Abstract
Despite advances of treatment for glioblastoma multiforme (GBM), patient prognosis remains poor. Although there is growing evidence that molecular targeting may translate into better survival for GBM, current clinical data show limited impact on survival. Recent progress in GBM genomics implicate several activated pathways and numerous mutated genes. This molecular diversity may partially explain therapeutic resistance, and several approaches have been postulated to target molecular changes. Furthermore, most drugs are unable to reach effective concentrations within the tumor due to elevated intratumoral pressure, restrictive vasculature and other limiting factors. Here we describe the preclinical and clinical developments in treatment strategies of GBM. We review the current clinical trials for GBM and discuss the challenges and future directions of targeted therapies.
Keywords: Glioblastoma, Molecular Targeting, Drug Discovery, Animal Models, Receptor Tyrosine Kinase, Anti-angiogenesis
An Overview of GBM and Treatments
In the United States, the majority of the estimated 20,000 newly diagnosed primary brain tumors annually are gliomas 1, which are named according to the cell type they most closely resemble and likely originated from. The main histological subtypes of gliomas include astrocytomas, oligodendrogliomas and ependymomas. Astrocytomas are graded from I to IV, with grade I and II as slow growing astrocytomas, grade III as anaplastic astrocytomas and grade IV consisting of glioblastoma multiforme (GBM) - the most common (65%) and malignant form of brain tumors. The prognosis of GBM patients is very poor, largely due to early invasion into the central nervous system, making a surgical cure nearly impossible. Only 10 % of those patients including all post-treatment living conditions survive 5 years after diagnosis 1, 4, despite continuous improvement of GBM therapy that currently consists of surgical resection, concurrent or sequential chemo-radiotherapy with temozolomide (TMZ) as the current established first line regimen 1.
GBM is typically characterized by complex chromosome abnormalities and extensive intratumour cytogenetic and histological heterogeneity. Indeed, cytogenetically related or unrelated clones coexist in different regions within the same GBM sample 2, 3. For example, amplification/overexpression of EGFR and EGFRvIII mutation can be found in scattered cell populations in the same GBM specimen 5, 6. In addition to the heterogeneity at genomic level, GBMs also present diversely differentiated tumor cells that may have been originated from the cancer stem cells population, which are multipotent and self-renewal immature tumor cells 8-10.
DNA-damaging drugs such as irinotecan (topoisomerase 1 inhibitor), etoposide (topoisomerase 2 inhibitor), doxorubicin (DNA-intercalating agent), BCNU and TMZ (DNA-alkylating agents), have been frequently used or tried for GBM 1. The lack of clinical success with traditional DNA-damaging chemotherapeutics in systemic use has led to the investigation of targeted therapy that are directed against certain tumoral features including tumor-specific markers, altered signaling or metabolic pathways, tumor vessels and microenvironment. Encouraged by the breakthrough of targeted agents such as imatinib (Gleevec) inhibiting the translocated Abl tyrosine kinase in chronic myelogenous leukemia (CML) 7, multiple concepts were proposed to incorporate targeted agents in GBM treatment. The success of targeted therapy entails several key steps, such as target identification, developing or identifying a small molecule or antibody against the target, relevant preclinical studies and ultimately, clinical trials. This is unfortunately a lengthy and expensive process and compared to other tumors, GBMs are less common, making them less attractive to for-profit entities 11. In this article, we review some of the steps leading to the drug discovery and development for the treatment of GBM.
Drug Discovery and Preclinical Development
Identification of Targets in GBM
A genome-wide analysis of over 20,000 genes from 22 GBM tumor genomes identified most mutations that likely drive glioblastoma formation 12. These DNA alterations were point mutations, small insertions/deletions and larger copy-number changes (genomic amplifications and deletions). It is likely that these represent the most common alterations that drive tumor formation. Analysis of the genes mutated showed that they clustered into pathways that drive cell growth, cell cycle control (such as p53 checkpoint) and other key pathways.
DNA alterations drive cancer formation, and their effect is often reflected in changed patterns of gene transcription. There are many ways to comprehensively assay mRNA levels, one of which is serial analysis of gene expression (SAGE), a technique to produce a snapshot of the messenger RNA population in a sample in the form of short sequence tags that correspond to fragments of those transcripts 13. SAGE has provided the basis of a widely used online public database, providing a comprehensive and convenient way to obtain expression data and cross-compare gene expression levels in different tumor samples or cell lines of brain tumors and other cancers to normal tissue 14 (http://cgap.nci.nih.gov/SAGE/AnatomicViewer).
The alterations involved in GBM development and growth point to the involvement of several key pathways (Figure 1): 1) the PI3K-Akt and RAS oncogenic pathway with EGFR/PI3K/PTEN/NF1/RAS alterations, 2) the TP53 tumor suppressor pathway with TP53/MDM2/MDM4/p14ARF alterations, 3) RB and cell cycle regulators with RB1/ CDK4/p16INK4A/CDKN2B alterations, and 4) the recently discovered metabolic pathway featured by IDH1/IDH2 alterations found in low grade astrocytomas (astrocytoma grade I and II) and secondary GBMs 12, 15, 16.
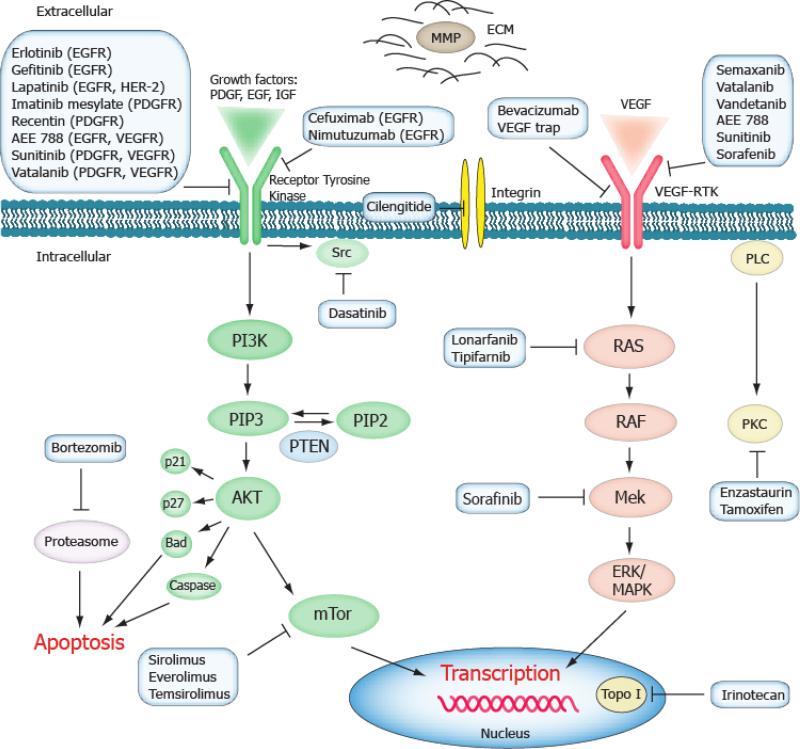
Figure 1.
Schematic overview of current molecular targeted therapies of GBM. Aberrant oncogenic RTK pathways are frequent therapeutic targets in GBM. The PI3K-Akt (green) and RAS (pink) oncogenic pathways are often targeted intracellularly with small molecules inhibitors. EGF, VEGF and PDGF as well as their receptors can be blocked by small molecules and monoclonal antibodies. Items in blue boxes include examples of drugs targeting on the respective pathways. Abbreviations: ECM: extracellular matrix, MMP: matrix metalloproteinase, Topo I: topoisomerase I.
Further potential targeting areas include DNA repair mechanisms, tumor hypoxia, tumor invasion regulation and microenvironments. The main role of poly ADP ribose polymerase (PARP) family proteins is to detect and signal single strand DNA breaks to the repair mechanism. PARP inhibitors can be used as monotherapy in tumor genetic backgrounds deficient in specific DNA repair pathways such as BCRA 1 and 2 17. Frequently, PARP inhibitors have been tried in combination with DNA-damaging agents, like TMZ, topoisomerase inhibitors and radiation that induce PARP-1 activity for the repair of DNA damage, and thereby sensitize tumor cells to these agents 17. Veliparib and BSI 201 are small molecule PARP inhibitors currently in clinical trials in combination with TMZ for primary GBM (Table 1).
Table 1.
