Abstract
Chikungunya fever, a re-emerging infection, is an arthropod-borne viral disease prevalent in different parts of the world, particularly Africa and South East Asia. Chikungunya virus envelope 2 protein is involved in binding to host receptors and it contains specific epitopes that elicit virus neutralizing antibodies. A highly immunogenic, recombinant Chikungunya virus envelope 2 protein was produced by bioreactor in Escherichia coli for development of a suitable diagnostic and vaccine candidate. This protein was refolded and further purified to achieve biologically active protein. The biological function of refolded and purified recombinant envelope 2 protein of Chikungunya virus was confirmed by its ability to generate envelope 2 specific antibodies with high titers in animal models. These findings suggest that recombinant envelope 2 protein of Chikungunya virus in combination with compatible adjuvant is highly immunogenic. Thus, recombinant envelope 2 protein can be a potential diagnostic reagent and vaccine candidate against Chikungunya virus infection.
Keywords: Chikungunya, Escherichia coli, fermentation, immunogenicity, Plackett-Burman design, vaccine, virus
Chikungunya fever is a mosquito-borne viral disease caused by the Chikungunya virus, which belongs to family Togaviridae and genus Alphavirus. The global prevalence of Chikungunya fever has increased dramatically in recent years.1 Currently, there is no specific treatment for Chikungunya virus infection, and no licensed vaccine is available for human use. The envelope 1 (E1) and envelope 2 (E2) are conserved among alphaviruses; hence the E1 and E2 glycoproteins serve as the major target antigen in the development of a diagnostic or subunit vaccine against Chikungunya virus infections. For this reason, the envelope 2 protein of Chikungunya virus is an important immunogen for a subunit vaccine, as well as a prospective diagnostic reagent for the improved clinical diagnosis of Chikungunya virus infections. Researchers developed experimental Chikungunya virus vaccine based on virus-like particles containing both E1 and E2 antigen, which induced a protective immune response in non-human primates.2 However, mammalian cells systems remain challenging in terms of up scaling for vaccine production. Therefore, a subunit vaccine approach may be better compatible with large-scale operations.3 Thus, there is a need to develop a production process for E2 protein to further study its vaccine potential. Escherichia coli is the commonly used host strain for recombinant protein production. The main challenge for large-scale protein production using E. coli is to achieve a high volumetric productivity of a target protein with a high quality and biological activity. The improvement of the cultivation techniques and the manipulation of the physiology of cells are crucial for obtaining a high protein yield.4 Generally, for processes using E. coli, the batch and fed-batch cultivation techniques at a small and a large scale have become the method of choice to increase the volumetric productivity of the target protein.5,6 Many biological products obtained from E. coli are produced in fermentors, including amino acids, poly (3-hydroxybutyrate), diagnostics, vaccines, and various therapeutic recombinant proteins.7 Generally, process development for protein production begins with screening and optimization stages, which are usually performed at small scale vessel such as shake flask cultures. The recombinant protein expression level in E. coli depends on various important parameters such as pH, temperature, medium composition, duration of induction, and concentration of inducer. These parameters can be optimized at small scale and then applied to large scale protein production.7 The scale up process of recombinant Chikungunya virus E2 protein production could be performed by fed-batch or batch fermentations in place of small scale shake flask cultures.8 To facilitate the purification of recombinant proteins, the proteins are commonly produced as fusion proteins that comprise of target protein fused with an affinity tag, such as the hexahistidine tag.9 The expression of recombinant proteins in E. coli often results in the form of inclusion bodies.10 The inclusion bodies must be solubilized and refolded to recover active protein for vaccine studies. Refolding is usually achieved by removing the chaotrope via buffer exchange after solubilizing the inclusion bodies, using dilution, dialysis, diafiltration, or chromatography. Protein refolding by liquid chromatography is an alternative to the other methods. Immobilized metal ion affinity chromatography (IMAC) has become a well-established and versatile technique for protein purification. It has recently been reported that IMAC has the potential to perform protein refolding with high recovery of purified recombinant proteins.9 The purity level of these proteins can be further improved by more chromatography steps, viz., ion exchange or size exclusion chromatography. In our previous study, we reported the media optimization, batch, and fed fermentation as well as affinity purification under denaturing conditions for production of recombinant envelope 2 protein of Chikungunya virus. Furthermore, we have evaluated the diagnostic potential of this protein for detection of Chikungunya virus infection in patient serum samples using ELISA.8 We have used commercially available media as well as the same medium with some additional nutrients for media optimization studies. The additional nutrients were selected based on earlier studies for recombinant proteins production in E. coli.7 High cell density fed-batch cultivation process for recombinant Chikungunya virus envelope 2 protein resulted in about 35 g/l dry cell weight. The E2 protein concentration was 190 mg/l after affinity chromatography purification under denaturing conditions from fed-batch culture. In a 6.6 L fermentor with 5 L working volume, a pH and DO stat feeding strategy was adopted where culture pH and DO served as an indicator of the nutrient status of the culture.8 Procedure for preparation of biologically active recombinant Chikungunya virus envelope 2 protein in E. coli is shown in Figure 1.
