Abstract
Major burns induce immune complications, which are associated with myeloid cell activation by ill-defined mechanisms. While γδ T-cells have been shown to be important in post-injury inflammation and wound healing, their role in the regulation of myeloid cells remains unknown. To study this wildtype (WT) and γδ T-cell deficient (δTCR−/−) mice were subjected to major burn (25% TBSA, 3rd degree) or sham treatment. At 3 days thereafter, skin samples were assayed for cytokine content or used to isolate single cells that were used for myeloid cell characterization by flow cytometry. The number of CD11b+ myeloid cells increased by ~75% in the wound skin of WT mice. This influx was due to increased myeloid-derived suppressor cells (MDSC; CD11b+ GR1+), who’s numbers increased 19-fold compared to sham skin. In contrast, macrophage (MØ; CD11b+ F4/80+) numbers decreased by ~50% after burn. In δTCR−/− mice, burn increased the myeloid cell numbers ~5-fold. The increase in myeloid cells at the injury site of δTCR−/− mice was due to both a MDSC (50-fold) and a MØ (2-fold) influx. Burn increased skin cytokine levels for a number of prototypic inflammatory cytokines (IL-1β, IL-6, TNF-α, MIP-1β etc). TNF-α, MIP-1 α and MIP-1β levels were further elevated (2–3 fold) in the injured skin of δTCR−/− mice, as compared with WT mice. In conclusion these data show that γδ T-cells regulate myeloid cell infiltration of the wound site and act to quell inflammation, thereby promoting the transition to the proliferative phase of wound healing.
Keywords: Injury, Inflammation, Macrophage, MDSC, Cytokines
Introduction
The morbidity and mortality associated with major burn can, in part, be attributed to various derangements of the immune system and inflammatory response that contributes to the subsequent development of SIRS and MOF (1, 2). Nonetheless, inflammation has a beneficial role at times and, in particular, plays a major role in the complex process of wound repair. The regulation and propagation of inflammatory responses is highly regulated and involves multiple immune cell types (i.e., T-cells, macrophages, neutrophils).
Numerous studies have implicated macrophages and other myeloid cells implicated in the post-burn immune dysfunction (2–5). In general these studies have support a concept of a “hyperactivation” of the myeloid cell with elevated release of various pro-inflammatory mediators. Nonetheless, these studies have primarily focused on circulating leukocytes or cells from primary immune organs, such as the spleen. While studies have examined wound macrophages function and phenotypes (6–8), detailed analysis of the myeloid cells at the healing burn wound site have not been conducted. Recent findings with a wound sponge model suggest an important role for myeloid cells and γδ T-cells in the burn wound healing response (9, 10). Nonetheless, this model system did not look at the cell directly infiltrating the burn wound.
T-cells expressing the γδ TCR normally represent a small percentage of cells in lymphoid tissues, but are abundant in the skin and other epithelial tissue beds (11). With regard to trauma, recent studies have shown the presence of activated γδ T-cells in the circulation of patients with severe SIRS, demonstrating the important role of these cells in the early response to severe injury (12) and preclinical studies have shown the presence of activated γδ T-cells in the circulation of burn injured mice (13). The current study was undertaken to better characterize the role of wound γδ T-cells in the regulation of the wound myeloid cell activity.
Materials and Methods
Mice
C57BL/6 wildtype (WT) and mice lacking γδ T-cells (δ TCR−/−; C57BL/6J-Tcrdtm1Mom) (male, 18–25 g, the Jackson Laboratory, Bar Harbor, ME) were used for all the experiments. Mice were allowed to acclimatize for at least one week prior to experimentation and maintained in ventilated cages under specific pathogen-free conditions. Animals were randomly assigned to either sham or burn group. All animal protocols were approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Texas Health Science Center at San Antonio. This study was conducted in compliance with the Animal Welfare Act, the implementing Animal Welfare Regulations, and the principles of the Guide for the Care and Use of Laboratory Animals.
Burn procedure
Mice received a scald burn as described previously (14). Briefly, the mice were anesthetized by intraperitoneal (i.p.) injection of ketamine/xylazine, and the dorsal surface was shaved. The anesthetized mouse was placed in a custom-built, insulated mold exposing 12.5% of their total body surface area (TBSA) along the right dorsum. The mold was immersed in 70°C water for 10 sec to produce a 3rd degree burn. The burn procedure was repeated on the left dorsal side yielding a total burn size of 25% TBSA. Previous studies have verified this injury to be a full thickness burn as defined by observed damage to the epidermal, dermal and sub-dermal layers (14). No analgesics were used post-burn, as they can impact the immune response to burn injury and other forms of trauma (15). The mice were then resuscitated with 1 ml of Ringer's lactate solution administered by i.p. injection and returned to their cages. The cages were placed on a heating pad until the mice were fully awake, at which time they were returned to the animal facility. Sham treatment consisted of anesthesia and resuscitation only.
Skin tissue collection, digestion and cell isolation
At 3 days after burn or sham procedure, skin samples were collected and wet weight was measured. Normal non-injured skin was collected from sham and burn mice and injured skin from burn site was collected from burn mice. Skin samples from the burn site included injured skin and the wound margin. All skin samples were excised, down to the level of the musculofascia, including the submucosal layer [which contains the majority of the infiltrating cells in the burn (16)], by sharp dissection.
Collected skin tissues were washed in PBS with 50 U/ml penicillin and 50 µg/ml streptomycin (GIBCO) in a 60 mm petri dish (Corning) and the skin was minced with scissors into small pieces of approximately 2–3 mm in size and placed into dispase II medium (0.05%, Roche) for overnight digestion at 4°C on an orbital rocker. The following day the skin samples were further minced into smaller pieces and then digested by agitating in trypsin-GNK (0.3%, Glucose/dextrose, NaCl and KCl buffer, Sigma) for 30 min at 37°C in a water bath shaker. Heat inactivated Fetal Bovine Serum (FBS, GIBCO) was added (10% total volume) to stop the digestion reaction and the dissociated cells were sieved through a 100 µm mesh. The cell suspension was centrifuged at 400 × g for 10 min and 4°C and resuspended in RPMI culture medium [RPMI with 10% FBS, 50 µM of 2-Mercaptoethanol (Sigma-Aldrich), 2 mM L-glutamine (GIBCO), 1 mM sodium pyruvate (GIBCO), 100 µM non-essential amino acids (GIBCO), 50 U/ml penicillin, 50 µg/ml streptomycin (GIBCO) and 10 U/ml murine recombinant IL-2 (BD Biosciences)]. Cells were cultured overnight at a density of 1×106/ml in a 12-well plate. The cultured cells were passed through a 70 µm mesh prior to staining for flow cytometry.
Cell phenotyping by flow cytometry
The cells were washed in staining buffer (PBS with 0.2 % BSA and 0.09% NaN3) and treated with Fc-blocking antibody (anti-CD16/CD32, BD Biosciences) for 15 min. The cells were stained with the following directly conjugated antibodies: anti-CD11b (PerCPCy5.5; Clone: M1/70), anti-F4/80 (PECy7; Clone: BM8), and anti-Ly6G (Gr1, efluor450; Clone: RB6.8C5). After 30 min of incubation on ice, the cells were washed and resuspended in staining buffer. Appropriate isotype controls were used for gate setting for all staining. All data were acquired using a LSRII (BD Biosciences) and analyzed using FlowJo (Tree Star) software. A minimum of 50,000 events was collected and live cells were gated according to forward- and side-scatter properties for the lymphocyte/monocytes gate. Data were analyzed as % cells and total cells per gram of wet weight of skin tissue.
Determination of skin cytokine levels
The Bioplex (Bio-Rad) system was used for cytokine level analysis in tissue lysates according to the manufacturer’s recommendations. The following factors were assessed: IL-1β, IL-6, IL-10, KC, MCP-1, MIP1α, MIP-1β, and TNF-α. Cytokine levels were normalized to skin total protein levels that were determined by BCA protein assay.
Statistical analyses
Data are expressed as mean ± SEM. Comparisons between groups were analyzed using ANOVA and SigmaPlot 11.0 software (Systat Software Inc, San Jose CA). Further post-hoc analysis employed the Dunnett’s method for multiple comparisons versus the control group. A p-value < 0.05 was considered to be statistically significant for all analyses.
