Abstract
The intracellular tyrosine kinase Lyn mediates inhibitory receptor function in B cells and myeloid cells, and Lyn−/− mice spontaneously develop an autoimmune and inflammatory disease that closely resembles human systemic lupus erythematosus. TLR signaling pathways have been implicated in the production of anti-nuclear antibodies in SLE and mouse models of it. We used a conditional allele of Myd88 to determine whether the autoimmunity of Lyn−/− mice is dependent on TLR/MyD88 signaling in B cells and/or in dendritic cells (DCs). The production of IgG anti-nuclear antibodies, as well as the deposition of these antibodies in the glomeruli of the kidneys, leading to glomerulonephritis in Lyn−/− mice were completely abolished by selective deletion of Myd88 in B cells and the autoantibody production and glomerulonepritis were delayed or decreased by deletion of Myd88 in DCs. The reduced autoantibody production in mice lacking MyD88 in B cells or DCs was accompanied by a dramatic decrease of the spontaneous germinal center (GC) response, suggesting that autoantibodies in Lyn−/− mice may depend on GC responses. Consistent with this view, IgG anti-nuclear antibodies were absent if T cells were deleted (TCRβ−/− TCRδ−/− mice) or if T cells were unable to contribute to GC responses due to mutation of the adaptor molecule SAP. Thus, the autoimmunity of Lyn−/− mice was dependent on T cells and on TLR/MyD88 signaling in B cells and in DCs, supporting a model whereby DC hyperactivity combines with defects in tolerance in B cells to lead to a T cell-dependent systemic autoimmunity in Lyn−/− mice.
INTRODUCTION
The human autoimmune disease systemic lupus erythematosus (SLE) is characterized by production of autoantibodies against multiple self-antigens, of which nuclear autoantigens such as double-stranded (ds) DNA and ribonucleoproteins (RNPs) are predominant (1). A similar spontaneously developing autoimmunity characterized by anti-nuclear antibody production is seen in a variety of genetically determined mouse models, some of which are multigenic and others of which result from spontaneous or targeted mutations of known genes (2). One of the better studied of the latter category is the Lyn−/− mouse, which develops a highly penetrant autoimmune and inflammatory disease characterized by anti-dsDNA IgG antibodies and glomerulonephritis (3-5). Lyn is a Src-family protein tyrosine kinase that is required for the function of a number of inhibitory receptors on B cells and myeloid cells. In B cells, the functions of both the sialic acid-binding Ig superfamily member CD22 and of the inhibitory FcγRIIB depend on the ability of Lyn to phosphorylate tyrosines in their cytoplasmic tails, catalyzing the recruitment to the membrane of the inhibitory phosphatases SHP-1 and SHIP-1 (4, 6, 7). Autoimmunity of Lyn-deficient mice likely involves a combination of compromised tolerance of B cells due to loss of these inhibitory pathways, and hyperactivity of myeloid cells, which drive activation of T cells and inflammatory disease (8-11).
Like most human autoimmune diseases, lupus has a strong genetic susceptibility component that is multigenic in the great majority of patients (1, 12). Among the genes that contribute to lupus susceptibility in humans are genes encoding components of Lyn inhibitory pathways. For example, some individuals of European descent have a single nucleotide polymorphism in the 5’ untranslated region of the LYN gene that is mildly protective for development of lupus (odds ratio 0.80) (12). More impressively, loss-of-function alleles of SIAE, which encodes a sialic acid acetyl esterase that is necessary to create the ligand for CD22, contributes a large increase in susceptibility for lupus and several other autoimmune diseases (odds ratio ~8) in a small but significant fraction of individuals (13). Given that Lyn+/− mice exhibit a mild lupus phenotype in mice (14), it is possible that additional less frequent alleles of Lyn than those examined in GWAS analysis and/or alleles of genes encoding the other components of Lyn-dependent inhibitory pathways contribute significantly to lupus susceptibility in humans.
Recent studies in several mouse models of lupus have implicated TLR9 and TLR7 in the spontaneous production of anti-dsDNA and anti-RNP IgG, respectively (15). For example, MRL/lpr mice are protected from development of glomerulonephritis when combined with loss-of-function mutation of TLR7, either alone or in combination with mutation of TLR9 (16). Similarly, deletion of the TLR signaling component MyD88 prevents spontaneous lupus-like disease in Lyn-deficient mice (17). Conversely, the Yaa autoimmune accelerator locus of mice turns out to be a duplication onto the Y chromosome of a small region of the X chromosome that includes TLR7, resulting in elevated expression of TLR7 (18-20). The possible relevance of TLR7 and TLR9 to lupus-like autoimmunity was initially suggested by in vitro studies of Marshak-Rothstein and coworkers demonstrating a marked synergy for B cell activation in vitro between BCR engagement and TLR9 or TLR7 engagement (15). This synergy has been shown to operate in vivo as well (21). While those studies strongly suggest that the contribution of TLR7 and TLR9 to lupus-like autoantibody production is by their action in nucleic-acid recognizing B cells, TLR7 and TLR9 are also potent activators of dendritic cells (DCs) and moreover, induce type 1 interferon production by plasmacytoid DCs (22). A number of studies have implicated type 1 interferons in the pathogenesis of human lupus (23), so it is also possible that the nucleic acid-recognizing TLRs play key roles in DCs for the development or propagation of lupus-like autoimmunity.
Recently, we have used cell type-specific deletion of the key TLR signaling component MyD88 to dissect the cellular basis for TLR9 stimulation of antibody responses to immunization. We found that in vivo IgG responses to soluble protein-CpG oligonucleotide conjugates were dependent primarily on TLR9/MyD88 signaling in DCs, whereas IgG responses to virus-like particles or inactivated influenza virus particles were primarily dependent on TLR/MyD88 signaling in B cells (24). In this study, we have addressed the role of MyD88 signaling in B cells and in DCs for the spontaneous development of lupus-like autoimmunity in Lyn−/− mice. We have found that anti-dsDNA and anti-RNP IgG in this model were likely produced by germinal center responses that were highly dependent on TLR/MyD88 signaling in B cells. TLR/MyD88 signaling in DCs also played a major, although less essential role in autoantibody production. In contrast, TLR/MyD88 signaling in DCs was essential for accumulation of activated T cells and splenomegaly, reinforcing the notion that Lyn-deficient autoimmune disease has both autoantibody and inflammatory components and that TLR/MyD88 signaling contributes to both disease manifestations.
MATERIALS AND METHODS
Mice
B6 (000664; C57BL/6J) mice were from Jackson Laboratory. Lyn−/−, Myd88fl mice (B6.129P2-Myd88tm1Defr), Myd88fl/fl Cd11c-Cre and Myd88fl/fl Cd79a-Cre mice were previously described (25-27), and have been backcrossed onto the C57BL/6 background for at least ten generations. These mice were bred to generate Lyn−/− Myd88fl/fl,Lyn−/− Myd88fl/fl Cd79a-Cre or Lyn−/− Myd88fl/fl Cd11c-Cre used in this study. Sex-matched littermates were used in all experiments, and data were pooled and analyzed according to the age group. Sap−/− mice (28) were obtained from Dr. Pamela Schwartzberg, National Institutes of Health, Bethesda, MD. tcrβ−/−tcrδ−/− mice (29, 30) were from Jackson Laboratory. Animals were housed in specific pathogen-free animal facilities of UCSF or IBP under conditions that meet respective institutional and national guidelines. Animal use in each facility was approved by the respective Institutional Animal Care and Use Committee.
ANA immunofluorescence
Serum was diluted at 1:40 or 1:160 in PBS containing 1% FBS and applied to fixed and permeabilized Hep-2 ANA slides (Bio-Rad Laboratories). After overnight incubation at 4°C, anti-nuclear antibodies were detected by Alexa 488®-conjugated goat anti-mouse IgG (Fc γ fragment specific) (Jackson ImmunoResearch). DAPI was included in the last wash of slides. Slides were visualized with a regular fluorescence microscope under 40X objective lens and imaged with an Olympus digital camera. Nuclear regions were defined with DAPI staining, and the average fluorescence intensity of nuclear regions from Alexa 488 was quantified with Image J (National Institute of Health, USA).
Serum Ig and autoantibody measurement
Levels of serum IgM and IgG were measured using ELISA-based quantification kits (Bethyl Laboratories) according to the manufacturer's instructions. For anti-dsDNA Ig ELISA, 96-well flat-bottom plates (BD Falcon) were coated with 20 ng/ well of linearized pUC19 plasmid in 100 mM Tris-HCl (pH7.3). For anti-smRNP Ig ELISA, plates were coated with 2U/ml of Smith antigen ribonucleoprotein complex antigen (Sm/RNP Ag; Immunovision) in carbonate buffer, pH 9.6. After overnight incubation at 4°C, plates were blocked with PBS containing 1% BSA and 0.05% Tween 20 for 1 hour. Serially diluted sera were added to the plates and incubated for 2 hours at room temperature. After wash with PBS containing 0.05% Tween 20, auto-antibodies were detected by horseradish peroxidase (HRP)-conjugated goat anti-mouse IgM antibody (μ chain specific; Bethyl Laboratories) or anti-mouse total IgG (Fcγ fragment specific, Bethyl Laboratories). The assays were developed by adding HRP substrate 3,3’,5,5’-tetramethylbenzidine (TMB) (Vector Laboratories), and stopped by addition of 2N sulfuric acid. The absorbance at 450 nm was measured with a microplate reader (Spectra Max Plus; MDS Analytical Technologies). For total IgM or IgG, the concentration was calculated according to standard samples supplied by Bethyl Laboratories. For anti-dsDNA or anti-Sm/RNP Ig, the relative amount was represented as absorbance values at the same dilutions (1:40 for IgG and 1:160 for IgM, respectively).