Figure 1. Procedure for preparation of recombinant CHIKV E2 protein for its immunogenicity.
A Plackett-Burman experimental design was used to test 11 factors for their effect on Chikungunya E2 protein expression. The 11 environmental factors tested for this design were included as following: culture temperature, pH, yeast extract concentration, concentration of M9 minimal salt, concentration of magnesium sulfate, tryptone concentration, concentration of ammonium sulfate, trace metals, induction time, and the concentration of IPTG inducer. Screening experiments were performed in shake flasks (500 ml). About 2 ml of inoculum culture was added to each of 12 flasks containing 100 ml of each medium as mentioned in Table 1. All cultures were grown for 4 h at 180–200 rpm. The culture was induced with 1 mM IPTG and further grown for 4 h. The culture medium components used, cultivation conditions adopted and the results are summarized in Table 1. Finally cell density (OD at 600 nm) and total protein were measured. The effect of each variable on total protein production is given in Table 2. The increase in the yeast extract concentration from 10 to 25 g/l had the maximum effect on the total protein production of recombinant Chikungunya virus E2 protein (Fig. 2). This was likely because yeast extract provided a source of amino acids and trace metals to enhance the cell growth rate. Cultures grown in media rich in yeast extract are known to produce high growth rates with a high rate of total protein synthesis. The cultivation temperature had a negative effect on the total protein expression of the recombinant protein (Table 2). The concentration of the inducer, magnesium sulfate, and ammonium sulfate had a very little effect on protein expression (Fig. 2). The post induction timing had strong effect on protein expression. The total protein concentration decreases when the time of induction is increased. Media supplementation with M9 minimal salt has been reported to increase cell growth and recombinant protein production.11 The M9 salt (1×) contained sufficient phosphate and further increase had not much effect on protein production (Table 2). The change in pH from 6.8 to 7.2 led to decrease in protein expression (Table 2). The Pareto chart shown in Figure 2 offers a convenient way to view the results of Plackett-Burman experimental design. The Pareto chart illustrates the order of significance of the experimental factors affecting the protein expression. The order of significance as shown in Figure 2 is yeast extract concentration, induction time, tryptone concentration, pH, trace metals, temperature, glycerol concentration, M9 salt concentration, ammonium sulfate concentration, magnesium sulfate concentration, and inducer concentration.
Table 1. Plackett Burman experimental design for evaluation of 11 variables with coded values for recombinant Chikungunya E2 protein and response of the design.
| Experiment | A | B | C | D | E | F | G | H | J | K | L | R1 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | - | - | - | + | + | + | + | - | + | + | + | 1200 |
| 2 | - | + | + | + | - | - | - | + | - | + | + | 1600 |
| 3 | - | - | + | - | + | + | - | + | + | + | - | 1376 |
| 4 | + | + | - | - | - | + | - | + | + | - | + | 1880 |
| 5 | + | - | - | - | + | - | + | + | - | + | + | 1856 |
| 6 | + | + | - | + | + | + | - | - | - | + | - | 1560 |
| 7 | + | - | + | + | - | + | + | + | - | - | - | 1792 |
| 8 | - | + | + | - | + | + | + | - | - | - | + | 1616 |
| 9 | - | - | - | - | - | - | - | - | - | - | - | 1688 |
| 10 | + | + | + | - | - | - | + | - | + | + | - | 1744 |
| 11 | - | + | - | + | + | - | + | + | + | - | - | 1370 |
| 12 | + | - | + | + | + | - | - | - | + | - | + | 1448 |
A, Yeast Extract concentration (g/l); B, Glycerol concentration (ml/l); C, Inducer concentration (mM); D, Induction time (h); E, pH; F, M9 salt concentration (x); G, MgSO4 concentration; H, Trace metal (ml/l); J, Tryptone concentration; K, Temperature (°C); L, (NH4)2SO4 concentration; R1, Total protein (mg/l)
Table 2. Analysis of medium optimization using Plackett Burman design for recombinant Chikungunya E2 protein production.
