometrial invasion), those authors observed the highest expression present in IC (IB according to the FIGO classification adopted in our study) tumors. Furthermore, we did not observe an association between RUNX1/AML1 protein and its gene expression. In fact, RUNX1 activity can be controlled by posttranslational modifications such as phosphorylation, acetylation, and ubiquitination,32-34 and recent study has shown that this gene may also be regulated by microRNA-27.35
The reason for the high expression of RUNX1/AML1 in EEC is not known, but Wall et al. showed that estradiol induced the expression of this protein in estradiol-responsive mice.36 However, as for ERM/ETV5, we did not find a positive association between exogenous estrogen exposure and high levels of RUNX1/AML1. Alternatively, this study suggests that RUNX1/AML1 expression is posttransductionally regulated and alterations in protein translation in EECs may lead to the high levels observed in the nuclei of endometrial tumor cells.
We conclude that ERM/ETV5 protein and its gene overexpression, and RUNX1/AML1 nuclear protein expression are common features for early development of EEC.
Materials and Methods
Patients and samples
The study comprised 219 patients with a confirmed histologically diagnosis of endometrioid endometrial carcinoma (EEC) who had undergone surgical treatment between 2007 and 2009 in the Brazilian National Cancer Institute (Rio de Janeiro, Brazil). Histological diagnosis was confirmed by two independent pathologists after surgical treatment. We also included 12 patients who underwent total hysterectomy for diagnosis different from endometrial cancer in the same period (they donated 6 samples of atrophic endometrium and 6 samples of proliferative endometrium). Clinicopathological data were collected from their medical records, including age at diagnosis, previous history of cancer in family, comorbidities (hypertension, heart disease, obesity, and overweight), nulliparity, oral contraceptive use, exogenous estrogen therapy, tumor staging (according to the International Federation of Gynecology and Obstetrics 2009 criteria), histological grade, recurrence, and death. The follow-up period was 55 ± 14 mo. Patients who had not undergone surgery as prime treatment, who had suspicions of hereditary cancer syndrome such as Lynch syndrome, and those who had a diagnosed second primary tumor were excluded. Tumor and normal endometrial samples were formalin-fixed paraffin-embedded (FFPE). The protocol was approved by the Institutional Ethics Committee and all patients signed a consent form.
Immunohistochemistry
Immunohistochemistry (IHC) was performed on 3 μm paraffin sections of 219 EEC cases and in 12 normal endometrial samples. For ERM/ETV5 antigen retrieval, sections were incubated in water bath while submerged in a target buffer solution (DAKO®), pH 9.0, for 40 min at 98 °C. For RUNX1/AML1 antigen retrieval, sections were incubated in a pressure cooker while submerged in a tris-EDTA (Tris 10 mM, EDTA 1 mM) buffer solution, pH 9.0, for 3 min at 120 °C. Sections were incubated with the primary polyclonal antibody against ERM/ETV5 (Santa Cruz Biotechnology®, sc-22807; diluted 1:300 in diluent solution) and with the primary monoclonal antibody against RUNX1/AML1 (Abcam®, ab54869, diluted 1:2000 in diluent solution), during 12 h at least. FFPE brain and placenta tissues served as positive controls of ERM/ETV5 and RUNX1/AML1, respectively. For a negative control, the primary antibody was replaced with the diluent solution. The detection system was the NovoLinkTM Max Polymer Detection System (Leica Biosystems®), following the protocol described by the manufacturer, using diaminobenzidine as substrate (DAKO®). Sections were counterstained with Harris’ hematoxylin.
The staining score evaluation was performed by two independent pathologists. For both proteins, scored cases were considered 1+ when positive staining was present in up to 35% of tumor region; 2+ when staining was present in >35% and ≤70% of tumor region; and 3+ when >70% of tumor region was positive.