Histology and immunofluorescence analysis
For kidney histological analysis, kidneys from both sides of a mouse were laterally cut in half, fixed in 10% formalin, embedded in paraffin and stained with hematoxylin and eosin (H&E) by the IBP Pathology Core. The presence and severity of nephritis was evaluated in a blinded fashion by one of the authors with stained sections that contained glomeruli away from the medulla. Scoring of glomerulonephritis and interstitial nephritis on a 0-3 scale (0=absent, 1=mild, 2=moderate, 3=severe) for glomeruli was based on glomerular size, glomerular hypercellularity, and presence of glomerular sclerosis, and for interstitial disease was based on the degree of inflammatory infiltrate and alteration in tissue architecture.
For histological analysis of the GC response, spleen samples from 5-7 month old mice were placed in Tissue Tek optimum cutting temperature compound (OCT) (Sakura) and were immediately frozen with a mixture of ethanol and dry ice. Cryostat sections (7 μm in thickness) were fixed in ice-cold acetone, and stained with biotin-conjugated rat anti-mouse IgD (eBioscience) and FITC-conjugated anti-mouse GL-7 (BD Bioscience). HRP-conjugated extravidin (Sigma) and alkaline phosphatase (AP) -conjugated anti-FITC (Jackson ImmunoResearch) were then used as secondary antibodies. Enzyme reactions were developed with conventional substrates for peroxidases (diaminobenzidine/H2O2; Sigma) and AP (Fast Red; Sigma). Sections were counterstained with hematoxylin (Fisher).
To detect C3 deposition, kidneys were placed in OCT and snap-frozen in methyl-butane chilled in liquid nitrogen. Cryostat sections (15μm in thickness) were fixed in ice-cold acetone, and stained with FITC-conjugated anti-mouse C3 (Cappel Laboratories) for one hour at room temperature. The average intensity of FITC in the glomerular regions was quantified by Image J.
Flow cytometry
Splenocytes were obtained by treating the organs with digestion medium (RPMI-1640, 25mM HEPES, 50 μg/ml Liberase™ (Roche) and 100μg/ml DNase I (Worthington)). Bone marrow cells were obtained by flushing tibia and femur with medium. Kidney cells were prepared as previously described (8). Briefly, kidneys were pressed through a 70 μm cell strainer (BD Falcon). After washing, the cells were resuspended in 33% Percoll solution and centrifuged at 2,000 rpm for 20 min at room temperature. Kidney-infiltrating cells were obtained from cell pellets. For staining for flow cytometry, single cell suspensions were blocked with anti-CD16/CD32 antibody, and then stained with fluorescent antibodies in ice-cold flow cytometry buffer (PBS supplemented with 2mM EDTA, 1% heat-inactivated FBS, and 0.02% sodium azide). The antibodies included FITC-labeled anti-Ly6G (1A8), anti-Ly6C (HK1.4), anti-CD44 (IM7), anti-GL-7, anti-CD43 (S7) antibodies, PE-labeled anti-CD86 (GL1), anti-PD-1(RMP1-30), anti-TCRβ (H57-597), anti-CD23 (B3B4) antibodies, PerCP-Cy5.5-labeled anti-F4/80 (BM8), anti-ICOS (7E.17G9), anti-IgM (RMM-1) antibodies, PE-Cy7-labeled anti-CD11b (M1/70), anti-CD95 (Jo2), anti-CD62L (MEL-14) antibodies, and APC or Alexa Fluro® 647-labeled anti-CD11c (HL3), anti-CD8 (53-6.7), anti-CD138 (281-2), anti-CD62L (MEL-14) antibodies, Alexa Fluro® 700-labeled anti-I-Ab(M5/114.15.2), anti-CD11b (M1/70), anti-CD45.2 (104) antibodies, APC-Cy7-labeled anti-B220 (RA3-6B2), anti-CD19 (6D5), Pacifica Blue-labeled anti-IgD (11-26c.2a). All fluorochrome-conjugated monoclonal antibodies were purchased from BD PharMingen, eBioscience or Biolegend. Staining with biotinylated anti-CD86 (GL1) or anti-CD93 (AA4.1) antibodies were following streptavidin-conjugated Pacific Orange (Invitrogen). After the final wash, the cells were resuspended in flow cytometry buffer containing 0.4μM 4',6-diamidino-2-phenylindole (DAPI) (Invitrogen). All data were collected on a LSRII flow cytometer (Becton Dickinson) and were analyzed with FlowJo software (TreeStar).
Immature, transitional, and mature B cell populations were identified in the spleen and bone marrow of mice using fluorescently conjugated antibodies against surface B220, CD23 (BD biosciences), CD93 (clone AA4.1, eBioscience), and IgM F(ab)’ monomer (Jackson Immunoresearch). In the spleen, surface CD93 was used to distinguish immature (B220+, CD93+) and mature (B220+, CD93−) B cells. Within the immature B cell population, surface CD23 and IgM levels were used to identify immature-transitional T1 (IgMhi, CD23lo-neg), T2 (IgMhi, CD23hi-int), and T3 (IgMlo, CD23hi-int). Within the mature B cell population, surface expression of CD23, CD21, IgM and CD43 were used to distinguish follicular (CD23+, CD21lo, IgMlo-int), marginal zone B cell (CD23lo-neg, CD21hi, IgMhi CD43−), and B1 B cells (CD23lo-neg, CD21neg-lo, IgMlo, CD43+). These markers were also used to distinguish immature and pro/pre-B cells (B220+, CD93+), and mature recirculating B cells (B220+ CD93−) in the bone marrow. Surface IgM and CD23 levels within the CD93+ population distinguish newly formed (NF) immature B cells (IgM+, CD23lo-neg), BM-T1 (IgMhi, CD23lo-neg) and BM-T2 (IgMhi, CD23int), and pro/pre-B cells (IgM−, CD23−). Absolute cell numbers within each population were back-calculated from total splenocytes, or total cells/femur and tibia.
RNA extractions and Quantitative RT-PCR
For quantifying cytokine expression, mouse spleens were harvested and snap frozen in liquid nitrogen. Total RNA was extracted with the RNeasy kit (QIAGEN) with on-column DNase digestion. cDNA was transcribed from total RNA with the iScript cDNA Synthesis Kit (Bio-Rad). The primer pairs for IL-12p40, IL-6, and BAFF were previously described (8). Transcripts were quantified by PCR with iTaq SYBR Green Supermix with ROX (Bio-Rad), and the levels of cytokine transcripts were normalized to the levels of HPRT mRNA.
Statistical analysis
Statistical significance for data with multiple groups was calculated with a one-way ANOVA, and if a significant difference was observed between groups, then the Bonferroni post-test was used to assess statistical difference between specific groups and either the wild type group or the Lyn−/− group. In cases where there was a large difference between the wild type group and the three groups with Lyn-deficiency (B cell number data, Fig. 3E, Fig. 6B, and Supplemental Fig. 1B), this analysis was inadequate to detect differences between the Lyn-deficiency groups since all were greatly different from wild type. In these cases, a secondary statistical analysis was performed with just the Lyn-deficiency groups. In the figures, this secondary statistical analysis is denoted by different symbols. When there were 3 such groups, this secondary analysis again employed the one-way ANOVA, but in cases where only two groups remained, then Mann-Whitney test was used. All P values of 0.05 or less were considered significant and are indicated on the figures.
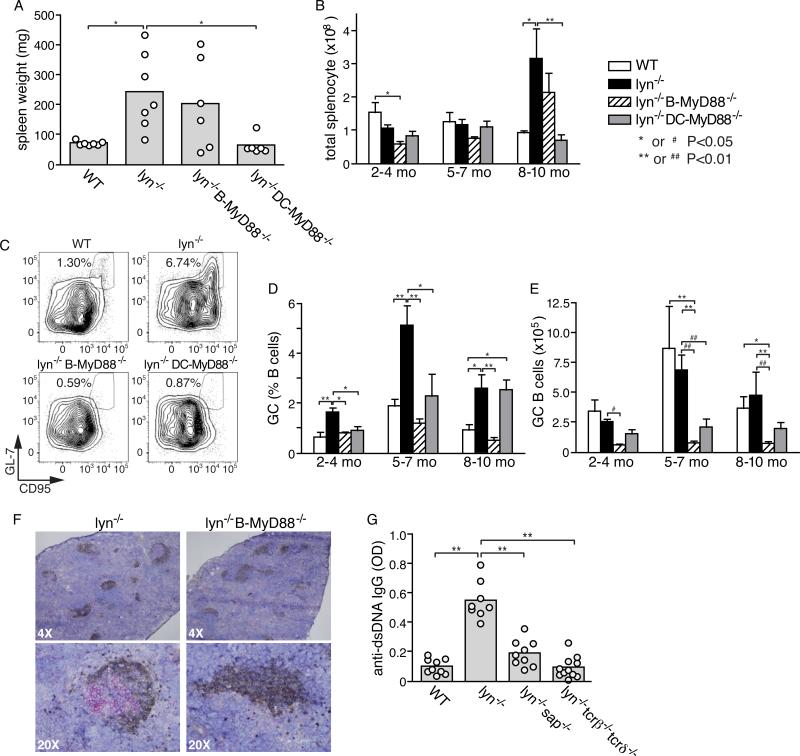
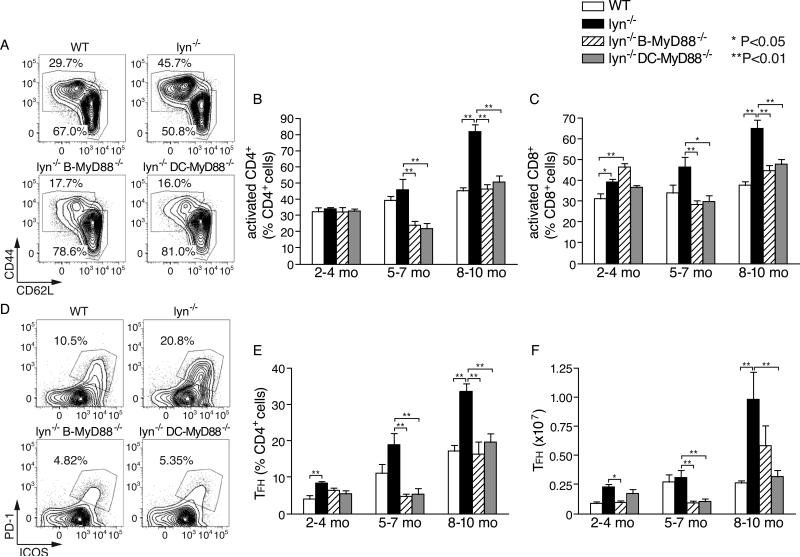
Figure 3. Role of germinal center response in autoantibody production by Lyn−/− mice and effect of ablation of MyD88 on germinal centers.