| Factors | Lower Level | Higher Level | Effects | Sum of square | % Explained |
|---|---|---|---|---|---|
| A | 10 | 25 | 238.33 | 170408.3 | 34.29 |
| B | 10 | 20 | 68.33 | 14008.3 | 2.82 |
| C | 0.5 | 1.0 | 3.67 | 40.3 | 0.01 |
| D | 4 | 6 | -198.33 | 118008.3 | 23.75 |
| E | 6.8 | 7.2 | -113.0 | 38307.0 | 7.71 |
| F | 1 | 2 | -47.0 | 6627.0 | 1.33 |
| G | 1.2 | 2.4 | 4.33 | 56.3 | 0.01 |
| H | 1 | 2 | 103.0 | 31827.0 | 6.41 |
| J | 5 | 12 | -182.33 | 99736.3 | 20.07 |
| K | 35 | 37 | -76.33 | 17480.3 | 3.52 |
| L | 1.2 | 2.4 | 11.67 | 408.33 | 0.08 |
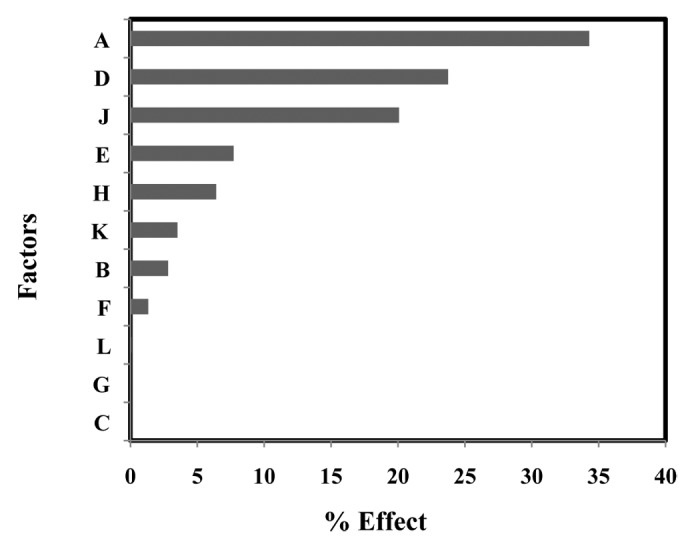
Figure 2. Pareto-Plot for Plackett-Burman parameter estimates for 11 components evaluated of recombinant Chikungunya virus E2 protein.
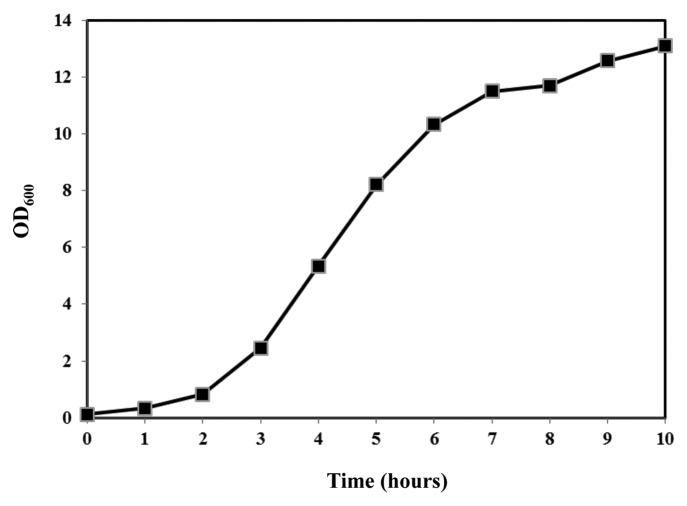
A pure and biologically active protein was required to conduct vaccine studies with recombinant Chikungunya virus E2 (rCHIKV E2) protein. We developed a batch fermentation process to produce rCHIKV E2 protein in large quantities for use in vaccine studies. Figure 1 provides an overall flow diagram for the production of rCHIKV E2 antigen for vaccine studies. Batch fermentations were performed to produce large amount of biomass for purification of rCHIKV E2 protein using 5 L working volume bioreactor The cultivation medium used for batch fermentation was modified M9 minimal salt medium (1× M9 minimal salt, 25 g yeast extract, 5 g tryptone, 10 ml glycerol, 1.2 g magnesium sulfate, 1.2 g ammonium sulfate, and 1 ml trace metals8 per liter). The conditions for batch fermentation were described elsewhere.5,8 The growth profile of batch process is shown in Figure 3. This fermentation process continued for ten hour which comprised of a pre-induction (six hours) and a post induction (four hours) phase. At the beginning of the fermentation DO was above 30% and it decreased during course of process. The DCW and OD600 increased continuously and reached a final value of 6.80 g/l and 13.10 (Fig. 3) respectively. The DCW at induction was 3.15 g/l. The successful production of this protein with a high yield in a bioreactor was initiated to foster further applications and research with Chikungunya vaccine. We developed an on-column refolding cum purification process for recombinant Chikungunya