Abstract
Early life events can alter gene expression through DNA methylation. The methylation status of the exon 17 promoter of the glucocorticoid receptor (Nr3c1 gene) in hippocampus associates with frequency of pup licking. Much of this work was conducted with male rats. Because dams more frequently lick male pups, this may contribute to sex differences in phenotypes through DNA methylation. Modifying litter gender composition (LGC), in which offspring of single-sex litters are compared to mixed-sex litters, alters maternal behavior. Previously, we demonstrated that LGC and sex affected pup licking times as well as anxiety and hippocampal DNA methylation of the Nr3c1 exon 17 promoter gene in adolescence. Now, we expand upon this work by examining effects in cerebellum and measuring mRNA levels. We also re-assessed DNA methylation in hippocampus using pyrosequencing and re-analyzed pup licking with the more commonly used frequency measure. Litters, culled to 8 pups on postnatal day 1 (PN1), were assigned to one of three conditions: all male, (n=10), all female, (n=12), or half of each sex (n=20). Licking was rated on PN4, 7, and 10. On PN35, hippocampal and cerebellar samples were obtained. Single-sex males were licked the least and mixed-sex males, the most. Hippocampal Nr3c1 mRNA levels were lowest in mixed females with no LGC or Sex effects in DNA methylation. Cerebellar DNA methylation levels were lowest in mixed males with no effect on mRNA levels. Maternal pup licking associated with DNA methylation of the Nr3c1 exon 17 promoter gene in cerebellum and with hippocampal mRNA.
Keywords: sex differences, female, epigenetics, mRNA, glucocorticoid receptors
1. Introduction
Understanding processes that contribute to vulnerability or resiliency to develop stress-related mental disorders is an important goal because information gained should help promote more effective prevention and treatment strategies. Risk factors appear to interact with gender as suggested by higher rates of some disorders (e.g., autism; addiction) in males and higher rates of other disorders (e.g., depression; anxiety) in females (Fombonne, 2003; Grant et al., 2009; Vigod and Stewart, 2009). Early life trauma increases susceptibility to depression, schizophrenia, post-traumatic stress disorder, addiction, and other disorders (Heim and Nemeroff, 2001; Yehuda et al., 2001; Gordon, 2002; Howes et al., 2004) and such effects can differ by gender (Goel and Bale, 2009). The mechanisms by which gender affects vulnerability to develop stress-related disorders may involve epigenetic mechanisms (Dudley et al., 2011; Jessen and Auger, 2011).
Early life manipulations can induce changes in gene expression that result in stable phenotypic alterations. DNA methylation is one type of epigenetic mechanism involved in cellular differentiation during development in which a methyl group is attached to the DNA preventing binding of transcriptional factors (Jones and Taylor, 1980). This silences the gene and can have enduring phenotypic effects (Razin, 1998). Epigenetic modification of gene expression and resulting changes in phenotype may be mediated via maternal care. Maternal behavior shapes the corticotrophin releasing factor-hypothalamic pituitary-adrenal (CRF-HPA) axis of adult offspring through epigenetic mechanisms (Champagne and Curley, 2009). Glucocorticoid receptor (GR) feedback is greater and HPA axis responses to stress are more moderate in offspring of dams that show high amounts of pup-licking and arched-back nursing (Liu et al., 1997; Francis et al., 1999; Champagne and Meaney, 2001). Variations in the maternal behavior of pup-licking associate with the methylation status of the Nr3c1 gene exon 17 promoter region in hippocampus, that contains GR, and is an important negative feedback mechanism for HPA axis activity. Consequently, GR gene expression is decreased and alterations in the hormonal and behavioral responses to stress are seen in the adult offspring (Champagne et al., 2003; Weaver et al., 2004). Regional differences in DNA methylation, histone H3K9 acetylation, and transcription across a seven million nucleotide region that contains the Nr3c1 gene associate with maternal behavior (McGowan et al., 2011).
Much of this work was performed with male rats (but see (Champagne et al., 2003; Weaver et al., 2004) and yet, several studies demonstrate that dams preferentially lick their male pups (Moore and Morelli, 1979; Richmond and Sachs, 1984; Moore and Chadwick-Dias, 1986; Alleva et al., 1989; Cirulli et al., 1997). This led us to hypothesize that sex differences in stress responses in adulthood may reflect sex-dependent effects of pup-licking and the resulting alterations in DNA methylation of the Nr3c1 gene exon 17 promoter region in relevant brain regions (Kosten et al., 2014). Indeed, female rats exhibit greater stress responsivity than male rats (Beatty and Beatty, 1970; Rivier, 1999; Kudielka and Kirschbaum, 2005) and the hippocampus shows sexual dimorphism (Herman and Cullinan, 1997; Rivier, 1999; McEwen, 2002). Not surprisingly, stress experienced in adulthood or during early life can have different effects depending upon the sex of the animal. For example, stress in adulthood enhances Pavlovian aversive conditioning in males but impairs conditioning in females (Wood and Shors, 1998; Shors et al., 2000; Cordero et al., 2003; Kim et al., 2006). Early life stress can have sex-dependent effects on measures of unconditioned fear or anxiety (McIntosh et al., 1999; Wigger and Neumann, 1999), performance on aversive conditioning tasks (Pryce et al., 2003; deJongh et al., 2005; Kosten et al., 2005; Kosten et al., 2006), and levels of hippocampal GRs (Sutanto et al., 1996; Avishai-Eliner et al., 1999).
Male, but not female, rats show altered GR expression in cerebellum that associates with impairments in aversive conditioning in response to early life stress (Wilber et al., 2007). The cerebellum, like the hippocampus, shows moderate to strong GR mRNA levels (Sousa et al., 1989) that can be down-regulated by stress experienced in adulthood in male rats (Kitraki et al., 1999) and is an important neural substrate for aversive conditioning (Steinmetz, 2000; Christian and Thompson, 2003). Finally, DNA methylation status of other genes in hippocampus, placenta, or other brain regions is differentially regulated by prenatal or postnatal influences in male versus female rodents (Mueller and Bale, 2008; Roth et al., 2009; Kurian et al., 2010; Edelmann and Auger, 2011; Hao et al., 2011).
Previously, we showed that altering the litter gender composition (LGC), such that litters are either single- or mixed-sex, influenced maternal care and led to modest changes in anxiety behavior and DNA methylation levels of some genes in hippocampus and other brain regions in adolescent rats (Hao et al., 2011; Kosten et al., 2014). Although LGC did not change the amount of time the dam spent licking her pups, these times were greater for male vs. female pups (Hao et al., 2011) consistent with previous studies (Moore and Morelli, 1979; Richmond and Sachs, 1984). We also found some modest effects of LGC as well as sex differences at some CpG sites for the exon 17 promoter region of the Nr3c1 gene in hippocampus using direct sequencing methods (Kosten et al., 2014). Now, we expand upon our prior work in the following ways. First, we include assessments of the cerebellum as well as hippocampus. Second, we used the pyrosequencing method in the current study because it is more sensitive and allows examination of a greater range of CpG sites compared to direct sequencing. Third, we measure mRNA levels to determine if alterations in DNA methylation correlated with Nr3c1 gene expression in the predicted manner (i.e., greater methylation should associated with less mRNA levels). Finally, we re-analyzed pup-licking using frequency measures, as opposed to total times, because this measure was used in the studies that showed an association between pup-licking and the CRF-HPA axis system including altered DNA methylation levels of the Nr3c1 exon 17 promoter gene in hippocampus (Liu et al., 1997; Francis et al., 1999; Champagne and Meaney, 2001; Weaver et al., 2004).