A and B, Effect of deletion of Myd88 in B cells or DCs for the splenic hypercellularity that accumulates over time in Lyn-deficient mice; shown are spleen weights of individual mice at 8-10 months of age for the indicated genotypes (A), and numbers of total splenocytes (mean±SE ) for mice of different age groups (B). C-E, analysis of germinal center B cells; representative flow cytometry profiles (C) and summarized data from different genotype mice of different ages, represented either as % of total splenic B cells that have a GC B cell phenotype (D) or as absolute number of GC B cells per spleen in the indicated mice (E). Data in D and E are expressed as mean±SE of five to eight mice per mouse group, and are representative of three separate experiments. F, representative immunohistological staining of germinal centers in the spleens of 5~7-month-old Lyn−/− and Lyn−/− B-MyD88−/− mice. IgD+ cells (brown) and GL-7+ cells (red) are shown with hematoxylin counterstaining. G, effect of genetic deletion of T cells or of the adaptor molecule SAP on anti-nuclear antibody production of Lyn−/− mice at 5-6 months of age. Shown are the relative amounts of anti-dsDNA IgG measured by ELISA in individual mice (circles) and means of each mouse group (bars). Primary statistical analysis by ANOVA is indicated by *=p<0.05 and **=p<0.01. As described in the Methods section, a secondary statistical analysis in which the wild type group was excluded was also performed on the data in E and is indicated by #=p<0.05 and ##=p<0.01.
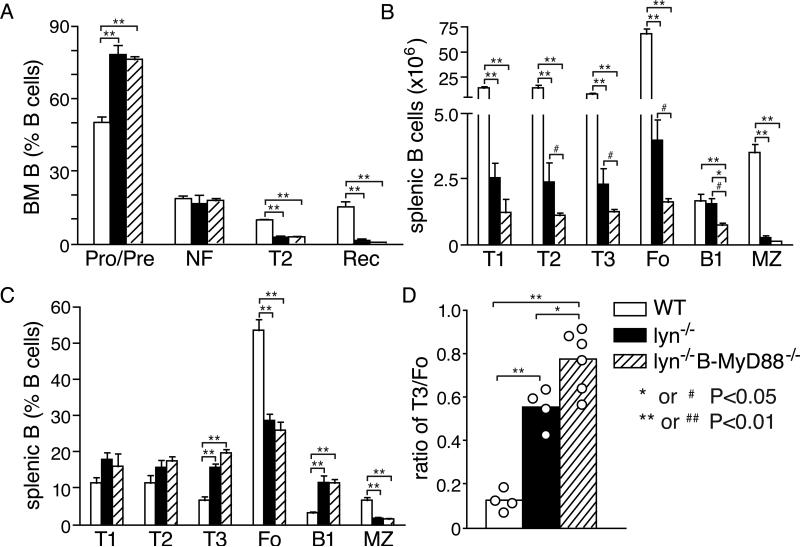
Figure 6. Effect of cell-intrinsic MyD88 signaling on B cell development in Lyn−/− mice.
Effect of ablation of Myd88 in B cells for the numbers of B cell precursors, immature B cells, and mature B cells in the bone marrow (A) or spleen (B, C) of 2-4 month old Lyn−/− mice. Also shown for comparison purposes are B cell populations from wild type mice. Shown are the percentage of bone marrow cells (A) that were CD19+CD93+IgMneg Pro- and Pre-B (Pro/Pre), CD19+CD93+IgM+CD23− newly-formed immature B (NF), CD19+CD93+IgM+CD23+ T2 B (T2) and CD19+CD93−IgM+CD23+ recirculating B cells. Also shown are the absolute numbers (B) and percentage (C) of CD19+CD93+IgM+CD23− T1 B (T1), CD19+CD93+IgMhiCD23+ T2 B (T2), CD19+CD93+IgMlowCD23+ T3 B (T3), CD19+CD93−IgM+CD23+ follicular B (Fo), CD19+, CD23lo-neg, CD21hi, IgMhi CD43− marginal zone (MZ), and CD19+, CD93−,CD23-CD21lo-neg, CD43+B1 B cells (B1) in the spleens. D, ratio of T3 B cells to follicular B cells in the spleens of Lyn−/− and Lyn−/−B-Myd88−/− mice. Data are expressed as mean±SE of four to six mice per group, and are representative of three separate experiments. Primary statistical analysis by ANOVA is indicated by *=p<0.05 and **=p<0.01. As described in the Methods section, a secondary statistical analysis in which the wild type group was excluded was also performed on the data in D and is indicated by #=p<0.05, ##=p<0.01.
RESULTS
Ablation of MyD88 signaling in either B cells or DCs attenuates autoimmunity in Lyn−/− mice
To examine the contribution of TLR signaling in B cells and DCs to the spontaneous autoimmunity caused by Lyn deficiency, mice with a conditional allele of Myd88 (Myd88fl) were crossed to Lyn−/− mice, and transgenic Cre alleles that express selectively in B cells (Cd79a-Cre) or in DCs (CD11c-Cre) were also introduced. Progenies of genotypes Lyn−/− Myd88fl/fl ,Lyn−/− Myd88fl/fl Cd79a-Cre or Lyn−/− Myd88fl/fl Cd11c-Cre (hereafter referred to as Lyn−/−, Lyn−/− B-Myd88−/−, and Lyn−/− DC-Myd88−/− mice, respectively) were generated and housed under specific pathogen-free (SPF) conditions, and examined for autoimmune phenotypes at different ages.
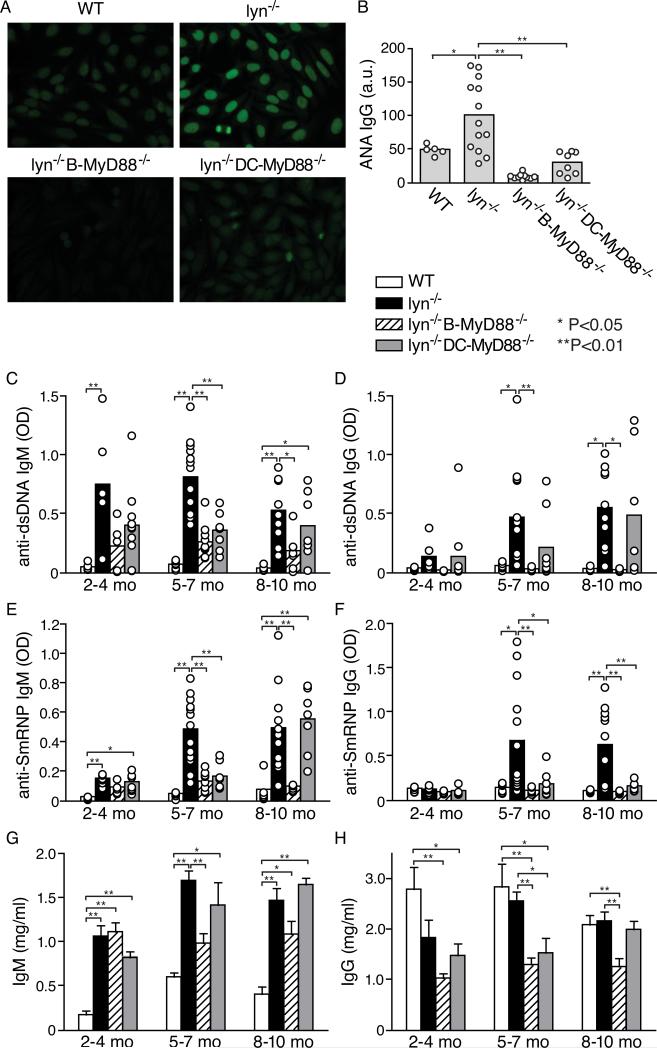
The development of autoantibodies by these mice was initially screened by anti-nuclear antibody (ANA) immunofluorescence with mouse sera collected at 5~7-month of age. As previously described (4, 5), most Lyn−/− mice exhibited elevated titers of ANAs by this age (Fig. 1A, 1B). The development of IgG ANAs was almost completely abolished in Lyn−/− B-Myd88−/− and was substantially attenuated in mice lacking MyD88 selectively in DCs (Fig. 1A, 1B).