Select targeted drugs of GBM in clinical trials.
| Drug | Class / MW | Target | Most recent GBM Trial / Initial or Recurrent GBM | Comments | References |
|---|---|---|---|---|---|
| Erlotinib (Tarceva, OSI-774) | Small molecule / 393 Da | EGFR | Phase II / initial & recurrent | Minimal efficacy as single agent; modest survival benefit with TMZ & radiation; ongoing trials in combination with other drugs; so far no significant efficacy has been reported in completed combination therapies. | 9, 70, 71, 86, 87 |
| Gefetinib (Iressa, ZD1839) | Small molecule / 447 Da | EGFR | Phase II / recurrent | Minimal efficacy as monotherapy compared to current standard RT/TMZ; combination therapies not effective either. | 88-90 |
| Lapatinib (Tykerb, GW572016) | Small molecule / 581 Da | EGFR, ErbB2 | Phase II / recurrent | No efficacy in a trial with small number of recurrent GBMs; one phase II trial is ongoing. | 91 |
| Sunitinib (Sutent, SU11248) | Small molecule / 398 Da w/o malate | PDGFR, VEGFR, c-Kit | Phase II / recurrent | Phase II trials under way. | |
| Sorafenib (Nexavar) | Small molecule / 465 Da | Raf, VEGFR, PDGFR | Phase II / initial & recurrent | Minimal efficacy compared to standard RT/TMZ; ongoing phase II trials in combination with other drugs | 92, 93 |
| Dasatinib (Sprycel) | Small molecule/ 488 Da | BCR-ABL, SRC family kinases | Phase I, II / initial & recurrent | SRC family kinases might promote the invasion of GBM cells; among 7 phase I/II trials of GBM, one was withdrawn and three were suspended. | 94 |
| Nimotuzumab | Humanized extracellular-binding antibody | EGFR | Phase II, III / initial & recurrent | Well tolerated in patients, modest (17.47 mo vs 14.6 mo) survival benefit in small subgroup of GBM or no survival benefit of GBM patients in Cuban patients compared to standard RT/TMZ. | 95,96 |
| Cetuximab (Erbitux) | Chimeric extracellular-binding antibody | EGFR | Phase I, II / initial & recurrent | Phase II trials ongoing; a small group of GBM patient responded in a phase II study; little additional efficacy in combination with irrenotecan and bevacizumab in a phase II trial. | 97, 98 |
| AMG 102 | Human HGF antibody | Hepatocyte growth factor (HGF) | Phase II / recurrent | Phase II trials ongoing. | 99 |
| Imatinib (Gleevec) | Small molecule / 494 Da | PDGFR, c-KIT, BCR-ABL | Phase I, II / recurrent | Minimal efficacy as single agent; after an initially promising phase II trial of imatinib in combination with hydoxyurea, a multicenter study and further trials failed to show meaningful anti-tumor efficacy; further trials of combination therapies are ongoing. | 100-104 |
| Tandutinib (MLN518) | Small molecule / 562 Da | PDGFR, FLT3, c-KIT | Phase II / recurrent | Phase II trials as single agent and in combination with Avastin are underway. | |
| Enzastaurin (LY317615) | Small molecule / 516 Da | PKC, PI3K/AKT pathway inhibitor | Phase I, II, III / initial & recurrent | Limited efficacy in recurrent GBM as monotherapy; in a phase III trial with recurrent GBM, it failed to show superior efficacy compared with lomustine. | 105, 106 |
| Sirolimus (Rapamycin) | Small molecule / 914 Da | mTOR inhibitor | Phase II / initial & recurrent | Not effective as single agent; other phase II trials in combination with EGFR/PI3K pathway inhibitors ongoing; limited efficacy in phase II trial in combination with erlotinib. | 87, 107 |
| Temsirolimus (Toricel, CCI-779) | Small molecule / 1030 Da | mTOR inhibitor, ester analog of sirolimus | Phase I, II / initial & recurrent | Limited or inclusive efficacy as single agent in recurrent GBM; Ongoing trials of combination therapies with EGFR/PI3K pathway inhibitors or Avastin. | 108, 109 |
| Everolimus (RAD-001, Zortress) | Small molecule / 958 Da | mTOR inhibitor, derivative of sirolimus | Phase II / initial & recurrent | No clear clinical benefit in combination with gefitinib in a pilot trial of recurrent GBM; multiple phase II trials of combination therapies ongoing. | 90 |
| Veliparib (ABT-888) | Small molecule / 244 Da | Poly ADP ribose polymerase (PARP) inhibitor | Phase II / initial & recurrent | Currently phase II trials ongoing. | |
| Iniparib (BSI 201) | Small molecule / 292 Da | PARP1 inhibitor | Phase I, II / primary | Currently phase I & II trial recruiting. | |
| Bortezomib (Velcade) | Small peptide / 384 Da | Proteasome inhibitor | Phase II / initial & recurrent | Phase I trials established the safe doses and showed low response rate in recurrent GBM but favorable tendency in initial GBM with standard RT/TMZ. | 110, 111 |
| Cilengitide | Cyclic peptide / 589 Da | αv integrins inhibitor, anti-angiogenesis | Phase II, III / initial & recurrent | Phase I trials found the drug well tolerated also with TMZ; modest efficacy as single agent in recurrent GBM; encouraging results of combining cilengitide with standard TMZ/RT in initial GBM with methylated MGMT promoter, on which a phase III trial is ongoing. | 76, 112 |
Tumor hypoxia can provide another highly interesting subject of therapies. Due to limited vasculature, most solid tumors including GBM form intratumoral necrosis with hypoxic condition. This induces either directly or indirectly activation of hypoxia-responding transcription factors, and changes the tumor biology and its microenvironment, leading to increased aggressiveness and the feared resistance to chemotherapy and radiation 18. In GBM, targeting of hypoxia/necrosis has not been established yet, however, potential targets include the various hypoxia-regulated molecules, among them hypoxia inducible factor-1 (HIF-1), carbonic anhydrase IX and glucose transporter 1 18.
Although GBM does usually not metastasize to the other organs, it invades into the brain tissue in a highly aggressive manner (Figure 3). Manipulation of microenvironment is required for growth and invasion, and a number of factors related to this can be subjects of GBM therapies. Transforming growth factor β (TGF-β) is a critical component of the GBM microenvironment that drives tumor cells toward more aggressive behaviors and supports their survival while simultaneously limiting suppression by the host 19. GBM invasion is also promoted by the tumor hypoxia and HIF-1, which upregulates a variety of genes whose products play a well-established role in GBM invasion that include CXCR4, stromal derived factor-1 (SDF-1), VEGF, and matrix metalloproteinase (MMP) 19.
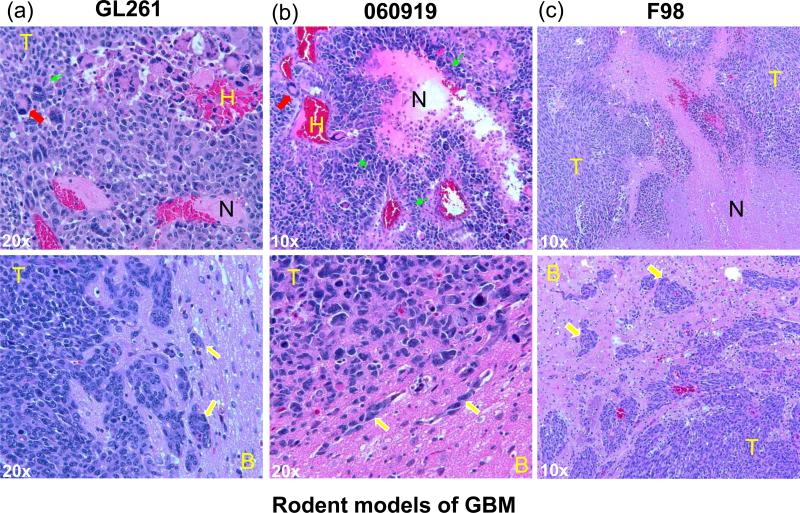
Figure 3.
Histological features of select rodent GBMs. (a) GL261, (b) 060919 and (c) F98 tumors were grown in the frontal lobe of C57BL6 mouse, athymic nude rat or F344 Fischer rat, respectively. Paraffin-embedded brain samples were stained with H&E. In 060919, the pseudopalisading necrosis is especially pronounced and histological features of necrosis, giant cells, hemorrhage and invasive growth closely resemble those in human GBM. Abbreviations: T: tumor, B: brain, N: necrosis, H: hemorrhage. Symbols: yellow arrow: invasion, red arrow: giant cell, green arrow: pseudopalisades.
Small Molecule Drugs
In cancer drug development, surface molecules such as receptors are relatively accessible for targeting. Many therapeutic approaches are aimed at EGFR that is overexpressed or amplified in GBM as well as its variant, EGFRVIII, which is a re-arranged and constitutively activated form of EGFR 20. Protein kinases including the intracellular kinase domains of growth receptors such as EGFR and PDGFR, are suitable for small molecule inhibitors and have been subject of most anti-GBM drug screening efforts 21. Most common chemical compounds targeting protein kinases have affinity for ATP binding sites or allosteric sites. Novel kinase inhibitors are typically developed by a combination of methods, including high-throughput screening based on biochemical or cellular assays, in silico structure-guided design, analogue synthesis and fragment expansion.