virus envelope 2 protein to obtain biologically active antigen for vaccine studies. Purification of rCHIKV E2 protein was performed from the cell pellet obtained from fermentation as method described earlier.5 The final product concentration of rCHIKV E2 protein following IMAC was 35 mg/l. Further purification using gel filtration chromatography using phosphate buffer saline resulted in 22 mg/l of rCHIKV E2 protein.
Figure 3. Batch fermentation profile (OD vs. time) of E. coli cells for rCHIKV E2 protein production using 5 L working volume bioreactor. Modified M9 minimal salt medium was used for batch cultivation. After 6 h of culture growth, 1 mM IPTG was added in bioreactor culture and growth continued for further 4 h.
This purified protein was used for immunization in rabbit. The techniques used for bleeding, and sacrifice of animals were strictly performed following mandates approved by the animal ethics committee (Committee for the Purpose of Control and Supervision of Experiments on Animals, Govt. of India). The biological activity of the rCHIKV E2 protein was determined by ELISA.5 The purified protein was used for raising hyper immune sera in rabbit. For this purpose, 200 µg of purified protein was injected per rabbit per dose. The protein was emulsified with FCA (Freund’s Complete Adjuvant) in 1:1 ratio. For subsequent booster doses, this protein was formulated with FIA (Freund’s Incomplete Adjuvant) instead of FCA. The control rabbit was injected with adjuvant only in phosphate buffer saline (PBS). About 1 ml of emulsified rCHIKV E2 protein (200 µg) was injected subcutaneously at interval of 0, 14, and 28 d. After 14th day of last inoculation the rabbits were bled and serum was collected. Sera collected from rabbit were tested for the recognition of rCHIKV E2 protein by ELISA. Microtiter ELISA plate (Nunc) was coated with this protein (300 ng per 100 µl per well) in coating buffer (0.05 M carbonate-bicarbonate buffer, pH 9.6) and incubated at 37 °C for 1h. The further procedure was similar to the methods described elsewhere.5 Goat Anti-rabbit (IgG) antibodies conjugated to horseradish peroxidase (1:5000 dilution) was used as secondary antibody and TMB as substrate. The absorbance was measured at 450 nm using an ELISA plate reader.