Figure 1. Roles of MyD88 signaling in B cells and in DCs for development of autoantibodies in Lyn−/− mice.
A and B, anti-nuclear IgG antibodies of individual mice were screened using sera of 5~7-month-old mice. Representative ANA fluorescence images of 1:40 diluted sera of the indicated genotype are shown (A), as well as summarized relative fluorescence intensity of sera from individual mice (circles) and means of each mouse group (bars) are shown (B). C-F, serum IgM and IgG antibodies to dsDNA (C,D) and to anti-SmRNP (E,F) of individual mice of the four different genotypes indicated and of different age groups were measured by ELISA. The relative amount of autoantibody in each mouse (open circles) and the mean of each mouse group (bars) are shown. G and H, total serum IgM and IgG levels of mice of the different genotypes and age groups were quantified by ELISA. Data are presented as mean±SE of wt (n=6, 6, 6 mice, respectively in each age group), Lyn−/− (n=6, 13, 11), Lyn−/− B-MyD88−/− (n=6,10,7) and Lyn−/−DC-MyD8−/− mice (n=7, 8, 7). Differences between wild type or Lyn−/− and other groups are indicated by brackets and * or ** if statistical analysis as described in Methods revealed p<0.05 (*) or p<0.01 (**).
Next, we used ELISA to examine development of antibodies to dsDNA and smRNP, two major types of ANAs seen in SLE patients (1) as well as in animal models of SLE. All Lyn−/− mice developed IgM autoantibodies against both of these nuclear antigens, typically by 4 months of age, and a substantial majority also developed IgG anti-dsDNA and IgG anti-smRNP. Deletion of MyD88 in DCs delayed production of these autoantibodies, but 8-10 month old mice still had substantial titers of IgG and IgM anti-dsDNA and IgM anti-smRNP. In contrast, deletion of Myd88 in B cells prevented production of IgG autoantibodies in Lyn−/− B-Myd88−/− mice at all time points tested and also attenuated production of IgM autoantibodies (Fig. 1C-F). Total serum IgM and IgG levels were somewhat reduced in these mice compared to Lyn−/− mice (Fig. 1G, 1H), but the differences were generally 2-fold or less, indicating that there was a selective defect in production of autoantibodies.
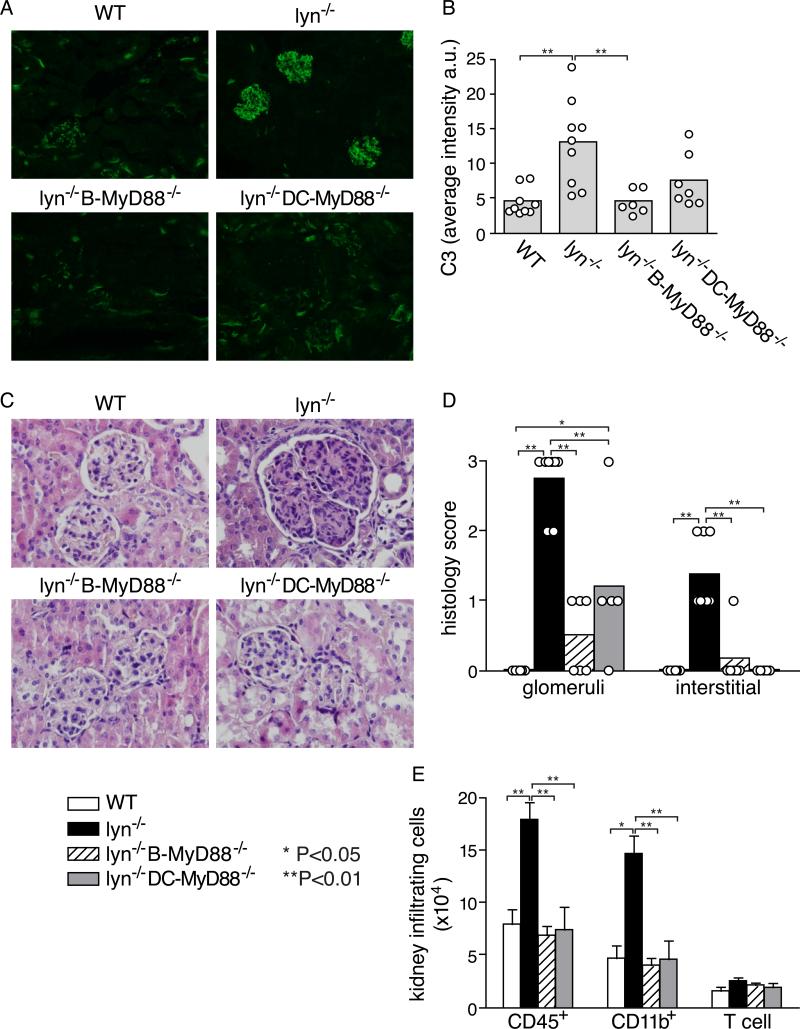
Anti-dsDNA IgG antibodies are often deposited in the glomeruli of the kidneys and can lead to glomerulonephritis, as has previously been observed in Lyn-deficient mice (8, 31). Indeed, immunofluorescent staining clearly revealed large amount of complement 3 (C3) deposition in the glomeruli of most of the 5~7-month-old Lyn−/− mice (Fig. 2A, 2B). In agreement with the almost complete abolishment of IgG autoantibodies in Lyn−/− B-Myd88−/− mice, C3 deposition was not evident in the glomeruli of these mice at a corresponding age. Moreover, the nephritis typically seen in old Lyn−/− mice by H & E staining of kidney tissue sections was substantially decreased but not completely absent in Lyn-deficient mice deleted for MyD88 in either B cells or DCs (Fig. 2C, 2D) and the numbers of infiltrating inflammatory cells (CD45+), mainly CD11b+ neutrophils and macrophages, were correspondingly reduced (Fig. 2E). The splenomegaly and myeloproliferation characteristic of aged Lyn−/− mice (> 6 months old) was corrected by deletion of Myd88 in DCs but apparently not by deletion of Myd88 in B cells (Fig. 3A, 3B, and Supplemental Fig. 1A). Together, these results are consistent with the observation that ablating MyD88 signaling in B cells or DCs reduces IgG autoantibody production in Lyn−/− mice, but also indicates that some of the inflammatory phenotypes are independent of autoantibody production.
Figure 2. Effect of ablation of MyD88 signaling in B cells and in DCs for complement deposition and inflammation in the kidneys of Lyn−/− mice.
A and B, deposition of C3 was detected by immunofluorescent staining of frozen kidney sections of 5-7 month old mice of the indicated genotype; representative images are shown (A) along with summarized data (B). C, kidney sections of the different genotype mice at 8-10 months of age were stained with hemoxylin and eosin (H&E) to assess pathological changes associated with glomerulonephritis, and D, sections from individual mice were graded in a blinded fashion on a scale of 0-3(0=absent, 1=mild, 2=moderate, 3=severe) for the degree of histological abnormality in the glomeruli, and for the degree of inflammation in the interstitial regions. E, Numbers of inflammatory cells in the kidneys were measured by flow cytometry in 6 mice of each genotype at 8-10-month of age. Data are presented as mean±SE, and are representative of two separate experiments. Statistical significance: *=p<0.05; **=p<0.01.
Autoantibody production in Lyn−/− mice requires SAP expression and MyD88 signaling
Antibodies against dsDNA obtained from MRL/lpr mice exhibit somatic mutations that increase reactivity to DNA (32), which is consistent with a germinal center (GC) origin for these autoantibody responses. However, Ig transgenic B cells with a rheumatoid factor specificity in these mice make an extrafollicular antibody response with substantial numbers of somatic mutations even in the absence of antigen-specific T-cell help (21, 33). Moreover, mice overexpressing BAFF also spontaneously produce ANAs in a T cell-independent fashion (34). Thus, there are precedents for extrafollicular production of anti-nuclear antibodies in some mouse models.
To address this issue, we first examined Lyn−/− mice for the frequency of GC phenotype B cells. The frequency of CD95+GL-7+ GC B cells relative to the number of splenic B cells was significantly increased in Lyn−/− mice compared with wild type mice already by 2~4-month and this increase became more dramatic at 5~7-month (Fig. 3C, 3D), correlating with the time course of development of IgG anti-nuclear antibodies. It should be noted, however, that Lyn−/− mice have substantially fewer splenic B cells than do wild type mice (Supplemental Fig. 1B), so the absolute numbers of GC B cells were similar in Lyn−/− mice and wild type mice (Fig. 3E). In agreement with flow cytometry analysis, staining of spleen sections also clearly revealed existence of IgDlow GL-7+ GC structures in multiple follicles of Lyn−/− mice (Fig. 3F) at 5~7-month of age. In contrast, GCs were not common in wild type mice housed in the same mouse room (data not shown). Interestingly, the spontaneous GC response seen in Lyn−/− mice was greatly reduced upon deletion of Myd88 in B cells (Fig. 3D, 3E). In contrast, the mesenteric LNs of Lyn−/− and Lyn−/− B-Myd88−/− mice contained similar numbers of GC B cells (data not shown), suggesting that the latter mice did not have a general defect in mounting GC reactions.
