Abstract
Herpesvirus infection reorganizes components of the nuclear lamina usually without loss of integrity of the nuclear membranes. We report that wild-type HSV infection can cause dissolution of the nuclear envelope in transformed mouse embryonic fibroblasts that do not express torsinA. Nuclear envelope breakdown is accompanied by an eight-fold inhibition of virus replication. Breakdown of the membrane is much more limited during infection with viruses that lack the gB and gH genes, suggesting that breakdown involves factors that promote fusion at the nuclear membrane. Nuclear envelope breakdown is also inhibited during infection with virus that does not express UL34, but is enhanced when the US3 gene is deleted, suggesting that envelope breakdown may be enhanced by nuclear lamina disruption. Nuclear envelope breakdown cannot compensate for deletion of the UL34 gene suggesting that mixing of nuclear and cytoplasmic contents is insufficient to bypass loss of the normal nuclear egress pathway.
Keywords: HSV-1, nuclear envelope, fusion, UL34, gB, gH, US3
INTRODUCTION
Herpesviruses morphogenesis starts in the nucleus by formation of DNA-filled capsids and is finalized in the cytoplasm where capsids acquire the tegument proteins and the final envelope. The primary mechanism for nuclear egress is an envelopment/de-envelopment process. Capsids leave the nucleus by budding into the INM (primary envelopment), which results in formation of enveloped virions inside the perinuclear space. These so-called primary virions then quickly resolve by fusing with the outer nuclear membrane (ONM) (de-envelopment) (Johnson and Baines, 2011; Roller, 2008). Several viral proteins are involved in envelopment/de-development at the NE. HSV-1 pUL34 and pUL31, form a complex at the INM (Reynolds et al., 2001; Reynolds et al., 2002). The alphaherpesviral pUL34/pUL31 complex has a multifunctional role during nuclear egress including lamina disruption (Bjerke and Roller, 2006; Leach et al., 2007; Mou et al., 2007; Park and Baines, 2006; Simpson-Holley et al., 2005) and wrapping the INM around the capsid (Klupp et al., 2007; Roller et al., 2010; Roller et al., 2011). De-envelopment of primary virions is facilitated by the pUS3 serine/threonine kinase and this function appears to be conserved within the alphaherpesviruses (Klupp et al., 2001; Mou et al., 2009; Reynolds et al., 2002; Ryckman and Roller, 2004; Schumacher et al., 2005). In HSV-1, the two glycoproteins gB and gH also participate in de-envelopment. Double deletion of gB and gH results in accumulation of primary virions inside a dilated perinuclear space (Farnsworth et al., 2007; Wisner et al., 2009; Wright et al., 2009). The fusogenic activity of gB is required for its de-envelopment function since mutations that interfere with gB fusion activity also interfere with de-envelopment when gH is also absent (Wright et al., 2009). pUS3 presumably has a regulatory role in de-envelopment that, in HSV-1, includes regulating the fusogenic activity of gB (Wisner et al., 2009). Interestingly, de-envelopment fusion in infection with the related alphaherpesvirus PrV does not require gB and/or gH, since single and double deletions egress from the nucleus as efficiently as wild-type virus, and accumulation of perinuclear virions is not observed (Klupp et al., 2008).
Alphaherpesvirus deletion recombinants that fail to express either of pUL31 or pUL34 still exhibit low levels of replication, suggesting that they can egress from the nucleus by a mechanism independent of pUL34 and pUL31 (Chang, 1997; Fuchs et al., 2002; Klupp et al., 2000; Roller et al., 2000). Furthermore, the requirement in HSV-1 for UL31 expression in nuclear egress is apparently cell-specific, indicating that that there is a nuclear egress mechanism that does not rely on the pUL31/pUL34 complex (Liang et al., 2004). Extensive passage of UL31 or UL34-null PrV recombinants resulted in variants (called ΔUL31Pass and ΔUL34pass) that produced virus at titers similar to the wild type PrV (Grimm et al., 2012; Klupp et al., 2011). Both viruses caused nuclear envelope breakdown (NEBD) in infected cells. The observation was quite striking since normally during herpesvirus infection the NE appears intact despite drastic changes in nuclear architecture that include chromatin marginalization, phosphorylation of the lamina components and expansion of the nucleus (Bjerke and Roller, 2006; Camozzi et al., 2008; Hamirally et al., 2009; Leach et al., 2007; Leach and Roller, 2010; Milbradt et al., 2007; Morris et al., 2007; Mou et al., 2007; Muranyi et al., 2002; Park and Baines, 2006; Reynolds et al., 2004; Scott and O’Hare, 2001; Simpson-Holley et al., 2005; Simpson-Holly et al., 2004). Although the mechanisms by which ΔUL31pass or ΔUL34Pass induced NE breakdown are not known, these studies imply that breakdown might provide an alternative pathway to envelopment/de-envelopment for capsids to exit the nucleus.