2. Methods
2.1 Subjects
Adult (90–110 days of age), Sprague-Dawley male and female rats (Charles River, MA) were used for breeding. All females were primiparous and were bred once. Males were allowed to mate up to three times before being retired. Rats were housed in sets of one male and two females until females appeared to be pregnant. At that time, females were housed individually in polypropylene cages in a vivarium that was temperature- and humidity-controlled and maintained on a 12:12 light-dark cycle with lights on at 0700. Food and water were available at all times. The Institutional Animal Care and Use Committee (IACUC) approved the experimental procedures in accordance with the National Institute of Health (NIH) guidelines.
2.2 Litter gender composition groups
Pregnant females were monitored daily and when a new litter was found before 1700, it was considered post-natal day 0 (PN0). Litters were weighed and sexed the following day, PN1, and culled to 8 pups each. Litters were assigned to be either single-sex (all male or all female pups) or mixed-sex (4 male and 4 female pups). Of the 42 litters assessed, 10 were all male, 12 were all female, and 20 were mixed-sex litters. Ten mixed-sex litters were designated to have a male pup sampled during the maternal behavior testing and 10 were designated to have a female pup sampled for this test. However, for DNA methylation analyses, one pup per sex was chosen from all 20 mixed-sex litters.
2.3 Mother-pup behaviors
Pup licking frequency was tabulated in 10-min sessions on PN4, PN7, and PN10 as described previously (Hao et al., 2011). Briefly, the dam was removed from the home cage and placed in an observation tank and 30-min later, one of her pups was introduced into the tank. These sessions were video-recorded and rated at a later time under blind conditions. The rater recorded the presence or absence of pup licking every 30-sec for the entire 10-min session. Frequency was defined as the total counts of the presence of this behavior on each of the three days. We also summed the total times (s) the pup spent moving towards its dam in each of these sessions.
2.4 DNA methylation and mRNA levels
On PN35, one rat per sex per litter was sacrificed and brain tissue samples from hippocampus and cerebellum were obtained as described previously (Hao et al., 2011). DNA was isolated from the brain tissues using the Gentra Purgene DNA isolation method (Qiagen, Valencia, CA) according to manufacturer’s protocol.
The nucleotide sequence of the rat Nr3c1 exon 17 promoter region [chr18:32462001-32462377, Rat Nov. 2004 (Baylor 3.4/rn4)] was downloaded from the UCSC Genome Browser website (genome.ucsc.edu). This sequence contains the region studied by Weaver and colleagues (Weaver et al., 2005) with an additional 100 nucleotides added to each end. The sequence was exported to PyroMark Assay Design 2.0 (Qiagen, Valencia, CA) for the design of the amplification and sequencing primers. Primers were synthesized (Midland Reagent Co., Midland, TX). Genomic DNA was sodium bisulfite-treated as previously described (Nielsen et al., 2009) and amplified with the primers M-RATGR17-F (5′-AGAGGGAGTGTTTGTAGTTTTGTTT-3′) and M-RATGRE17-R1-B (5′-biotin-CTTTAATTTCTCTTCTCCCAAACTC-3′). Amplification was performed with 4 μl bisulfite-treated DNA, 1 μM of each primer, 200 μM each of dATP, dCTP, dGTP, and TTP, 18 mM ammonium sulfate, 2 mM MgSO4, 0.5 units Platinum® Taq DNA Polymerase High Fidelity (Invitrogen), and 60 mM Tris–SO4 (pH 8.9) in 25 μl. Amplification consisted of 5-min at 95°C, 40 cycles of 15-s at 95°C, 15-s at 56°C, and 30 sec at 72°C, followed by a final elongation step at 72°C for 7-min. Briefly, the single-stranded biotinylated product was purified by mixing 5 μl of the amplification mixture, 2 μl Streptavidin Sepharose HP (Amersham Biosciences, Uppsala, Sweden), 40 μl binding buffer in 80 μl final volume according to the manufacturer’s protocol. The Sepharose beads containing the immobilized biotinylated PCR product were purified, washed, denatured in 0.2 M NaOH, and washed again using the Pyrosequencing Vacuum Prep Tool (Qiagen) according to the manufacturer’s protocol. The biotinylated DNA was re-suspended in 12 μl of annealing buffer containing 0.3 μM pyrosequencing primer. Two pyrosequencing primers were utilized: M-RATGR17-S1 (5′-AGTTTTTTTGTTAGTGTGAT-3′), and M-RATGR17-S2 (5′-GGGGGTTTTGGTTGT-3′). DNA methylation levels were determined using a PyroMark Q96 MD Pyrosequencing System (Qiagen). Nucleotides are numbered relative to the transcription start site of exon 17 of rat Nr3c1. See Fig 1. The rat exon 17 promoter region was analyzed for predicted transcription factor binding sites using TESS: Transcription Element Search System (Schug and Overton, 1977) and AliBaba2.1 (http://www.gene-regulation.com/pub/programs/alibaba2/index.html).
Figure 1.
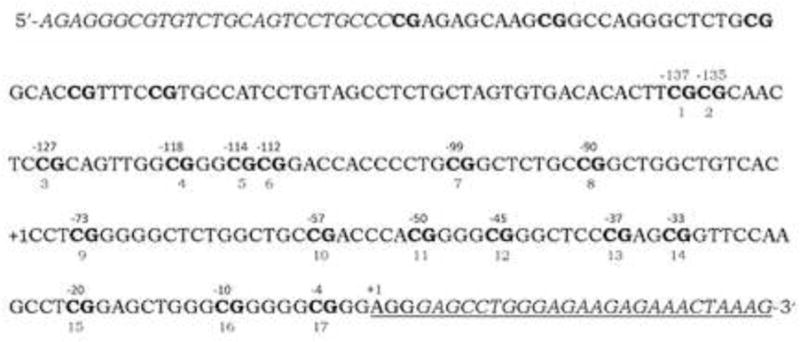
The sequence of the amplified Nr3c1 gene exon 17 promoter region is displayed with the CpG sites shown in bold. The position of the cytosine of the CpG sites analyzed in this study relative to the transcription start site of exon 17 are indicated above the DNA sequence. The CpG site numbers used by Meaney, Weaver, et al. are shown below the sequence. Sequences corresponding to the PCR primers are in italics.
Quantitative PCR (qPCR) was performed using cDNA reverse transcribed from 1μg total RNA (QuantiTect Reverse Transcription Kit, Qiagen). TaqMan gene expression assays (Applied Biosystems) were used for Nr3c1 (Rn00561369_m1) and for the 18S RNA (Rn03928990_g1) internal control. For each sample, 1 μl 20X TaqMan gene expression assay was mixed with 10 μl TaqMan Gene Expression Master Mix, 25 ng cDNA, and water to yield 20μl final volume and analyzed per manufacturer recommendation on a ViiA™ 7 Real-Time PCR System (Applied Biosystems). Three 25 ng cDNA replicates per sample were made in a 96 well plate. One sample was used as an intra-plate control. PCR was performed by incubation at 50°C for 2 min and 95°C for 10 min, followed by 40 cycles of 95°C for 15 sec and 60°C for 45 sec. Comparative CT (ddCT) method was used to determine Nr3c1 RNA concentration relative to the 18S RNA (Applied_Biosystems, 2001). Data were analyzed with the Sequence Detection System Software (Applied Biosystems).
2.5 Data analysis
Frequency of pup licking was tabulated across each 10-min session and analyzed using a 2 × 2 × 3 ANOVA representing the between-group factors of litter gender composition (LGC; single- vs. mixed-sex), Sex of pup, and the within-group factor of Day. Total time (sec) that the pup moved towards its dam was measured and analyzed similarly. DNA methylation levels were analyzed using 2 × 2 × 17 ANOVA in separate analyses by brain region. These factors represent the two levels each of LGC (mixed- and single-sex) and Sex (male, female), with repeated measures on the 17 CpG sites. The mRNA level data were analyzed using a 2 × 2 ANOVA (LGC × Sex). Significant main effects of LGC and Sex or a significant interaction of these terms were followed by post-hoc statistics (Newman-Keuls) using the Statistica software package. The P value was set at 0.05.