Results
CD11b myeloid cells infiltrate the burn wound in a γδ T-cell dependent manner
Skin cells were studied at 3 days after sham or burn procedure. The data in Table 1 shows the total number of cells, (irrespective of myeloid lineage) isolated from the skin of sham and burn mice. The cell numbers are normalized to the wet weight of the tissue (g). In WT mice, the number of cells collected from burn wound skin was significantly less than that obtained from sham skin or uninjured skin from burn mice. In contrast, total cell numbers were comparable in skin from δTCR−/− mice, irrespective of burn injury (Table 1).
Table 1.
Skin cell count (×106 cells/g wet weight of skin) after isolation
| Sham | Burn Uninjured | Burn Wound | |
|---|---|---|---|
| WT | 9.7 ± 1.9a | 9.8 ± 1.0 | 3.4 ± 0.9b |
| δ TCR−/− | 4.6 ± 1.7 | 8.2 ± 1.0 | 5.2 ± 0.6 |
Three days after sham or burn procedure, skin cells from WT or δ TCR−/− mice were isolated and were normalized as per gram wet weight of the skin.
Data are mean ± SEM for 3–7 mice/group
p<0.05 vs. sham and burn uninjured skin of the WT mice.
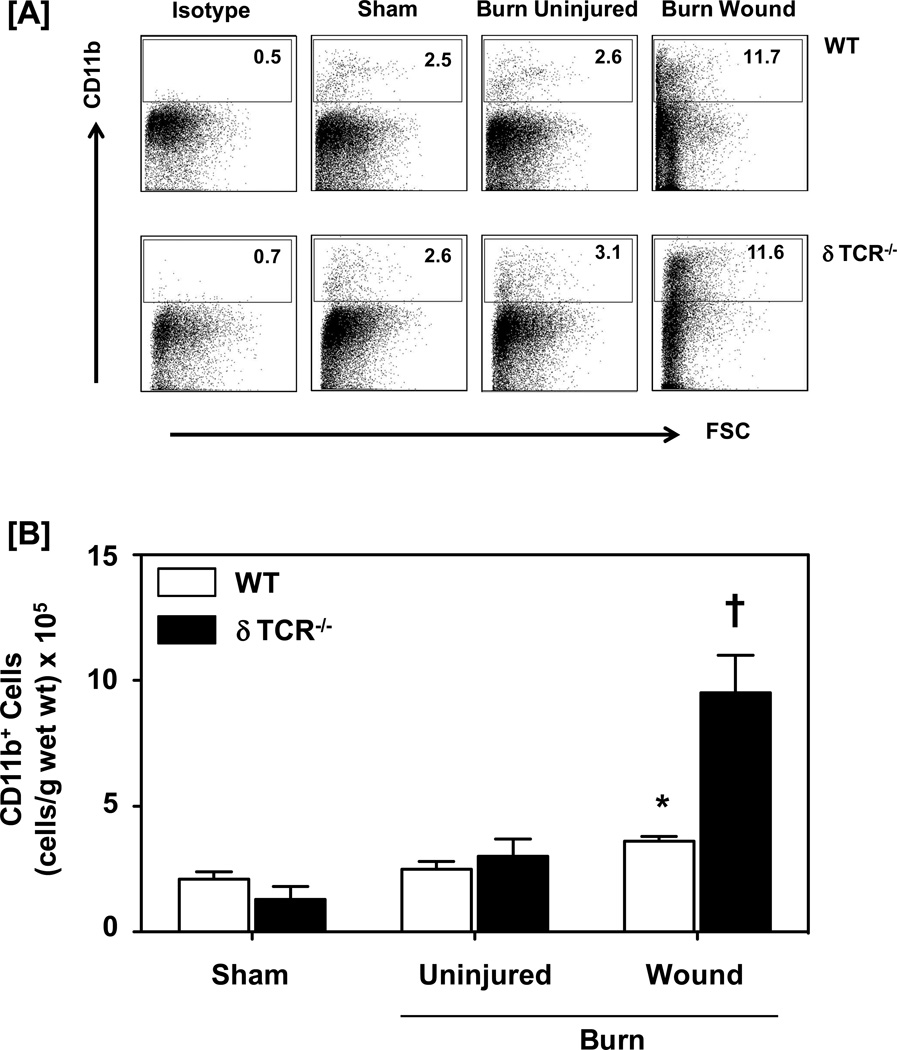
To investigate whether γδ T-cells impact the infiltration by myeloid cells after burn, skin cells were isolated after 3 days of sham or burn procedure. The 3 day time point was chosen based on our previous studies (9, 16) that 3 days post-injury was the time of maximal cellular infiltration and observed differences in growth factors at the wound site in δTCR−/− mice as compared to wildtype mice. Myeloid cells were characterized in the lymphocyte/monocytes gated population based on their CD11b, F4/80 and/or Gr1 surface expression. Characterization of different myeloid cells demonstrated a significant influx by these cells in the burn wound, as compared with sham skin and uninjured skin from burn mice. In WT mice, the percentage of CD11b+ cells is increased from 2.5% for sham to 11.7% for cells from the burn wound (Fig. 1a). The percentages of CD11b+ cells in uninjured skin from burn mice were comparable to that of sham mice (2.6%). When cell numbers were normalized to wet weight of the skin, increases in CD11b+ cells at the wound site were also evident (Fig. 1b). In mice deficient in γδ T-cells (δTCR−/−), the percentages of CD11b+ myeloid cells were comparable to that for WT mice for sham, uninjured and wound skin (Fig 1a). In contrast, when cell percentages were normalized to gram wet weight, a profound 7-fold increase in the numbers of CD11b+ myeloid cells in the burn wound was observed as compared with sham skin, which was significantly greater than that observed in WT mice (Fig 1b).
Figure 1.
Impact of γδ T-cells on wound CD11b+ myeloid cells. Three days after sham or burn procedure, skin cells from WT or δ TCR−/− mice were prepared and studied for CD11b+ myeloid cell characterization using flow cytometry. [A] Gating strategy. CD11b+ cells from the lymphocyte/myeloid cell gate of WT (Fig. 1A, Upper Panel) and δ TCR−/− (Fig. 1A, Lower Panel) mice. Representative dot plots are shown from sham, burn uninjured and burn injured skin cells. The numbers indicate the percentages of CD11b+ cells as determined by flow cytometry. [B] The number of CD11b+ cells as normalized to gram wet weight of the skin tissue. Data are mean ± SEM for 3–7 mice/group; * p<0.05 vs. uninjured skin of the respective WT or δ TCR−/− mice. † p<0.05 vs. burn wound of the respective WT mice.
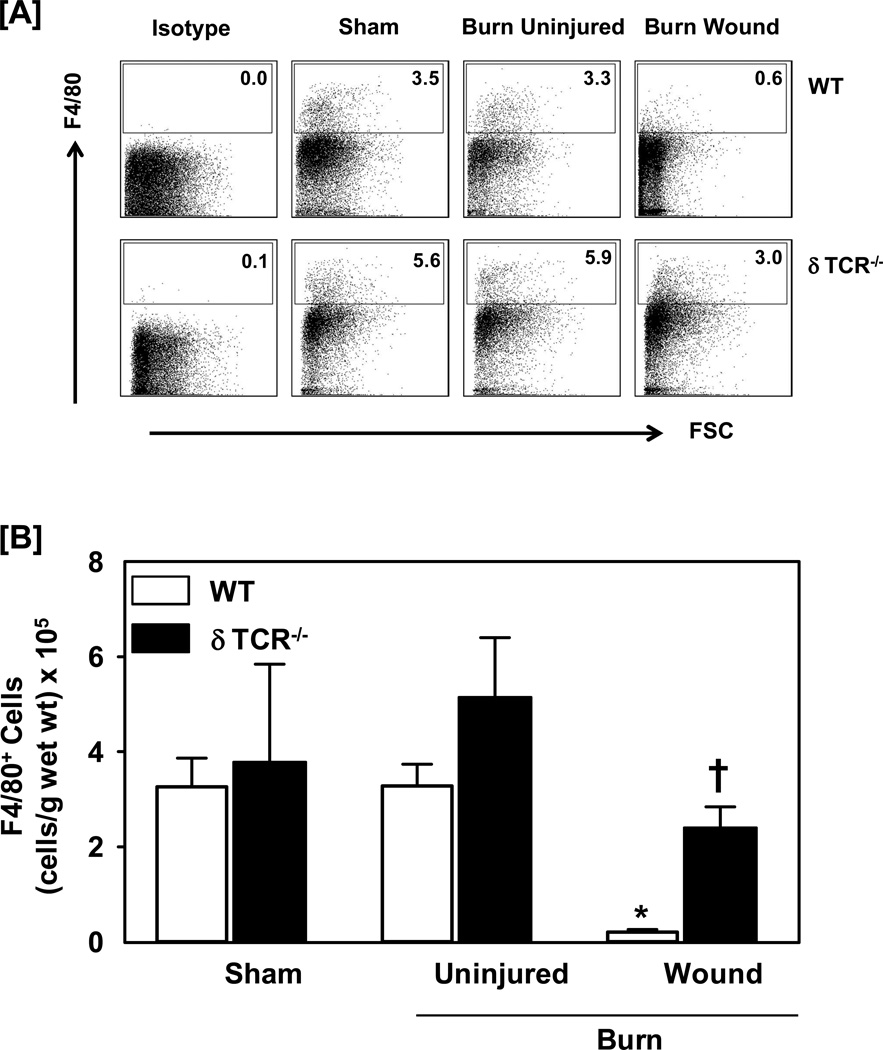
A significant decrease in the percentage of F4/80+ myeloid cells was observed in the burn wound, as compared with both sham skin and uninjured skin from burn mice (Fig. 2). In WT mice, the percentage of F4/80+ cells from sham skin was comparable to that of uninjured skin from burn mice. When the percentages were normalized to cells numbers, the pattern for cellular infiltration remained comparable (Fig. 2b). In δTCR−/− mice, the percentages and numbers of F4/80+ cells were comparable for sham and uninjured skin for burn mice. While the percentages and total numbers of F4/80+ cells at the wound site of δTCR−/− mice were decreased as compared with uninjured skin, the decrease was significantly less than that observed in WT mice (Fig. 2).