For intracranial tumors, sufficient tumor drug delivery perhaps presents more of a challenge than with other tumors. Several parameters need to be considered including a possibly elevated intratumoral pressure, reduced blood flow to regions of the tumor, a normal or abnormal blood-brain barrier formed by the tumor endothelial cells.
In contrast to the intact blood brain barrier and the close to normal capillaries in the low grade astrocytomas, the blood-brain tumor barrier is usually more permeable in high grade malignant brain tumors. This increased permeability is due to the anatomic defects between the endothelial cells and the tumor barrier in the new tumor microvasculature induced by vascular endothelial growth factor (VEGF), which allow for the transvascular passage of larger molecules with the pore size ranging from 7 to 100 nm depending on the models and studies 22, 23. Some orthotopic GBM rodent models such as RG-2 and D54 can form highly fenestrated tumor capillaries that measured up to 1 μm 24. The altered barrier in high grade gliomas not only allows more effective use of small molecule drugs, but it also enables or enhances the delivery of macromolecules, such as nanoparticles and liposomes into the brain tumors. By a so-called enhanced permeability and retention (EPR) effect, tumor vasculature allows macromolecules leaking from the fenestrated vessels to accumulate in the interstitial space in tumor tissues 25.
Drug Screening Assays
Once a molecular target is selected, an in vitro enzymatic assay can be setup against a pool of drug candidates or small molecule library, to directly assess the impact of the compounds to the target protein. A hit determined by an in vitro screening may display specificity to the target, but its general cellular toxicity cannot be determined. An alternative approach is to use a cell-based assay, such as an isogenic cell screening system, in which the response to small molecules is compared between a cancer cell line with a mutation and a matched cell line without that mutation 26. Isogenic screening of cell lines with EGFR/PI3K/AKT/PTEN pathway mutations provides a model of this strategy. For example, DLD1 isogenic cell lines carrying wildtype PI3K-CA or a gain-of-function mutation of PIK3-CA were first created by homologous recombination 27, and assayed with a panel of PI3K/AKT inhibitors 28. A specific AKT1 inhibitor A-443654 was found to selectively inhibit the cell line containing the PI3K-CA mutation and subsequent in vivo studies confirmed its efficacy. In another study, D54 GBM cell line was overexpressed with EGFRVIII and transfected with yellow fluorescence protein (YFP), while the parental D54 cells expressing blue fluorescence protein (BFP) served as the reference cell line 29. D54-EGFRVIII-YFP and D54-BFP cells were mixed and screened against a NCI small molecule diversity library, and the selective suppression of D54-EGFRVIII-YFP cells was observed with compounds that preferentially targeted on tumor cells harboring the EGFRVIII-induced pathway. Cell-based screening can also be used to find small molecules that target cancer cells with loss of tumor suppressors. In a study of TP53 inactivation in colon cancer cells, isogenic cell lines that differed in TP53 status were exposed to ionizing radiation 30. Gene-expression analysis revealed a consistent upregulation of polo-like kinase 1 (PLK1) controlling the G2/M transition in the cells whose TP53 genes were inactivated, compared with those with WT TP53 genes. It was subsequently determined that the PLK1 inhibitor BI-2536 specifically inhibited the cells with TP53 inactivation 30. Similar concepts can be applied to targets not only in aberrant oncogenic pathways, but also in cell populations involved in glioblastomas, such as the tumor stem cells. For example, recent studies used a primitive neural stem cell line as the matched normal cell line against glioblastoma stem cell lines in screening with chemical compounds, to achieve selected killing of tumor stem cells 31.
Preclinical Animal Models
Preclinical animal studies are the make-or-break point in the process of finding innovative drugs for GBM therapy. One major in vivo screening strategy involves transplanting various rodent tumor cell lines into the appropriate immunocompetent host (syngeneic) to provide an accurate picture of potential immune responses. Among the various tumor implantation sites (e.g. orthotopic, flank) orthotopic models (i.e. intracranial brain tumor models) offer the most realistic setting to assess the drug delivery aspects because they match the microenvironment in the brain to grow a histologically accurate GBM and offer a realistic tumor vasculature with the blood-brain tumor barrier. However, the use of tumor cell lines in in vitro drug testing with the constant selective pressure raises concerns that cells used for transplantation experiments do not represent the heterogeneity of the original tumor. An interesting and highly relevant animal model is therefore the spontaneous high grade gliomas that occur in certain dog breeds such as Boxers and Boston terriers. These breeds are predisposed to develop spontaneous GBMs that closely resemble the pathology of human tumors and can sometimes be used as clinical trial subjects with pet owner consent 32, 33. However, the adverse factors including the cost, availability and reproducibility make this biologically attractive large animal model a rare choice.
As with most cancers various rodent models remain the most common for brain tumor studies. Compared to mice, rats have larger size of brain (~ 1200mg vs ~ 400mg), which can therefore grow larger brain tumors and allow for more precise stereotactic implantation, facilitating therapeutic and monitoring procedures such as convection enhanced delivery (CED) and micro-dialysis. On the other hand, rats incur more expenses in purchase and maintenance, and increase the cost of drug use due to the approximate 10 times body weight compared to mice (~250 mg vs ~25 mg). Genetic engineering of mouse glioma models has been an ever expanding field promoted by improved understanding of the underlining genetic disorders of the disease 34, 35. However, considering the genetic and histological heterogeneity that a human GBM displays, our laboratory often chooses syngeneic models induced by mutagens that maintain the genetic complexity and xenograft models with human tumor cells.
The F98 glioma syngeneic model and another similar RG2 glioma model were produced by i.v. administration of N-ethyl-N-nitrosourea (ENU), a highly potent mutagen, to a pregnant Fischer 344 rat 36. Similar to human GBM, F98 cells carry the deletion of p16INK4A and overexpress PDGF-beta and Ras along with elevated levels of EGFR, cyclin D1 and D2 compared to rat astrocytes as the reference non-tumor population 37, 38. A fully grown intracerebral F98 tumor shows mixed population of spindle-shaped cells with hemorrhage and necrosis, and displays a highly invasive growth pattern (Fig. 3c) with very low immunogenicity 39, 40. F98 is also refractory to a number of systemic chemotherapies including paclitaxel and carboplatin and poorly responsive to irradiation alone, suggesting that it closely represents some of the challenges in treating high grade gliomas 36.
The C6 glioma model was produced by administering the mutagen N-nitroso-N-methylurea (MNU) to outbred Wistar rats 41. C6 cells carry the deletion of p16INK4A with no expression of p16 and p19ARF mRNA and also have increased expression of PDGF-beta, IGF-1, EGFR and Erb3/Her3 37, 42. Since produced in outbred Wistar rats and no truly syngeneic host can be found, C6 glioma turned out to be immunogenic in Wistar and other rats and is seriously limited in survival studies 43.
9L gliosarcoma was produced in Fischer 344 rats by i.v. injection of MNU 44. It has a mutated TP53 gene and elevated EGFR expression 38, 45. Intracerebral implantation of 9L cells gives rise to rapidly growing tumors with spindle-shaped cells of a sarcomatoid (sarcoma-like) appearance, while the tumor margins are sharply delineated with little invasion into the surrounding brain tissues 36. 9L gliosarcoma is responsive to radiation and a number of immuno- and chemotherapies, and can be highly immunogenic as revealed by animals immunized by X-irradiated 9L cells, which became subsequently resistant to tumor challenge 36.
GL261 mouse glioma was induced originally by intracranial injection of 3-methylcholantrene into C57BL/6 mice 46. Another syngeneic mouse glioma of C57BL/6 host, GL26, showed similar overall characteristics and growth patterns 46. GL261 cells possess a homozygous TP53 mutation, elevated c-myc and no detectable MHCII expression 47. They are moderately immunogenic as revealed by vaccination experiments with irradiated GL261 cells, in which the simultaneous and post-implantational vaccination did not impair the tumor challenge, while the pre-implantational vaccination did 47. Intracranial GL261 tumors showed rapid growth and invasive growth pattern (Fig. 2a), and were limitedly responsive to radiation alone 47. This model has been frequently used in tumor vaccination and immune therapy studies and is a valuable platform given the limited number of available syngeneic mouse glioma models.
