Abstract
Historically, the identification of bacterial or yeast isolates has been based on phenotypic characteristics such as growth on defined media, colony morphology, Gram stain, and various biochemical reactions, with significant delay in diagnosis. Clinical microbiology as a medical specialty has embraced advances in molecular technology for rapid species identification with broad-range 16S rDNA polymerase chain reaction (PCR) and matrix-assisted laser desorption and/or ionization time of flight (MALDI-TOF) mass spectrometry demonstrated as accurate, rapid, and cost-effective methods for the identification of most, but not all, bacteria and yeasts. Protracted conventional incubation times previously necessary to identify certain species have been mitigated, affording patients quicker diagnosis with associated reduction in exposure to empiric broad-spectrum antimicrobial therapy and shortened hospital stay. This short commentary details such molecular advances and their implications in the clinical microbiology setting.
Keywords: clinical microbiology, MALDI, PCR, patient care, impact
Introduction
Europe and North America have experienced significant declines in mortality secondary to infectious diseases.1 Indeed, the incidence of severe sepsis, associated with multiorgan failure, in the European Union is currently estimated as 90.4 cases per 100 000 population.2 Global developments in medicine, including advances in education and training for medical students, continuous professional development for qualified physicians, modern cleaner hospitals with single en-suite accommodation, improved infection control practices, newer antimicrobial therapies, advanced molecular technology used in the laboratory, and sepsis management protocols have all contributed to the decline in sepsis-related deaths.3 Previous studies have shown a direct link between outcomes from infectious illnesses and time to pathogen identification.4 Consequently, laboratory testing volumes are increasing by 10–15% per year internationally, driven partly by infection control demands, with enhanced screening for methicillin-resistant Staphylococcus aureus and multi-drug resistant organisms such as vancomycin-resistant enterococci, extended spectrum β-lactamases, and carbapenemase-resistant enterobacteriaceae.5
Clinical microbiology has evolved in response to clinical needs and laboratories have adapted to meet demand for testing. The laboratory service expected by physicians and patients has changed dramatically. Most Western tertiary referral hospitals operate a laboratory service capable of providing an on-call 24 hour service enabling clinical teams to obtain results as soon they become available. Patients, many empowered and self-educated through the vast array of information readily available on the Internet, expect rapid reporting of results with minimal delay from time of presentation to diagnosis and discharge. Patients are also increasingly aware of the dangers of acquiring nosocomial illnesses, and there are also many added economic and financial pressures being brought to bear on clinical services. As a consequence, approximately 80% of patient management decisions are influenced by laboratory testing6 and reducing time to identification within the laboratory is increasingly a priority.
The Complementary Roles of the Clinical Microbiologist and the Microbiology Laboratory
The primary roles of the clinical microbiology team are to guide and support physicians in community or hospital settings, to select appropriate diagnostic investigations and antimicrobials, as warranted, and to achieve the best possible outcome for patients. For any patient, an array of samples may be sent for laboratory analysis with the objective of identifying causative pathogens. Specimens may be analyzed following sampling from diverse physiological sources including, but not limited to, cerebro-spinal fluid, blood, “sterile” body fluids, tissue and pus, urine, intravascular cathether tips, prosthetic devices, and the respiratory tract. Equipped with expertise and experience, clinical microbiologists are responsible for construction of differential diagnoses and provision of advice on required testing. However, skilled medical scientists are critical to the processes of appropriate growth media selection, innoculation and incubation of specimens, and analysis and interpretation of complex analytical data.
Patient-Centered Care
Patients presenting for medical attention with signs of infection, particularly evidence of fever, with or without hemodynamic instability, should have at least one set of blood cultures taken using an appropriate aseptic technique. Blood samples forwarded by physicians to the laboratory, that flag as positive when incubated, are typically the first engagement of clinical microbiologists with patients who may require either commencement or escalation of antimicrobial therapy. However, blood cultures are problematic in diagnosis of sepsis due to the potential presence of unculturable organisms, underfilling of blood collection vessels, protracted incubation times, and false positives caused by leucocytosis or contamination with commensal flora (e.g., coagulase negative staphylococci). In addition, microorganism detection and susceptibility testing may require up to 48 hours, delaying definitive diagnoses for patients.