Figure 2.
Impact of γδ T-cells on wound F4/80+ myeloid cells. Three days after sham or burn procedure, skin cells from WT or δ TCR−/− mice were prepared and studied for F4/80+ myeloid cell characterization using flow cytometry. [A] Gating strategy. F4/80+ cells from the lymphocyte/myeloid cell gate of WT (Fig. 1A, Upper Panel) and δ TCR−/− (Fig. 1A, Lower Panel) mice. Representative dot plots are shown from sham, burn uninjured and burn injured skin cells. The numbers indicate the percentages of respective population as determined by flow cytometry. [B] The number of F4/80+ cells as normalized to gram wet weight of the skin tissue. Data are mean ± SEM for 3–7 mice/group; * p<0.05 vs. uninjured skin of the respective WT or δ TCR−/− mice. † p<0.05 vs. burn wound of the respective WT mice.
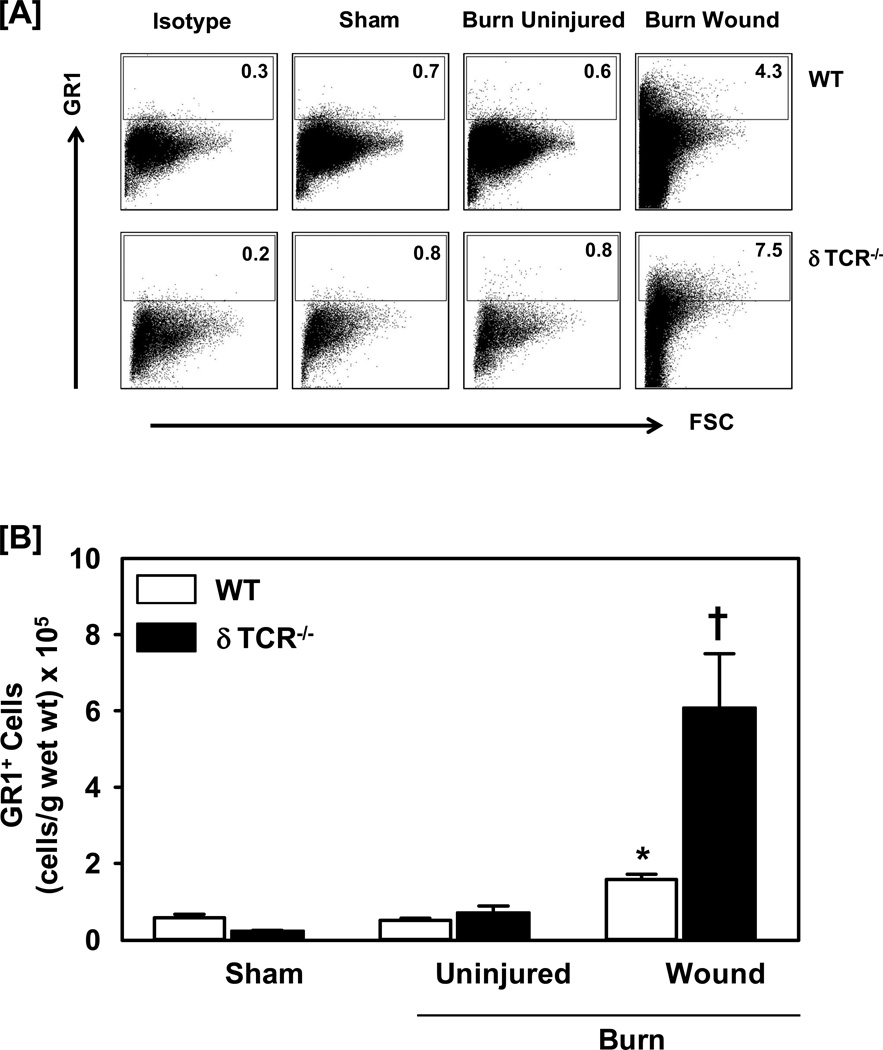
Further characterization of myeloid cells for Gr1 expression revealed a significant influx by these cells in the burn wound, as compared with sham skin and uninjured skin from burn mice (Fig. 3). In WT mice, the percentage and absolute numbers of Gr1+ cells for sham skin was comparable to that of uninjured skin from burn mice (less than 1%; Fig 3a and 3b, respectively). Both, the percentages and cell numbers were significantly increased up to 6-folds in the skin of burn wound as compared with sham skin. In parallel to the response to burn in WT mice, the percentages of Gr1+ cells in the burn wound of δTCR−/− were significantly increased as compared with sham skin; however, they were markedly greater than that observed in WT mice (9-folds for δTCR−/− vs. 6-folds for WT mice). When the percentages were normalized to cell numbers, the increased infiltration at the wound site in δTCR−/− mice was even more pronounced as compared to its sham group and greater than that observed in WT mice (Fig 3b).
Figure 3.
Impact of γδ T-cells on wound Gr1+ myeloid cells. Three days after sham or burn procedure, skin cells from WT or δ TCR−/− mice were prepared and studied for Gr1+ myeloid cell characterization using flow cytometry. [A] Gating strategy. Gr1+ cells from the lymphocyte/myeloid cell gate of WT (Fig. 1A, Upper Panel) and δ TCR−/− (Fig. 1A, Lower Panel) mice. Representative dot plots are shown from sham, burn uninjured and burn injured skin cells. The numbers indicate the percentages of respective population as determined by flow cytometry. [B] The number of Gr1+ cells as normalized to gram wet weight of the skin tissue. Data are mean ± SEM for 3–7 mice/group; * p<0.05 vs. uninjured skin of the respective WT or δ TCR−/− mice. † p<0.05 vs. burn wound of the respective WT mice.