The TOR1A gene encodes TorsinA, a member of the AAA+ ATPase superfamily of proteins that perform diverse cellular activities (Ozelius et al., 1997; White and Lauring, 2007). TorsinA is a widely expressed, peripheral membrane protein (i.e., having no transmembrane domain) localized in the lumen of the ER and in the space between the inner and outer nuclear membranes (Callan et al., 2007; Jungwirth et al., 2010; Kustedjo et al., 2000; Vander Heyden et al., 2011). The TorsinA mRNA encodes a polypeptide of 332 amino acids with a cleaved signal sequence that is thought to form hexamers in the ER lumen. Mutation in the TOR1A gene that leads to a loss of a single glutamic acid residue, Glu302 or Glu303 near the C-terminus of TorsinA (called the E mutation) is associated with dominantly-inherited early-onset torsion dystonia (Ozelius et al., 1997) and reviewed in (Granata et al., 2009; Granata and Warner, 2010). Several functions, including a role as a molecular chaperone and a homeostatic regulator of an induced ER stress response have been suggested for TorsinA, and chaperone activity has been demonstrated in vitro (Burdette et al., 2010; Caldwell et al., 2003; Chen et al., 2010; Hewett et al., 2003; Hewett et al., 2007; McLean et al., 2002). Although TorsinA is ubiquitously expressed, pathology is limited to neuronal cells and in transgenic mice with defects in TorsinA expression or function that pathology is accompanied by changes in the architecture of the nuclear envelope including loss of proper spacing between the INM and the ONM and formation of intranuclear membrane inclusions (Gonzalez-Alegre and Paulson, 2004; Goodchild et al., 2005; Jungwirth et al., 2010; Kustedjo et al., 2000). TorsinA has been found to interact with other host cell proteins that may participate in its function in maintenance of NE architecture, including LAP1 and nesprin 3A (Goodchild and Dauer, 2005; Naismith et al., 2009; Nery et al., 2008).
Overexpression of wild-type torsinA (TA), but not a dominant negative mutant form, impaired herpes simplex virus type 1 (HSV-1) replication and caused a defect in capsid nuclear egress (Maric et al., 2011). To continue our studies on importance of TorsinA for HSV-1 we used transformed wild-type and Tor1a−/−mouse embryonic fibroblasts (MEFs). We show here that wild type HSV-1 can induce NEBD in Tor1a −/− mouse embryonic fibroblast cell line that is morphologically highly similar to that seen in infection with mutant PrV. This demonstrates that, in some host cell environments, extensive NEBD is one possible consequence of a wild-type virus infection. We have used recombinant mutant viruses to explore the mechanism of NEBD and find that it is strongly enhanced by UL34 expression and by expression of the HSV glycoproteins gH and gB that have been implicated in de-envelopment fusion during nuclear egress.
MATERIAL AND METHODS
Cells and viruses
Tor1a−/− and littermate Tor1a +/+ mouse embryonic fibroblast (MEFs) were previously described (Kim et al., 2010). MEFs were maintained in DMEM media supplemented with 15% FBS and Pen-Strep. Vero and HEK 293T cells were maintained as described previously. An infection-inducible clonal Vero cell line that expresses pUL34 (CX cells) was generated as previously described (Roller et al., 2010). The properties of HSV-1(F), vRR1202 (HSV-1(F) with deletion in US3 gene), vRR1072 (UL34-null virus) and its repair viruses (vRR1072rep) were previously described and characterized (Roller et al., 2000; Ryckman and Roller, 2004). Bacterial artificial chromosome (BAC) containing the HSV-1(F) genome, BAC with deletion in gB (gB-null), gH (gH-null) or double deletions in gB and gH (gB/gH-null) were described previously (Farnsworth et al., 2007). We refer to HSV-1(F) reconstituted from the BAC as HSV-1(F)BAC to distinguish it from the non-recombinant HSV-1(F) described by Ejercito et al. (Ejercito et al., 1968).