RT-qPCR
In order to validate the differential expression of both genes, FFPE of EEC and normal endometrial epithelium samples were sectioned in 10 μm. Total RNA was extracted using PureLinkTM FFPE Total RNA Isolation Kit (Invitrogen®), following the protocol described by the manufacturer. RNA was isolated in column and in 50 μL of 65 °C preheated RNase free water. All RNA samples were quantified by spectrophotometry. One hundred and five of 219 RNA EEC samples and 6 of 12 normal endometrial epithelium samples had satisfactory amount of RNA for performing cDNA synthesis. All samples cDNA was synthesized from 1 μg RNA by the SuperScriptTM II Reverse Transcriptase (Invitrogen®) protocol.
ERM/ETV5 and RUNX1/AML1 expression analysis was performed using Rotor-Gene Q (Qiagen®) thermocycler with the following primers spanning exon-exon junctions as follows: ETV5-Sense: 5′ ACTGGAAGGC AAAGTCAAAC A 3′, ETV5-Anti-Sense: 5′ GCTGGGTCAT CAAGAAGGGT GA 3′; RUNX1-Sense: 5′ AACCCTCAGC CTCAGAGTCA 3′, RUNX1-Anti-Sense: 5′ ACAGAAGGAG AGGCAATGGA 3′; β-actin Sense: F: 5′ CCAGATCATG TTTGAGACCT T 3′, β-actin Anti-Sense: 5′ CGGAGTCCAT CACGATGCCA G 3′. Each reaction consisted of 7.5 μL of Quantifast SYBR Green PCR Master Mix (Qiagen®), 25 pmols of primers, and 1 μL of cDNA. The amplification reaction was performed as follows: 5 min for DNA predenaturation at 95 °C, followed by 40 cycles of hybridization and complementary chain synthesis for 5 s at 95 °C and 10 s at 60 °C.
ERM/ETV5 and RUNX1/AML1 gene expression was normalized by the expression of β-actin. For mRNA relative quantitation, ΔCt method was used and its parameter was defined as the difference between the average of the gene of interest (three experiments) and the housekeeping gene.
Statistical analysis
Frequencies of clinicopathological data and IHC staining score of ERM/ETV5 and RUNX1/AML1 were calculated. For continuous variables, we performed a descriptive analysis of central and dispersion tendencies. To assess the relationship between IHC staining score and clinicopathological features, we used the chi-square test. To assess the relationship among ERM/ETV5 and RUNX1/AML1 gene expression, FIGO staging and IHC staining score, we used Kruskal–Wallis test. The Kaplan–Meier method was used to evaluate overall survival and disease-free survival, based on a statistically significant confidence interval of 95% and P value < 0.05. Finally, in order to assess the impact of ERM/ETV5 and RUNX1/AML1 detection profile on overall survival and its statistical significance, Kaplan–Meier test was performed. Cox regression was performed with all clinicopathological parameters to adjust the effect of clinical stage and age. All statistical analysis was performed with GraphPad Prism 5.0 (GraphPad Software Inc.) and SPSS 17.0. The final values were considered of statistical significance when P < 0.05.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was supported by grants from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Brazil) and Fundação de Amparo a Pesquisa do Estado do Rio de Janeiro (FAPERJ, Brazil).