Why the Long Delay?
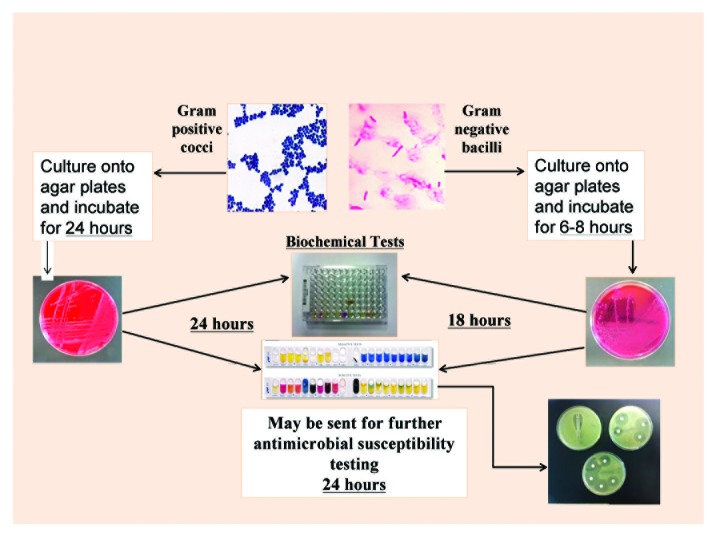
Processing of microbiology samples can be a lengthy process (Fig. 1). Once a blood sample is confirmed as positive in the laboratory, preliminary organism identification—usually performed by medical scientists—is determined by Gram stain, traditional culture techniques, and colony morphology. Microscopic examination of stained smears of tissue or biological fluid is relatively quick and requires minimal resource inputs. However, such assessment may have low sensitivity and specificity as, historically it has been grounded in subjective criteria such as odor, color, and experience of phenotypic patterns derived using a variety of confirmatory biochemical tests performed at the laboratory bench. Subsequent, authorized clinical diagnosis is reliant on the organism being culturable on solid media following 24 hours (aerobic) or 48 hours (anaerobic) incubation. Frequently, incubation can prolong the process by 24–48 hours.
Figure 1. Conventional laboratory identification process.
The duration of processes involved in reaching definitive laboratory diagnoses via these conventional culture methods results in widespread utilization of empiric broad-spectrum antimicrobial therapy in stabilizing of deteriorating patients. Clearly, the intention is to administer a broad spectrum of bacteriostatic and/or bacteriocidal agents sufficient to treat the unidentified pathogen, but, in reality, this approach is often associated with development of antimicrobial resistance, Clostridium difficile overgrowth, and iatrogenic complications for patients.7,8 Typically, empiric antimicrobial therapy is rationalized to a narrower spectrum agent once microbiology laboratory results become available.
Can Laboratory Results Be Expedited?
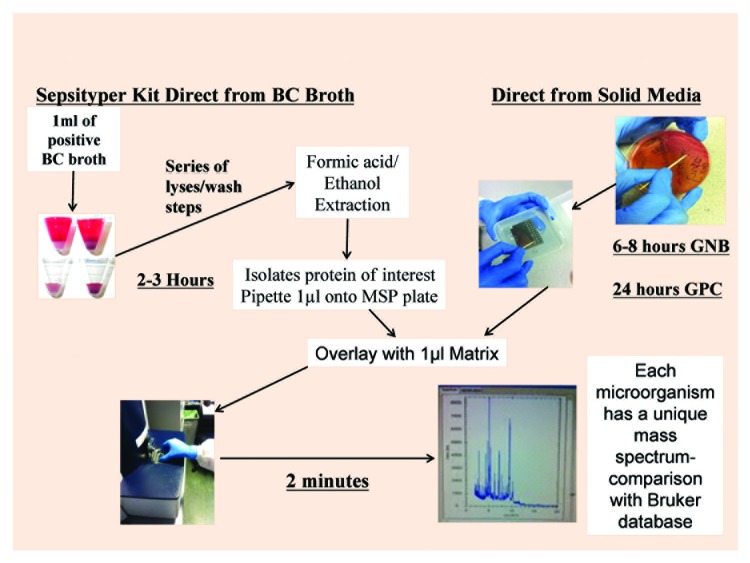
Matrix-assisted laser desorption ionisation–time of flight mass spectrometry (MALDI-TOF MS)9-11 has facilitated substantial movement toward same-day clinical decision-making underpinned by molecular microbiology (Fig. 2).12 In use, MALDI-TOF MS determines characteristic protein patterns derived from microbe composition and allows interrogation of well-developed databases for identification. This can occur once an incubated sample grows to colony level (approximately 105 cfu) and can be transferred to a slide. Laser analysis then takes less than 10 min, significantly reducing organism identification times compared with conventional microbiology.