To examine whether helper T cells and the germinal center reaction contribute to the generation of IgG anti-nuclear antibodies, we crossed Lyn−/− mice to mice deleted for TCR genes (tcrβ−/−tcrδ−/−) or to mice lacking the SAP signaling adaptor protein, which is critical for follicular helper T cells (Tfh) to interact with GC B cells and promote germinal center antibody responses (35, 36). Lyn-deficient mice lacking T cells or lacking the SAP adaptor molecule made greatly attenuated levels of anti-dsDNA IgG antibodies measured by ELISA (Fig. 3G). These genetic results strongly support the hypothesis that there is a GC-origin of lupus-like autoantibodies in Lyn-deficient mice.
Activation and expansion of self-reactive T cells in Lyn−/− mice requires MyD88 signaling in DCs and B cells
Lyn−/− mice exhibit a dramatic expansion in activated T cells as autoimmune disease progresses (8, 37). Therefore, we next investigated the roles MyD88 in DCs and B cells in the hyperreactivity of T cells in Lyn−/− mice. As previously reported (8), Lyn−/− mice accumulate increasing numbers of CD4+ and CD8+ T cells with an activated or effector memory phenotype (CD44+CD62Llow) as they age. As Lyn-deficient mice lacking MyD88 in B cells or in DCs aged, these populations did not increase at all (CD4+ T cells) or nearly as much (CD8+ T cells) (Fig. 4A-C; Supplemental Fig. 1C, 1D). Thus, increased spontaneous activation of T cells in Lyn−/− mice was largely dependent on MyD88 signaling in both DCs and B cells.
Figure 4. Roles of MyD88 signaling in DCs and B cells for the activation and expansion of T cells in Lyn−/− mice.
A-C, age-dependent accumulation of activated phenotype T cells in Lyn−/− mice and effect of ablation of Myd88 in B cells or DCs. Representative flow cytometry plots of activated CD4+ T cells (CD44hi CD62Llow) in spleens of 5-7 month-old mice of the indicated genotype (A) and summarized data for CD4+ T cells (B) and CD8+ T cells (C) from mice of different ages. D-F, age-dependent accumulation of Tfh phenotype cells in Lyn−/− mice. Representative flow cytometry plots of Tfh cells (PD-1+ ICOS+) for gated CD4+ T cells in the spleen (D), and summarized data (E, F). Data in B, C, E and F are expressed as mean±SE of five to eight mice per group, and are representative of three separate experiments. Statistical differences are indicated as *=p<0.05; **=p<0.01.
Many of the activated CD4+ T cells also expressed ICOS and PD-1, two markers that have been used to identify T follicular helper (Tfh) cells. The percentage and absolute number of those cells was already slightly elevated in Lyn−/− mice at 2~4 month of age compared to wild type mice, and increased especially in older mice (Fig. 4E, 4F). In another recent study the number of Tfh at 4-5 months of age was found to be roughly normal in Lyn−/− mice (38). Importantly, deletion of Myd88 in either DCs or B cells substantially reduced expansion of these cells in Lyn−/− mice (Fig. 4E, 4F).
Splenic B cells and DCs in Lyn−/− mice have elevated expression of MHC II and CD86 that is not dependent on MyD88 signaling
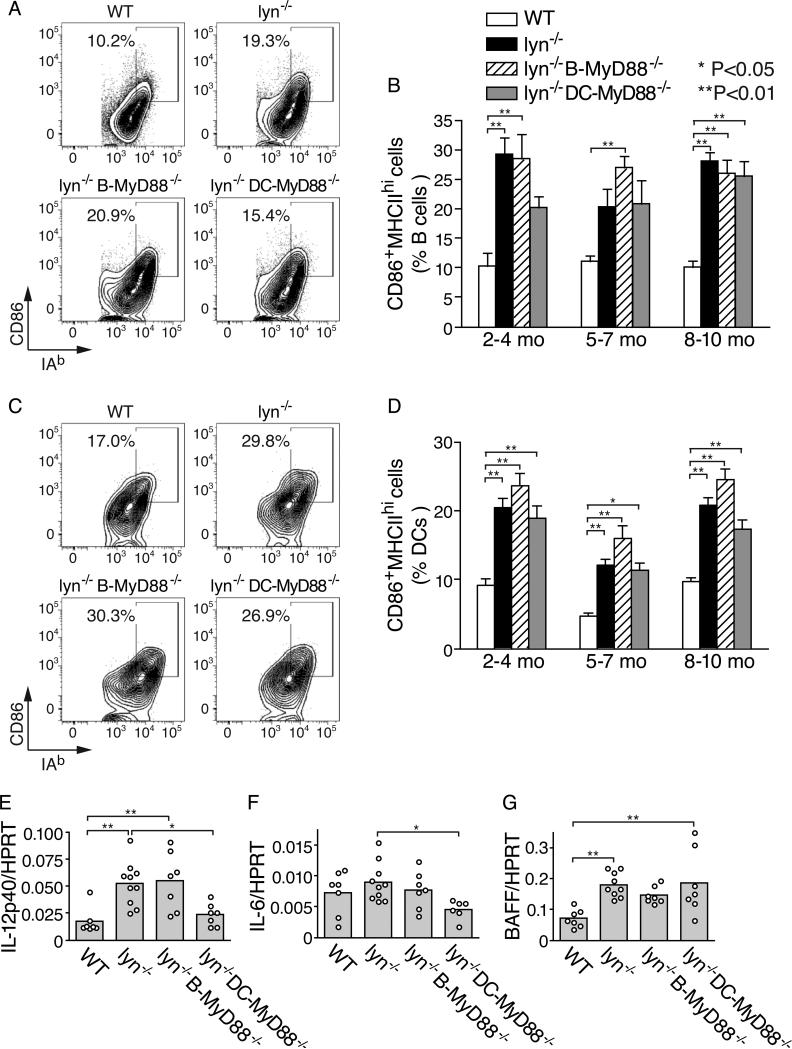
TLR agonists have well established adjuvant properties for promoting T cell responses (39). To investigate the possibility that TLRs of DCs or B cells promote their ability to present self-antigens to T cells, we examined the expression of MHC II and of the co-stimulatory molecule CD86 on splenic B cells and on DCs of Lyn−/− mice with or without DC or B cell MyD88. In Lyn−/− mice, an increased proportion of B cells exhibited elevated levels of MHC II and CD86 by 2-4 months of age (Fig. 5A, 5B). The number of such B cells was not dependent on expression of MyD88 in B cells (Fig. 5A, 5B), suggesting that loss of Lyn-based attenuation of BCR signaling in B cells may be sufficient for this phenotypic alteration. Similarly, DCs in young Lyn−/− mice had spontaneously elevated MHC class II expression and CD86 expression. Deletion of Myd88 in DCs had little effect on their expression of MHCII and CD86 (Fig. 5C, 5D). Together, these data indicate that the requirement for MyD88 in B cells and in DCs for development of autoimmunity in Lyn−/− mice was not caused by changes in the expression of MHC II and CD86.
Figure 5. Role of MyD88 signaling for the activation of B cells and DCs in Lyn−/− mice.
A and B, characterization of the fraction of splenic B cells with an activated phenotype in Lyn−/− mice. Representative flow cytometry plots examining the expression of MHC II and CD86 on gated CD19+ B cells in the spleen (A) and summary data for the percentage of B cells in the spleen with an activated MHC II+ CD86+ phenotype (B). C and D, characterization of the fraction of dendritic cells in the spleen with an activated phenotype. Representative flow cytometry plots for characterization of activated phenotype of splenic DCs gated on CD11chiMHC II+ cells (C), and summary data for the percentage of DCs in the spleen with an activated MHC II+ CD86+ phenotype (D). Data in (B) and (D) are expressed as mean±SE of five to eight mice per mouse group, and are representative of three separate experiments. E-G, expression of IL-12p40 (E), IL-6 (F), and BAFF (G) mRNAs in total splenocytes from two month old mice were quantified by real-time PCR. Data of each mouse (closed circles) and the mean of each mouse group (bars) are presented as relative abundance of each cytokine mRNA normalized to HPRT mRNA. Statistical differences are indicated as *=p<0.05; **=p<0.01.
Next, we examined the role of MyD88 signaling in DCs and B cells in Lyn−/− mice for the induction of cytokines that have been implicated in the pathogenesis of autoimmunity and inflammation in this model (8, 40). In total splenocytes from Lyn−/− mice at 2 months of age, increased mRNA expression of the cytokines IL-12p40, and BAFF was readily detected (Fig. 5E-G), demonstrating that enhanced inflammatory responses develop even before development of overt autoimmunity in these mice. Interestingly, deletion of Myd88 in DCs substantially reduced the expression of IL-12p40 and IL-6 in the spleens of young Lyn−/− mice (Fig. 5E, 5F), suggesting that Lyn-deficient DCs are sensitized to have enhanced responses to endogenous or ubiquitous TLR ligands, and are a major producer of pro-inflammatory cytokines in these mice. This is in agreement with other recent reports describing a pro-inflammatory role of Lyn-deficient DCs (10, 11). As mentioned above, the incidence of splenomegaly was greatly reduced in aged (~10 month) Lyn−/− DC-MyD88−/− mice compared with Lyn−/− mice (Fig. 3A, 3B). In comparison, deletion of B cell Myd88 had a small or no effect on the expression of these three cytokines (Fig. 5E-G).