Plasmids
C-terminally GFP-tagged wild type human TorsinA (GFP-TA) and N-terminally myc-tagged mouse TorsinA (myc-TA) were a kind gift from Phyllis Hanson, Washington University School of Medicine, St. Louis (Naismith et al., 2004). pVETL vector expressing CMV-promoter driven GFP and U6-promoter driven short hairpin RNA (shRNA) targeting human and mouse TOR1A genes (shTA) or nonsense gene (shcontrol) have been previously described (Gonzalez-Alegre et al., 2005).
Viral growth assays
Replication of HSV-1(F), vRR1072 and vRR1072rep viruses on MEFs was determined at MOI of 5. After allowing infection to proceed for 1 hour, the inoculum was removed and cells were washed once briefly with 1 ml of 50 mM Na Citrate, 4 mM KCl pH 3.0. After removal of this first wash, 1 ml of fresh low pH citrate buffer was added and left on the cells for 1 minute to inactivate residual virus. The low pH buffer was removed and cells were washed twice with cell growth medium and then maintained for the remainder of the infection in their growth medium. Tor1a+/+ and Tor1a−/− MEFs were infected 12 h after plating to minimize the impact of different generation times of the two cell lines. Virus yields were determined by plaque assay on Vero (for HSV-1(F) and pRR1072rep viruses) or CX cells (for vRR1072 virus) as described (Roller et al., 2000). Statistical significance p values were calculated using a two-tailed unpaired Student’s t test.
Localization of HSV-1 scaffold protein
Cells were infected at an MOI of 5 and 12 h later were fixed in 4% formaldehyde in PBS for 10 min, washed once in PBS and then blocked and permeabilized by incubation in IF buffer (PBS containing 1% TritonX-100, 1% Sodium Deoxycholate, and 1% bovine serum albumin) for at least 1 h. Primary anti-scaffold antibody (AbD serotech, MCA406) was diluted 1:1000 in IF buffer AlexaFluor conjugated secondary antibody (Invitrogen) was diluted 1:1000 in IF buffer. The nucleus was counterstained by incubating the samples with TO-PRO-3 (Invitrogen) diluted 1:2000 in PBS for 15 min. SlowFade Antifade kit (Invitrogen) was used to mount coverslips on glass slides. All images were obtained using a Zeiss 510 confocal microscope. Confocal images of up to 25 randomly selected areas were taken. An area contained up to 15 cells. Cell scoring is described below and the data are presented as percentage of cells with each score.
Immunofluorescence
Cells were infected at an MOI of 5 and 14 hours later were fixed and stained for scaffold protein as described above, and for pUL34 using 1:1000 anti-UL34 antibody and 1:1000 AlexaFluor647 secondary antibody.
Transmission EM of infected cells
Confluent monolayers of cells were infected with HSV-1(F) at a multiplicity of ten for 20 h and then fixed by incubation in 2.5% glutaraldehyde in 0.1M cacodylate buffer (pH7.4) for 2 h. Cells were post-fixed in 1% osmium tetroxide, washed in cacodylate buffer, embedded in Spurr’s resin and cut into 95 nm sections. Sections were mounted on grids, stained with uranyl acetate and lead citrate and examined with a JEOL 1250 transmission electron microscope.
Subcellular fractionation
Approximately 3×106 MEFs were seeded in Petri dishes and infected with mock or HSV-1(F) and an MOI of 5. At 12 h.p.i. cells were scraped from the plates, washed one time with PBS, and resuspended in 500 μL of buffer A (10 mM HEPES pH 7.5, 2 mM MgCl2, 15 mM KCl, 0.1 mM EDTA, 0.1 mM EGTA, protease inhibitors cocktail (Sigma, S8820)) for 15 minutes on ice. The suspension was then gently mixed with 25 μL of 10% NP-40 and centrifuged at 7500 rpm for 5 min. The collected supernatant represented the cytosolic fraction. The pellet was washed one time with buffer A and then resuspended with 165 μL buffer C (25 mM HEPES pH 7.5, 400 mM NaCl, 1mM EDTA, protease inhibitors cocktail) and incubated for 30 minutes on ice with occasional mixing. The soluble nuclear supernatant fraction was collected following centrifugation at 7500 rpm for 5 min.
Sample preparation for SDS-PAGE
To determine expression of viral proteins in MEFs, Tor1a+/+ and Tor1a−/− MEFs were infected with HSV-1(F) at an MOI of 5 and at 12 h.p.i. cells were scraped, washed one time with PBS and lysed in 1X SDS sample buffer (Thermo Scientific, 39001). To analyze accumulation of myc-TorsinA in stably expressing cell lines, cells were scraped, washed one time in PBS and lysed in lysis buffer (10 mM Tris/HCl pH 7.5, 150 mM NaCl, 0.5 mM EDTA, 0.5% NP-40 with added protease inhibitors) on ice for 30 min. The lysates were centrifuged at high speed and the supernatants were then mixed with an equal volume of 2X SDS sample buffer. All SDS containing samples were boiled and total protein concentration was determined with BioRad Dc Protein Assay. β-mercaptoethanol (Sigma) was added to samples before loading equal amounts of protein on the SDS-PAGE.