Figure 2. MALDI-TOF system.
Increasingly utilized clinically for identification of bacteria8,13,14 and yeasts,15 MALDI-TOF MS has potential for accurate identification of viruses.16 Its utilization in clinical laboratories has resulted in reports of previously undetected organisms, such as new species of anaerobes17,18 (e.g., Prevotella spp. and Anaerococcus spp.). However, while MALDI-TOF MS has a reported reliability of >95% (of routine isolates grown on solid media in the laboratory),19 its limitations include inability to differentiate genetically similar organisms, such as Streptococcus pneumoniae from other members of the Streptococcus mitis group,20 and Shigella species from Eschericheria coli.21
Despite this, MALDI-TOF MS analysis can reduce manual workload for medical scientists and improve throughput of laboratory samples.22 Exemplifying this, previously technically-challenging identification of microorganisms from sputum of cystic fibrosis patients,8 utilizing multiple selective media and prolonged incubation, may now be completed within 48 hours of sampling.23 More specifically, a 2012 Canadian study24 reported a mean reduction in turnaround time of 34.3 hours when definitive identification of isolates directly from blood samples was possible, albeit that this reduction was lower, but still clinically important, at 26.5 hours when incubation was necessary before MALDI-TOF MS could be utilized. These advances are especially relevant clinically as identification of Gram-negative and Gram-positive bacteria as well as yeasts can now be obtained directly from 7–8 ml of blood when using systems such as the MALDI Sepsityper® Kit (BrukerDaltonics 2010), thereby eliminating incubation times associated with growth on solid media (Fig. 2).25
In an attempt to replicate the reductions observed in Canada (described above), 150 blood samples, flagged as positive for presence of microorganisms using the BacT/ALERT® system (bioMérieux®), were analyzed at University Hospital Limerick (UHL) over a 6 week period using the MALDI Sepsityper® Kit and conventional microbiological techniques, and results compared with respect to turnaround time (TAT) of positive blood cultures (Table 1). We observed that for polymicrobial blood cultures, MALDI-TOF resulted in TAT being reduced by a mean of 17.5 hours, mean reduction of 14.47 hours for Gram-positive cultures and mean reduction of 23.19 hours for Gram-negative cultures.
Table 1. Turnaround time (TAT)* for microbial identification**.
| MALDI-TOF | Conventional microbiology | Reduction of mean TAT | |||
|---|---|---|---|---|---|
| Mean | Min- Max | Mean | Min- Max | ||
| Gram-positive and Gram-negative |
11.12*** | 2.0‒27 | 28.62 | 18.0‒60.0 | 17.5 |
| Gram-positive only | 13.41 | 2.0‒27.0 | 27.88 | 20.0‒55.0 | 14.47 |
| Gram-negative only | 5.36 | 2.0‒20.0 | 28.55 | 18.0‒60.0 | 23.19 |
*TAT = turnaround time. **Over a 6 wk period, 150 blood samples at University Hospital Limerick, Ireland. ***All values are in hours
The impact of our study was that, of the patients from whom these blood samples were taken, 15 patients (30.0%) experienced a change of therapy in a timelier manner, 12 patients (24%) were changed to a more appropriate antibiotic, while one patient (2%) had antibiotic therapy discontinued. These changes correspond with findings elsewhere that rapid detection of microbes facilitates the introduction of appropriate narrow spectrum targeted therapy and reduction of patient length of stay or, indeed, avoidance altogether of admission via outpatient antibiotic therapy (OPAT). Related sophisticated use of MALDI-TOF further improves antibiotic usage metrics through detection of false positive blood samples associated with environmental or skin contaminants.26
Further, MALDI-TOF is both cost and environmentally effective when compared with conventional microbiology. Gaillot et al.27 demonstrated 89.3% cost savings in the first year of MALDI-TOF use, whereby cost per isolate decreased from $5.80 to $0.50 and waste reduced from >1400 kg to <50 kg, in addition to lesser need for DNA and/or RNA sequencing and, overall, significantly enhanced time to bacterial identification. At the time of writing, microbiological testing at UHL was predominantly based on the bioMérieux® ARIS system costing approximately €3.50 per identification, and with ca. €17 000 annual expenditure on supplementary identification methods (bioMérieux®API® and RemelRapID®).