Gamma delta (γδ) T-cells suppress the infiltration of the burn wound with F4/80+CD11b+ macrophages
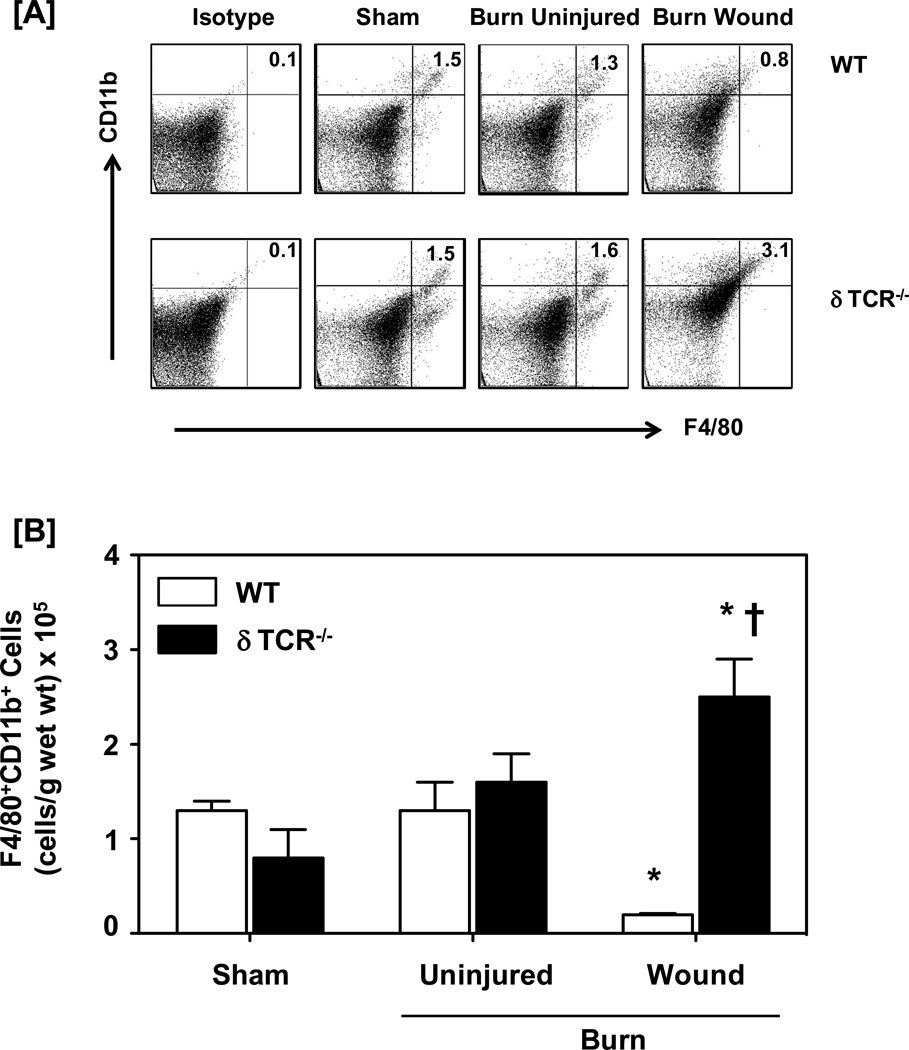
The percentage and absolute number of F4/80+ CD11b+ cells (i.e., macrophages) in the skin of the burn wound from WT mice significantly decreased by approximately 50% as compared with sham skin (Fig. 4). In sharp contrast to this response to burn in the WT mice, the percentage and absolute numbers of macrophages in the burn wound of δTCR−/− were significantly increased as compared with sham skin. Absolute cell numbers were increased 3-fold over that of sham skin and 13-fold over that of burn wound skin from WT mice (Fig. 4b).
Figure 4.
Impact of γδ T-cells on wound F4/80+CD11b+ macrophages. Three days after sham or burn procedure, skin cells from WT or δ TCR−/− mice were prepared and studied for F4/80+CD11b+ macrophage cell characterization using flow cytometry. [A] Gating strategy. F4/80+CD11b+ cells from the lymphocyte/myeloid cell gate of WT (Fig. 1A, Upper Panel) and δ TCR−/− (Fig. 1A, Lower Panel) mice. Representative dot plots are shown from sham, burn uninjured and burn injured skin cells. The numbers indicate the percentages of respective population as determined by flow cytometry. [B] The number of F4/80+CD11b+ cells as normalized to gram wet weight of the skin tissue. Data are mean ± SEM for 3–7 mice/group; * p<0.05 vs. uninjured skin of the respective WT or δ TCR−/− mice. † p<0.05 vs. burn wound of the respective WT mice.
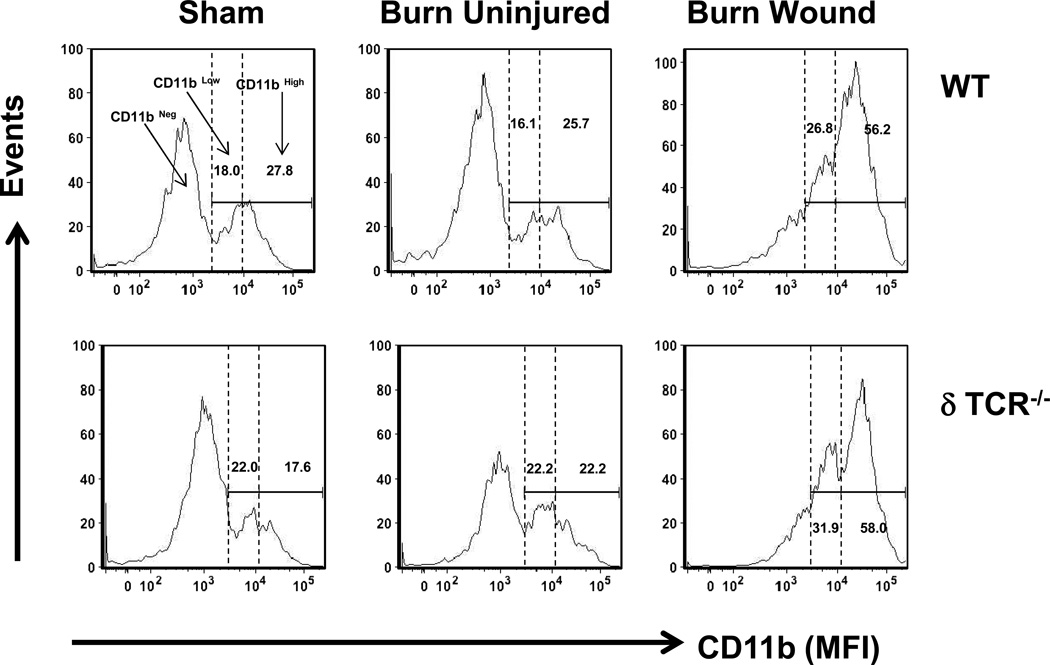
Macrophages (F4/80+ CD11b+) were further investigated in terms of their CD11b surface expression (Table 2). Three populations of cells were identified in the sham skin, uninjured skin and wound skin from burn mice (Fig 5). The populations were defined as F4/80+CD11bneg (based on the CD11b isotype control; up to ~ 103 logs on x-axis), F4/80+CD11blow (from ~103 logs to 104 logs on x-axis) and F4/80+CD11bhigh (from 104 logs until end of x-axis). In WT mice, the percentage of F4/80+CD11blow and F4/80+CD11bhigh cells for sham skin was comparable to that of uninjured skin from burn mice. However, the percentages of both CD11blow, as well as CD11bhigh macrophages was significantly increased in burn wound skin, as compared with sham skin (Fig 5 and Table 2). In parallel to increased percentage of these cells in the burn wound, there was a dramatic shift from the CD11blow population to the CD11bhigh population. In mice lacking γδ T-cells (δTCR−/− mice), the pattern for both CD11blow to CD11bhigh expressing macrophages in skin of sham mice, uninjured skin and burn wound skin from burn mice was comparable as that of observed in WT mice (Fig 5 and Table 2).
Table 2.
Percentages of F4/80+ macrophages based on their CD11b expression
| F480+ CD11bLow | F480+ CD11bHigh | |||||
|---|---|---|---|---|---|---|
| Sham | Burn Uninjured |
Burn Wound | Sham | Burn Uninjured |
Burn Wound | |
| WT | 18.0 ± 1.6a | 16.1 ± 1.8 | 26.8 ± 2.0* | 27.8 ± 5.5 | 25.7 ± 3.5 | 56.2 ± 0.6b |
| δ TCR−/− | 22.0 ± 6.1 | 22.2 ± 0.6 | 31.9 ± 1.8b | 17.6 ± 2.8 | 22.2 ± 1.3 | 58.0 ± 1.7b |
Three days after sham or burn procedure, skin cells from WT or δ TCR−/− mice were prepared and studied for CD11blow and CD11bhigh expression on F4/80+ macrophages using flow cytometry.
Data are mean ± SEM for 3–7 mice/group
p<0.05 vs. uninjured skin of the respective WT or δ TCR−/− mice.
Figure 5.
Characterization of F4/80+ macrophages based on the CD11blow and CD11bhigh expression. Three days after sham or burn procedure, skin cells from WT or δ TCR−/− mice were prepared and studied for F4/80+CD11b+ macrophage cell characterization using flow cytometry. [A] Gating strategy. F4/80+CD11b+ cells from the lymphocyte/myeloid cell gate of WT (Fig. 1A, Upper Panel) and δ TCR−/− (Fig. 1A, Lower Panel) mice. Representative histograms are shown from sham, burn uninjured and burn injured skin cells. The numbers indicate the percentages of respective population as determined by flow cytometry. Data are mean ± SEM for 3–7 mice/group; * p<0.05 vs. uninjured skin of the respective WT or δ TCR−/− mice. † p<0.05 vs. burn wound of the respective WT mice.