Immunoblotting
After electrophoresis, proteins were transferred to nitrocellulose membranes and membranes were then blocked overnight in 5% nonfat milk in T-TBS (10 mM NaCl, 20 mM Tris HCl and 0.05% Tween 20). Primary antibodies were diluted in 5% nonfat milk in T-TBS as follows: monoclonal anti-pUS11 (1:1000), chicken polyclonal antibody anti-pUL34 (1:1000) (Roller et al., 2000), rabbit polyclonal anti-US3 (kind gift of Bernard Roizman) (1:500), mouse monoclonal anti-gC (1:1000) (Virusys, H1A022), mouse monoclonal anti-gD/DL6 (1:5000), mouse polyclonal anti-gB/SS55 (1:500) (kindly provided by Gary H. Cohen and Roselyn J. Eisenberg, University of Pennsylvania), monoclonal mouse anti-GAPDH (1:1000) (Millipore, MAB374), monoclonal mouse anti-scaffold (1:1000) (AbD serotech, MCA406), mouse anti-α-tubulin (1:1000) (Li-COR, 926–42213), polyclonal rabbit anti-GFP (Invitrogen, A6455). Secondary alkaline phosphatase conjugated anti-mouse (Sigma, A3562), anti-rabbit (Sigma, A3687) and anti-chicken (Aves Lab, AP1001) antibodies were incubated with the appropriate blot for 1 h.
RESULTS
HSV-1 replication is impaired in transformed Tor1a−/− MEFs
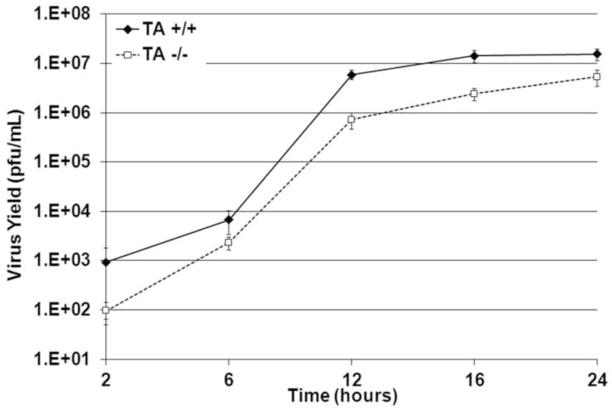
We previously showed that overexpression of TA inhibits HSV-1 production in two different cell lines (Maric et al., 2011). As part of an effort to determine whether TA expression was necessary for efficient HSV-1 replication, we tested viral single-step growth on transformed MEFs from Tor1a−/− and Tor1a+/+ mouse embryos (Figure 1). Viral yields in Tor1a−/− MEFs were significantly lower at 12–24 h.p.i. (12 (p<0.003), 16 (p<0.008) and 24 (p<0.018) h.p.i.) in comparison to Tor1a+/+ MEFs. At 12 h.p.i. virus production was more than 8-fold less efficient in Tor1a−/− than in Tor1a+/+ MEFs. At later times during infection the difference in HSV-1 replication decreased to about than 3-fold.
Figure 1.
HSV-1 replication is inhibited in transformed Tor1a−/− MEFs. Single-step growth kinetics of HSV-1 were measured on TA MEFs. Tor1a+/+ and Tor1a−/− MEFs were infected with HSV-1(F) at an MOI of 5 and at indicated points virus infectivity of whole cell cultures was determined by plaque assay on Vero cells. Points plotted are the mean of three independent experiments; error bars represent the standard deviation. The difference in infectivity at 2 h.p.i. reflects differences in the amount of residual infectivity left from the inoculum after washes in citrate buffer.
HSV-1 infection induces NE breakdown in transformed Tor1a−/− MEFs
Since TorsinA is a NE and ER-resident protein, we asked if its loss interferes with HSV-1 morphogenesis. To ensure that events prior to capsid assembly are unaffected by TorsinA loss, Tor1a+/+ and Tor1a−/− MEFs were infected with HSV-1(F) at an MOI of 5 and at 12 h.p.i. accumulation of late (VP5 and pUL34), true late gene products (pUS11, gC) and processing of viral glycoproteins (gC, gD, gB) were analyzed by immunoblotting (Fig. 2A). Both cells lines supported accumulation and processing of viral proteins to similar extent, indicating that absence of TorsinA does not interfere with early events in virus life cycle (entry, gene expression, and replication). The impairment in HSV-1 replication is thus likely associated with capsid assembly and/or subsequent events.