PCR and Patients
Polymerase chain reaction (PCR) is used extensively for identification of multiple pathogens, commonly including methicillin-resistant Staphylococcus aureus, Clostridium difficile, Streptococcus agalacticae (group B Streptococcus), and bacterial causes of meningitis. At UHL, polymerase chain reaction (PCR) is used extensively in testing for Chlamydia trachomatis and Neisseria gonorrhoeae. Between January and December 2013, 13,338 clinical samples, on average 1100 per month, were analyzed using PCR (Table 2). Such testing introduced a “same-day service,” with results available for clinicians within hours of a patient’s attendance at an on-site sexual health clinic, facilitating early commencement of directed-therapy and reduced patient anxiety. We also use PCR in evaluating success or otherwise of antiviral therapies and for long-term follow-up of patients with HIV and Hepatitis B and C attending our local services.
Table 2. PCR analysis at University Hospital Limericka.
| Annual | Monthly | ||
|---|---|---|---|
| Mean | Min - Max | ||
| Total samples tested | 15732 | 1311 | 2.0‒27 |
| Samples tested for potential STIb only | 13338 | 1112 | 769‒1148 |
| For Clostridium difficile (toxin)c | 1714d | 429 | 415‒447 |
| For Salmonella, Shigella, Campylobacter, VTECc | 2680e | 670 | 544‒941 |
a January–December 2013; discrete clinical samples tested. bSTI: sexually transmitted infection, specifically Chlamydia trachomatis and Neisseria gonorrhoeae. cIntroduced at UHL in September 2013. dProjected annual total based on 4 mo analysis: 5142. eProjected annual total based on 4 mo analysis: 8040
Decision-making regarding respiratory viruses, for example, respiratory syncitial virus (RSV) in pediatric patients has been reduced at UHL from up to a week to just hours, with significant benefits for hospital bed management and prompt initiation of antiviral therapy as necessary, and as reported elsewhere.28 Detection of Herpes viruses and a variety of enteroviruses via PCR is performed on all cerebrospinal fluid samples received in the laboratory, thus supporting appropriate timely management of common viral central nervous system infections.29
In our hands, PCR for analysis of stool samples has reduced turnaround time for samples by up to 24 h. C. difficile toxin testing via PCR was introduced at UHL in September 2013, with over 400 stool samples now analyzed each month. At a practical level, given the transmissibility of C. difficile, the need for urgent isolation, commencement of treatment, and the potential for serious complications such as toxic megacolon, the introduction of PCR has considerably improved our patient service.
PCR technology continues to evolve and ready analysis of samples obtained directly from clinical specimens such as pleural fluid, central spinal fluid, blood, joint aspirates, heart valves, and abscess aspirates is increasingly commonplace.30 Regularly, broad-range 16S rDNA PCR is utilized in the context of culture negative samples (arguably rendered unculturable by the use of empiric antibiotics) where clinical suspicion exists but conventional microbiology has not confirmed infection.31
Practical Issues Associated with Molecular Microbiology
No diagnostic technology is universally applicable. For example, important pathogens such as Streptococcus pnuemoniae, β-hemolytic streptococci, and enteric pathogens such as Shigella spp. and Eschericheria coli remain difficult to identify using MALDI-TOF MS. For this reason, microbiologists remain reliant on traditional methods of identification and for antimicrobial susceptibility testing. On a financial note, there are also capital funding considerations in adopting molecular technologies. Many clinical laboratories will require refurbishment, or new builds, before being suitable to accommodate molecular equipment such as MALDI-TOF MS or to facilitate laboratory automation using suites such as Becton Dickinson’s Kiestra™. Indeed, movement toward sophistication of laboratory methods should ideally to be integrated with electronic patient records (i.e., [national] unique patient identification numbers) to avoid duplication of unnecessary tests caused by patients having duplicate or triplicate patient laboratory registration numbers. At least in Ireland, such a system does not yet exist. An equally important consideration is the requirement for upskilling of staff and maintenance of expensive equipment in the context of stringent laboratory accreditation requirements, such as compliance with ISO standard 17025.