3. Results
3.1 Mother-pup behaviors
Frequencies of pup licking tabulated on PN4, PN7, and PN10 are presented in Fig. 2 for male and female pups. As seen in Fig. 2, dams of mixed-sex litters more frequently lick their male pups than do the dams of single-sex litters. Licking received by female pups does not differ by LGC. These statements are supported by the significant main effect of LGC, F=(1, 36)=8.10; P<0.01, and by the significant interaction of LGC × Sex, F=(1,36)=8.30; P<0.01. Post-hoc statistics show that the LGC × Sex interaction is significant on PN4 and PN7 (P’s<0.05) that likely reflects the differences between the mixed- and single-sex males. On PN10, there is a significant LGC effect (P<0.05) that likely reflects that pups of both sexes from mixed-litters receive more licking than pups from single-sex litters. The main effects of Sex and Day and all other interactions failed to reach significance (P’s>0.10).
Figure 2.
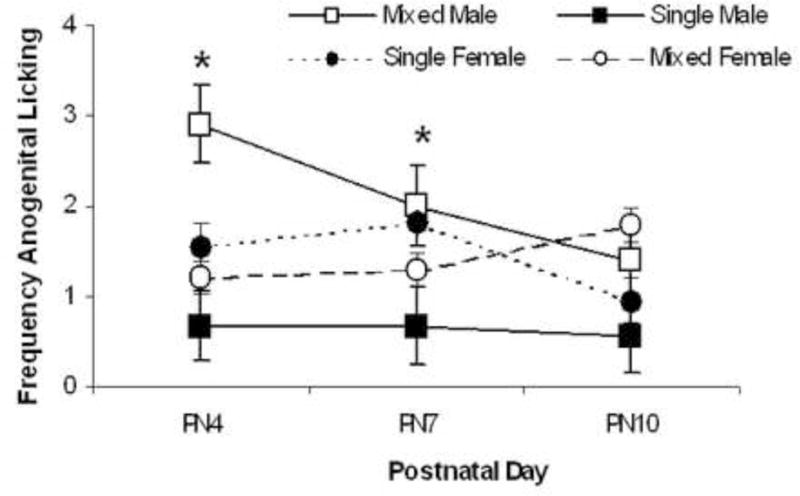
Mean ± S.E.M frequencies of pup licking across the three, 10-min sessions rated on postnatal (PN) days 4, 7, and 10 are shown for male pups (squares) and female pups (circles). Data from pups raised in mixed-sex litters are depicted by closed symbols and data from pups raised in single-sex litters are depicted by open symbols. Male pups from mixed-sex litters are licked the most whereas male pups from single-sex litters are licked the least, particularly on PN4 and PN7. # LGC; % LGC × Sex; P’s<0.01.
The times that the pups spent moving towards its dam recorded on PN4, PN7, and PN10 are shown in Table 1. Male pups spend more time moving towards their dams than female pups as supported by the significant main effect of Sex, F(1,38)=3.55; P=0.05. These times also varied by postnatal Day, F(2,76)=3.45; P<0.05, that likely reflects that these times are greatest on PN7. LGC does not affect this behavior, P>0.10.
Table 1.
Mean ± S.E.M. time (sec) pup spent moving towards its dam by postnatal day and LGC and Sex groups.
| DAY | MIXED-MALES | SINGLE-MALES | MIXED-FEMALES | SINGLE-FEMALES |
|---|---|---|---|---|
| PN4 | 70 ± 18 | 68 ± 20 | 28 ± 12 | 56 ± 15 |
| PN7 | 109 ± 19 | 80 ± 46 | 90 ± 21 | 46 ± 13 |
| PN10 | 71 ± 18 | 61 ± 25 | 46 ± 11 | 24 ± 7 |
3.2 DNA methylation levels of the Nr3c1 gene exon 17 promoter region
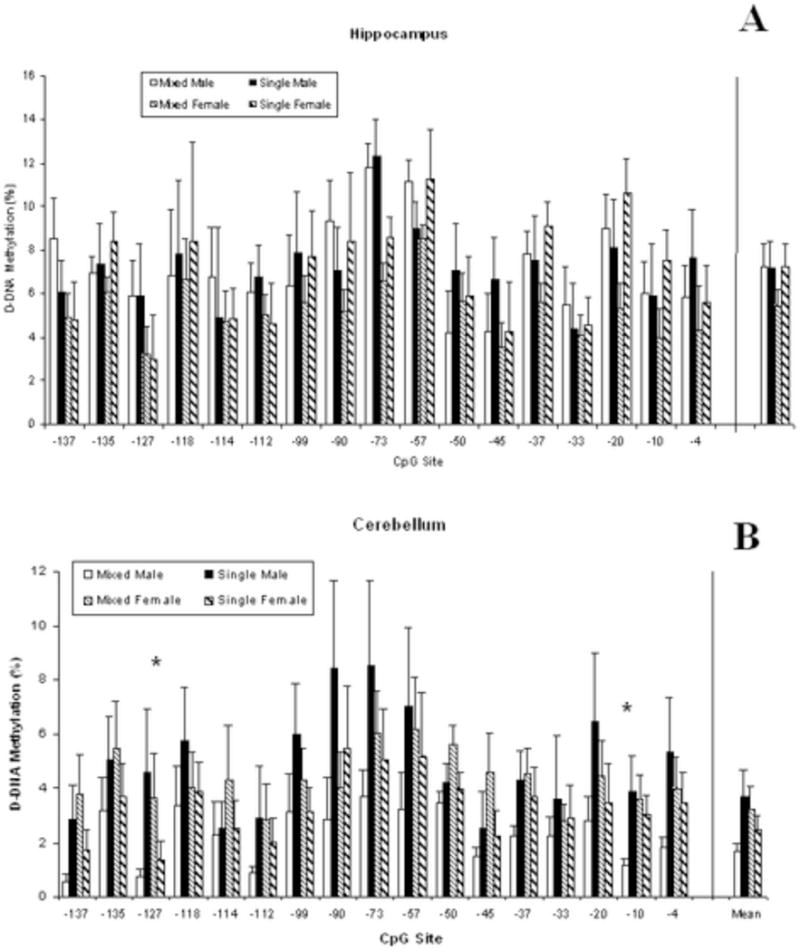
Mean DNA methylation levels of the Nr3c1 gene exon 17 promoter region in hippocampus are shown in Fig 3a by the 17 CpG sites. DNA methylation levels are not altered by LGC or by Sex. Although the LGC and Sex main effects do not reach significance, P’s >0.10, methylation levels do vary by CpG site, F(16,912)=5.06; P<0.001. The overall mean across CpG sites is seen at the far right of Fig. 3a.
Figure 3.