Figure 2.
HSV-1-induced nuclear envelope breakdown in Tor1a−/− MEFs. (A) Accumulation and processing of viral proteins in TA MEFs. Tor1a+/+ and Tor1a−/− MEFs were infected with HSV-1(F) at an MOI of 5 and 12 h later, whole cell lysates were subject to immunoblot analysis with antibodies to proteins indicated on left. Blots shown are representative of three experiments. (B) Ultrastructural analyses of TA MEFs. Shown are micrographs of Tor1a+/+ (b) and Tor1a−/− (a, c, d) MEFs infected with mock (a) or HSV-1(F) at an MOI of 10 (b, c, d) and 20 h later fixed and processed for TEM. The scale in μm is indicated at the lower left of each panel and is the same for all panels. In (b) white arrowheads point to NE perforations which likely represent the NPC. White arrows in (c) indicate large NE breaks and black arrowhead in (c, d) point to tubular membranous structures in place of the NE.
To examine the defects in TorsinA-null MEFs, mock- or HSV-1(F)-infected Tor1a+/+ and Tor1a−/− MEFs were examined by TEM (Fig. 2B). All stages of virus assembly were observed in both MEF cell lines and the quantification of virus particles in 10 randomly selected cell sections is provided in Table 1. About 3 fold fewer virus particles were counted in Tor1a−/− in comparison to Tor1a+/+ MEFs and this reduction was correlated with fewer capsids found in the nucleus and the cytoplasm of Tor1a−/− MEFs. While quantitative measurement of capsid production is uncertain in analysis of EM, due to the low number of cells examined, these results suggest the possibility of a defect in assembly and/or stability of capsids in Tor1a−/− MEFs, which might explain the lower virus titer in these cells. Ultrastructural analysis also revealed breakdown of the NE in HSV-1 infected Tor1a−/− MEFs (Fig. 2Bc, d). In 3 out of 10 examined cell sections between 0.25 and 1 μm long perforations at the NE were observed (Fig. 2Bc white arrows). These perforations were even larger in an additional 4 out of 10 cell sections where substantial areas of cells without NE were observed and borders between the nucleus and the cytoplasm were diminished (Fig. 2Bd). Instead of NE, tubular or cisternal structures ranging in length from 0.2 to 2 μm were observed suggesting vesicularization of the NE (Fig. 2Dc, d black arrowheads). The NE of uninfected Tor1a−/− cells is intact and without blebs due to compensatory effect of torsinB (Fig. 2Ba), as shown previously (Jungwirth et al., 2010). The NE in 10 randomly selected infected Tor1a+/+ MEF cell sections was intact with occasional small perforations (less than 0.1 μm) that most likely represent the nuclear pore complex (NPC) (Fig. 2Bb white arrowheads).
Table 1.
Quantification of virus particles in HSV-1(F) infected Tor1a+/+ and Tor1a−/− MEFsa
| No.(%) of nuclear capsids | No. (%) of perinuclear virions | No. (%) of cytoplasmic virus particles | No. (%) of surface virions | Total No. (%)of counted particles | ||
|---|---|---|---|---|---|---|
| Naked capsids | Enveloped virions | |||||
| Tor1a+/+ | 867 (68.1) | 5 (0.4) | 181 (14.2) | 184 (14.4) | 37 (2.9) | 1274 (100) |
| Tor1a−/− | 307* (66.2) | 11 (2.4) | 58 (12.5) | 81 (17.5) | 7 (1.5) | 464 (100) |
TEM images of ten randomly selected cells with at least one capsid or margination of chromatin were taken and subsequently counted for virus particles.
In cases where boundaries between the nucleus and cytoplasm were diminished due to NE breakdown capsids were counted as nuclear when no obvious cytoplasmic structures were visible in capsids proximity.