Training in the Molecular Era
There are challenges for clinical microbiologists and physicians in keeping pace with rapidly changing discoveries in molecular diagnostic techniques, for instance, the potential use of MALDI-TOF or high-resolution melting PCR analysis in assessment of antimicrobial susceptibility and isolate profiling.32,33 Training programs for specialist physicians in clinical microbiology will need to take greater account of this and allow time for medical professionals to become and remain familiar with emerging developments, both their advantages and, from a patient safety perspective, their limitations. A further risk to be mitigated is the potential de-skilling of medical scientists with the advent of, and over-reliance on, modern technologies.
Conclusions
Clinical microbiology is pivotal to patient outcomes in providing highly accurate diagnostic and supportive advisory services to clinicians across all specialties in acute settings and primary care. The introduction of molecular technology and automation fundamentally changes the way in which laboratory diagnoses are reached, providing superior laboratory services to physicians and patients. Molecular diagnostics afford improved sensitivity and specificity, and the possibility of rapid diagnoses, with turnaround times of hours rather than days, with subsequent reduced length of hospital stay. The increase in antimicrobial resistance globally reinforces the need for more rapid diagnostics, facilitating judicious use of effective antimicrobials and mitigated risk of hospital-acquired infections and, in summary, improving patient-centered care.
Disclosure of Potential Conflicts of Interest
No potential conflict of interest was disclosed.
Acknowledgments
We are grateful to the staff of the microbiology laboratory, and to Mike O’Nolan (IT Department), at University Hospital Limerick for their contributions to this commentary.
References
- 1.Armstrong GL, Conn LA, Pinner RW. Trends in infectious disease mortality in the United States during the 20th century. JAMA. 1999;281:61–6. doi: 10.1001/jama.281.1.61. [DOI] [PubMed] [Google Scholar]
- 2.Daniels R. Surviving the first hours in sepsis: getting the basics right (an intensivist’s perspective) J Antimicrob Chemother. 2011;66(Suppl 2):ii11–23. doi: 10.1093/jac/dkq515. [DOI] [PubMed] [Google Scholar]
- 3.Phua J, Ho BC, Tee A, Chan KP, Johan A, Loo S, So CR, Chia N, Tan AY, Tham HM, et al. The impact of clinical protocols in the management of severe sepsis: a prospective cohort study. Anaesth Intensive Care. 2012;40:663–74. doi: 10.1177/0310057X1204000413. [DOI] [PubMed] [Google Scholar]
- 4.Yang S, Rothman RE. PCR-based diagnostics for infectious diseases: uses, limitations, and future applications in acute-care settings. Lancet Infect Dis. 2004;4:337–48. doi: 10.1016/S1473-3099(04)01044-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bourbeau PP, Ledeboer NA. Automation in clinical microbiology. J Clin Microbiol. 2013;51:1658–65. doi: 10.1128/JCM.00301-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dokouhaki P, Blondeau JM. Advances in laboratory diagnostic technologies in clinical microbiology and what this means for clinical practice. Clin Pract. 2012;9:347–52. doi: 10.2217/cpr.12.32. [DOI] [Google Scholar]
- 7.Duszyńska W. Strategies of empiric antibiotic therapy in severe sepsis. Anaesthesiol Intensive Ther. 2012;44:96–103. [PubMed] [Google Scholar]
- 8.Carleton A, Casserly B, Power L, Linnane B, O’flaherty G, Powell J, Hartnett P, Collins J, Murphy P, Kenna D, et al. Clustered multidrug‐resistant Bordetella petrii in adult cystic fibrosis patients in Ireland: case report and review of antimicrobial therapies. JMM Case Reports. 2014;1:1–6. [Google Scholar]