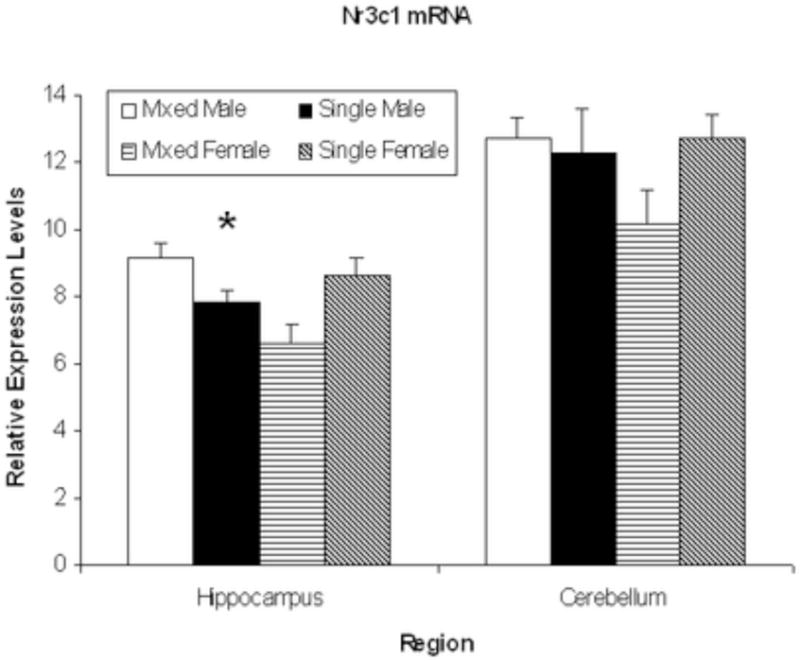
Mean ± S.E.M. relative expression levels of the Nr3c1 gene for hippocampus and cerebellum sites are shown for mixed-sex male (open bars), single-sex male (solid bars,) mixed-sex female (horizontal striped bars), and single-sex female (diagonal striped bars) offspring. There were no group differences in cerebellum. In hippocampus, females from mixed-sex litters had lower mRNA levels than females from single-sex litters and males from mixed-sex litters. * LGC × Sex, P<0.05.
Mean DNA methylation levels of the Nr3c1 gene exon 17 gene promoter region in cerebellum are shown in Fig 3b by the 17 CpG sites. Although neither main effect reaches significance, P’s>0.10, there is a significant interaction of LGC × Sex, F(1, 57)=5.44; P<0.05. As seen in Fig 3b, males from mixed-sex litters show significantly lower DNA methylation levels than both mixed-sex females and single-sex males, P’s<0.05. Methylation levels also vary by CpG site, F(16,912)=4.42; P<0.001. Post-hoc statistics for individual CpG sites show a significant LGC × Sex interaction at the −127 and−10 CpG sites, F(1,57)=4.00 and 4.78; P’s<0.05. This reflects that methylation levels are highest in males from single-sex litters.
3.3 mRNA levels of the Nr3c1 gene
The mRNA levels of the Nr3c1 gene are shown in Fig 4. There is a significant LGC × Sex interaction for hippocampal mRNA levels, F(1,50)=33.96; P<0.01, although neither main effect reaches significance, P’s>0.10. As seen in Fig 4, mixed-sex females have lower mRNA levels than both mixed-sex males and single-sex females, P’s<0.05. The mRNA levels of this gene in cerebellum, also shown in Fig 4, do not differ by LGC or Sex nor show a significant interaction effect, P’s >0.10.
Figure 4.
Mean ± S.E.M. % DNA methylation levels of the Nr3c1 gene exon 17 promoter across the 17 CpG sites for mixed-sex male (open bars), single-sex male (solid bars,) mixed-sex female (horizontal striped bars) and single-sex female (diagonal striped bars) offspring for the hippocampus (A) and cerebellum (B). The mean across all 17 CpG sites is shown at the far right of each figure. There were no group differences in hippocampus. In cerebellum, males from mixed-sex litters show higher DNA methylation levels than single-sex males and mixed-sex females. * LGC × Sex, P<0.05.
4. Discussion
The results of the present study show that altering litter gender composition (LGC) has sex-dependent effects on both the behavior of the dam towards her pup and on DNA methylation levels of the Nr3c1 gene exon 17 promoter region in the cerebellum of the offspring. Specifically, male pups raised in single-sex litters receive the least amount of licking from the dam. Male pups raised in mixed-sex litters receive the most licking and also show hypomethylation of the Nr3c1 exon 17 promoter region in cerebellum. While there is no effect of sex or LGC on DNA methylation levels of this promoter region in hippocampus, mRNA levels are significantly affected by LGC such that mixed-sex female offspring show lower levels than both mixed-sex males and single-sex females. There is some support for our original hypothesis that predicted sex differences in these measures. That is, we find sex differences among the mixed-sex litters, the typical litter condition, such that males have lower DNA methylation levels in cerebellum and higher mRNA levels in hippocampus compared to female rats.
Data from the present study replicate and extend prior work that shows that the behavior of a dam towards her pups is sex-dependent in rats (Moore and Morelli, 1979; Richmond and Sachs, 1984; Moore and Chadwick-Dias, 1986; vanHasselt et al., 2012) and in mice (Alleva et al., 1989; Cirulli et al., 1997). That is, dams lick their male pups more than their female pups. However, we find that if the dam’s litter has only male pups, she licks them the least although this difference in licking received between males of single- versus mixed-sex litters declines over days (see Fig 2). This LGC effect in males may reflect that having pups of both sexes in a litter makes it easier for the dam to discriminate the sex of the pup so she can lick the male pups more. It appears that this sex discrimination does not depend upon the pup’s behavior as male pups from both litter types spend more time moving towards their dam than female pups. However, other behaviors, such as ultrasonic vocalization patterns that were not assessed in the present study, may have been affected by the LGC manipulation.
The behavior of arched-back nursing is commonly assessed in studies of maternal behavior and epigenetic factors (e.g., Weaver, et al. 2004; Champagne et al., 2006). We had hoped to measure this behavior but it occurred too infrequently perhaps due to the short observation time period. These former studies as well as prior LGC studies of maternal care, observed mother-pup behaviors in the home cage. In contrast, we employed a procedure in which the dam was removed from the home cage and one pup of a predetermined sex was introduced for the 10-min observation period. We chose this method because it allowed us to examine the factors of sex and LGC separately as well as their interaction. Although the observation period used in the present study was relatively short, removal of pups and then re-introducing them to the dam likely stimulated a bout of maternal behavior (Grota and Ader, 1969; Kosten and Kehoe, 2010). In spite of these methodological differences, the data obtained from the present study are consistent with those obtained from previous LGC studies that used home cage observation methodology (Moore and Morelli, 1979; Alleva et al., 1989; Cirulli et al., 1997).
Either natural variations in maternal care or changes in care induced by neonatal “handling” affect stress responsivity in the adult such that greater arched-back nursing and licking/grooming received as a pup relates to more moderate stress responsivity in adulthood (Francis and Meaney, 1999; Meaney, 2001). This effect may be mediated by epigenetic changes in gene expression. Indeed, maternal care associates with DNA methylation levels of the exon 17 promoter region of the glucocorticoid receptor Nr3c1 gene and glucocorticoid receptor (GR) gene expression in hippocampus of male offspring (Weaver et al., 2004). That is, offspring of high-licking/grooming dams have lower stress responsivity and DNA methylation levels of the Nr3c1 exon 17 promoter. In humans, childhood abuse associates with lower GR mRNA levels and higher cytosine methylation of the NR3C1 promoter, that includes the 1F exon, in post-mortem hippocampal tissue (McGowan et al., 2009). In addition to this postnatal effect, prenatal exposure to maternal depression or anxiety increases methylation of NR3C1 at the predicted NGFI-A binding site in cord blood of infants (Oberlander et al., 2008). Yet, major depressive disorder does not link to higher methylation levels of the exon 1F, which was found to be unmethylated in hippocampus of both depressed and non-depressed humans, even though NGFI-A was down-regulated and GR transcript levels lower in the depressed sample (Alt et al., 2010).
Based on results of the rat studies, most of which were conducted with all-male litters, we predicted that female offspring would show higher DNA methylation levels of the Nr3c1 exon 17 promoter in hippocampus than male offspring because they receive less licking from their dams (Moore and Morelli, 1979; Richmond and Sachs, 1984). However, we failed to find an association between degree of licking and DNA methylation levels of the Nr3c1 exon 17 promoter in hippocampus across sexes. Another early life manipulation, maternal separation, also failed to affect DNA methylation levels of Nr3c1 exon 17 promoter in hippocampus of male rats (Daniels et al., 2009) even though this manipulation also increases maternal licking (Pryce et al., 2001; Marmendal et al., 2004). Yet, prenatal stress in mice increased DNA methylation levels of the Nr3c1 gene exon 17 promoter in hypothalamus of male offspring, but not in female offspring (Mueller and Bale, 2008).