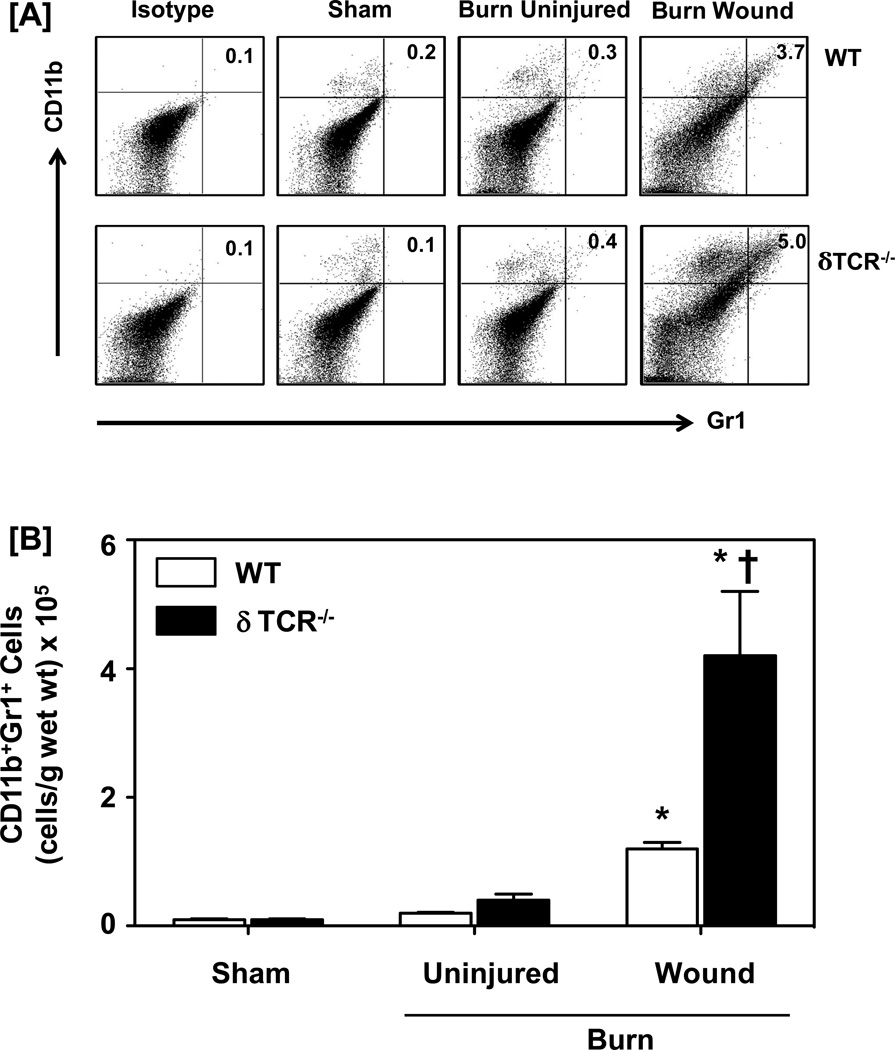
Gamma delta (γδ) T-cells suppress the infiltration of the burn wound with myeloid derived suppressor cells (CD11b+Gr1+ MDSCs)
In contrast to macrophages, CD11b+ GR1+ cells (i.e., MDSCs; the upper right quadrant of dot-plots; Fig. 6a) were negligible in sham skin and uninjured skin from WT and δTCR−/− mice. In WT mice the percentages and number of CD11b+ GR1+ cells in the skin of the burn wound significantly increased approximately 20-fold as compared with sham skin (Fig. 6). The percentage and numbers of MDSCs in the burn wound of δTCR−/− mice were also significantly increased as compared with sham skin; however the increases were significantly greater (~50-fold) than that observed in WT mice. The number of MDSCs in the burn wound of δTCR−/− mice was also greater (4-fold) than that observed in WT mice (Fig. 6b).
Figure 6.
Impact of γδ T-cells on wound CD11b+Gr1+ myeloid-derived suppressor cells. Three days after sham or burn procedure, skin cells from WT or δ TCR−/− mice were prepared and studied for CD11b+Gr1+ MDSCs characterization using flow cytometry. [A] Gating strategy. CD11b+Gr1+ cells from the lymphocyte/myeloid cell gate of WT (Fig. 1A, Upper Panel) and δ TCR−/− (Fig. 1A, Lower Panel) mice. Representative dot plots are shown from sham, burn uninjured and burn injured skin cells. The numbers indicate the percentages of respective population as determined by flow cytometry. [B] The number of CD11b+ Gr1+ cells as normalized to gram wet weight of the skin tissue. Data are mean ± SEM for 3–7 mice/group; * p<0.05 vs. uninjured skin of the respective WT or δ TCR−/− mice. † p<0.05 vs. burn wound of the respective WT mice.
The percentage for the CD11b+ GR1− myeloid cells (population shown in the upper left quadrant of dot-plots in Fig 6; Table 3) from WT mice significantly increased by 2-fold in the skin of the burn wound as compared with sham skin (Table 3). However, absolute number of CD11b+ GR1− cells in the burn wound was comparable to that of the sham skin. The percentages for the CD11b+ GR1− myeloid cells in the burn wound of δTCR−/− mice were significantly increased as compared with sham skin, and was significantly greater (~5-folds) than that observed in WT mice. While the absolute numbers of CD11b+ GR1− cells in WT mice were comparable irrespective of the injury; a 7-fold increase was observed in the burn wound of δTCR−/− mice as compared to the sham skin. Further characterization of the CD11b+ GR1− cells in terms of their F4/80 expression revealed that most of these cells in the WT sham skin or uninjured skin form the burn mice were F4/80+ (i.e., CD11b+ GR1−F4/80+, ~80–96%; Table 4). However, the percentage of CD11b+ GR1-F4/80+ cells was significantly decreased in the burn wound skin (19% in burn wound vs. 87% in sham skin). In δTCR−/− mice, the percentages of CD11b+ GR1−F4/80+ cells were comparable to that for WT mice for sham and uninjured skin for burn mice: 96% for sham skin; 86% for uninjured skin from burn mice. In parallel to WT mice, the percentage of CD11b+ GR1−F4/80+ cells in the burn wound skin of δTCR−/− mice were decreased significantly as compared to sham skin; however, the decrease was far less than that observed in WT mice (Table 4).
Table 3.
Characterization of CD11b+ Gr1− myeloid cells
| Percentage (%) | Cell number (× 105 cells/g wet wt. of skin) |
|||||
|---|---|---|---|---|---|---|
| Sham | Burn Uninjured |
Burn Wound | Sham | Burn Uninjured |
Burn Wound |
|
| WT | 2.6 ± 0.4a | 2.5 ± 0.2 | 5.3 ± 0.4* | 2.2 ± 0.1 | 2.4 ± 0.6 | 2.0 ± 0.3 |
| δ TCR−/− | 2.6 ± 0.2 | 3.3 ± 0.2 | 11.6 ± 1.1bc | 1.4 ± 0.2 | 3.4 ± 0.7 | 9.3 ± 1.4bc |
Three days after sham or burn procedure, skin cells from WT or δ TCR−/− mice were studied for CD11b and Gr1 expression to characterize CD11b+Gr1− myeloid cells using flow cytometry.
Data are mean ± SEM for 3–7 mice/group;
p<0.05 vs. uninjured skin of the respective WT or δ TCR−/− mice.
p<0.05 vs. injured skin of the respective WT mice.
Table 4.
Characterization of CD11b+Gr1− myeloid cells based on their F4/80 expression
| Sham | Burn Uninjured | Burn Wound | |
|---|---|---|---|
| WT | 86.7 ± 1.3a | 79.1 ± 3.3 | 19.1 ± 3.1b |
| δ TCR−/− | 95.9 ± 1.0 | 85.9 ± 1.9 | 46.8 ± 4.3bc |
Three days after sham or burn procedure, skin cells from WT or δ TCR−/− mice were prepared and studied for CD11b, Gr1 and F4/80 expression to characterize CD11b+Gr1− myeloid cells using flow cytometry. Gating was on the CD11b+ Gr1− cell population.
Data are mean ± SEM for 3–7 mice/group;
p<0.05 vs. uninjured skin of the respective WT or δ TCR−/− mice.
p<0.05 vs. injured skin of the respective WT mice.