- 9.Carbonnelle E, Mesquita C, Bille E, Day N, Dauphin B, Beretti JL, Ferroni A, Gutmann L, Nassif X. MALDI-TOF mass spectrometry tools for bacterial identification in clinical microbiology laboratory. Clin Biochem. 2011;44:104–9. doi: 10.1016/j.clinbiochem.2010.06.017. [DOI] [PubMed] [Google Scholar]
- 10.Seng P, Rolain JM, Fournier PE, La Scola B, Drancourt M, Raoult D. MALDI-TOF-mass spectrometry applications in clinical microbiology. Future Microbiol. 2010;5:1733–54. doi: 10.2217/fmb.10.127. [DOI] [PubMed] [Google Scholar]
- 11.Neville SA, Lecordier A, Ziochos H, Chater MJ, Gosbell IB, Maley MW, van Hal SJ. Utility of matrix-assisted laser desorption ionization-time of flight mass spectrometry following introduction for routine laboratory bacterial identification. J Clin Microbiol. 2011;49:2980–4. doi: 10.1128/JCM.00431-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bullman S, Lucey B, Sleator RD. Molecular diagnostics: the changing culture of medical microbiology. Bioeng Bugs. 2012;3:1–7. doi: 10.4161/bbug.3.1.19011. [DOI] [PubMed] [Google Scholar]
- 13.Bizzini A, Durussel C, Bille J, Greub G, Prod’hom G. Performance of matrix-assisted laser desorption ionization-time of flight mass spectrometry for identification of bacterial strains routinely isolated in a clinical microbiology laboratory. J Clin Microbiol. 2010;48:1549–54. doi: 10.1128/JCM.01794-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Loonen AJ, Jansz AR, Bergland JN, Valkenburg M, Wolffs PF, van den Brule AJ. Comparative study using phenotypic, genotypic, and proteomics methods for identification of coagulase-negative staphylococci. J Clin Microbiol. 2012;50:1437–9. doi: 10.1128/JCM.06746-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Santos C, Paterson RR, Venâncio A, Lima N. Filamentous fungal characterizations by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. J Appl Microbiol. 2010;108:375–85. doi: 10.1111/j.1365-2672.2009.04448.x. [DOI] [PubMed] [Google Scholar]
- 16.Cobo F. Application of maldi-tof mass spectrometry in clinical virology: a review. Open Virol J. 2013;7:84–90. doi: 10.2174/1874357920130927003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.La Scola B, Fournier PE, Raoult D. Burden of emerging anaerobes in the MALDI-TOF and 16S rRNA gene sequencing era. Anaerobe. 2011;17:106–12. doi: 10.1016/j.anaerobe.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 18.Barreau M, Pagnier I, La Scola B. Improving the identification of anaerobes in the clinical microbiology laboratory through MALDI-TOF mass spectrometry. Anaerobe. 2013;22:123–5. doi: 10.1016/j.anaerobe.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 19.Carbonnelle E, Grohs P, Jacquier H, Day N, Tenza S, Dewailly A, Vissouarn O, Rottman M, Herrmann JL, Podglajen I, et al. Robustness of two MALDI-TOF mass spectrometry systems for bacterial identification. J Microbiol Methods. 2012;89:133–6. doi: 10.1016/j.mimet.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 20.Werno AM, Christner M, Anderson TP, Murdoch DR. Differentiation of Streptococcus pneumoniae from nonpneumococcal streptococci of the Streptococcus mitis group by matrix-assisted laser desorption ionization-time of flight mass spectrometry. J Clin Microbiol. 2012;50:2863–7. doi: 10.1128/JCM.00508-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tan KE, Ellis BC, Lee R, Stamper PD, Zhang SX, Carroll KC. Prospective evaluation of a matrix-assisted laser desorption ionization-time of flight mass spectrometry system in a hospital clinical microbiology laboratory for identification of bacteria and yeasts: a bench-by-bench study for assessing the impact on time to identification and cost-effectiveness. J Clin Microbiol. 