Previously, we found that female rats did have higher DNA methylation levels than male rats at two of 10 CpG sites in hippocampus assessed with the direct sequencing method (Kosten et al., 2014). We found no sex difference in DNA methylation levels in hippocampus in the present study that used the pyrosequencing method. This may be due to the differences in experimental procedures between studies used to assess DNA methylation. The direct sequencing method used the same primers that were used to amplify the bisulfate-treated DNA to sequence the amplification product and the results were presented as the mean level of DNA methylation determined by sequencing in the forward and reverse directions. In the pyrosequencing method, different primers were used to amplify the DNA than were used in the direct sequencing method. Additionally, single-stranded amplified DNA was purified and sequenced internally with two different primers. Other studies have shown that sequencing of cloned bisulfite-treated DNA yields similar results compared to direct sequencing (Nielsen et al., 2009) and to pyrosequencing (Shiao et al., 2005; Reed et al., 2010).
In contrast to the lack of sex and LGC effects in hippocampus, we did find that mixed-sex, male rats, the group that received the highest licking frequency, had lower DNA methylation levels of the Nr3c1 exon 17 gene promoter in cerebellum compared to mixed-sex, female rats and to single-sex male rats. This effect in cerebellum was what we predicted would be seen in hippocampus. In contrast to the hippocampus, much less is known about GR in cerebellum although these receptors are found in this area, particularly in granule and Purkinje cell layers (Sousa et al., 1989; Ahima and Harlan, 1990; Morimoto et al., 1996). In fact, we chose to examine this region because it has morphological similarities to the hippocampus and, like the hippocampus, contributes to Pavlovian aversive conditioning (Steinmetz, 2000; Christian and Thompson, 2003). Nr3c1 gene expression in granule and Purkinje cell layers is down-regulated by stress experienced in adulthood (Kitraki et al., 1999) and GR expression in the posterior interpositus nucleus is increased in adult, male rats with maternal separation experience (Wilber et al., 2007). The latter effect was not seen in female rats and former effect was not assessed in female rats.
Differentially methylated CpG were found at −127 and at −10 of the Nr3c1 gene exon 17 promoter in the cerebellum. Examination of these sites in silico revealed that the −10 CpG site is located in the putative transcription factor (TF) binding sites for Egr1, Egr2, Sp1, and Wt1. No TF binding sites were predicted for the −127 CpG site. In the present study, the −10 site was hypermethylated in cerebellum of single males compared to mixed males. This is of interest since we found previously that the Egr1 gene promoter region was hypomethylated in the NAc of single males compared to the other LGC groups (Kosten et al., 2014). Hypomethylation of the −10 site may alter transcription of the Nr3c1 exon 17 gene promoter as it has been shown previously that DNA methylation alters the binding of several TFs (Hwang et al., 2010; Chen et al., 2013). Although we did find differences in DNA methylation at specific sites in the Nr3c1 gene exon 17 promoter in the cerebellum, we found no change in GR expression. This may be because the probe we used to measure gene expression is specific for the Nr3c1 mRNA exon 2-exon 3 splice junction. Therefore, we have quantitated the expression of mature Nr3c1 transcripts, not only those transcribed from exon 17. Since 11 transcription start sites have been identified in the promoter of Nr3c1, alternative promoters may be used in the cerebellum (McCormick et al., 2000). In addition, the changes in methylation seen in the cerebellum may be a provide memory that reflect events that occurred earlier in development. For example, tyrosine amino transferase (Tat) expression is transiently increased in glucocorticoid-stimulated rat hepatic cells, returning to baseline after three months (Thomassin et al., 2001). This glucocorticoid stimulation hypomethylates the Tat promoter, a state that is stable over a three-month period. Subsequent stimulation with glucocorticoids results in a 3–5 fold greater expression of Tat, presumably due to the hypomethylation. Additionally, hypomethylation of a specific CpG site in the corticotropin-releasing hormone (Crh) gene promoter by maternal deprivation alters expression after prolonged acute stress in adult rats (Chen et al., 2012). Hence, the DNA methylation differences we observe may constitute a memory that allows for priming for later neuronal activation (Baker-Andresen et al., 2013).
Although we find that the same group (males from mixed-sex litters) that received the most licking as pups showed the lowest DNA methylation levels in cerebellum, these measures do not correlate with each other. Seven TSSs of Nr3c1 are shown to be utilized in the hippocampus (McCormick et al., 2000), but TSSs usage has not been determined in the cerebellum. In the hippocampus, exon 17 utilization accounts for only 8% of the Nr3c1 mRNA, so it may be that the change in DNA methylation observed at the exon 17 contributes only a minor fraction to total Nr3c1 mRNA levels. Future studies on the expression from the alternate TSSs in cerebellum could shed light on this question. Yet, other studies have shown causal, not just correlational, evidence that maternal behavior of licking or grooming affects DNA methylation levels of Nr3c1 or ER! promoter regions in offspring (Weaver et al., 2004; Weaver et al., 2005; Edelmann and Auger, 2011). Our findings of sex differences in DNA methylation levels of the Nr3c1 gene exon 17 promoter region in cerebellum may relate to the sex-dependent effects of stress experienced in adulthood (Wood and Shors, 1998; Shors et al., 2000; Cordero et al., 2003; Kim et al., 2006) or during early life (Pryce et al., 2003; deJongh et al., 2005; Kosten et al., 2005; Kosten et al., 2006) on performance in Pavlovian aversive conditioning tasks. Overall, research suggests that sex or gender may affect vulnerability or resiliency to develop stress-related disorders via epigenetic mechanisms (Dudley et al., 2011; Jessen and Auger, 2011).
5. Conclusions
The results of the present study add to the growing literature demonstrating a link between maternal behavior and altered DNA methylation levels of various genes in several brain regions. In addition to alterations in the methylation status of the Nr3c1 exon 17 promoter in cerebellum (current findings), hypothalamus (Mueller and Bale, 2008), and hippocampus (Weaver et al., 2004), there are reports of other epigenetic changes due to early life events. Previously, we showed that LGC interacted with sex to alter DNA methylation levels of the Oprm1 gene in hippocampus and nucleus accumbens (NAc) (Hao et al., 2011). This gene is also differentially methylated in NAc of offspring of dams maintained on a high-fat diet during pregnancy and lactation (Vucetic et al., 2010). DNA methylation levels of ER! promoter in medial preoptic area differ between female offspring of high-licking dams versus offspring of low-licking dams (Champagne et al., 2006). Methylation levels of the Bdnf gene promoter in prefrontal cortex are altered in offspring of dams exposed to the stress of limited nesting material (Roth et al., 2009) although levels in hypothalamus were not affected by prenatal stress (Mueller and Bale, 2008) and did not differ by LGC or sex in NAc (Kosten et al., 2014). These studies point to the importance of examining effects in offspring of both sexes particularly given the differential rates of various behavioral disorders between genders (Fombonne, 2003; Grant et al., 2009; Vigod and Stewart, 2009).
Table 2.
Mean ± S.E.M. of the mean percent DNA methylation levels of the Nr3c1 gene exon 17 promoter region across the 17 CpG sites are shown
| BRAIN AREA | MIXED-MALES | SINGLE-MALES | MIXED-FEMALES | SINGLE-FEMALES |
|---|---|---|---|---|
| Hippocampus | 9.14 ± 0.49 | 7.82 ± 0.38 | 6.63 ± 0.58 | 8.66 ± 0.52 |
| Cerebellum | 12.7 ± 0.64 | 12.3 ± 1.3 | 10.2 ± 0.97 | 12.7 ± 0.72 |
Highlights.