Gamma delta (γδ) T-cells suppress aspects of the myeloid-mediated burn wound inflammatory response
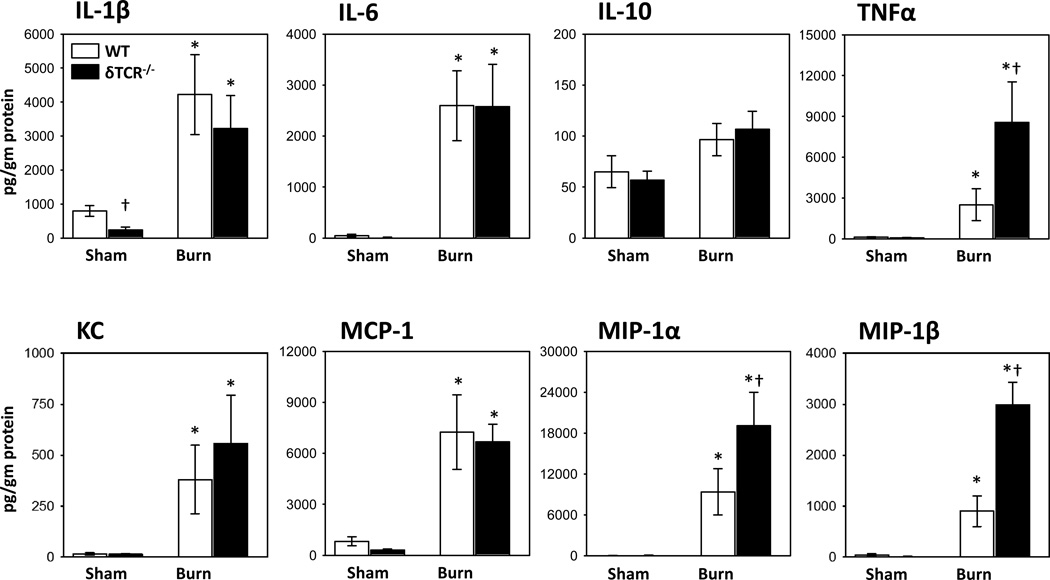
The burn wound inflammatory response was assessed by measuring the cytokine content of skin lysates. As expected a number of pro-inflammatory cytokines were elevated in the burn wound of WT mice (Fig. 7). In particular a 50-fold increase in the levels of IL-6 and a 150-fold increase in the levels of MIP-1α were observed in the burn wound, as compared with sham skin. IL-10 was the only cytokine measured that did not increase in the burn wound as compared with sham skin. A number of the inflammatory cytokines were further increased in the burn wound of δTCR−/−, as the levels of MIP-1α, MIP-1β, and TNF-α in the burn wound of δTCR−/− mice were ~2–3-fold greater than that of wounds from WT mice.
Figure 7.
Cytokine content in uninjured and burn skin lysates. Three days after sham or burn procedure, skin cells from WT or δ TCR−/− mice were prepared and assessed for IL-1β, IL-6, IL-10 and TNF-α expression by Luminex as described in Materials and Methods. Data are mean ± SEM for 3–7 mice/group; * p<0.05 vs. uninjured skin of the respective WT or δ TCR−/− mice. † p<0.05 vs. injured skin of the respective WT mice.
Discussion
Major burn is associated with immunoinflammatory and wound healing complications (4, 9, 10, 17–19). However, while inflammatory complications are deleterious, inflammation also plays an important role in the progression of the injury healing process, via the recruitment of immune cells to the injury site (9, 17). These immune cells, that include myeloid cells (i.e. neutrophils and macrophages) and T-cells, release a wide range of factors, including cytokines, chemokines and growth factors that are essential for proper wound healing (17, 20–22). Our group has previously shown that γδ T-cells play a pivotal role after burn in the regulation of inflammation and wound healing (9, 16, 19). Interestingly, γδ T-cells also have been shown to induce macrophage infiltration of the wound site (23), which are central in the immune complications associated with burn (2, 19). The current study was conducted to assess the role of γδ T-cells in the regulation of myeloid cells at the burn wound site. Our findings herein demonstrate that the γδ T-cells are critical in the regulation of myeloid cell trafficking at the burn wound site. In the absence of γδ T-cells, (δTCR−/− mice) CD11b+, F4/80+ and Gr1+ myeloid cell numbers were markedly increased over that observed in WT burn mice. This increased influx of myeloid cells included both CD11b+F4/80+ macrophages and CD11b+Gr1+ myeloid-derived suppressor cells (MDSCs).
Wound healing after burn is an intricate process orchestrated by the complex interplay of myeloid cells, T-cells and other immune cells (10). Previous studies have shown a role for myeloid cells in the immune response to burn, trauma and sepsis (17, 19, 24–26). Gr-1, CD11b, and F4/80 antigens have been shown to be expressed on the surface of immature myeloid cells and monocytes. In the present study, characterization of the wound infiltrating cells revealed that CD11b+, F4/80+ and Gr1+ myeloid cells were increased over that observed in uninjured skin. Further characterization of myeloid cells demonstrated that these cells were comprised of both traditional macrophages CD11b+F4/80+), as well as CD11b+Gr1+ myeloid cells. These myeloid cells have been shown to increase in animal models and patients with cancer, injury and infection (5, 17, 26, 27). Cairns et al. have also shown an accumulation in the periphery of CD11b+F4/80+ macrophages after burn injury (28). Further characterization of F4/80+ based on the CD11blow and CD11bhigh expression revealed that after injury both of these population were increased significantly at the burn wound site; however, there was a profound shift towards a CD11bhigh expressing population. Holt et al. have also identified two distinct macrophage populations in mouse liver after acetaminophen (APAP) challenge (29). While they observed CD11blowF4/80high macrophages in PBS-treated control mice, CD11bhighF4/80low macrophages were present in the mice challenged with APAP. In another study, Arnold et al. demonstrated a change in the phonotype of recruited monocytes during the resolution of inflammation and tissue repair (30). They demonstrated that the recruited macrophages at the tissue injury site were changed from inflammatory to anti-inflammatory phenotype, which was tissue-protective. The different subsets observed in our study may represent activated resident macrophages that have increased the expression of CD11b, or alternatively they may be derived from circulating monocytes that are recruited at the wound site after burn.
A profound increase in CD11b+Gr1+ myeloid cells was observed at the wound site after burn. In this regard, MDSCs are a heterogeneous population of myeloid cells that are characterized by the co-expression of CD11b and Gr1 (26, 31), thus this cellular infiltration is likely composed of MDSCs. Gr1 and CD11b are co-expressed on both neutrophils and on MDSCs; however, neutrophils do not express F4/80, a macrophage-associated molecule (32). On the contrary, MDSC have been shown to express F4/80 (33). Therefore, the cells characterized herein are consistent with a MDSC phenotype. Nonetheless, MDSCs are a heterogeneous population and a limitation of the experiments presented is the absence of analysis of more specific MDSC markers, such as CD31 and the absence of HLA-DR expression. Subsequent studies will need to address analysis of these markers, as well as the presence of suppressor activity by the burn wound infiltrating cells in order to confirm that they are truly MDSCs. MDSCs have only recently been reported in the trauma and sepsis literature (10, 17, 24–26). Mendoza et al. have shown the proliferation of CD11b+Gr1+ MDSCs within secondary lymphoid organs in a radiation and burn injury mouse model (17), whereas other researchers have shown an increase in CD11b+Gr1+ splenic MDSCs after traumatic injury in the mouse (26, 34). With regard to burn, Noel et al. described an increase CD11b+Gr1+ population in spleen of burn mice (18). While these studies demonstrate a role for MDSCs after injury, they have focused on lymphoid organs and not the injury site. In contrast, our recent findings in a wound sponge model showed an infiltration of CD11b+Gr1+ cells at 3 days, irrespective of burn injury (10). The specific factors involved in MDSC trafficking to the burn wound remain to be elucidated. Our previous studies have shown that the chemokine levels are reduced in burn-injured γδ T-cell deficient mice (41). It is probably that suppressed chemokine levels in the burn wound of γδ T-cell deficient mice are in part causative for the changes in myeloid cell infiltration that were observed.
The expansion of MDSCs has shown to be beneficial by increasing immune surveillance and innate immune responses in different injury models (17, 25). In addition to their suppressive effects on adaptive immune responses, MDSCs have also been reported to regulate innate immune responses by modulating macrophage cytokine production.
Noel et al. (42) have shown that infiltrating monocytes in the spleens of burned mice had increased inflammatory properties, including TNF-α production. In the study herein, we also observed elevated TNF-α levels, which may be due to the infiltration MDSCs.