Selection of an antigen is an important aspect for the development of diagnostic, prophylactic or therapeutic candidates. The envelope protein of the Chikungunya virus is the major antigen used to elicit neutralizing antibody response and protective immunity in hosts. In the previous studies, investigators have expressed the E2 protein at small scale and evaluated the protection using these proteins.3,12-14 Production of important foreign proteins in E. coli expression system has been highly successful in recent past. The ability to produce a vaccine candidate with a process that is amenable to scale-up, reproducible, and capable of producing reasonable number of doses of the vaccine is important for process development.15 Based on the earlier studies on rCHIKV E2 protein, we envisioned producing this vaccine candidate in a bioreactor. In this study, we describe the process development by fermentation, purification and immunogenicity of a candidate vaccine recombinant rCHIKV E2 antigen. We developed a purification process for rCHIKV E2 protein to obtain biologically active protein suitable for vaccine studies. The simultaneous refolding using affinity chromatography removed a majority of contaminants as well as denaturants. To further purify this protein, gel filtration chromatography was developed. About 22 mg of pure, refolded, and biologically active rCHIKV E2 protein was obtained form 1 L of batch fermentation culture. Efforts are being made to further improve the final yield. The characterization of rCHIKV E2 protein by ELISA with sera raised in rabbit confirms that E2 protein is reactive (Fig. 4). Our initial evaluation indicated that the rCHIKV E2 protein in combination with adjuvant elicited humoral immune response in rabbit. Absorbance of adjuvant control sera tested at 1:1600 dilution was 0.343. The rCHIKV E2 antigen specific antibody titer was found to be more than 51 200 (Fig. 4). The result shows that rCHIKV E2 protein produced in bioreactor is able to elicit E2 specific antibody responses. The end point titers of the FCA group sera are shown in Figure 4. The rCHIKV E2 antigen with adjuvant was highly immunogenic and yielded good ELISA titers. This rCHIKV E2 protein could also be used for the detection of antibody in Chikungunya virus infection in patients’ serum samples.8 The humoral response was characterized by high titers of antibodies (Fig. 4). These antibodies could also neutralize Chikungunya virus. Neutralizing antibodies could bind to virus and prevent virus from binding to host cell receptors. The present study reveals the vaccine potential of recombinant CHIKV E2 protein against Chikungunya virus. An ideal subunit vaccine using envelope 2 protein of Chikungunya virus needs to be correctly folded and should be well characterized. This is the first report on the use of a bioreactor produced, refolded and purified E2 protein which has been used for the immunomodulatory studies in combination with adjuvant and evaluation of humoral immune response.
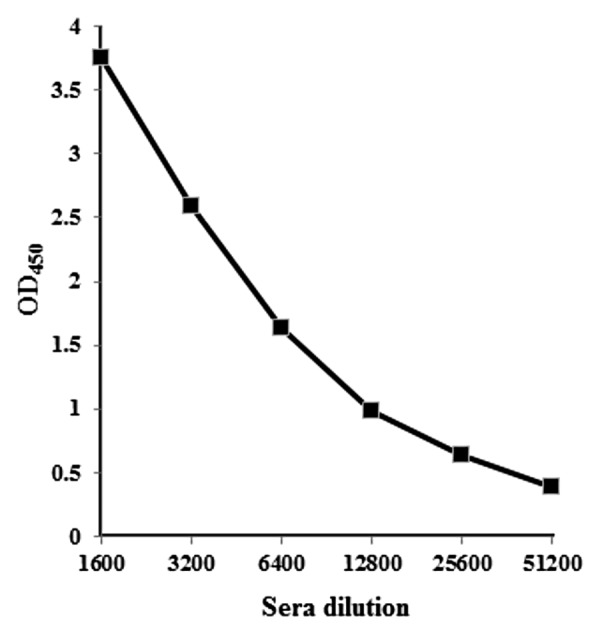
Figure 4. Endpoint titers for recognition of rCHIKV E2 protein. Sera from rabbit collected at days 42 were tested for recognition of recombinant rCHIKV E2 protein at various dilutions by ELISA.
In conclusion, we have developed a scalable process for production of recombinant Chikungunya virus envelope 2 protein. A simple, two-step purification strategy involving immobilized metal affinity chromatography with simultaneous refolding followed by gel filtration chromatography was used to purify rCHIKV E2 protein. The characterization of purified and refolded rCHIKV E2 protein for its immunogenicity established its application in further vaccine development. These findings suggest that rCHIKV E2 protein in combination with compatible adjuvant is immunogenic and could elicit neutralizing antibodies, which proves that refolded and purified rCHIKV E2 protein could be a potential vaccine and diagnostic candidate against Chikungunya virus infection.
Disclosure of Potential Conflicts of Interest
No potential conflict of interest was disclosed.
Acknowledgments
The authors are thankful to Prof MP Kaushik, Director, DRDE, Gwalior, for his keen interest, constant support, and providing necessary facilities for this study.