Effect of cell intrinsic MyD88 signaling on peripheral numbers of B cells in Lyn−/− mice
Lyn-deficiency has substantial B cell intrinsic effects on the numbers of B cells in the spleen (8), decreasing their overall numbers (Supplemental Fig. 1B), apparently due both to enhanced cell death at the T1 or T2 immature stages in the spleen and to decreased maturation to the follicular mature state and/or decreased survival of these cells (41-43). Interestingly, while deletion of Myd88 in Lyn−/− B cells did not affect bone marrow populations of B cell precursors or immature B cells compared to Lyn−/− mice (Fig. 6A), the decreased number of B cells in the spleens of young Lyn−/− mice was further exacerbated by deletion of Myd88 in B cells (Fig. 6B). The reduction of B cells in Lyn−/−B-Myd88−/− mice began at the T1 stage in the spleen, persisted in subsequent developmental stages until maturation (Fig. 6B, 6C). Despite these decreases in mature B cell number, the numbers of plasma cells in the spleen were elevated in Lyn−/− and Lyn−/−B-Myd88−/− mice compared to wild type mice (Supplemental Fig. 1E). These results suggest that MyD88 signaling in B cells promotes their survival at the immature T1 stage in the spleen, but does not greatly affect maturation or survival at subsequent stages of development in the spleen and is not required for differentiation into plasma cells, in agreement with the circulating levels of total IgM and IgG in these mice (Fig. 1G, 1H). We did observe a modest increase in the percentage of CD93+CD23+IgMlow T3 B cells and a small decrease in the percentage of follicular B cells in the spleens of Lyn−/−B-Myd88−/− mice, such that the ratio of T3 to follicular B cells was slightly but significantly increased by loss of MyD88 in B cells (Fig. 6D). The T3 B cell population was previously shown to include many anergic auto-reactive B cells (44). Our data suggests that intrinsic MyD88 signaling may keep some self-reactive B cells from becoming anergic, although other explanations are possible.
DISCUSSION
Lyn is an intracellular protein tyrosine kinase that is critical for inhibitory receptor function in B cells and in DCs, and Lyn−/− mice spontaneously develop a lupus-like autoimmune and inflammatory disease (4, 5). To address the mechanism by which nucleic acid-recognizing TLRs contribute to spontaneous production of anti-nuclear antibodies in the Lyn−/− mouse model of SLE, we have used cell-type specific deletion of the gene encoding MyD88, a key adaptor molecule required for intracellular signaling by most TLRs, including TLR7 and TLR9. Deletion of Myd88 selectively in B cells completely blocked production of the anti-dsDNA and anti-RNP IgG antibodies, which are among the most characteristic autoantibodies seen in human SLE and are rarely produced in other human diseases (1), and ameliorated glomerulonephritis. In addition, deletion of Myd88 selectively in DCs delayed production of IgG anti-dsDNA, blocked production of IgG anti-smRNP, largely abrogated accumulation of activated phenotype T cells in the spleen, and also ameliorated glomerulonephritis. These results demonstrate that TLR/MyD88 signaling is required in both B cells and DCs for development of autoimmune disease in these mice.
The demonstration that MyD88/TLR signaling in B cells is required for anti-nuclear IgG production in Lyn−/− mice adds to the accumulating evidence indicating that dual stimulation of DNA- and RNP-specific B cells by the BCR and by TLR9 or TLR7, respectively, is a key underlying mechanism in the breakdown of tolerance to nuclear self antigens in mouse models of lupus (15, 45, 46). While TLR signaling can boost antibody responses in multiple ways (47), we have recently shown that it can boost germinal center responses dramatically in response to virus-like particles and inactivated virions (24). It is likely that an analogous mechanism, perhaps in response to fragments from apoptotic cells, participates in the production of anti-dsDNA and anti-RNP IgG antibodies in Lyn−/− mice since it was largely dependent on MyD88 expression in B cells, on the presence of T cells and on the expression of SAP, which is required for the germinal center responses (Figure 3G). SAP is an adaptor for SLAM family adhesion molecules and its function in T cells is important at early stages of the germinal center response by acting to stabilize interactions between activated B cells and cognate helper T cells (36). Moreover, a recent report from another group found that Lyn−/− IL-21−/− mice failed to produce anti-dsDNA IgG antibodies, which is also consistent with an important role for the GC response in the production of these antibodies, given the known role of IL-21 in the GC response(38). Curiously, in that study glomerulonephritis was not ameliorated despite a substantial decrease in most IgG autoantibodies. Thus, a number of results strongly implicate the germinal center response in the production of anti-nuclear IgG antibodies in Lyn-deficient mice.
Our finding of a requirement for T cells in the autoimmunity of Lyn−/− mice is consistent with previous data demonstrating that the T cell production of interferon-γ plays a critical role in the autoimmune phenomena of Lyn-deficient mice (8) and that IL-10 from B cells decreases the inflammatory reaction generated by T cells in these mice (9). Interestingly, a previous study found that blockade of T cell costimulation via CD28 using CTLA4-Ig found that IgG ANAs were blocked but there was now production of IgA (48). The mechanism of IgA anti-nuclear antibody production in these treated mice is unclear at this time.
The germinal center response has been implicated in lupus-like autoantibody production in several other mouse models of SLE, including the NZB x NZW F1 mouse (46, 49, 50). It should be noted, however that in BAFF transgenic mice (34) and the rheumatoid factor Ig transgene combined with MRL/lpr, autoantibody production occurs independently of T cells (21). Therefore, the nature of the genetic alterations causing susceptibility to SLE apparently can affect to some degree the underlying immunological mechanism of autoantibody production. These results suggest that human SLE may also be mechanistically diverse and therefore it will be of interest to develop methods for analyzing immune cells from SLE patients to assess such possible heterogeneity as this may be relevant to their treatment.
In previous studies of the role of MyD88 signaling in B cells vs. DCs for antibody responses to antigen-CpG oligonucleotide conjugates, we found that the cellular requirement for an optimal IgG response to the antigen depended on the physical form of the antigen (24). When soluble protein antigens such as ovalbumin or the ragweed pollen antigen Amb a1 were used, MyD88 in DCs was required and MyD88 in B cells did not contribute to the response. In contrast, when virus-like particles containing TLR9 or TLR7 ligands or chemically inactivated influenza virus particles were used as immunogen, the IgG response to viral coat proteins was greatly augmented by MyD88 signaling in B cells, but not by MyD88 signaling in DCs. Moreover, for virus-like particles, the magnitude of the TLR effect was dependent on the epitope density of the antigen on the particle, indicating that stronger BCR signaling enabled TLR7 or TLR9 in B cells to enhance the response (24). This enhancement occurred by promotion of the germinal center component of the response (24). Conversely, weak BCR signaling, as likely occurs with the soluble protein antigens, did not enable B cell TLR9 to boost the IgG response. Based on the results obtained here and the results obtained previously, we propose that the true autoantigen in SLE is a particulate form of chromatin and/or ribonucleoproteins, such as occurs on apoptotic fragments released from dying cells. This proposal is consistent with the fact that many autoantibodies obtained from mouse SLE-prone stains bind to apoptotic blebs (51).
The observation mentioned above that strong BCR signaling is required to enable TLR7 or TLR9 signaling in B cells to enhance their germinal center response suggests why Lyn-deficiency of B cells creates a strong susceptibility for development of anti-dsDNA and anti-RNP IgG antibodies. Low affinity DNA- or RNP-reactive mature or anergic follicular B cells in wild type mice presumably have weak BCR signaling that is attenuated by the Lyn/CD22/SHP-1 feedback inhibitory pathway and therefore even if they acutely encounter apoptotic blebs, their low level of BCR signaling does not synergize with TLR7 or TLR9 signaling to promote a germinal center response. In contrast, Lyn-deficient B cells of the same specificity have exaggerated BCR signaling and therefore, we hypothesize they can enter into and participate in germinal center responses, leading to production of class-switched and affinity-matured pathogenic autoantibodies.
Several recent studies have implicated dysregulation of DCs by loss of Lyn as also being an important contributor to the autoimmune phenomena in Lyn−/− mice (10, 11). Consistent with this view is our demonstration that MyD88 signaling in DCs contributes importantly to the autoimmune phenotypes of Lyn−/− mice. The characteristic expansion of activated phenotype T cells that is seen in Lyn−/− mice as they get older was abrogated by deletion of Myd88 in DCs (Fig. 4), indicating that TLR/MyD88 signaling in DCs provides a necessary activation signal that synergizes with the effects of loss of Lyn-dependent inhibitory signaling in these cells. This interpretation is supported by the recent report that mice in which Lyn is deleted only in DCs also develop a severe lupus-like autoimmunity (11). Thus, we propose that Lyn−/− mice develop a severe lupus-like autoimmunity and inflammatory disease due to the combination of at least two defects: the deficiency of Lyn in B cells compromises their cell-intrinsic tolerance mechanisms by allowing TLR7 and TLR9 to promote activation of self DNA- and RNP- reactive B cells; and the dysregulation of Lyn-deficient DCs leads to excessive activation of T cells, which in turn can promote increased affinity IgG responses of the DNA- and RNP-specific B cells. In addition, Lyn-deficient DCs and the T cells activated by them provide a self-reinforcing inflammatory response that can also contribute to inflammatory disease (8, 11). This latter component of the disease of Lyn-deficient mice is especially evident if Lyn is selectively deleted in DCs (11), or if the B cells are unable to produce IL-10 to inhibit it (9).