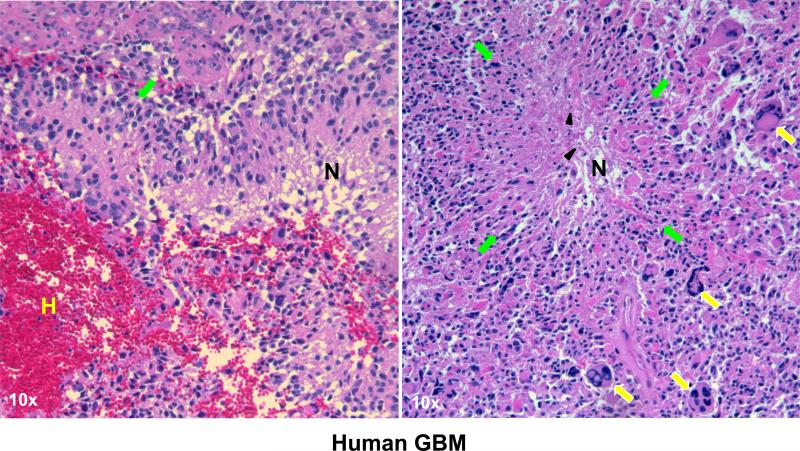
Figure 2.
Histological features of human GBMs. Paraffin-embeded human GBM samples were stained with H&E. Human GBMs are characterized by pseudopalisading necrosis (N) in a garlandlike arrangement of hypercellular tumor nuclei (pseudopalisades: green arrows) lining up around tumor necrosis (N) containing pyknotic nuclei (black arrowheads). Further features include hemorrhage (H) and multi-nucleated giant cells (yellow arrows).
In the 1990s, human tumor cell lines entered the field for large scale drug screening. Therefore, xenograft models were routinely employed by implanting and growing a human GBM sample or cell line into the brain of an athymic nude mouse or SCID mouse. Human GBMs can be serially passaged as mouse xenograft in the brain or flank without being subjected to artificial cultural selection, thereby preserving GBM properties such as EGFR amplification and CD133-expressing population 48, 49. Xenograft models of traditional GBM cell lines, grown in serum-containing media, have been used in brain tumor studies. One example is U87MG, which is used for preclinical tests due to its reliable tumor intake and narrow survival window. However, U87MG and other similar traditional GBM cell lines, form homogeneous and less invasive bulky tumor masses that do not resemble GBM histology and are perfused by leaky vessels, rendering them more accessible for systemic drug delivery than invasive human GBM cells 35. In contrast, neural stem cell (NSC)-like GBM neurosphere cell lines generated by EGF- and bFGF-containing serum free media have been shown to more closely mirror the phenotype and genotype of primary tumors (Fig. 2) 50. As example, 060919, one of the GBM neurosphere lines established in our laboratory, can form highly invasive and neo-vascularized xenograft intracranial tumors that well recapitulate the morphology of human GBMs featured by brain tissue infiltrations, heterogenic population, hemorrhages, neoplastic giant cells, necrotic/hypoxic tissues and pseudopalisading cells surrounding the tumor necrosis (Fig. 2 and 3b) 28, 51. These neurosphere GBM xenograft models or xenografts maintained by serial transplantation may offer more reliable preclinical test ground for GBM therapeutics. The most important caveat to this model is the defects in the immune system of SCID and nude animals, which limit the ability to test the effect of immuno-modulatory agents, and the DNA repair damage that may diminish their capacity to tolerate treatments 52.
Current Targeted Therapies and Clinical Trials
Inhibitors of Receptor Tyrosine Kinase (RTK) Pathways
Genomic amplification and mutation of the EGFR gene occur in about 40% of GBMs12, 15. In addition, PDGFRA and MET showed 8% and 4% genetic aberrations in GBMs, respectively15. PTEN phosphatase, the tumor suppressor of RTK/PI3K pathway, is mutated or deleted in 30% of GBMs 12. Activated RTKs stimulate the RAS/RAF/MAPK and PI3-K/Akt pathways resulting in tumorigenic cell proliferation (Fig. 1). Inhibitors of these pathways have been extensively tested in various clinical trials, in both recurrent GBM and primary GBM in addition to the standard of care. The current standard of care procedures consist of surgical resection and radiation therapy (RT) plus concomitant and adjuvant TMZ treatment as the first line therapy for primary GBM 53. In addition, Gliadel, a dissolvable polymer wafer, can optionally be used for local delivery of BCNU (carmustine) to GBM after resection and is associated with increased survival 54. For recurrent GBM, Avastin has been introduced as a common second line therapy 55. Table 1 and Figure 1 provide an overview of targeted therapeutics in clinical trials for GBM. So far, no clear survival benefits have been demonstrated beyond the accepted standard of care of surgical resection and radiation plus TMZ and the common optional Gliadel® 53, 54.
Anti-angiogenic Drugs
Anti-VEGF therapies have been widely tested in clinical trials and cancer therapies (Table 2). Avastin, a humanized monoclonal antibody against VEGF, is approved as second line treatment for recurrent GBM while its use for treatment for initial GBM is currently undergoing phase III trials. Although in Europe the marketing of Avastin in GBM is pending further demonstration of efficacy, due to its expedited approval and wide use in the US, Avastin becomes an interesting case study of anti-angiogenic strategies in GBM. Avastin has demonstrated improved radiographic response and 6 month progression-free suvival (PFS6), but with modest or little overall survival benefit, either as a single agent or in combination with irinotecan (CPT-11), a topoisomerase 1 inhibitor mainly used in colon cancer therapy 56-60. It is possible that similar to dexamethasone, part of the impressive radiographic response after Avastin administration is due to alleviated brain edema without much actual change in tumor mass. While Avastin markedly increased the radiographic PFS6 up to 25–42.6% from 10–15% of prior salvage chemotherapy, a disparity to the overall survival of those patients was noted and attributed to the difficulty of contrast-enhanced MRI in reflecting the real tumor mass, especially as those phase II trials were conducted in single-armed fashions and used various historical records as untreated control 58-61. Furthermore, the lack of salvage therapies to treat GBM regrowth after Avastin's transient tumor control presents other challenges such as rebound of intracranial edema and changed tumor features as detailed below 58, 62, 63.
Table 2.
Select anti-agiogenic drugs of GBM in clinical trials.
| Drug | Class / MW | Target | Most Recent GBM Trial / Initial or Recurrent GBM | Comments | References |
|---|---|---|---|---|---|
| Bevacizumab (Avastin) | Anti-VEGF antibody | VEGF | Phase II, III / initial & recurrent | FDA approved for treating recurrent GBM due to high response rates; modest survival benefit as mono-therapy; many phase II trials underway as combination therapies; phase III trials treating initial GBM with standard RT/TMZ ongoing. | 113, 114 |
| Vatalanib (PTK787, PTK/ZK) | Small molecule / 347 Da | VEGFR, c- KIT, PDGFR | Phase I, II / initial & recurrent | Well tolerated in treating initial and recurrent GBM; a phase II trial with intial GBM was discontinued due to industrial decision, showing limited efficacy with a small number of patients; multiple phase II trials also as combination therapies ongoing. | 115, 116 |
| Cediranib (Recentin, AZD2171) | Small molecule kinase inhibitor / 451 Da | VEGFR, PDGFR, FGFR1, c- KIT | Phase I, II / initial & recurrent | Initial human trial showed normalization of tumor vessels and reduction of brain edema; increased tumor infiltration was detected; multiple phase II trials ongoing also as combination therapies. | 66, 69 |
| Pazopanib (Votrient) | Small molecule kinase inhibitor / 438 Da | VEGFR, PDGFR, c- KIT | Phase II / recurrent | No survival benefit as single agent in recurrent GBM, while showing MRI responses. | 117 |
| Vandetanib (Zactima, ZD6474) | Small molecule kinase inhibitor / 475 Da | VEGFR, EGFR | Phase I, II / initial & recurrent | Safe to use with standard RT/TMZ in initial GBMs in a phase I study; multiple phase I and II trials underway as mono and combination therapies. | 118 |
| Aflibercept | Protein / 97 kD | VEGF trap | Phase I, II / initial & recurrent | Working as a decoy receptor of VEGF; a phase I trial with standard RT/TMZ of initial GBMs and a phase II trial with recurrent GBMs,ongoing. | 119 |
| AEE-788 | VEDGR, EGFR/ErbB 2 | Phase I, II / recurrent | Completed phase I/II trial of AEE788 as single agent in recurrent GBM; ongoing phase I/II trial in combination with everolimus in recurrent GBM. |
The role of Avastin in tumor cell invasion has been controversial. Avastin treatment promoted GBM infiltration in the U87 xenograft model and was associated with diffusing invasive recurrence pattern of some GBM cases 64, 65. Another anti-angiogenic VEGFR inhibitor, Cediranib, increased tumor infiltration in a phase II trial for recurrent GBM 66. These come in line with the findings and hypothesis that an angiogenesis-independent tumor population or mechanism in GBM may exist, which can be promoted by anti-angiogenic therapies and responsible for the induced infiltrative tumor phenotype, as reviewed by Miletic et al.67.