TorsinA independence in NEBD
We used several approaches to determine whether the NEBD phenotype observed in transformed Tor1a−/− MEFs was due to the absence of TA expression, including testing for the phenotype in fresh primary Tor1a−/− MEFs and in MEFs in which TA expression was restored by stable transfection of a TA expression construct. We did not observe NEBD in fresh primary MEFs, and NEBD was still observed to the same extent when TorsinA expression was restored by stable transfection (not shown) indicating either that the phenotype was unrelated to loss of TorsinA expression, or was the result of a stable adaptation to loss of TorsinA
Mechanism of NEBD
Regardless of its relationship to TorsinA expression we were interested in the mechanism by which herpesvirus infection might induce NEBD. We developed two assays for NEBD based on the localization of the viral scaffold protein and capsid maturation protease. The maturation protease and scaffold proteins VP21 and VP22a (respectively) share an open reading frame and are recognized by the same monoclonal antibody. The two proteins are involved in capsid maturation and are normally highly concentrated inside the nucleus (Yang and Baines, 2008). We reasoned that breaks in the NE, should result in relocalization of scaffold/protease in the cytoplasm.
We first used sub-cellular fractionation to determine whether scaffold proteins become less concentrated in the nucleus in infected Tor1a−/− MEFs. Nuclear and cytoplasmic fractions of mock- or HSV-1-infected Tor1a+/+ and Tor1a−/− MEFs were analyzed by immunoblotting for scaffold proteins, and for laminB, and alpha tubulin, which mark the nuclear and cytoplasmic fractions, respectively (Fig. 3A). As expected, more scaffold proteins accumulated in the nuclear than in the cytoplasmic fraction in Tor1a+/+ MEFs. The scaffold localization was reversed in Tor1a−/− MEFs, in which most of the scaffold protein accumulated in the cytoplasmic fraction. LaminB was confined to the nuclear fraction in all conditions because it is retained by association with other lamina proteins. The presence of laminB only in the nuclear fraction shows that the fractionation procedure effectively separated nucleus and cytoplasm without contamination of the cytoplasmic fraction. The presence of tubulin in the nuclear fraction however, indicates some contamination with cytoplasmic fraction.
Figure 3.
Localization of the scaffold proteins. (A) Tor1a+/+ and Tor1a−/− MEFs were mock or infected with HSV-1(F) at an MOI of 5 and 12 h later biochemically fractionated into cytoplasmic (C) and nuclear (N) fractions, as described in Material and methods. Fractions were then analyzed by immunoblot with anti-scaffold antibody. Blotting for laminB1 and alpha tubulin served an indicator for purity of nuclear and cytoplasmic fractions respectively. (B) Tor1a+/+ and Tor1a−/− MEFs were infected with HSV-1(F) at an m.o.i. of 5 and at 12 h.p.i visualized for scaffold proteins (red) and nucleus (blue). Scaffold localizations were scored as described in the text. The graph shown is representative of three experiments with similar results.
To quantify frequency of NEBD using scaffold protein localization, Tor1a+/+ and Tor1a−/−MEFs were infected with HSV-1(F) and at 12 h.p.i. immunofluorescently stained to detect scaffold and counter stained for nuclei with TO-PRO-3. For each experiment, confocal images of between 100 and 115 cells per cell line were taken. Examples that show all of the scored localizations (not representative fields) for Tor1a+/+ and Tor1a−/− MEFs are shown in Fig. 3B. Scaffold localizations received the following scores: “nucleus” – the scaffold proteins were exclusively localized in the nucleus (examples are indicated in Fig. 3B with white arrowheads); “mainly nucleus” – more scaffold accumulated in the nucleus than in the cytoplasm (Fig. 3B grey arrow); “cytoplasm” – the scaffold was distributed equally between the nucleus and the cytoplasm or majority of the scaffold accumulated in the cytoplasm (Fig. 3B white arrow). The scaffold was found exclusively in the nucleus in vast majority (>95%) of Tor1a+/+ MEFs, but in less than half of the Tor1a−/− MEFs (< 41%). In three independent experiments the standard deviation for scaffold localization scores in Tor1a+/+ and Tor1a−/− MEFs was 5 and 15 % respectively. Thus, in the majority of infected Tor1a−/− cells, the otherwise nuclear protein was also detected in the cytoplasm to varying degrees.