Studies in mouse models indicate that the presence of genetic susceptibility loci is required for spontaneous breakdown of tolerance to nuclear autoantigens. While most natural susceptibility loci in human and mouse remain poorly understood, a subset of identified loci alter regulation of BCR signaling, including ablation of Lyn (4, 31), B cell-specific deletion of SHP-1 (52), deletion of CD22 (53), and a point mutation of CD45 that increases the activity of some Src-family tyrosine kinases while decreasing the activity of Lyn (54) (55). Strikingly, Lyn, CD22 and SHP-1 work together in a feedback inhibitory pathway to limit BCR signaling, especially in mature B cells (4, 56). Genetic analysis in human SLE patients suggests that this pathway is likely compromised in some SLE patients (12, 13). Thus, the Lyn−/− mouse model of lupus is highly relevant to a subset of human SLE patients. Our results indicate that TLR/MyD88 signaling is likely to be necessary for anti-nuclear antibody production in this subset of patients.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank Derek Rookhuizen for helpful discussions related to analysis of Tfh cells by flow cytometry, and Yongmei Hu for assistance with animal husbandry.
Footnotes
This work was supported by research grants from NIH to A.L. DeFranco (R01AI072058 and P01AI078869), from NIH to Clifford A. Lowell (P01AI078869 and R01AI068150), and by the National Natural Science Foundation of China to B. Hou (31170848) and to Z. Hua (31200669). B. Hou is supported by an award from National Thousand Talent Plan of China (2011).
DISCLOSURES
The authors have no financial conflicts of interest.
REFERENCES
- 1.Rahman A, Isenberg DA. Systemic lupus erythematosus. N. Engl. J. Med. 2008;358:929–939. doi: 10.1056/NEJMra071297. [DOI] [PubMed] [Google Scholar]
- 2.Pathak S, Mohan C. Cellular and molecular pathogenesis of system lupus erythematosus: lessons from animal models. Arthritis Res. Ther. 2011;13:241. doi: 10.1186/ar3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hibbs ML, Tarlinton DM, Armes J, Grail D, Hodgson G, Maglitto R, Stacker SA, Dunn AR. Multiple defects in the immune system of Lyn-deficient mice, culminating in autoimmune disease. Cell. 1995;83:301–311. doi: 10.1016/0092-8674(95)90171-x. [DOI] [PubMed] [Google Scholar]
- 4.Xu Y, Harder KW, Huntington ND, Hibbs ML, Tarlinton DM. Lyn tyrosine kinase: accentuating the positive and the negative. Immunity. 2005;22:9–18. doi: 10.1016/j.immuni.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 5.Scapini P, Pereira S, Zhang H, Lowell CA. Multiple roles of Lyn kinase in myeloid cell signaling and function. Immunol Rev. 2009;228:23–40. doi: 10.1111/j.1600-065X.2008.00758.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith KG, Tarlinton DM, Doody GM, Hibbs ML, Fearon DT. Inhibition of the B cell by CD22: a requirement for Lyn. J Exp Med. 1998;187:807–811. doi: 10.1084/jem.187.5.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chan VW, Lowell CA, DeFranco AL. Defective negative regulation of antigen receptor signaling in Lyn-deficient B lymphocytes. Curr Biol. 1998;7:545–553. doi: 10.1016/s0960-9822(98)70223-4. [DOI] [PubMed] [Google Scholar]
- 8.Scapini P, Hu Y, Chu CL, Migone TS, Defranco AL, Cassatella MA, Lowell CA. Myeloid cells, BAFF, and IFN-gamma establish an inflammatory loop that exacerbates autoimmunity in Lyn-deficient mice. J Exp Med. 2010;207:1757–1773. doi: 10.1084/jem.20100086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scapini P, Lamagna C, Hu Y, Lee K, Tang Q, DeFranco AL, Lowell CA. B cell-derived IL-10 suppresses inflammatory disease in Lyn-deficient mice. Proc Natl Acad Sci U S A. 2011;108:E823–833. doi: 10.1073/pnas.1107913108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krebs DL, Chehal MK, Sio A, Huntington ND, Da ML, Ziltener P, Inglese M, Kountouri N, Priatel JJ, Jones J, Tarlinton DM, Anderson GP, Hibbs ML, Harder KW. Lyn-dependent signaling regulates the innate immune response by controlling dendritic cell activation of NK cells. J Immunol. 2012;188:5094–5105. doi: 10.4049/jimmunol.1103395. [DOI] [PubMed] [Google Scholar]
- 11.Lamagna C, Scapini P, van Ziffle J, DeFranco AL, Lowell CA. Hyperactivated MyD88 signaling in dendritic cells, through specific deletion of Lyn kinase, causes severe autoimmunity and inflammation. Proc Natl Acad Sci U S A. 2013;110:E3311–3320. doi: 10.1073/pnas.1300617110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lu R, Vidal GS, Kelly JA, Delgado-Vega AM, Howard XK, Macwana SR, Dominguez N, Klein W, Burrell C, Harley IT, Kaufman KM, Bruner GR, Moser KL, Gaffney PM, Gilkeson GS, Wakeland EK, Li Q-Z, Langefeld CD, Marion MC, Divers J, Alarcon GS, Brown EE, Kimberly RP, Edberg JC, Ramsey-Goldman R, Reveille JD, McGwin G, Vila LM, Petri MA, Bae S-C, Cho S-K, Bang S-Y, Kim I, Choi C-B, Martin J, Vyse TJ, Merrill JT, Harley JB, Alarcon-Riquelme ME, B. a. G. M. Collaborations. Nath SK, James JA, Guthridge JM. Genetic associations of LYN with systemic lupus erythematosus. Genes Immun. 2009;10:397–403. doi: 10.1038/gene.2009.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Surolia I, Pirnie SP, Chellappa V, Taylor KN, Cariappa A, Moya J, Liu H, Bell DW, Driscoll DR, Diederichs S, Haider K, Netravali I, Le S, Elia R, Dow E, Lee A, Freudenberg J, De Jager P, Chretien Y, Varki A, MacDonald ME, Gillis T, Behrens TW, Bloch D, Collier D, Korzenik J, Podolsky DK, Hafler D, Murali M, Sands B, Stone JH, Gregersen PK, Pillai S. Functionally defective germline variants of sialic acid acetylesterase in autoimmunity. Nature. 2010;466:243–247. doi: 10.1038/nature09115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsantikos E, Maxwell MJ, Kountouri N, Harder KW, Tarlinton DM, Hibbs ML. Genetic interdependence of Lyn and negative regulators of B cell receptor signaling in autoimmune disease development. J Immunol. 2012;189:1726–1736. doi: 10.4049/jimmunol.1103427. [DOI] [PubMed] [Google Scholar]
- 15.Green NM, Marshak-Rothstein A. Toll-like receptor driven B cell activation in the induction of systemic autoimmunity. Semin Immunol. 2011;23:106–112. doi: 10.1016/j.smim.2011.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nickerson KM, Christensen SR, Shupe J, Kashgarian M, Kim D, Elkon K, Shlomchik MJ. TLR9 regulates TLR7- and MyD88-dependent autoantibody production and disease in a murine model of lupus. J. Immunol. 2010;184:1840–1848. doi: 10.4049/jimmunol.0902592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Silver KL, Crockford TL, Bouriez-Jones T, Milling S, Lambe T, Cornall RJ. MyD88-dependent autoimmune disease in Lyn-deficient mice. Eur. J. Immunol. 2007;37:2734–2743. doi: 10.1002/eji.200737293. [DOI] [PubMed] [Google Scholar]
- 18.Pisitkun P, Deane JA, Difilippantonio MJ, Tarasenko T, Satterthwaite AB, Bolland S. Autoreactive B cell responses to RNA-related antigens due to TLR7 gene duplication. Science. 2006;312:1669–1672. doi: 10.1126/science.1124978. [DOI] [PubMed] [Google Scholar]
- 19.Subramanian S, Tus K, Li Q-Z, Wang A, Tian X-H, Zhou J, Liang C, Bartov G, McDaniel LD, Zhou XJ, Schultz RA, Wakeland EK. A Tlr7 translocation accelerates systemic autoimmunity in murine lupus. Proc. Natl. Acad. Sci. USA. 2006;103:9970–9975. doi: 10.1073/pnas.0603912103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ehlers M, Fukuyama H, McGaha TL, Aderem A, Ravetch JV. TLR9/MyD88 signaling is required for class switching to pathogenic IgG2a and 2b autoantibodies in SLE. J. Exp. Med. 2006;203:553–561. doi: 10.1084/jem.20052438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Herlands RA, Christensen SR, Sweet RA, Hershberg U, Shlomchik MJ. T cell-independent and Toll-like receptor-dependent antigen-driven activation of autoreactive B cells. Immunity. 2008;29:249–260. doi: 10.1016/j.immuni.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gilliet M, Cao W, Liu YJ. Plasmacytoid dendritic cells: sensing nucleic acids in viral infection and autoimmune diseases. Nat Rev Immunol. 2008;8:594–606. doi: 10.1038/nri2358. [DOI] [PubMed] [Google Scholar]