Glossary
Abbreviations:
- EEC
endometrioid endometrial carcinomas
- FFPE
formalin-fixed paraffin-embedded
- FIGO
International Federation of Gynecology and Obstetrics
- IHC
immunohistochemistry
- RT-qPCR
reverse transcription polymerase chain reaction
References
- 1.Dobrzycka B, Terlikowski SJ. Biomarkers as prognostic factors in endometrial cancer. Folia Histochem Cytobiol. 2010;48:319–22. doi: 10.2478/v10042-10-0061-8. [DOI] [PubMed] [Google Scholar]
- 2.Jaakkola S, Lyytinen HK, Dyba T, Ylikorkala O, Pukkala E. Endometrial cancer associated with various forms of postmenopausal hormone therapy: a case control study. Int J Cancer. 2011;128:1644–51. doi: 10.1002/ijc.25762. [DOI] [PubMed] [Google Scholar]
- 3.Cibula D, Gompel A, Mueck AO, La Vecchia C, Hannaford PC, Skouby SO, Zikan M, Dusek L. Hormonal contraception and risk of cancer. Hum Reprod Update. 2010;16:631–50. doi: 10.1093/humupd/dmq022. [DOI] [PubMed] [Google Scholar]
- 4.Dossus L, Rinaldi S, Becker S, Lukanova A, Tjonneland A, Olsen A, Stegger J, Overvad K, Chabbert-Buffet N, Jimenez-Corona A, et al. Obesity, inflammatory markers, and endometrial cancer risk: a prospective case-control study. Endocr Relat Cancer. 2010;17:1007–19. doi: 10.1677/ERC-10-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lewin SN. Revised FIGO staging system for endometrial cancer. Clin Obstet Gynecol. 2011;54:215–8. doi: 10.1097/GRF.0b013e3182185baa. [DOI] [PubMed] [Google Scholar]
- 6.Engelsen IB, Akslen LA, Salvesen HB. Biologic markers in endometrial cancer treatment. APMIS. 2009;117:693–707. doi: 10.1111/j.1600-0463.2009.02467.x. [DOI] [PubMed] [Google Scholar]
- 7.Uharcek P. Prognostic factors in endometrial carcinoma. J Obstet Gynaecol Res. 2008;34:776–83. doi: 10.1111/j.1447-0756.2008.00796.x. [DOI] [PubMed] [Google Scholar]
- 8.Abal M, Llauradó M, Doll A, Monge M, Colas E, González M, Rigau M, Alazzouzi H, Demajo S, Castellví J, et al. Molecular determinants of invasion in endometrial cancer. Clin Transl Oncol. 2007;9:272–7. doi: 10.1007/s12094-007-0054-z. [DOI] [PubMed] [Google Scholar]
- 9.Bokhman JV. Two pathogenetic types of endometrial carcinoma. Gynecol Oncol. 1983;15:10–7. doi: 10.1016/0090-8258(83)90111-7. [DOI] [PubMed] [Google Scholar]
- 10.Fader AN, Arriba LN, Frasure HE, von Gruenigen VE. Endometrial cancer and obesity: epidemiology, biomarkers, prevention and survivorship. Gynecol Oncol. 2009;114:121–7. doi: 10.1016/j.ygyno.2009.03.039. [DOI] [PubMed] [Google Scholar]
- 11.Bansal N, Yendluri V, Wenham RM. The molecular biology of endometrial cancers and the implications for pathogenesis, classification, and targeted therapies. Cancer Control. 2009;16:8–13. doi: 10.1177/107327480901600102. [DOI] [PubMed] [Google Scholar]
- 12.Zighelboim I, Goodfellow PJ, Gao F, Gibb RK, Powell MA, Rader JS, Mutch DG. Microsatellite instability and epigenetic inactivation of MLH1 and outcome of patients with endometrial carcinomas of the endometrioid type. J Clin Oncol. 2007;25:2042–8. doi: 10.1200/JCO.2006.08.2107. [DOI] [PubMed] [Google Scholar]
- 13.Peltomäki P. Deficient DNA mismatch repair: a common etiologic factor for colon cancer. Hum Mol Genet. 2001;10:735–40. doi: 10.1093/hmg/10.7.735. [DOI] [PubMed] [Google Scholar]