Another question that arises with the use of Avastin is if vascular “normalization” impairs brain penetration of other potential adjuvant therapeutics. Vascular normalization is a hypothesis that certain antiangiogenic agents can transiently “normalize” the abnormal structure and function of tumor vasculature to render it more efficient for blood and oxygen supplies. Blocking VEGF with Avastin or other anti-angiogenic drugs such as cediranib have been shown to induce vascular normalization, leading to a decreased vascular permeability in GBM 68-70. Although it has also been suggested that the reduced intratumoral pressure and restored vasculature may potentially be beneficial to drug delivery, a definitive answer remains pending with numerous ongoing trials of combination therapies with Avastin. Among the available clinical trial data, a side by side phase II trial of Avastin vs. Avastin plus irenotecan in recurrent GBM did not display a significant survival benefit in terms of PFS6 and median overall survival by adding irenotecan 59, while the most recent phase II trial of Avastin in combination with erlotinib failed to show a clear clinical benefit over other Avastin monotherapies 58, 59, 71. In addition, the latest phase II trial of Avastin plus TMZ during and after RT for primary GBM showed largely unchanged overall survival compared to standard of care with TMZ and RT, but improved PFS based on radiographic evaluation and clinical indications, which might be attributed to the edema reduction by Avastin 72. Cediranib, an VEGFR inhibitor, alone or in combination with lomustine failed to significantly improve overall survival and PFS in comparison to lomustine alone in recurrent GBM 73. Thus, clinical evidences so far indicate no overall survival benefit of anti-angiogenic agents such as Avastin in combination with other chemotherapeutics compared to the respective single agent controls in the studies. This may suggest a negative effect of anti-angiogenic drugs in the drug delivery of chemotherapeutics. With the recent FDA recommendation of revoking approval of Avastin in treating breast cancer, its application in various cancers including GBM will likely be more carefully reviewed in the US. A consensus regarding its efficacy and cost-risk/benefit ratio as single agent or in combination therapies is expected to emerge soon with numerous pre-clinical and clinical studies currently underway.
Challenges and Future Directions
Among the major oncogenic pathways in GBM, TP53/MDM2/MDM4/p14ARF tumor suppressor pathway and cell cycle regulators with RB1/ CDK4/p16INK4A/CDKN2B are largely untapped in targeted therapies, mostly due to the difficulties in designing small molecules effective for these mostly intracellular loss-of-function targets. A phase I trial of adenovirus carrying wildtype TP53 gene via intratumoral administration found only limited transduction in short distance of injection site 74. These areas remain a challenge in GBM targeting strategy.
In the preclinical front, drug screening needs to be more focused on the compounds with potentially better tumor delivery. A pre-selection of small molecules based on the molecular weight, polar surface area and lipophilicity would potentially improve the success rate of in vivo testing later on. Furthermore, packaging drugs in nano-scale particles, such as long circulating liposomes or liposomes with tumor-targeting surface ligands, can take advantage of the trapping effect of the highly fenestrated vasculature of GBM and greatly increase intratumoral drug concentration.
GBMs are known of complex heterogeneity at the genomic and differentiontion levels. Not surprisingly, perhaps with the exception of Avastin, so far the targeted therapies with single agents have disappointed in delivering clear survival benefit in GBM patients most likely due to multiple driver mutations in the various cell populations within a tumor. Combinations of multiple RTK inhibitors has been proposed for GBM similar to many other tumors 75, and have been under various clinical trials.
In dealing with the major challenges of GBM, namely intratumoral heterogeneity and invasive growth pattern, two concepts of targeted therapy emerge as possible future directions: go personal versus go universal. Personalized tumor therapy has been proposed for GBM; for example, determining the O-6-methylguanine methyltransferase (MGMT) status to determine response to TMZ 76, 77. A recent study of personalized tumor markers using personalized analysis of rearranged ends (PARE) offered a clinically feasible approach in profiling certain types of genetic aberrations individually as well as accurately tracking recurrence 78. A specific molecular profile of individual tumors could eventually be beneficial in designing a tailored therapeutic approach to maximize therapeutic efficacy of existing targeted drugs in GBM patients. However, there is still a lack of effective therapies for GBM and no present incentive for parsing GBMs into different treatment groups.
The ultimate quest of the search for tumor markers in benefit of therapies would be finding a targetable molecular feature that reflects the fundamental differences between tumor and normal cells. In contrast to the diagnostic tumor markers, such as the circulating tumor DNA and tumor antigens, an effective universal therapeutic marker needs to be accessible and technically targetable, as well as presented in all tumor cells capable of tumor propagation. With CD133 as the prominent surface marker, glioma stem cells have been proposed as the primary population of GBM initiation and a major culprit conveying resistance to radiotherapy 79, 80. Considerable efforts have been made to target this multipotent and self-renewal population during last few years, which have not delivered convincing results yet 8. Another possible venue of searching for targetable tumor properties would be tumor metabolic pathways. Since tumors use altered metabolic arrangements, such as high glucose uptake, elevated aerobic glycolysis and reduced oxidative phosphorylation (Warbug effect), compared with those of normal differentiated cells in the body 81, tumor metabolic presents a widely open field with very promising potential. For example, recent data showed somatic mutations in isocitrate dehydrogenase 1 (IDH1) in low grade astrocytomas and secondary GBMs 12. This enzyme converts isocitrate to α-ketoglutarate and its gain-of-function mutations produced instead 2-hydoxyglutarate, an oncometabolite 82. This altered metabolic pattern can be exploited for potential targeted therapeutic intervention 83. Besides the scientific and technical difficulties discussed above, development of a GBM therapy also faces challenges in high cost of clinical trials and extensive regulatory procedures. In order to initiate a first-in-human drug in human trial, an application of investigational new drug (IND) needs to be submitted to FDA, which requires the information including mechanism of action and pharmacodynamics, pharmacokinetics, safety pharmacology, general pharmacology and toxicology studies, and determination of a safe starting dose based on rodent data for the first-inhuman phase I trial 84. There are three types (investigator, emergency use and treatment), and two categories (commercial and research) of IND 85. Pharmaceutical sponsors can pursue full “traditional” IND or expedited IND. In 2005, FDA introduced a new category of expedited IND, namely the exploratory IND, with the purpose of allowing for early clinical testing of one or several new chemical entities based on a reduced pre-clinical package. Once a suitable candidate is determined, a full traditional IND has to be submitted 85. Designed for the limited dosing and duration (microdosing), an exploratory IND study could improve the quality of internal decision making by sponsors based on the exploratory human data obtained early, before the substantial investments are made for a traditional phase I trial 85.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Khasraw M, Lassman AB. Advances in the treatment of malignant gliomas. Curr Oncol Rep. 2010;12:26–33. doi: 10.1007/s11912-009-0077-4. [DOI] [PubMed] [Google Scholar]
- 2.Harada K, et al. Intratumoral cytogenetic heterogeneity detected by comparative genomic hybridization and laser scanning cytometry in human gliomas. Cancer Res. 1998;58:4694–4700. [PubMed] [Google Scholar]