References
- 1.Reddy V, Ravi V, Desai A, Parida M, Powers AM, Johnson BW. Utility of IgM ELISA, TaqMan real-time PCR, reverse transcription PCR, and RT-LAMP assay for the diagnosis of Chikungunya fever. J Med Virol. 2012;84:1771–8. doi: 10.1002/jmv.23406. [DOI] [PubMed] [Google Scholar]
- 2.Akahata W, Yang ZY, Andersen H, Sun S, Holdaway HA, Kong WP, Lewis MG, Higgs S, Rossmann MG, Rao S, et al. A virus-like particle vaccine for epidemic Chikungunya virus protects nonhuman primates against infection. Nat Med. 2010;16:334–8. doi: 10.1038/nm.2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Metz SW, Geertsema C, Martina BE, Andrade P, Heldens JG, van Oers MM, Goldbach RW, Vlak JM, Pijlman GP. Functional processing and secretion of Chikungunya virus E1 and E2 glycoproteins in insect cells. Virol J. 2011;8:353. doi: 10.1186/1743-422X-8-353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Glazyrina J, Krause M, Junne S, Glauche F, Storm D, Neubauer P. Glucose-limited high cell density cultivations from small to pilot plant scale using an enzyme-controlled glucose delivery system. N Biotechnol. 2012;29:235–42. doi: 10.1016/j.nbt.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 5.Tripathi NK, Shrivastava A, Biswal KC, Rao PV. Development of a pilot-scale production process and characterization of a recombinant Japanese encephalitis virus envelope domain III protein expressed in Escherichia coli. Appl Microbiol Biotechnol. 2012;95:1179–89. doi: 10.1007/s00253-012-4100-6. [DOI] [PubMed] [Google Scholar]
- 6.Tripathi NK, Kumar JS, Biswal KC, Rao PV. Production of recombinant nonstructural 1 protein in Escherichia coli for early detection of Japanese encephalitis virus infection. Microb Biotechnol. 2012;5:599–606. doi: 10.1111/j.1751-7915.2012.00344.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang CJ, Lin H, Yang X. Industrial production of recombinant therapeutics in Escherichia coli and its recent advancements. J Ind Microbiol Biotechnol. 2012;39:383–99. doi: 10.1007/s10295-011-1082-9. [DOI] [PubMed] [Google Scholar]
- 8.Tripathi NK, Priya R, Shrivastava A. Production of recombinant Chikungunya virus envelope 2 protein in Escherichia coli. Appl Microbiol Biotechnol. 2013 doi: 10.1007/s00253-013-5426-4. [DOI] [PubMed] [Google Scholar]
- 9.Wang C, Wang L, Geng X. Optimization of refolding with simultaneous purification of recombinant human granulocyte colony-stimulating factor from Escherichia coli by immobilized metal ion affinity chromatography. Biochem Eng J. 2009;43:197–202. doi: 10.1016/j.bej.2008.09.018. [DOI] [Google Scholar]
- 10.Panda AK. Bioprocessing of therapeutic proteins from the inclusion bodies of Escherichia coli. Adv Biochem Eng Biotechnol. 2003;85:43–93. doi: 10.1007/3-540-36466-8_3. [DOI] [PubMed] [Google Scholar]
- 11.Manderson D, Dempster R, Chisti Y. Production of an active recombinant Aspin antigen in Escherichia coli for identifying animals resistant to nematode infection. Enzyme Microb Technol. 2006;38:591–8. doi: 10.1016/j.enzmictec.2005.03.029. [DOI] [Google Scholar]
- 12.Kumar M, Sudeep AB, Arankalle VA. Evaluation of recombinant E2 protein-based and whole-virus inactivated candidate vaccines against chikungunya virus. Vaccine. 2012;30:6142–9. doi: 10.1016/j.vaccine.2012.07.072. [DOI] [PubMed] [Google Scholar]
- 13.Khan M, Dhanwani R, Rao PV, Parida M. Subunit vaccine formulations based on recombinant envelope proteins of Chikungunya virus elicit balanced Th1/Th2 response and virus-neutralizing antibodies in mice. Virus Res. 2012;167:236–46. doi: 10.1016/j.virusres.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 14.Metz SW, Martina BE, van den Doel P, Geertsema C, Osterhaus AD, Vlak JM, Pijlman GP. Chikungunya virus-like particles are more immunogenic in a lethal AG129 mouse model compared to glycoprotein E1 or E2 subunits. Vaccine. 2013;31:6092–6. doi: 10.1016/j.vaccine.2013.09.045. [DOI] [PubMed] [Google Scholar]
- 15.Bell BA, Wood JF, Bansal R, Ragab H, Cargo J, 3rd, Washington MA, Wood CL, Ware LA, Ockenhouse CF, Yadava A. Process development for the production of an E. coli produced clinical grade recombinant malaria vaccine for Plasmodium vivax. Vaccine. 2009;27:1448–53. doi: 10.1016/j.vaccine.2008.12.027. [DOI] [PubMed] [Google Scholar]