- 14.Moreno-Bueno G, Hardisson D, Sánchez C, Sarrió D, Cassia R, García-Rostán G, Prat J, Guo M, Herman JG, Matías-Guiu X, et al. Abnormalities of the APC/beta-catenin pathway in endometrial cancer. Oncogene. 2002;21:7981–90. doi: 10.1038/sj.onc.1205924. [DOI] [PubMed] [Google Scholar]
- 15.Planagumà J, Díaz-Fuertes M, Gil-Moreno A, Abal M, Monge M, García A, Baró T, Thomson TM, Xercavins J, Alameda F, et al. A differential gene expression profile reveals overexpression of RUNX1/AML1 in invasive endometrioid carcinoma. Cancer Res. 2004;64:8846–53. doi: 10.1158/0008-5472.CAN-04-2066. [DOI] [PubMed] [Google Scholar]
- 16.Planagumà J, Abal M, Gil-Moreno A, Díaz-Fuertes M, Monge M, García A, Baró T, Xercavins J, Reventós J, Alameda F. Up-regulation of ERM/ETV5 correlates with the degree of myometrial infiltration in endometrioid endometrial carcinoma. J Pathol. 2005;207:422–9. doi: 10.1002/path.1853. [DOI] [PubMed] [Google Scholar]
- 17.de Launoit Y, Baert JL, Chotteau A, Monte D, Defossez PA, Coutte L, Pelczar H, Leenders F. Structure-function relationships of the PEA3 group of Ets-related transcription factors. Biochem Mol Med. 1997;61:127–35. doi: 10.1006/bmme.1997.2605. [DOI] [PubMed] [Google Scholar]
- 18.Seth A, Watson DK. ETS transcription factors and their emerging roles in human cancer. Eur J Cancer. 2005;41:2462–78. doi: 10.1016/j.ejca.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 19.Doll A, Gonzalez M, Abal M, Llaurado M, Rigau M, Colas E, Monge M, Xercavins J, Capella G, Diaz B, et al. An orthotopic endometrial cancer mouse model demonstrates a role for RUNX1 in distant metastasis. Int J Cancer. 2009;125:257–63. doi: 10.1002/ijc.24330. [DOI] [PubMed] [Google Scholar]
- 20.Monge M, Colas E, Doll A, Gonzalez M, Gil-Moreno A, Planaguma J, Quiles M, Arbos MA, Garcia A, Castellvi J, et al. ERM/ETV5 up-regulation plays a role during myometrial infiltration through matrix metalloproteinase-2 activation in endometrial cancer. Cancer Res. 2007;67:6753–9. doi: 10.1158/0008-5472.CAN-06-4487. [DOI] [PubMed] [Google Scholar]
- 21.Chotteau-Lelièvre A, Révillion F, Lhotellier V, Hornez L, Desbiens X, Cabaret V, de Launoit Y, Peyrat JP. Prognostic value of ERM gene expression in human primary breast cancers. Clin Cancer Res. 2004;10:7297–303. doi: 10.1158/1078-0432.CCR-04-0593. [DOI] [PubMed] [Google Scholar]
- 22.Wasylyk B, Hagman J, Gutierrez-Hartmann A. Ets transcription factors: nuclear effectors of the Ras-MAP-kinase signaling pathway. Trends Biochem Sci. 1998;23:213–6. doi: 10.1016/S0968-0004(98)01211-0. [DOI] [PubMed] [Google Scholar]
- 23.Keld R, Guo B, Downey P, Cummins R, Gulmann C, Ang YS, Sharrocks AD. PEA3/ETV4-related transcription factors coupled with active ERK signalling are associated with poor prognosis in gastric adenocarcinoma. Br J Cancer. 2011;105:124–30. doi: 10.1038/bjc.2011.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dedes KJ, Wetterskog D, Ashworth A, Kaye SB, Reis-Filho JS. Emerging therapeutic targets in endometrial cancer. Nat Rev Clin Oncol. 2011;8:261–71. doi: 10.1038/nrclinonc.2010.216. [DOI] [PubMed] [Google Scholar]