- 3.Walker C, et al. Phenotype versus genotype in gliomas displaying inter- or intratumoral histological heterogeneity. Clin Cancer Res. 2003;9:4841–4851. [PubMed] [Google Scholar]
- 4.Nobusawa S, et al. Intratumoral patterns of genomic imbalance in glioblastomas. Brain Pathol. 2010;20:936–944. doi: 10.1111/j.1750-3639.2010.00395.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nishikawa R, et al. Immunohistochemical analysis of the mutant epidermal growth factor, deltaEGFR, in glioblastoma. Brain Tumor Pathol. 2004;21:53–56. doi: 10.1007/BF02484510. [DOI] [PubMed] [Google Scholar]
- 6.Jeuken J, et al. Robust detection of EGFR copy number changes and EGFR variant III: technical aspects and relevance for glioma diagnostics. Brain Pathol. 2009;19:661–671. doi: 10.1111/j.1750-3639.2009.00320.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Druker BJ, et al. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med. 2001;344:1031–1037. doi: 10.1056/NEJM200104053441401. [DOI] [PubMed] [Google Scholar]
- 8.Denysenko T, et al. Glioblastoma cancer stem cells: heterogeneity, microenvironment and related therapeutic strategies. Cell Biochem Funct. 2010;28:343–351. doi: 10.1002/cbf.1666. [DOI] [PubMed] [Google Scholar]
- 9.van den Bent MJ, et al. Randomized phase II trial of erlotinib versus temozolomide or carmustine in recurrent glioblastoma: EORTC brain tumor group study 26034. J Clin Oncol. 2009;27:1268–1274. doi: 10.1200/JCO.2008.17.5984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sarin H. Recent progress towards development of effective systemic chemotherapy for the treatment of malignant brain tumors. J Transl Med. 2009;7:77. doi: 10.1186/1479-5876-7-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pope WB, Itagaki MW. Characterizing brain tumor research: the role of the National Institutes of Health. AJNR Am J Neuroradiol. 2010;31:605–609. doi: 10.3174/ajnr.A1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parsons DW, et al. An integrated genomic analysis of human glioblastoma multiforme. Science. 2008;321:1807–1812. doi: 10.1126/science.1164382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Velculescu VE, et al. Serial analysis of gene expression. Science. 1995;270:484–487. doi: 10.1126/science.270.5235.484. [DOI] [PubMed] [Google Scholar]
- 14.Boon K, et al. An anatomy of normal and malignant gene expression. Proc Natl Acad Sci U S A. 2002;99:11287–11292. doi: 10.1073/pnas.152324199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rao SK, et al. A survey of glioblastoma genomic amplifications and deletions. J Neurooncol. 2010;96:169–179. doi: 10.1007/s11060-009-9959-4. [DOI] [PubMed] [Google Scholar]
- 16.Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455:1061–1068. doi: 10.1038/nature07385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Papeo G, et al. Poly(ADP-ribose) polymerase inhibition in cancer therapy: are we close to maturity? Expert Opin Ther Pat. 2009;19:1377–1400. doi: 10.1517/13543770903215883. [DOI] [PubMed] [Google Scholar]
- 18.Jensen RL. Brain tumor hypoxia: tumorigenesis, angiogenesis, imaging, pseudoprogression, and as a therapeutic target. J Neurooncol. 2009;92:317–335. doi: 10.1007/s11060-009-9827-2. [DOI] [PubMed] [Google Scholar]
- 19.Barcellos-Hoff MH, et al. Therapeutic targets in malignant glioblastoma microenvironment. Semin Radiat Oncol. 2009;19:163–170. doi: 10.1016/j.semradonc.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wong AJ, et al. Structural alterations of the epidermal growth factor receptor gene in human gliomas. Proc Natl Acad Sci U S A. 1992;89:2965–2969. doi: 10.1073/pnas.89.7.2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chico LK, et al. Targeting protein kinases in central nervous system disorders. Nat Rev Drug Discov. 2009;8:892–909. doi: 10.1038/nrd2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hobbs SK, et al. Regulation of transport pathways in tumor vessels: role of tumor type and microenvironment. Proc Natl Acad Sci U S A. 1998;95:4607–4612. doi: 10.1073/pnas.95.8.4607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sarin H, et al. Physiologic upper limit of pore size in the blood-tumor barrier of malignant solid tumors. J Transl Med. 2009;7:51. doi: 10.1186/1479-5876-7-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Newton HB. Advances in strategies to improve drug delivery to brain tumors. Expert Rev Neurother. 2006;6:1495–1509. doi: 10.1586/14737175.6.10.1495. [DOI] [PubMed] [Google Scholar]
- 25.Chen ZG. Small-molecule delivery by nanoparticles for anticancer therapy. Trends Mol Med. 2010;16:594–602. doi: 10.1016/j.molmed.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Strausberg RL, et al. Oncogenomics and the development of new cancer therapies. Nature. 2004;429:469–474. doi: 10.1038/nature02627. [DOI] [PubMed] [Google Scholar]
- 27.Samuels Y, et al. Mutant PIK3CA promotes cell growth and invasion of human cancer cells. Cancer Cell. 2005;7:561–573. doi: 10.1016/j.ccr.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 28.Gallia GL, et al. Inhibition of Akt inhibits growth of glioblastoma and glioblastoma stem-like cells. Mol Cancer Ther. 2009;8:386–393. doi: 10.1158/1535-7163.MCT-08-0680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Trembath DG, et al. A novel small molecule that selectively inhibits glioblastoma cells expressing EGFRvIII. Mol Cancer. 2007;6:30. doi: 10.1186/1476-4598-6-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sur S, et al. A panel of isogenic human cancer cells suggests a therapeutic approach for cancers with inactivated p53. Proc Natl Acad Sci U S A. 2009;106:3964–3969. doi: 10.1073/pnas.0813333106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Danovi D, et al. Imaging-based chemical screens using normal and glioma-derived neural stem cells. Biochem Soc Trans. 2010;38:1067–1071. doi: 10.1042/BST0381067. [DOI] [PubMed] [Google Scholar]
- 32.Heidner GL, et al. Analysis of survival in a retrospective study of 86 dogs with brain tumors. J Vet Intern Med. 1991;5:219–226. doi: 10.1111/j.1939-1676.1991.tb00952.x. [DOI] [PubMed] [Google Scholar]
- 33.Candolfi M, et al. Intracranial glioblastoma models in preclinical neuro oncology: neuropathological characterization and tumor progression. J Neurooncol. 2007;85:133–148. doi: 10.1007/s11060-007-9400-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fomchenko EI, Holland EC. Mouse models of brain tumors and their applications in preclinical trials. Clin Cancer Res. 2006;12:5288–5297. doi: 10.1158/1078-0432.CCR-06-0438. [DOI] [PubMed] [Google Scholar]
- 35.de Vries NA, et al. High-grade glioma mouse models and their applicability for preclinical testing. Cancer Treat Rev. 2009;35:714–723. doi: 10.1016/j.ctrv.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 36.Barth RF, Kaur B. Rat brain tumor models in experimental neuro-oncology: the C6, 9L, T9, RG2, F98, BT4C, RT-2 and CNS-1 gliomas. J Neurooncol. 2009;94:299–312. doi: 10.1007/s11060-009-9875-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schlegel J, et al. The p16/Cdkn2a/Ink4a gene is frequently deleted in nitrosourea-induced rat glial tumors. Pathobiology. 1999;67:202–206. doi: 10.1159/000028073. [DOI] [PubMed] [Google Scholar]
- 38.Sibenaller ZA, et al. Genetic characterization of commonly used glioma cell lines in the rat animal model system. Neurosurg Focus. 2005;19:E1. doi: 10.3171/foc.2005.19.4.2. [DOI] [PubMed] [Google Scholar]
- 39.Clavreul A, et al. Effects of syngeneic cellular vaccinations alone or in combination with GM-CSF on the weakly immunogenic F98 glioma model. J Neurooncol. 2006;79:9–17. doi: 10.1007/s11060-005-9115-8. [DOI] [PubMed] [Google Scholar]
- 40.Mathieu D, et al. Standardization and detailed characterization of the syngeneic Fischer/F98 glioma model. Can J Neurol Sci. 2007;34:296–306. doi: 10.1017/s0317167100006715. [DOI] [PubMed] [Google Scholar]
- 41.Schmidek HH, et al. Morphological studies of rat brain tumors induced by N-nitrosomethylurea. J Neurosurg. 1971;34:335–340. doi: 10.3171/jns.1971.34.3.0335. [DOI] [PubMed] [Google Scholar]
- 42.Guo P, et al. Platelet-derived growth factor-B enhances glioma angiogenesis by stimulating vascular endothelial growth factor expression in tumor endothelia and by promoting pericyte recruitment. Am J Pathol. 2003;162:1083–1093. doi: 10.1016/S0002-9440(10)63905-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Parsa AT, et al. Limitations of the C6/Wistar rat intracerebral glioma model: implications for evaluating immunotherapy. Neurosurgery. 2000;47:993–999. doi: 10.1097/00006123-200010000-00050. discussion 999-1000. [DOI] [PubMed] [Google Scholar]