- 23.Banchereau J, Pascual V. Type I interferon in systemic lupus erythematosus and other autoimmune diseases. Immunity. 2006;25:383–392. doi: 10.1016/j.immuni.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 24.Hou B, Saudan P, Ott G, Wheeler ML, Ji M, Kuzmich L, Lee LM, Coffman RL, Bachmann MF, DeFranco AL. Selective utilization of Toll-like receptor and MyD88 signaling in B cells for enhancement of the antiviral germinal center response. Immunity. 2011;34:375–384. doi: 10.1016/j.immuni.2011.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hou B, Reizis B, DeFranco AL. Toll-like receptors activate innate and adaptive immunity by using dendritic cell-intrinsic and -extrinsic mechanisms. Immunity. 2008;29:272–282. doi: 10.1016/j.immuni.2008.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Caton ML, Smith-Raska MR, Reizis B. Notch-RBP-J signaling controls the homeostasis of CD8-dendritic cells in the spleen. J Exp Med. 2007;204:1653–1664. doi: 10.1084/jem.20062648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hobeika E, Thiemann S, Storch B, Jumaa H, Nielsen PJ, Pelanda R, Reth M. Testing gene function early in the B cell lineage in mb1-cre mice. Proc Natl Acad Sci U S A. 2006;103:13789–13794. doi: 10.1073/pnas.0605944103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Czar MJ, Kersh EN, Mijares LA, Lanier G, Lewis J, Yap G, Chen A, Sher A, Duckett CS, Ahmed R, Schwartzberg PL. Altered lymphocyte responses and cytokine production in mice deficient in the X-linked lymphoproliferative disease gene SH2D1A/DSHP/SAP. Proc Natl Acad Sci U S A. 2001;98:7449–7454. doi: 10.1073/pnas.131193098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Itohara S, Mombaerts P, Lafaille J, Iaocmini J, Nelson A, Clarke AR, Hooper ML, Farr A, Tonegawa S. T cell receptor delta gene mutant mice: independent generation of alpha beta T cells and programmed rearrangements of gamma delta TCR genes. Cell. 1993;72:337–348. doi: 10.1016/0092-8674(93)90112-4. [DOI] [PubMed] [Google Scholar]
- 30.Mombaerts P, Clarke AR, Hooper ML, Tonegawa S. Creation of a large genomice deletion at the T-cell receptor beta-subunit locus in embryonic stem cells by gene targeting. Proc Natl Acad Sci U S A. 1991;88:3084–3087. doi: 10.1073/pnas.88.8.3084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yu CC, Yen TS, Lowell CA, DeFranco AL. Lupus-like kidney disease in mice deficient in the Src family tyrosine kinases Lyn and Fyn. Curr. Biol. 2001;11:34–38. doi: 10.1016/s0960-9822(00)00024-5. [DOI] [PubMed] [Google Scholar]
- 32.Radic MZ, Weigert M. Genetic and structural evidence for antigen selection of anti-DNA antibodies. Annu Rev Immunol. 1994;12:487–520. doi: 10.1146/annurev.iy.12.040194.002415. [DOI] [PubMed] [Google Scholar]
- 33.William J, Euler C, Christensen SR, Shlomchik MJ. Evolution of autoantibody responses via somatic hypermutation outside of germinal centers. Science. 2002;297:2066–2070. doi: 10.1126/science.1073924. [DOI] [PubMed] [Google Scholar]
- 34.Groom JR, Fletcher CA, Walters SN, Grey ST, Watt SV, Sweet MJ, Smyth MJ, Mackay CR, Mackay F. BAFF and MyD88 signals promote a lupuslike disease independent of T cells. J Exp Med. 2007;204:1959–1971. doi: 10.1084/jem.20062567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Crotty S, Kersh EN, Cannons J, Schwartzberg PL, Ahmed R. SAP is required for generating long-term humoral immunity. Nature. 2003;421:282–287. doi: 10.1038/nature01318. [DOI] [PubMed] [Google Scholar]
- 36.Cannons JL, Tangye SG, Schwartzberg PL. SLAM family receptors and SAP adaptors in immunity. Annu. Rev. Immunol. 2011;29:665–705. doi: 10.1146/annurev-immunol-030409-101302. [DOI] [PubMed] [Google Scholar]
- 37.Tsantikos E, Quilici C, Harder KW, Wang B, Zhu HJ, Anderson GP, Tarlinton DM, Hibbs ML. Perturbation of the CD4 T cell compartment and expansion of regulatory T cells in autoimmune-prone Lyn-deficient mice. J Immunol. 2009;183:2484–2494. doi: 10.4049/jimmunol.0804346. [DOI] [PubMed] [Google Scholar]
- 38.Gutierrez T, Mayeux JM, Ortega SB, Karandikar NJ, Li QZ, Rakheja D, Zhou XJ, Satterthwaite AB. IL-21 promotes the production of anti-DNA IgG but is dispensable for kidney damage in lyn−/− mice. Eur J Immunol. 2013;43:382–393. doi: 10.1002/eji.201142095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Coffman RL, Sher A, Seder RA. Vaccine adjuvants: putting innate immunity to work. Immunity. 2010;29:492–503. doi: 10.1016/j.immuni.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tsantikos E, Oracki SA, Quilici C, Anderson GP, Tarlinton DM, Hibbs ML. Autoimmune disease in Lyn-deficient mice is dependent on an inflammatory environment established by IL-6. J Immunol. 2010;184:1348–1360. doi: 10.4049/jimmunol.0901878. [DOI] [PubMed] [Google Scholar]
- 41.Gross AJ, Proekt I, DeFranco AL. Elevated BCR signaling and decreased survival of Lyn-deficient transitional and follicular B cells. Eur J Immunol. 2011;41:3645–3655. doi: 10.1002/eji.201141708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shahaf G, Gross AJ, Sternberg-Simon M, Kaplan D, Defranco AL, Mehr R. Lyn deficiency affects B-cell maturation as well as survival. Eur J Immunol. 2012;42:511–521. doi: 10.1002/eji.201141940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Meade J FC, Turner M, E. JI. The tyrosine kinase Lyn is required for B cell development beyond the T1 stage in the spleen: rescue by over-expression of Bcl-2. Eur J Immunol. 2002;32:1029–1034. doi: 10.1002/1521-4141(200204)32:4<1029::AID-IMMU1029>3.0.CO;2-M. Apr;32(4):1029-34. [DOI] [PubMed] [Google Scholar]
- 44.Merrell KT, Benschop RJ, Gauld SB, Aviszus K, Decote-Ricardo D, Wysocki LJ, Cambier JC. Identification of anergic B cells within a wild-type repertoire. Immunity. 2006;25:953–962. doi: 10.1016/j.immuni.2006.10.017. [DOI] [PubMed] [Google Scholar]
- 45.Hwang SH, Lee H, Yamamoto M, Jones LA, Dayalan J, Hopkins R, Zhou XJ, Yarovinsky F, Connolly JE, Curotto de Lafaille MA, Wakeland EK, Fairhurst AM. B cell TLR expression drives anti-RNA autoantibody production and exacerbates disease in systemic lupus erythematosus-prone mice. J. Immunol. 2012;189:5786–5796. doi: 10.4049/jimmunol.1202195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Walsh ER, Pisitkun P, Voynova E, Deane JA, Scott BL, Caspi RR, Bolland S. Dual signaling by innate and adaptive immune receptors is required for TLR7-induced B-cell-mediated autoimmunity. Proc Natl Acad Sci U S A. 2012;109:16276–16281. doi: 10.1073/pnas.1209372109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rawlings DJ, Schwartz MA, Jackson SW, Meyer-Bahlburg A. Integration of B cell responses through Toll-like receptors and antigen receptors. Nat Rev Immunol. 2012;12:282–294. doi: 10.1038/nri3190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Oracki SA, Tsantikos E, Quilici C, Light A, Schmidt T, Lew AM, Martin JE, Smith KG, Hibbs ML, Tarlinton DM. CTLA4Ig alters the course of autoimmune disease development in Lyn−/− mice. J Immunol. 2010;184:757–763. doi: 10.4049/jimmunol.0804349. [DOI] [PubMed] [Google Scholar]
- 49.Wofsy D. Treatment of murine lupus with anti-CD4 monoclonal antibodies. Immunol Ser. 1993;59:221–236. [PubMed] [Google Scholar]
- 50.Shlomchik MJ. Activating systemic autoimmunity: B's, T's, and tolls. Curr Opin Immunol. 2009;21:626–633. doi: 10.1016/j.coi.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cline AM, Radic MZ. Murine lupus autoantibodies identify distinct subsets of apoptotic bodies. Autoimmunity. 2004;37:85–93. doi: 10.1080/0891693042000196219. [DOI] [PubMed] [Google Scholar]
- 52.Pao LI, Lam KP, Henderson JM, Kutok JL, Alimzhanov M, Nitschke L, Thomas ML, Neel BG, Rajewsky K. B cell-specific deletion of protein-tyrosine phosphatase Shp1 promotes B-1a cell development and causes systemic autoimmunity. Immunity. 2007;27:35–48. doi: 10.1016/j.immuni.2007.04.016. [DOI] [PubMed] [Google Scholar]
- 53.Jellusova J, Wellmann U, Amann K, Winkler TH, Nitschke L. CD22 x Siglec-G double-deficient mice have massively increased B1 cell numbers and develop systemic autoimmunity. J Immunol. 2010;184:3618–3627. doi: 10.4049/jimmunol.0902711. [DOI] [PubMed] [Google Scholar]
- 54.Hermiston ML, Tan AL, Gupta VA, Majeti R, Weiss A. The juxtamembrane wedge negatively regulates CD45 function in B cells. Immunity. 2005;23:635–647. doi: 10.1016/j.immuni.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 55.Zikherman J, Parameswaran R, Hermiston M, Weiss A. The structural wedge domain of the receptor-like tyrosine phosphatase CD45 enforces B cell tolerance by regulating substrate specificity. J. Immunol. 2013;190:2527–2535. doi: 10.4049/jimmunol.1202928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gross AJ, Lyandres JR, Panigrahi AK, Prak ET, DeFranco AL. Developmental acquisition of the Lyn-CD22-SHP-1 inhibitory pathway promotes B cell tolerance. J. Immunol. 2009;182:5382–5392. doi: 10.4049/jimmunol.0803941. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.