Sex of pup and altering the sex composition of the litter affects frequency of pup licking and mRNA and DNA methylation levels of the glucocorticoid gene in adolescent rat brains
Male rat pups raised in mixed-sex litters are licked more frequently by their dams than male pups raised in single-sex litters
Mixed-sex male adolescent rats have the lowest DNA methylation levels of the Nr3c1 exon 17 promoter gene in cerebellum
Mixed-sex female adolescent rats have the lowest mRNA levels of the Nr3c1 gene in hippocampus
Acknowledgments
This research was supported by a grant and from NIH (DA 020117) and by the Toomim Family Fund. This material is the result of work supported with resources and use of facilities at the Michael E. DeBakey Veteran’s Affairs Medical Center, Houston, TX. None of these funding sources or resources had any role in study design, data collection or analysis and interpretation of the data, writing the manuscript or the decision on which journal to submit the article for publication.
We greatly acknowledge the technical assistance provided by W. Huang, Y. Hao, J. Taylor, and A. Yarnall.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Statement of Interest
The authors have no conflicts to declare.
References
- Ahima RS, Harlan RE. Charting of type II glucocorticoid receptor-like immunoreactiviy in the rat central nervous system. Neuroscience. 1990;39:579–604. doi: 10.1016/0306-4522(90)90244-x. [DOI] [PubMed] [Google Scholar]
- Alleva E, Caprioli A, Laviola G. Litter gender composition affects maternal behavior of the primiparous mouse dam (mus musculus) Journal of Comparative Psychology. 1989;103:83–87. doi: 10.1037/0735-7036.103.1.83. [DOI] [PubMed] [Google Scholar]
- Alt SR, Turner JD, Klok MD, Meijer OC, Lakke EA, Derijk RH, Muller CP. Differential expression of glucocorticoid receptor transcripts in major depressive disorder is not epigenetically programmed. Psychoneuroendocrinology. 2010;35:544–556. doi: 10.1016/j.psyneuen.2009.09.001. [DOI] [PubMed] [Google Scholar]
- Applied_Biosystems. User Bulletin #2: ABI Prism 7700 sequence detection system, relative quantitation of gene expression. 2001. In. [Google Scholar]
- Avishai-Eliner S, Hatalski CG, Tabachnik E, Eghbal-Ahmadi M, Baram TZ. Differential regulation of glucocorticoid receptor messenger RNA (GR-mRNA) by maternal deprivation in immature rat hypothalamus and limbic regions. Brain Research Developmental Brain Research. 1999;114:265–268. doi: 10.1016/s0165-3806(99)00031-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker-Andresen D, Ratnu VS, Bredy TW. Dynamic DNA methylation: a prime candidate for genomic metaplasticity and behavioral adaptation. Trends in Neurosciences. 2013;36:3–13. doi: 10.1016/j.tins.2012.09.003. [DOI] [PubMed] [Google Scholar]
- Beatty WW, Beatty PA. Hormonal determinants of sex differences in avoidance behavior and reactivity to electric shock in the rat. Journal of Comparative and Physiological Psychology. 1970;73:446–455. doi: 10.1037/h0030216. [DOI] [PubMed] [Google Scholar]
- Champagne F, Meaney MJ. Like mother, like daughter: evidence for non-genomic transmission of parental behavior and stress responsivity. Progress in Brain Research. 2001;133:287–302. doi: 10.1016/s0079-6123(01)33022-4. [DOI] [PubMed] [Google Scholar]
- Champagne FA, Curley JP. Epigenetic mechanisms mediating the long-term effects of maternal care on development. Neuroscience and Biobehavioral Reviews. 2009;33:593–600. doi: 10.1016/j.neubiorev.2007.10.009. [DOI] [PubMed] [Google Scholar]
- Champagne FA, Francis DD, Mar A, Meaney MJ. Variations in maternal care in the rat as a mediating influence for the effects of environment on development. Physiology & Behavior. 2003;79:359–371. doi: 10.1016/s0031-9384(03)00149-5. [DOI] [PubMed] [Google Scholar]
- Champagne FA, Weaver ICG, Diorio J, Dymov S, Szyf M, Meaney MJ. Maternal care associated with methylation of the estrogen receptor-a1b promoter and estrogen receptor-a expression in the medial preoptic area of female offspring. Endocrinology. 2006;147:2909–2915. doi: 10.1210/en.2005-1119. [DOI] [PubMed] [Google Scholar]
- Chen J, Evans AN, Liu Y, Honda M, Saavedra JM, Aguilera G. Maternal deprivation in rats is associated with corticotrophin-releasing hormone (CRH) promoter hypomethylation and enhances CRH transcriptional responses to stress in adulthood. Journal of Neuroendocrinology. 2012;24:1055–1064. doi: 10.1111/j.1365-2826.2012.02306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Xiong F, Zhang L. Promoter methylation of Egr-1 site contributes to fetal hypoxia-mediated PKCε gene repression in the developing heart. American Journal of Physiology: Regul Inegr Comp Physiol. 2013;304:R683–689. doi: 10.1152/ajpregu.00461.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian KM, Thompson RF. Neural substrates of eyeblink conditioning: acquisition and retention. Learning and Memory. 2003;11:427–455. doi: 10.1101/lm.59603. [DOI] [PubMed] [Google Scholar]
- Cirulli F, Adriani W, Laviola G. Sexual segregation in infant mice: behavioural and neuroendocrine responses to d-amphetamine administration. Psychopharmacology. 1997;134:140–152. doi: 10.1007/s002130050435. [DOI] [PubMed] [Google Scholar]
- Cordero MI, Venero C, Kruyt ND, Sandi C. Prior exposure to a single stress session facilitates subsequent contextual fear conditioning in rats: Evidence for a role of corticosterone. Hormones and Behavior. 2003;44:338–345. doi: 10.1016/s0018-506x(03)00160-0. [DOI] [PubMed] [Google Scholar]
- Daniels WMU, Fairbairn LR, vanTilburg G, McEvoy CRE, Zigmond MJ, Russell VA, Stein DJ. Maternal separation alters nerve growth factor and corticosterone levels but not the DNA methylation status of the exon 17 glucocorticoid receptor promotor region. Metabolic Brain Disease. 2009;24:615–627. doi: 10.1007/s11011-009-9163-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- deJongh R, Geyer MA, Olivier B, Groenink L. The effects of sex and neonatal maternal separation on fear-potentiated and light-enhanced startle. Behavioural Brain Research. 2005;161:190–196. doi: 10.1016/j.bbr.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Dudley KJ, Li X, Kobor MS, Kippin TE, Bredy TW. Epigenetic mechanisms mediating vulnerability and resilience to psychiatric disorders. Neuroscience and Biobehavioral Reviews. 2011;35:1544–1551. doi: 10.1016/j.neubiorev.2010.12.016. [DOI] [PubMed] [Google Scholar]
- Edelmann MN, Auger AP. Epigenetic impact of simulated maternal grooming on estrogen receptor alpha within the developing amygdala. Brain, Behavior, and Immunity. 2011;25:1299–1304. doi: 10.1016/j.bbi.2011.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fombonne E. Epidemiological surveys of autism and other pervasive developmental disorders: an update. Journal of Autism and Developmental Disorders. 2003;33:365–382. doi: 10.1023/a:1025054610557. [DOI] [PubMed] [Google Scholar]
- Francis DD, Meaney MJ. Maternal care and the development of stress responses. Current Opinion in Neurobiology. 1999;9:128–134. doi: 10.1016/s0959-4388(99)80016-6. [DOI] [PubMed] [Google Scholar]