- 44.Benda P, et al. Morphological and immunochemical studies of rat glial tumors and clonal strains propagated in culture. J Neurosurg. 1971;34:310–323. doi: 10.3171/jns.1971.34.3.0310. [DOI] [PubMed] [Google Scholar]
- 45.Asai A, et al. Negative effects of wild-type p53 and s-Myc on cellular growth and tumorigenicity of glioma cells. Implication of the tumor suppressor genes for gene therapy. J Neurooncol. 1994;19:259–268. doi: 10.1007/BF01053280. [DOI] [PubMed] [Google Scholar]
- 46.Ausman JI, et al. Studies on the chemotherapy of experimental brain tumors: development of an experimental model. Cancer Res. 1970;30:2394–2400. [PubMed] [Google Scholar]
- 47.Szatmari T, et al. Detailed characterization of the mouse glioma 261 tumor model for experimental glioblastoma therapy. Cancer Sci. 2006;97:546–553. doi: 10.1111/j.1349-7006.2006.00208.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pandita A, et al. Contrasting in vivo and in vitro fates of glioblastoma cell subpopulations with amplified EGFR. Genes Chromosomes Cancer. 2004;39:29–36. doi: 10.1002/gcc.10300. [DOI] [PubMed] [Google Scholar]
- 49.Shu Q, et al. Direct orthotopic transplantation of fresh surgical specimen preserves CD133+ tumor cells in clinically relevant mouse models of medulloblastoma and glioma. Stem Cells. 2008;26:1414–1424. doi: 10.1634/stemcells.2007-1009. [DOI] [PubMed] [Google Scholar]
- 50.Lee J, et al. Tumor stem cells derived from glioblastomas cultured in bFGF and EGF more closely mirror the phenotype and genotype of primary tumors than do serum-cultured cell lines. Cancer Cell. 2006;9:391–403. doi: 10.1016/j.ccr.2006.03.030. [DOI] [PubMed] [Google Scholar]
- 51.Siu IM, et al. Establishment of a human glioblastoma stemlike brainstem rodent tumor model. J Neurosurg Pediatr. 2010;6:92–97. doi: 10.3171/2010.3.PEDS09366. [DOI] [PubMed] [Google Scholar]
- 52.Fulop GM, Phillips RA. The scid mutation in mice causes a general defect in DNA repair. Nature. 1990;347:479–482. doi: 10.1038/347479a0. [DOI] [PubMed] [Google Scholar]
- 53.Stupp R, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 54.Attenello FJ, et al. Use of Gliadel (BCNU) wafer in the surgical treatment of malignant glioma: a 10-year institutional experience. Ann Surg Oncol. 2008;15:2887–2893. doi: 10.1245/s10434-008-0048-2. [DOI] [PubMed] [Google Scholar]
- 55.Norden AD, et al. Bevacizumab for recurrent malignant gliomas: efficacy, toxicity, and patterns of recurrence. Neurology. 2008;70:779–787. doi: 10.1212/01.wnl.0000304121.57857.38. [DOI] [PubMed] [Google Scholar]
- 56.Vredenburgh JJ, et al. Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. J Clin Oncol. 2007;25:4722–4729. doi: 10.1200/JCO.2007.12.2440. [DOI] [PubMed] [Google Scholar]
- 57.Zuniga RM, et al. Efficacy, safety and patterns of response and recurrence in patients with recurrent high-grade gliomas treated with bevacizumab plus irinotecan. J Neurooncol. 2009;91:329–336. doi: 10.1007/s11060-008-9718-y. [DOI] [PubMed] [Google Scholar]
- 58.Kreisl TN, et al. Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol. 2009;27:740–745. doi: 10.1200/JCO.2008.16.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Friedman HS, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27:4733–4740. doi: 10.1200/JCO.2008.19.8721. [DOI] [PubMed] [Google Scholar]
- 60.Raizer JJ, et al. A phase 2 trial of single-agent bevacizumab given in an every-3-week schedule for patients with recurrent high-grade gliomas. Cancer. 2010;116:5297–5305. doi: 10.1002/cncr.25462. [DOI] [PubMed] [Google Scholar]
- 61.Lamborn KR, et al. Progression-free survival: an important end point in evaluating therapy for recurrent high-grade gliomas. Neuro Oncol. 2008;10:162–170. doi: 10.1215/15228517-2007-062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Iwamoto FM, et al. Patterns of relapse and prognosis after bevacizumab failure in recurrent glioblastoma. Neurology. 2009;73:1200–1206. doi: 10.1212/WNL.0b013e3181bc0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Quant EC, et al. Role of a second chemotherapy in recurrent malignant glioma patients who progress on bevacizumab. Neuro Oncol. 2009;11:550–555. doi: 10.1215/15228517-2009-006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.de Groot JF, et al. Tumor invasion after treatment of glioblastoma with bevacizumab: radiographic and pathologic correlation in humans and mice. Neuro Oncol. 2010;12:233–242. doi: 10.1093/neuonc/nop027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Narayana A, et al. Bevacizumab in recurrent high-grade pediatric gliomas. Neuro Oncol. 2010;12:985–990. doi: 10.1093/neuonc/noq033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gerstner ER, et al. Infiltrative patterns of glioblastoma spread detected via diffusion MRI after treatment with cediranib. Neuro Oncol. 2010;12:466–472. doi: 10.1093/neuonc/nop051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Miletic H, et al. Anti-VEGF therapies for malignant glioma: treatment effects and escape mechanisms. Expert Opin Ther Targets. 2009;13:455–468. doi: 10.1517/14728220902806444. [DOI] [PubMed] [Google Scholar]
- 68.Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307:58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 69.Batchelor TT, et al. AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell. 2007;11:83–95. doi: 10.1016/j.ccr.2006.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.de Groot JF, et al. Phase II study of carboplatin and erlotinib (Tarceva, OSI-774) in patients with recurrent glioblastoma. J Neurooncol. 2008;90:89–97. doi: 10.1007/s11060-008-9637-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sathornsumetee S, et al. Phase II trial of bevacizumab and erlotinib in patients with recurrent malignant glioma. Neuro Oncol. 2010;12:1300–1310. doi: 10.1093/neuonc/noq099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lai A, et al. Phase II Study of Bevacizumab Plus Temozolomide During and After Radiation Therapy for Patients With Newly Diagnosed Glioblastoma Multiforme. J Clin Oncol. 2011;29:142–148. doi: 10.1200/JCO.2010.30.2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Stupp R, Weller M. 2010: neuro-oncology is moving! Curr Opin Neurol. 2010;23:553–555. doi: 10.1097/WCO.0b013e3283407eed. [DOI] [PubMed] [Google Scholar]
- 74.Lang FF, et al. Phase I trial of adenovirus-mediated p53 gene therapy for recurrent glioma: biological and clinical results. J Clin Oncol. 2003;21:2508–2518. doi: 10.1200/JCO.2003.21.13.2508. [DOI] [PubMed] [Google Scholar]
- 75.Stommel JM, et al. Coactivation of receptor tyrosine kinases affects the response of tumor cells to targeted therapies. Science. 2007;318:287–290. doi: 10.1126/science.1142946. [DOI] [PubMed] [Google Scholar]
- 76.Stupp R, et al. Phase I/IIa study of cilengitide and temozolomide with concomitant radiotherapy followed by cilengitide and temozolomide maintenance therapy in patients with newly diagnosed glioblastoma. J Clin Oncol. 2010;28:2712–2718. doi: 10.1200/JCO.2009.26.6650. [DOI] [PubMed] [Google Scholar]
- 77.Weller M, et al. MGMT promoter methylation in malignant gliomas: ready for personalized medicine? Nat Rev Neurol. 2010;6:39–51. doi: 10.1038/nrneurol.2009.197. [DOI] [PubMed] [Google Scholar]
- 78.Leary RJ, et al. Development of personalized tumor biomarkers using massively parallel sequencing. Sci Transl Med. 2010;2:20ra14. doi: 10.1126/scitranslmed.3000702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Singh SK, et al. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 80.Bao S, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756–760. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- 81.Levine AJ, Puzio-Kuter AM. The control of the metabolic switch in cancers by oncogenes and tumor suppressor genes. Science. 2010;330:1340–1344. doi: 10.1126/science.1193494. [DOI] [PubMed] [Google Scholar]
- 82.Dang L, et al. Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature. 2009;462:739–744. doi: 10.1038/nature08617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Seltzer MJ, et al. Inhibition of glutaminase preferentially slows growth of glioma cells with mutant IDH1. Cancer Res. 2010;70:8981–8987. doi: 10.1158/0008-5472.CAN-10-1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Senderowicz AM. Information needed to conduct first-in-human oncology trials in the United States: a view from a former FDA medical reviewer. Clin Cancer Res. 16:1719–1725. doi: 10.1158/1078-0432.CCR-09-2766. [DOI] [PubMed] [Google Scholar]