Our findings suggest that early after burn (i.e., 3 days) there is a transition in the myeloid cell population at the injury site from a traditional macrophage phenotype (F4/80+) to a MDSC phenotype (i.e., Gr1+), as in WT mice, MDSC numbers increased and the numbers of F4/80+ cells decreased.
The lack of γδ T-cells profoundly influenced the myeloid cell populations at the wound site. Relative to WT mice, the numbers of CD11b+, F480+ and Gr1+ myeloid cells markedly increased after burn. This supports the concept that γδ T-cells at the burn wound site can act to suppress myeloid cell influx. In sharp contrast, Jameson et al. have shown that γδ T-cells are essential in the rapid migration of macrophages to the wound site in a murine punch wound model (23). These differences between our study and Jameson et al. may be in part related to the type of injury (burn vs. punch wound) and the overall systemic inflammatory response associated with burn as opposed to an isolated punch wound injury that would induce a minimal systemic response. We have previously shown that punch wound closure rates are suppressed in burn mice (35), supporting the concept that the wound inflammatory response differs between these 2 models.
Nitric oxide (NO) is known to promote angiogenesis (36), therefore it can be speculated that the appearance of NO producing MDSCs at the wound site 3 days after burn helps transition the wound from an inflammatory to the proliferative stage of healing. Gamma delta T-cells also regulate iNOS expression at the burn wound site, since iNOS expression in the injured tissue was significantly decreased in the absence of γδ T-cells (37). The lack of NO-mediated suppression in burn δTCR−/− mice may allow for expansion of the myeloid cell populations at the wound site. Nitric oxide is also involved in several inflammatory pathways of the, including granulation tissue formation, epithelial proliferation, collagen synthesis and angiogenesis and thereby helps accelerate the wound recovery (36). Alternatively, γδ T-cells produce a number of chemokines for the recruitment other immune cells, and the lack of infiltration by these cells may influence the myeloid cell numbers and phenotype.
While gamma-delta T-cells are the predominant dermal T-cells in the mouse skin; in humans, the majority of the T-cells are of αβ TCR lineage (19, 38). Nonetheless, it is clear from different clinical studies that, γδ T-cells, in human skin, play an important role in dermal pathologies, such as systemic lupus erythemoatosis (SLE), leprosy, leishmaniasis, malignancies (39, 40). Thus, while the absolute numbers of γδ T-cells in human skin may be less than that observed in rodents; they are an active cell population in humans, and their role in human dermal pathology is clearly evident.
In the current study, in parallel to the infiltration of myeloid cells and myeloid-derived suppressor cells, we also observed an increase in a number of inflammatory cytokines and chemokines such as, IL-1β, IL-6, TNF-α, MIP-1α, MIP-1β and MCP-1 at the burn site. The levels of epidermal TNF-α, MIP-1 α and MIP-1β were further elevated in the injured skin of δ TCR−/− mice as compared with WT mice. This data is consistent with our previous findings by Oppeltz et al. (37). In contrast, a study by Daniel et al. showed a profound attenuation in cytokine/chemokine levels at the wound site in δ TCR−/− mice (9). This suggests that γδ T-cell-mediated regulation of resident immune cells (in the current study) and infiltrating cells in the study by Daniel et al. (9) are markedly different.
The role of γδ T-cells in the recruitment of inflammatory cells to the injury site after burn has been previously described, as γδ T-cell deficient mice displayed a significant reduction in the cellular infiltration of the wounds and decreased growth factor (9, 16). Our recent study provides evidence that γδ T-cells also regulate T-cell infiltration of the burn wound, as infiltration of the wound with αβ T-cells was markedly attenuated in γδ T-cell deficient mice (43). Thus, these studies support the concept that γδ T-cells play a central role in regulating burn wound infiltration, inflammation and healing.
In conclusion, γδ T-cells play in important role in myeloid cell recruitment to the wound site early post burn and appear to act to transition the wound from an inflammatory stage to a proliferative stage of healing. Based on these findings and that wound healing after burn is a relevant clinical problem, and clearer understanding of potential targets of therapeutic intervention (i.e., γδ T-cells and MDSCs) may provide improvements in burn care leading to decreased morbidity and mortality.
Acknowledgements
MR was responsible for the animal experiments, cell isolation, FACs, data analysis and drafting of the manuscript. QZ were responsible for the animal experiments and cell isolation. MGS was responsible for scientific conception, design and interpretation and assisted in the final drafting of the manuscript. All authors read and approved the final version of the manuscript. The opinions or assertions contained herein are the private views of the author and are not to be construed as official or as reflecting the views of the Department of the Army or the Department of Defense.
Funding: This work was supported by grant funding from the National Institutes of Health (GM079122).
Footnotes
Conflict of Interest: The authors have no relevant conflicts of interest.
These finding were presented in part at the Experimental Biology 2013 in Boston, MA.
References
- 1.Flohe SB, Flohe S, Schade FU. Invited review: deterioration of the immune system after trauma: signals and cellular mechanisms. Innate Immun. 2008;14(6):333–344. doi: 10.1177/1753425908100016. [DOI] [PubMed] [Google Scholar]
- 2.Schwacha MG. Macrophages and post-burn immune dysfunction. Burns. 2003;29(1):1–14. doi: 10.1016/s0305-4179(02)00187-0. [DOI] [PubMed] [Google Scholar]
- 3.Alexander M, Chaudry IH, Schwacha MG. Relationships between burn size, immunosuppression, and macrophage hyperactivity in a murine model of thermal injury. Cell Immunol. 2002;220(1):63–69. doi: 10.1016/s0008-8749(03)00024-8. [DOI] [PubMed] [Google Scholar]
- 4.Heinrich SA, Messingham KA, Gregory MS, Colantoni A, Ferreira AM, Dipietro LA, Kovacs EJ. Elevated monocyte chemoattractant protein-1 levels following thermal injury precede monocyte recruitment to the wound site and are controlled, in part, by tumor necrosis factor-alpha. Wound Repair Regen. 2003;11(2):110–119. doi: 10.1046/j.1524-475x.2003.11206.x. [DOI] [PubMed] [Google Scholar]
- 5.O'Leary FM, Tajima G, Delisle AJ, Ikeda K, Dolan SM, Hanschen M, Mannick JA, Lederer JA. Injury-induced GR-1+ macrophage expansion and activation occurs independently of CD4 T-cell influence. Shock. 2011;36(2):162–169. doi: 10.1097/SHK.0b013e31821af669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brancato SK, Albina JE. Wound macrophages as key regulators of repair: origin, phenotype, and function. Am J Pathol. 2011;178(1):19–25. doi: 10.1016/j.ajpath.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.He L, Marneros AG. Macrophages are essential for the early wound healing response and the formation of a fibrovascular scar. Am J Pathol. 2013;182(6):2407–2417. doi: 10.1016/j.ajpath.2013.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Novak ML, Koh TJ. Macrophage phenotypes during tissue repair. J Leukoc Biol. 2013;93(6):875–881. doi: 10.1189/jlb.1012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Daniel T, Thobe BM, Chaudry IH, Choudhry MA, Hubbard WJ, Schwacha MG. Regulation of the postburn wound inflammatory response by gammadelta T-cells. Shock. 2007;28(3):278–283. doi: 10.1097/shk.0b013e318034264c. [DOI] [PubMed] [Google Scholar]
- 10.Schwacha MG, Thobe BM, Daniel T, Hubbard WJ. Impact of thermal injury on wound infiltration and the dermal inflammatory response. J Surg Res. 2010;158(1):112–120. doi: 10.1016/j.jss.2008.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haas W, Pereira P, Tonegawa S. Gamma/delta cells. Annu Rev Immunol. 1993;11:637–685. doi: 10.1146/annurev.iy.11.040193.003225. [DOI] [PubMed] [Google Scholar]