- 25.Colas E, Muinelo-Romay L, Alonso-Alconada L, Llaurado M, Monge M, Barbazan J, Gonzalez M, Schoumacher M, Pedrola N, Ertekin T, et al. ETV5 cooperates with LPP as a sensor of extracellular signals and promotes EMT in endometrial carcinomas. Oncogene. 2012;31:4778–88. doi: 10.1038/onc.2011.632. [DOI] [PubMed] [Google Scholar]
- 26.Monge M, Colas E, Doll A, Gil-Moreno A, Castellvi J, Diaz B, Gonzalez M, Lopez-Lopez R, Xercavins J, Carreras R, et al. Proteomic approach to ETV5 during endometrial carcinoma invasion reveals a link to oxidative stress. Carcinogenesis. 2009;30:1288–97. doi: 10.1093/carcin/bgp119. [DOI] [PubMed] [Google Scholar]
- 27.Bertucci F, Houlgatte R, Benziane A, Granjeaud S, Adélaïde J, Tagett R, Loriod B, Jacquemier J, Viens P, Jordan B, et al. Gene expression profiling of primary breast carcinomas using arrays of candidate genes. Hum Mol Genet. 2000;9:2981–91. doi: 10.1093/hmg/9.20.2981. [DOI] [PubMed] [Google Scholar]
- 28.Shiozawa T, Miyamoto T, Kashima H, Nakayama K, Nikaido T, Konishi I. Estrogen-induced proliferation of normal endometrial glandular cells is initiated by transcriptional activation of cyclin D1 via binding of c-Jun to an AP-1 sequence. Oncogene. 2004;23:8603–10. doi: 10.1038/sj.onc.1207849. [DOI] [PubMed] [Google Scholar]
- 29.Nakae K, Nakajima K, Inazawa J, Kitaoka T, Hirano T. ERM, a PEA3 subfamily of Ets transcription factors, can cooperate with c-Jun. J Biol Chem. 1995;270:23795–800. doi: 10.1074/jbc.270.40.23795. [DOI] [PubMed] [Google Scholar]
- 30.Fujimoto J, Hori M, Ichigo S, Morishita S, Tamaya T. Clinical implication of fos and jun expressions and protein kinase activity in endometrial cancers. Eur J Gynaecol Oncol. 1995;16:138–46. [PubMed] [Google Scholar]
- 31.Ito Y. Oncogenic potential of the RUNX gene family: ‘overview’. Oncogene. 2004;23:4198–208. doi: 10.1038/sj.onc.1207755. [DOI] [PubMed] [Google Scholar]
- 32.Yamaguchi Y, Kurokawa M, Imai Y, Izutsu K, Asai T, Ichikawa M, Yamamoto G, Nitta E, Yamagata T, Sasaki K, et al. AML1 is functionally regulated through p300-mediated acetylation on specific lysine residues. J Biol Chem. 2004;279:15630–8. doi: 10.1074/jbc.M400355200. [DOI] [PubMed] [Google Scholar]
- 33.Wang S, Zhang Y, Soosairajah J, Kraft AS. Regulation of RUNX1/AML1 during the G2/M transition. Leuk Res. 2007;31:839–51. doi: 10.1016/j.leukres.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 34.Huang G, Shigesada K, Ito K, Wee HJ, Yokomizo T, Ito Y. Dimerization with PEBP2beta protects RUNX1/AML1 from ubiquitin-proteasome-mediated degradation. EMBO J. 2001;20:723–33. doi: 10.1093/emboj/20.4.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Feng J, Iwama A, Satake M, Kohu K. MicroRNA-27 enhances differentiation of myeloblasts into granulocytes by post-transcriptionally downregulating Runx1. Br J Haematol. 2009;145:412–23. doi: 10.1111/j.1365-2141.2009.07632.x. [DOI] [PubMed] [Google Scholar]
- 36.Wall EH, Hewitt SC, Liu L, del Rio R, Case LK, Lin CY, Korach KS, Teuscher C. Genetic control of estrogen-regulated transcriptional and cellular responses in mouse uterus. FASEB J. 2013;27:1874–86. doi: 10.1096/fj.12-213462. [DOI] [PMC free article] [PubMed] [Google Scholar]