- Francis DD, Diorio J, Liu D, Meaney MJ. Nongenomic transmission across generations of maternal behavior and stress responses in the rat. Science. 1999;286:1155–1158. doi: 10.1126/science.286.5442.1155. [DOI] [PubMed] [Google Scholar]
- Goel N, Bale TL. Examining the intersection of sex and stress in modelling neuropsychiatric disorders. Journal of Neuroendocrinology. 2009;21:415–420. doi: 10.1111/j.1365-2826.2009.01843.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon HW. Early environmental stress and biological vulnerability to drug abuse. Psychoneuroendocrinology. 2002;27:115–126. doi: 10.1016/s0306-4530(01)00039-7. [DOI] [PubMed] [Google Scholar]
- Grant BF, Goldstein RB, Chou SP, Huang B, Stinson FS, Dawson DA, Saha TD, Smith SM, Pulay AJ, Pickering RP, Ruan WJ, Compton WM. Sociodemographic and psychopathologic predictors of first incidence of DSM-IV substance use, mood and anxiety disorders: results from the Wave 2 National Epidemiologic Survey on Alcohol and Related Conditions. Molecular Psychiatry. 2009;14:1051–1066. doi: 10.1038/mp.2008.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grota LJ, Ader R. Continuous recording of maternal behavior in Rattus norvegicus. Animal Behavior. 1969;17:722–729. doi: 10.1016/0003-3472(70)90083-7. [DOI] [PubMed] [Google Scholar]
- Hao Y, Huang W, Nielsen DA, Kosten TA. Litter gender composition and sex affect maternal behavior and DNA methylation levels of the Oprm1 gene in rat offspring. Frontiers in Child and Neurodevelopmental Psychiatry. 2011;2:21–12. doi: 10.3389/fpsyt.2011.00021. Article 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim C, Nemeroff CB. The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biological Psychiatry. 2001;49:1023–1039. doi: 10.1016/s0006-3223(01)01157-x. [DOI] [PubMed] [Google Scholar]
- Herman JP, Cullinan WE. Neurocircuitry of stress: central control of the hypothalamic-pituitary-adrenocortical axis. Trends in Neurosciences. 1997;20:78–84. doi: 10.1016/s0166-2236(96)10069-2. [DOI] [PubMed] [Google Scholar]
- Howes OD, McDonald C, Cannon M, Arseneault L, Boydell J, Murray RM. Pathways to schizophrenia: the impact of environmental factors. International Journal of Neuropsychopharmacology. 2004;7:S7–S13. doi: 10.1017/S1461145704004122. [DOI] [PubMed] [Google Scholar]
- Hwang CK, Kim CS, Kim K, Law PY, Wei LN, Loh HH. Up-regulation of the mu-opioid receptor gene is mediated through chromatin remodeling and transcriptional factors in differentiated neuronal cells. Molecular Pharmacology. 2010;78:58–68. doi: 10.1124/mol.110.064311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessen HM, Auger AP. Sex differences in epigenetic mechanisms may underlie risk and resilience for mental health disorders. Epigenetics. 2011;6:857–861. doi: 10.4161/epi.6.7.16517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones PA, Taylor SM. Cellular differentiation, cytidine analogs and DNA methylation. Cell. 1980;20:85–93. doi: 10.1016/0092-8674(80)90237-8. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Song EY, Kosten TA. Stress effects in the hippocampus: synaptic plasticity and memory. Stress. 2006;9:1–11. doi: 10.1080/10253890600678004. [DOI] [PubMed] [Google Scholar]
- Kitraki E, Karandrea D, Kittas C. Long-lasting effects of stress on glucocorticoid receptor gene expression in rat brain. Neuroendocrinology. 1999;69:331–338. doi: 10.1159/000054435. [DOI] [PubMed] [Google Scholar]
- Kosten TA, Kehoe P. The immediate and enduring effects of neonatal isolation on maternal behavior in rats. International Journal of Developmental Neuroscience. 2010;28:53–61. doi: 10.1016/j.ijdevneu.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosten TA, Lee HJ, Kim JJ. Early life stress impairs fear conditioning in adult male and female rats. Brain Research. 2006;1087:142–150. doi: 10.1016/j.brainres.2006.03.009. [DOI] [PubMed] [Google Scholar]
- Kosten TA, Huang W, Nielsen DA. Sex and litter effects on anxiety and DNA methylation levels of stress and neurotrophin genes in adolescent rats. Developmental Psychobiology. 2014;56:392–406. doi: 10.1002/dev.21106. [DOI] [PubMed] [Google Scholar]
- Kosten TA, Miserendino MJD, Bombace JC, Lee HJ, Kim JJ. Sex-selective effects of neonatal isolation on fear conditioning and foot shock sensitivity. Behavioural Brain Research. 2005;157:235–244. doi: 10.1016/j.bbr.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Kirschbaum C. Sex differences in HPA axis responses to stress: a review. Biological Psychiatry. 2005;69:113–132. doi: 10.1016/j.biopsycho.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Kurian JR, Olesen KM, Auger AP. Sex differences in epigenetic regulation of the estrogen receptor-alpha promoter within the developing preoptic area. Endocrinology. 2010;151:2297–2305. doi: 10.1210/en.2009-0649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D, Freedman A, Sharma S, Pearson D, Plotsky PM, Meaney MJ. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science. 1997;277:1659–1662. doi: 10.1126/science.277.5332.1659. [DOI] [PubMed] [Google Scholar]
- Marmendal M, Roman E, Eriksson CJP, Nylander I, Fahlke C. Maternal separation alters maternal care, but has minor effects on behavior and brain opioid peptides in adult offspring. Developmental Psychobiology. 2004;45:140–152. doi: 10.1002/dev.20027. [DOI] [PubMed] [Google Scholar]
- McCormick JA, Lyons V, Jacobson MD, Nobe J, Biorio J, Nyirenda M, Weaver S, Ester W, Yau JL, Meaney MJ, Seckl JR, Chapman KE. 5′-heterogeneity of glucocorticoid receptor messenger RNA is tissue specific: differential regulation of variant transcripts by early-life events. Molecular Endocrinology. 2000;14:506–517. doi: 10.1210/mend.14.4.0438. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Sex, stress and the hippocampus: allostasis, allostatic load and the aging process. Neurobiology of Aging. 2002;23:921–939. doi: 10.1016/s0197-4580(02)00027-1. [DOI] [PubMed] [Google Scholar]
- McGowan PO, Suderman M, Sasaki A, Huang TC. Broad epigenetic signature of maternal care in the brain of adult rats. PLoS. 2011;28:e14739. doi: 10.1371/journal.pone.0014739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan PO, Sasaki A, D’Alessio AC, Dymov S, Labone B, Szyf M, Turecki G, Meaney MJ. Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nature Neuroscience. 2009;12:342–348. doi: 10.1038/nn.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh J, Anisman H, Merali Z. Short- and long-period of neonatal maternal separation differentially affect anxiety and feeding in adult rats: gender-dependent effects. Brain Research Developmental Brain Research. 1999;113:91–106. doi: 10.1016/s0165-3806(99)00005-x. [DOI] [PubMed] [Google Scholar]
- Meaney MJ. Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annual Review of Neuroscience. 2001;24:1161–1192. doi: 10.1146/annurev.neuro.24.1.1161. [DOI] [PubMed] [Google Scholar]
- Moore CL, Morelli GA. Mother rats interact differently with male and female offspring. Journal of Comparative and Physiological Psychology. 1979;93:667–684. doi: 10.1037/h0077599. [DOI] [PubMed] [Google Scholar]