2012;50:3301–8. doi: 10.1128/JCM.01405-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Loonen AJ, Jansz AR, Stalpers J, Wolffs PF, van den Brule AJ. An evaluation of three processing methods and the effect of reduced culture times for faster direct identification of pathogens from BacT/ALERT blood cultures by MALDI-TOF MS. Eur J Clin Microbiol Infect Dis. 2012;31:1575–83. doi: 10.1007/s10096-011-1480-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baillie S, Ireland K, Warwick S, Wareham D, Wilks M. Matrix-assisted laser desorption/ionisation-time of flight mass spectrometry: rapid identification of bacteria isolated from patients with cystic fibrosis. Br J Biomed Sci. 2013;70:144–8. doi: 10.1080/09674845.2013.11669948. [DOI] [PubMed] [Google Scholar]
- 24.Lagacé-Wiens PR, Adam HJ, Karlowsky JA, Nichol KA, Pang PF, Guenther J, Webb AA, Miller C, Alfa MJ. Identification of blood culture isolates directly from positive blood cultures by use of matrix-assisted laser desorption ionization-time of flight mass spectrometry and a commercial extraction system: analysis of performance, cost, and turnaround time. J Clin Microbiol. 2012;50:3324–8. doi: 10.1128/JCM.01479-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schieffer KM, Tan KE, Stamper PD, Somogyi A, Andrea SB, Wakefield T, Romagnoli M, Chapin KC, Wolk DM, Carroll KC. Multicenter evaluation of the Sepsityper™ extraction kit and MALDI-TOF MS for direct identification of positive blood culture isolates using the BD BACTEC™ FX and VersaTREK(®) diagnostic blood culture systems. J Appl Microbiol. 2014;116:934–41. doi: 10.1111/jam.12434. [DOI] [PubMed] [Google Scholar]
- 26.Tenover FC. Potential impact of rapid diagnostic tests on improving antimicrobial use. Ann N Y Acad Sci. 2010;1213:70–80. doi: 10.1111/j.1749-6632.2010.05827.x. [DOI] [PubMed] [Google Scholar]
- 27.Gaillot O, Blondiaux N, Loïez C, Wallet F, Lemaître N, Herwegh S, Courcol RJ. Cost-effectiveness of switch to matrix-assisted laser desorption ionization-time of flight mass spectrometry for routine bacterial identification. J Clin Microbiol. 2011;49:4412. doi: 10.1128/JCM.05429-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Steed LL, Ball RT., Jr. Respiratory virus detection: beyond influenza and RSV into emerging infectious diseases. J S C Med Assoc. 2013;109:85–7. [PubMed] [Google Scholar]
- 29.López Roa P, Alonso R, de Egea V, Usubillaga R, Muñoz P, Bouza E. PCR for detection of herpes simplex virus in cerebrospinal fluid: alternative acceptance criteria for diagnostic workup. J Clin Microbiol. 2013;51:2880–3. doi: 10.1128/JCM.00950-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jensen KH, Dargis R, Christensen JJ, Kemp M. Ribosomal PCR and DNA sequencing for detection and identification of bacteria: experience from 6 years of routine analyses of patient samples. APMIS. 2014;122:248–55. doi: 10.1111/apm.12139. [DOI] [PubMed] [Google Scholar]
- 31.Duffett S, Missaghi B, Daley P. Culture-negative endocarditis diagnosed using 16S DNA polymerase chain reaction. Can J Infect Dis Med Microbiol. 2012;23:216–8. doi: 10.1155/2012/312607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kostrzewa M, Sparbier K, Maier T, Schubert S. MALDI-TOF MS: an upcoming tool for rapid detection of antibiotic resistance in microorganisms. Proteomics Clin Appl. 2013;7:767–78. doi: 10.1002/prca.201300042. [DOI] [PubMed] [Google Scholar]
- 33.Gabriel EM, Douarre PE, Fitzgibbon S, Clair J, Lucey B, Coffey A, O’Mahony JM. High-resolution melting analysis for rapid detection of linezolid resistance (mediated by G2576T mutation) in Staphylococcus epidermidis. J Microbiol Methods. 2012;90:134–6. doi: 10.1016/j.mimet.2012.04.002. [DOI] [PubMed] [Google Scholar]