- 12.Matsushima A, Ogura H, Fujita K, Koh T, Tanaka H, Sumi Y, Yoshiya K, Hosotsubo H, Kuwagata Y, Shimazu T, Sugimoto H. Early activation of gammadelta T lymphocytes in patients with severe systemic inflammatory response syndrome. Shock. 2004;22(1):11–15. doi: 10.1097/01.shk.0000129203.84330.b3. [DOI] [PubMed] [Google Scholar]
- 13.Schwacha MG, Daniel T. Up-regulation of cell surface Toll-like receptors on circulating gammadelta T-cells following burn injury. Cytokine. 2008;44(3):328–334. doi: 10.1016/j.cyto.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schwacha MG, Knoferl MW, Chaudry IH. Does burn wound excision after thermal injury attenuate subsequent macrophage hyperactivity and immunosuppression? Shock. 2000;14(6):623–628. doi: 10.1097/00024382-200014060-00009. [DOI] [PubMed] [Google Scholar]
- 15.Alexander M, Daniel T, Chaudry IH, Schwacha MG. Opiate analgesics contribute to the development of post-injury immunosuppression. J Surg Res. 2005;129(1):161–168. doi: 10.1016/j.jss.2005.04.028. [DOI] [PubMed] [Google Scholar]
- 16.Alexander M, Daniel T, Chaudry IH, Choudhry MA, Schwacha MG. T cells of the gammadelta T-cell receptor lineage play an important role in the postburn wound healing process. J Burn Care Res. 2006;27(1):18–25. doi: 10.1097/01.bcr.0000188325.71515.19. [DOI] [PubMed] [Google Scholar]
- 17.Mendoza AE, Neely CJ, Charles AG, Kartchner LB, Brickey WJ, Khoury AL, Sempowski GD, Ting JP, Cairns BA, Maile R. Radiation combined with thermal injury induces immature myeloid cells. Shock. 2012;38(5):532–542. doi: 10.1097/SHK.0b013e31826c5b19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Noel JG, Guo X, Wells-Byrum D, Schwemberger S, Caldwell CC, Ogle CK. Effect of thermal injury on splenic myelopoiesis. Shock. 2005;23(2):115–122. doi: 10.1097/01.shk.0000154239.00887.18. [DOI] [PubMed] [Google Scholar]
- 19.Schwacha MG. Gammadelta T-cells: potential regulators of the post-burn inflammatory response. Burns. 2009;35(3):318–326. doi: 10.1016/j.burns.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barrientos S, Stojadinovic O, Golinko MS, Brem H, Tomic-Canic M. Growth factors and cytokines in wound healing. Wound Repair Regen. 2008;16(5):585–601. doi: 10.1111/j.1524-475X.2008.00410.x. [DOI] [PubMed] [Google Scholar]
- 21.Norling LV, Perretti M. Control of myeloid cell trafficking in resolution. J Innate Immun. 2013;5(4):367–376. doi: 10.1159/000350612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ortega-Gomez A, Perretti M, Soehnlein O. Resolution of inflammation: an integrated view. EMBO Mol Med. 2013;5(5):661–674. doi: 10.1002/emmm.201202382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jameson JM, Cauvi G, Sharp LL, Witherden DA, Havran WL. Gammadelta T cell-induced hyaluronan production by epithelial cells regulates inflammation. J Exp Med. 2005;201(8):1269–1279. doi: 10.1084/jem.20042057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brudecki L, Ferguson DA, McCall CE, El Gazzar M. Myeloid-derived suppressor cells evolve during sepsis and can enhance or attenuate the systemic inflammatory response. Infect Immun. 2012;80(6):2026–2034. doi: 10.1128/IAI.00239-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cuenca AG, Delano MJ, Kelly-Scumpia KM, Moreno C, Scumpia PO, Laface DM, Heyworth PG, Efron PA, Moldawer LL. A paradoxical role for myeloid-derived suppressor cells in sepsis and trauma. Mol Med. 2011;17(3–4):281–292. doi: 10.2119/molmed.2010.00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Makarenkova VP, Bansal V, Matta BM, Perez LA, Ochoa JB. CD11b+/Gr-1+ myeloid suppressor cells cause T cell dysfunction after traumatic stress. J Immunol. 2006;176(4):2085–2094. doi: 10.4049/jimmunol.176.4.2085. [DOI] [PubMed] [Google Scholar]
- 27.Tajima G, Delisle AJ, Hoang K, O'Leary FM, Ikeda K, Hanschen M, Stoecklein VM, Lederer JA. Immune system phenotyping of radiation and radiation combined injury in outbred mice. Radiat Res. 2013;179(1):101–112. doi: 10.1667/RR3120.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cairns BA, Barnes CM, Mlot S, Meyer AA, Maile R. Toll-like receptor 2 and 4 ligation results in complex altered cytokine profiles early and late after burn injury. J Trauma. 2008;64(4):1069–1077. doi: 10.1097/TA.0b013e318166b7d9. discussion 1077-1068. [DOI] [PubMed] [Google Scholar]
- 29.Holt MP, Cheng L, Ju C. Identification and characterization of infiltrating macrophages in acetaminophen-induced liver injury. J Leukoc Biol. 2008;84(6):1410–1421. doi: 10.1189/jlb.0308173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arnold L, Henry A, Poron F, Baba-Amer Y, van Rooijen N, Plonquet A, Gherardi RK, Chazaud B. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J Exp Med. 2007;204(5):1057–1069. doi: 10.1084/jem.20070075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9(3):162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hidalgo A, Chang J, Jang JE, Peired AJ, Chiang EY, Frenette PS. Heterotypic interactions enabled by polarized neutrophil microdomains mediate thromboinflammatory injury. Nat Med. 2009;15(4):384–391. doi: 10.1038/nm.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Youn JI, Nagaraj S, Collazo M, Gabrilovich DI. Subsets of myeloid-derived suppressor cells in tumor-bearing mice. J Immunol. 2008;181(8):5791–5802. doi: 10.4049/jimmunol.181.8.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang K, Bai X, Li R, Xiao Z, Chen J, Yang F, Li Z. Endogenous glucocorticoids promote the expansion of myeloid-derived suppressor cells in a murine model of trauma. Int J Mol Med. 2012;30(2):277–282. doi: 10.3892/ijmm.2012.1014. [DOI] [PubMed] [Google Scholar]
- 35.Schwacha MG, Nickel E, Daniel T. Burn injury-induced alterations in wound inflammation and healing are associated with suppressed hypoxia inducible factor-1alpha expression. Mol Med. 2008;14(9–10):628–633. doi: 10.2119/2008-00069.Schwacha. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhu H, Ka B, Murad F. Nitric oxide accelerates the recovery from burn wounds. World J Surg. 2007;31(4):624–631. doi: 10.1007/s00268-007-0727-3. [DOI] [PubMed] [Google Scholar]
- 37.Oppeltz RF, Rani M, Zhang Q, Schwacha MG. Gamma delta (gammadelta) T-cells are critical in the up-regulation of inducible nitric oxide synthase at the burn wound site. Cytokine. 2012;60(2):528–534. doi: 10.1016/j.cyto.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Elbe A, Foster CA, Stingl G. T-cell receptor alpha beta and gamma delta T cells in rat and human skin--are they equivalent? Semin Immunol. 1996;8(6):341–349. doi: 10.1006/smim.1996.0045. [DOI] [PubMed] [Google Scholar]
- 39.Robak E, Niewiadomska H, Robak T, Bartkowiak J, Blonski JZ, Wozniacka A, Pomorski L, Sysa-Jedrezejowska A. Lymphocyctes Tgammadelta in clinically normal skin and peripheral blood of patients with systemic lupus erythematosus and their correlation with disease activity. Mediators Inflamm. 2001;10(4):179–189. doi: 10.1080/09629350124724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Holtmeier W, Pfander M, Zollner TM, Kaufmann R, Caspary WF. Distinct TCR delta repertoires are present in the cutaneous lesions and inflamed duodenum of patients with dermatitis herpetiformis. Exp Dermatol. 2002;11(6):527–531. doi: 10.1034/j.1600-0625.2002.110605.x. [DOI] [PubMed] [Google Scholar]
- 41.Toth B, Alexander M, Daniel T, Chaudry IH, Hubbard WJ, Schwacha MG. The tole of gammadelta T cells in the regulation of neutrophil-mediated tissue damage after thermal injury. J Leukoc Biol. 2004;76(3):545–552. doi: 10.1189/jlb.0404219. [DOI] [PubMed] [Google Scholar]
- 42.Noel JG, Guo X, Wang Q, Schwemberger S, Byrum D, Ogle C. Postburn monocytes are the major producers of TNF-alpha in the heterogeneous splenic macrophage populaiton. Shock. 2007;27(3):312–319. doi: 10.1097/01.shk.0000239753.75088.5e. [DOI] [PubMed] [Google Scholar]
- 43.Rani M, Zhang Q, Scherer MR, Cap AP, Schwacha MG. Activated skin γδ T-cells regulate T-cell infiltration of the wound site after burn. Innate Immnunity. doi: 10.1177/1753425913519350. (epub ahead of print PMID: 24412847). [DOI] [PubMed] [Google Scholar]