- Moore CL, Chadwick-Dias AM. Behavioral responses of infant rats to maternal licking: variations with age and sex. Developmental Psychobiology. 1986;19:427–438. doi: 10.1002/dev.420190504. [DOI] [PubMed] [Google Scholar]
- Morimoto M, Morita N, Ozawa H, Yokoyama K. Distribution of glucocorticoid receptor immunoreactivity and mRNA in the rat brain: an immunohistochemical and in situ hybridization study. Neuroscience Research. 1996;26:235–269. doi: 10.1016/s0168-0102(96)01105-4. [DOI] [PubMed] [Google Scholar]
- Mueller BR, Bale TL. Sex-specific programming of offspring emotionality after stress early in pregnancy. Journal of Neuroscience. 2008;28:9055–9065. doi: 10.1523/JNEUROSCI.1424-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen DA, Yuferov V, Hamon S, Jackson C, Ho A, Ott J, Kreek MJ. Increased OPRM1 DNA methylation in lymphocytes of methadone-maintained former heroin addicts. Neuropsychopharmacology. 2009;34:867–873. doi: 10.1038/npp.2008.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberlander TF, Weinberg J, Papsdorf M, Grunau R, Misri S, Devlin AM. Prenatal exposure to maternal depression, neonatal methylation of human glucocorticoid receptor gene (NR3C1) and infant cortisol stress responses. Epigenetics. 2008;3:97–106. doi: 10.4161/epi.3.2.6034. [DOI] [PubMed] [Google Scholar]
- Pryce CR, Bettschen D, Feldon J. Comparison of the effects of early handling and early deprivation on maternal care in the rat. Developmental Psychobiology. 2001;38:239–251. doi: 10.1002/dev.1018. [DOI] [PubMed] [Google Scholar]
- Pryce CR, Bettschen D, Nanz-Bahr NI, Feldon J. Comparison of the effects of early handling and early deprivation on conditioned stimulus, context, and spatial learning and memory in adult rats. Behavioral Neuroscience. 2003;117:883–893. doi: 10.1037/0735-7044.117.5.883. [DOI] [PubMed] [Google Scholar]
- Razin A. CpG methylation, chromatin structure and gene silencing-a three-way connection. EMBO J. 1998;17:4905–4908. doi: 10.1093/emboj/17.17.4905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed K, Poulin ML, Yan L, Parissenti AM. Comparison of bisulfite sequencing PCR with pyrosequencing for measuring differences in DNA methylation. Analytical Biochemistry. 2010;397:96–106. doi: 10.1016/j.ab.2009.10.021. [DOI] [PubMed] [Google Scholar]
- Richmond G, Sachs BD. Maternal discrimination of pup sex in rats. Developmental Psychobiology. 1984;17:87–89. doi: 10.1002/dev.420170108. [DOI] [PubMed] [Google Scholar]
- Rivier C. Gender, sex steroids, corticotropin-releasing factor, nitric oxide, and the HPA response to stress. Pharmacology, Biochemistry and Behavior. 1999;64:739–751. doi: 10.1016/s0091-3057(99)00148-3. [DOI] [PubMed] [Google Scholar]
- Roth TL, Lubin FD, Funk AJ, Sweatt JD. Lasting epigenetic influence of early-life adversity on the BDNF gene. Biological Psychiatry. 2009;65:760–769. doi: 10.1016/j.biopsych.2008.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schug J, Overton GC. Computational Biology and Informatics Library. University of Pennsylvania; 1977. TESS: Transcription element search software on the http://www.cbil.upenn.edu/cgi-bin/tess/tess. [Google Scholar]
- Shiao YH, Crawford EB, Anderson LM, Patel P, Ko K. Allele-specific germ cell epimutation in the space promoter of the 45S ribosomal RNA gene after Cr(III) exposure. Toxicology and Applied Pharmacology. 2005;205:209–209. doi: 10.1016/j.taap.2004.10.017. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Beylin AV, Wood GE, Gould E. The modulation of Pavlovian memory. Behavioural Brain Research. 2000;110:39–52. doi: 10.1016/s0166-4328(99)00183-7. [DOI] [PubMed] [Google Scholar]
- Sousa RJ, Tannery NH, Lafer EM. In situ hybridization mapping of glucocorticoid receptor messenger ribonucleic acid in rat brain. Molecular Endocrinology. 1989;3:481–494. doi: 10.1210/mend-3-3-481. [DOI] [PubMed] [Google Scholar]
- Steinmetz JE. Brain substrates of classical eyeblink conditioning: A highly localized but also distributed system. Behavioural Brain research. 2000;110:13–24. doi: 10.1016/s0166-4328(99)00181-3. [DOI] [PubMed] [Google Scholar]
- Sutanto W, Rosenfeld P, deKloet ER, Levine S. Long-term effects of neonatal maternal deprivation and ACTH on hippocampal mineralocorticoid and glucocorticoid receptors. Developmental Brain Research. 1996;92:156–164. doi: 10.1016/0165-3806(95)00213-8. [DOI] [PubMed] [Google Scholar]
- Thomassin H, Flavin M, Espinas ML, Grange T. Glucocorticoid-induced DNA demethylation and gene memory during development. The EMBO Journal. 2001;20:1974–1983. doi: 10.1093/emboj/20.8.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- vanHasselt FN, Cornelisse S, Zhang TY, Meaney MJ, Velzing EH, Krugers HJ, Joels M. Adult hippocampal glucocorticoid receptor expression and dentate synaptic plasticity correlate with maternal care received by individuals early in life. Hippocampus. 2012;22:255–266. doi: 10.1002/hipo.20892. [DOI] [PubMed] [Google Scholar]
- Vigod SN, Stewart DE. Emergent research in the cause of mental illness in women across the lifespan. Current Opinion in Psychiatry. 2009;22:396–400. doi: 10.1097/YCO.0b013e3283297127. [DOI] [PubMed] [Google Scholar]
- Vucetic Z, Kimmel J, Totoki K, Hollenbeck E, Reyes TM. Maternal high-fat diet alters methylation and gene expression of dopamine and opioid-related genes. Endocrinology. 2010;151:4756–4764. doi: 10.1210/en.2010-0505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver IC, Champagne FA, Brown SE, Dymov S, Sharma S, Meaney MJ, Szyf M. Reversal of maternal programming of stress responses in adult offspring through methyl supplementation: altering epigenetic marking later in life. Journal of Neuroscience. 2005;25:11045–11054. doi: 10.1523/JNEUROSCI.3652-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver IC, Cervoni N, Champagne FA, D’Alessio AC, Sharma S, Seckl JR, Dymov S, Szyf M, Meaney MJ. Epigenetic programming by maternal behavior. Nature Neuroscience. 2004;7:847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- Wigger A, Neumann ID. Periodic maternal deprivation induces gender-dependent alterations in behavioral and neuroendocrine responses to emotional stress in adult rats. Physiology & Behavior. 1999;66:293–302. doi: 10.1016/s0031-9384(98)00300-x. [DOI] [PubMed] [Google Scholar]
- Wilber AA, Southwood CJ, Sokoloff G, Steinmetz JE, Wellman CL. Neonatal maternal separation alters adult eyeblink conditioning and glucocorticoid receptor expression in the interpositus nucleus of the cerebellum. Developmental Neurobiology. 2007;67:1751–1764. doi: 10.1002/dneu.20549. [DOI] [PubMed] [Google Scholar]
- Wood GE, Shors TJ. Stress facilitates classical conditioning in males, but impairs classical conditioning in females through activational effects of ovarian hormones. Proceedings of the National Academy of Science. 1998;95:4066–4071. doi: 10.1073/pnas.95.7.4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehuda R, Halligan SL, Grossman R. Childhood trauma and risk for PTSD: relationship to intergenerational effects of trauma, parental PTSD, and cortisol excretion. Development & Psychopathology. 2001;13:733–753. doi: 10.1017/s0954579401003170. [DOI] [PubMed] [Google Scholar]