- 85.Sarapa N. Exploratory IND: a new regulatory strategy for early clinical drug development in the United States. Ernst Schering Res Found Workshop. 2007:151–163. doi: 10.1007/978-3-540-49529-1_11. [DOI] [PubMed] [Google Scholar]
- 86.Brown PD, et al. Phase I/II trial of erlotinib and temozolomide with radiation therapy in the treatment of newly diagnosed glioblastoma multiforme: North Central Cancer Treatment Group Study N0177. J Clin Oncol. 2008;26:5603–5609. doi: 10.1200/JCO.2008.18.0612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Reardon DA, et al. Phase 2 trial of erlotinib plus sirolimus in adults with recurrent glioblastoma. J Neurooncol. 2010;96:219–230. doi: 10.1007/s11060-009-9950-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rich JN, et al. Phase II trial of gefitinib in recurrent glioblastoma. J Clin Oncol. 2004;22:133–142. doi: 10.1200/JCO.2004.08.110. [DOI] [PubMed] [Google Scholar]
- 89.Franceschi E, et al. Gefitinib in patients with progressive high-grade gliomas: a multicentre phase II study by Gruppo Italiano Cooperativo di Neuro-Oncologia (GICNO). Br J Cancer. 2007;96:1047–1051. doi: 10.1038/sj.bjc.6603669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kreisl TN, et al. A pilot study of everolimus and gefitinib in the treatment of recurrent glioblastoma (GBM). J Neurooncol. 2009;92:99–105. doi: 10.1007/s11060-008-9741-z. [DOI] [PubMed] [Google Scholar]
- 91.Thiessen B, et al. A phase I/II trial of GW572016 (lapatinib) in recurrent glioblastoma multiforme: clinical outcomes, pharmacokinetics and molecular correlation. Cancer Chemother Pharmacol. 2009;65:353–361. doi: 10.1007/s00280-009-1041-6. [DOI] [PubMed] [Google Scholar]
- 92.Hainsworth JD, et al. Concurrent radiotherapy and temozolomide followed by temozolomide and sorafenib in the first-line treatment of patients with glioblastoma multiforme. Cancer. 2010;116:3663–3669. doi: 10.1002/cncr.25275. [DOI] [PubMed] [Google Scholar]
- 93.Reardon DA, et al. Effect of CYP3A-inducing anti-epileptics on sorafenib exposure: results of a phase II study of sorafenib plus daily temozolomide in adults with recurrent glioblastoma. J Neurooncol. 2011;101:57–66. doi: 10.1007/s11060-010-0217-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Du J, et al. Bead-based profiling of tyrosine kinase phosphorylation identifies SRC as a potential target for glioblastoma therapy. Nat Biotechnol. 2009;27:77–83. doi: 10.1038/nbt.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ramakrishnan MS, et al. Nimotuzumab, a promising therapeutic monoclonal for treatment of tumors of epithelial origin. MAbs. 2009;1:41–48. doi: 10.4161/mabs.1.1.7509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ramos TC, et al. Treatment of high-grade glioma patients with the humanized anti-epidermal growth factor receptor (EGFR) antibody h-R3: report from a phase I/II trial. Cancer Biol Ther. 2006;5:375–379. doi: 10.4161/cbt.5.4.2522. [DOI] [PubMed] [Google Scholar]
- 97.Belda-Iniesta C, et al. Long term responses with cetuximab therapy in glioblastoma multiforme. Cancer Biol Ther. 2006;5:912–914. doi: 10.4161/cbt.5.8.3118. [DOI] [PubMed] [Google Scholar]
- 98.Hasselbalch B, et al. Cetuximab, bevacizumab, and irinotecan for patients with primary glioblastoma and progression after radiation therapy and temozolomide: a phase II trial. Neuro Oncol. 2010;12:508–516. doi: 10.1093/neuonc/nop063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jun HT, et al. AMG 102, a fully human anti-hepatocyte growth factor/scatter factor neutralizing antibody, enhances the efficacy of temozolomide or docetaxel in U-87 MG cells and xenografts. Clin Cancer Res. 2007;13:6735–6742. doi: 10.1158/1078-0432.CCR-06-2969. [DOI] [PubMed] [Google Scholar]
- 100.Reardon DA, et al. Multicentre phase II studies evaluating imatinib plus hydroxyurea in patients with progressive glioblastoma. Br J Cancer. 2009;101:1995–2004. doi: 10.1038/sj.bjc.6605411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Dresemann G, et al. Imatinib in combination with hydroxyurea versus hydroxyurea alone as oral therapy in patients with progressive pretreated glioblastoma resistant to standard dose temozolomide. J Neurooncol. 2010;96:393–402. doi: 10.1007/s11060-009-9976-3. [DOI] [PubMed] [Google Scholar]
- 102.Wen PY, et al. Phase I/II study of imatinib mesylate for recurrent malignant gliomas: North American Brain Tumor Consortium Study 99-08. Clin Cancer Res. 2006;12:4899–4907. doi: 10.1158/1078-0432.CCR-06-0773. [DOI] [PubMed] [Google Scholar]
- 103.Razis E, et al. Phase II study of neoadjuvant imatinib in glioblastoma: evaluation of clinical and molecular effects of the treatment. Clin Cancer Res. 2009;15:6258–6266. doi: 10.1158/1078-0432.CCR-08-1867. [DOI] [PubMed] [Google Scholar]
- 104.Reardon DA, et al. Phase II study of imatinib mesylate plus hydroxyurea in adults with recurrent glioblastoma multiforme. J Clin Oncol. 2005;23:9359–9368. doi: 10.1200/JCO.2005.03.2185. [DOI] [PubMed] [Google Scholar]
- 105.Wick W, et al. Phase III study of enzastaurin compared with lomustine in the treatment of recurrent intracranial glioblastoma. J Clin Oncol. 2010;28:1168–1174. doi: 10.1200/JCO.2009.23.2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kreisl TN, et al. A phase I/II trial of enzastaurin in patients with recurrent high-grade gliomas. Neuro Oncol. 2010;12:181–189. doi: 10.1093/neuonc/nop042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Akhavan D, et al. mTOR signaling in glioblastoma: lessons learned from bench to bedside. Neuro Oncol. 2010;12:882–889. doi: 10.1093/neuonc/noq052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Galanis E, et al. Phase II trial of temsirolimus (CCI-779) in recurrent glioblastoma multiforme: a North Central Cancer Treatment Group Study. J Clin Oncol. 2005;23:5294–5304. doi: 10.1200/JCO.2005.23.622. [DOI] [PubMed] [Google Scholar]
- 109.Chang SM, et al. Phase II study of CCI-779 in patients with recurrent glioblastoma multiforme. Invest New Drugs. 2005;23:357–361. doi: 10.1007/s10637-005-1444-0. [DOI] [PubMed] [Google Scholar]
- 110.Phuphanich S, et al. Phase 1 clinical trial of bortezomib in adults with recurrent malignant glioma. J Neurooncol. 2010;100:95–103. doi: 10.1007/s11060-010-0143-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kubicek GJ, et al. Phase I trial using proteasome inhibitor bortezomib and concurrent temozolomide and radiotherapy for central nervous system malignancies. Int J Radiat Oncol Biol Phys. 2009;74:433–439. doi: 10.1016/j.ijrobp.2008.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Reardon DA, et al. Randomized phase II study of cilengitide, an integrin-targeting arginine-glycine-aspartic acid peptide, in recurrent glioblastoma multiforme. J Clin Oncol. 2008;26:5610–5617. doi: 10.1200/JCO.2008.16.7510. [DOI] [PubMed] [Google Scholar]
- 113.Chi AS, et al. Antiangiogenic strategies for treatment of malignant gliomas. Neurotherapeutics. 2009;6:513–526. doi: 10.1016/j.nurt.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Huang TT, et al. Targeted therapy for malignant glioma patients: lessons learned and the road ahead. Neurotherapeutics. 2009;6:500–512. doi: 10.1016/j.nurt.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Reardon DA, et al. Phase I pharmacokinetic study of the vascular endothelial growth factor receptor tyrosine kinase inhibitor vatalanib (PTK787) plus imatinib and hydroxyurea for malignant glioma. Cancer. 2009;115:2188–2198. doi: 10.1002/cncr.24213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Brandes AA, et al. EORTC study 26041-22041: phase I/II study on concomitant and adjuvant temozolomide (TMZ) and radiotherapy (RT) with PTK787/ZK222584 (PTK/ZK) in newly diagnosed glioblastoma. Eur J Cancer. 2010;46:348–354. doi: 10.1016/j.ejca.2009.10.029. [DOI] [PubMed] [Google Scholar]
- 117.Iwamoto FM, et al. Phase II trial of pazopanib (GW786034), an oral multi-targeted angiogenesis inhibitor, for adults with recurrent glioblastoma (North American Brain Tumor Consortium Study 06-02). Neuro Oncol. 2010;12:855–861. doi: 10.1093/neuonc/noq025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Drappatz J, et al. Phase I study of vandetanib with radiotherapy and temozolomide for newly diagnosed glioblastoma. Int J Radiat Oncol Biol Phys. 2010;78:85–90. doi: 10.1016/j.ijrobp.2009.07.1741. [DOI] [PubMed] [Google Scholar]
- 119.Gomez-Manzano C, et al. VEGF Trap induces antiglioma effect at different stages of disease. Neuro Oncol. 2008;10:940–945. doi: 10.1215/15228517-2008-061. [DOI] [PMC free article] [PubMed] [Google Scholar]