The apparent vesicularization of the NE during breakdown suggested that virus–induced membrane fusion between the INM and ONM might be responsible for NEBD. Accordingly, we determined whether gB, gH, and pUS3 expression were required. We also tested importance of the primary envelopment machinery in NE breakdown by determining whether there is a requirement for UL34 expression. Tor1a+/+ and Tor1a−/− MEFs were infected with a BAC-derived HSV-1(F), or with BAC-derived HSV-1(F) recombinant viruses with single deletions in gB (gB-null), gH (gH-null) or double deletion in gB and gH (gB/gH-null). In addition, TA MEFs we also infected with HSV-1(F), vRR1072 (HSV-1 with deletion in UL34 gene), and vRR1202 (HSV-1 with deletion in US3). The localization of scaffold proteins at 12 h.p.i. was analyzed with confocal microscopy (Fig. 4). Less than 5 % of Tor1a+/+ MEFs infected with any of the viruses showed cytoplasmic staining of the scaffold. Tor1a−/− MEFs infected with HSV-1(F), BAC, gB-null or gH-null infected Tor1a−/− MEFs al showed relocalization of scaffold with more than half of cells showing cytoplasmic staining (scored as “mainly nucleus” and “cytoplasm”), indicating a high rate of the NE breakdown. In Tor1a−/− MEFs infected with gB/gH-null or UL34-null, however, fewer cells showed cytoplasmic scaffold localization in comparison to wild type virus. Notably, there were about 5-fold fewer cells with considerable accumulation of the scaffold in the cytoplasm (scored as “cytoplasm”). These results indicate that pUL34, gB and gH promote NE breakdown and that gB and gH function in NE breakdown in redundant fashion. On the other hand, more scaffold accumulated in the cytoplasm in US3-null than in HSV-1(F) infected Tor1a−/− MEFs. In US3-null infected Tor1a−/− MEFs close to twice as many cells showed cytoplasmic staining of scaffold compared to HSV-1(F), indicating that pUS3 has an inhibitory effect on NE breakdown.
Figure 4.
Viral products involved in the NE breakdown. Tor1a−/− and Tor1a+/+ MEFs were infected at an m.o.i. of 5 with viruses indicated on the bottom of the graphs and at 12 h.p.i localization of scaffold proteins was analyzed with confocal microscopy. The results were separated into two graphs for the ease of data presentation. Data are representative of three independent experiments.
The similar effect of pUL34 and gB/gH deletions on NEBD suggests the possibility that gB/gH deletion might alter the localization or expression of pUL34. To test for change in localization, immunofluorescent assays for pUL34 and for scaffolding protein were performed in Tor1a+/+ and −/− MEFs that were infected for 14 hours with WT or mutant BAC-derived viruses (Figure 5A–D). pUL34 localization to the nuclear envelope was relatively poor in Tor1a+/+ cells infected with wild-type virus (Figure 5A). Although a distinct pUL34 nuclear rim could be detected in about half of the cells, it was always accompanied by extensive cytoplasmic staining and, in about half of the cells, concentration at the nuclear rim could not be detected at all. pUL34 localization was similar in Tor1a−/− cells, except that pUL34 was more often found in concentrations around the periphery of the nucleus. Deletion of gB and gH together, did not alter the localization of pUL34 in either Tor1a+/+ or Tor1a−/− cells (Figure 5C and D), indicating that the diminished nuclear breakdown seen in the deletion virus-infected cells was not due to mis-localization of pUL34. We also determined the level of pUL34 and pUS3 expression in MEFs infected with glycoprotein deletion viruses by immunoblotting of infected cell lysates (Figure 5E). The gB/gH null virus-infected cells reproducibly showed a small decrease in pUL34 accumulation compared to wild-type and the single deletion viruses, but expression was identical between Tor1a+/+ and Tor1a−/− cells, indicating that the loss of NEBD in gB/gH null infection was not due to loss of pUL34 expression. Expression of pUS3 was also unchanged as a result of the gB/gH deletion. Similarly, we tested to determine whether the inhibition of NEBD in UL34-null infection might be due to alterations in gB or gH expression, and found that gB and gH expression were unchanged in UL34-null infected cells (Figure 5E).
Figure 5.

Localization of pUL34 and expression of viral gene products in deletion virus-infected cells. (A–D) Digital images of MEFs infected at an m.o.i. of 5 for 14 h with WT or gB/gH-null viruses are shown. Cells were stained using primary antibodies directed against scaffolding protein (red) and pUL34 (far red rendered in blue). (E) Digital images of immunoblots of whole cell lysates of Tor1a+/+ (indicated with a + above the lane) or Tor1a−/− cells (indicated with a – above the lane) that had been infected for 16 hours with the indicated viruses at an m.o.i. of 5 The protein probed for in each of the blots is shown to the left of each panel.
UL34-null virus replication is not rescued in Tor1a−/− MEFs
UL34-null virus is unable to undergo primary envelopment at the INM and the virus titers in single-step growth are reduced three to four log orders of magnitude (Chang, 1997; Fuchs et al., 2002; Klupp et al., 2000; Klupp et al., 2011; Roller et al., 2000; Ye and Roizman, 2000). A phenotypic revertant of a UL34-null recombinant of PrV (called ΔUL34Pass) was isolated following extensive passage on non-complementing cells. This revertant caused NE disintegration in infected cells (Klupp et al., 2011), indicating that NE breakdown might provide another pathway for capsids to reach the cytoplasm for final maturation. Since the NE breakdown in UL34Pass infected cells was very similar to what we observed in HSV-1(F) infected Tor1a−/− MEFs, we asked if, in our system, NE disintegration would provide a growth advantage for the UL34-null virus. As shown in Fig. 4, UL34-null virus was still able to induce NE breakdown although to a lesser extent that HSV-1(F).
To measure growth of UL34 deletion virus, Tor1a+/+ and Tor1a−/− MEFs were infected with HSV-1(F), vRR1072 and vRR1072Rep (UL34-null repair virus) at an MOI of 5 and 16 h.p.i virus titer were determined (Fig. 6). As expected, deletion of the UL34 gene reduced virus titers about 1000-fold compared to WT infection in Tor1a+/+ cells. The UL34-null virus grew just as poorly on the Tor1a−/− cells, indicating that NEBD provided no apparent growth advantage.
Figure 6.

Replication of UL34-null virus in TA MEFs. Tor1a−/− and Tor1a+/+ MEFs were infected with the indicated viruses at an MOI of 5. At 16 h.p.i virus yields of total culture were determined by a plaque assay on Vero cells for HSV-1(F) and vRR1072 Rep or CX cells (Vero pUL34 complementing cell lines) for vRR1072. The values plotted are averages of three separate experiments. Standard deviations are indicated by error bars.
DISCUSSION
The NE breakdown in MEFs
Although the repertoire of HSV infected cell types that have been examined by TEM is limited, there are no previous reports of extensive NE breakdown in wild type virus infected cells. This can be seen as surprising, given the extensive reorganization of cellular components that are important for nuclear structure in herpesvirus infected cells. Nuclear breakdown has now been observed in two herpesvirus infection systems – the one described here, and NEBD induced by UL31-null and UL34-null PrV variants. This suggests that herpesviruses may walk a very fine line that ordinarily preserves the integrity of the NE, but allows capsid access to the inner nuclear membrane and sufficient flexibility of the NE to permit capsid envelopment.
That NEBD in response to wild type virus infection has only been observed in transformed Tor1a−/− MEFs and not in other MEF cell lines, and the functional connection between TA and nuclear envelope architecture suggest a connection with TA expression. However, the observations that fresh, primary Tor1a−/− MEFs do not show NE breakdown, and that NE breakdown is not prevented by exogenous expression of TA indicate that the connection is not simple, and are consistent with the possibility that NE breakdown is a peculiarity of this cell line unrelated to the lack of TA expression. Tor1a−/− mice are not viable past parturition (Goodchild et al., 2005). Although there are no obvious cellular abnormalities in MEFs from Tor1a−/− embryos, it is possible that subtle growth defects result in selection for compensatory adaptations over the time required for transformation and amplification of this cell line, and that some stable adaptation to TA deletion predisposes these cells to NE breakdown during HSV infection. The nature of these adaptations is uncertain, but since TorsinA is thought to be a molecular chaperone, it is possible that changes in the expression or activity of other ER-resident chaperones might compensate for TorsinA deficiency, and thereby alter the activity of other cellular proteins involved in maintenance of nuclear structure during HSV infection.
Mechanisms for the NE breakdown
The observation that two different herpesviruses can cause extensive NE breakdown in some circumstances raises the question of whether there is some conserved mechanism. Our results suggest roles for both nuclear lamina disruption and membrane fusion during infection-induced NE breakdown.
The nuclear lamina provides structural support, mechanical stiffness and elasticity to the nuclear membrane (Burke and Stewart, 2013). Not surprisingly, there is a strong correlation between lamina and NE integrity. The lamina becomes disrupted in instances of (i) NE breakdown during cell division, (ii) transient NE ruptures in interphase cancer cells or cells from laminopathy patients (De Vos et al., 2011; Vargas et al., 2012) and (iii) transient NE breaks caused by parvovirus and HIV-1 infections (Cohen et al., 2011; de Noronha et al., 2001). HSV-1 pUL34 and pUS3 are both involved in lamina phosphorylation and reorganization (Bjerke et al., 2003; Leach et al., 2007; Leach and Roller, 2010; Mou et al., 2007; Park and Baines, 2006). UL34-null infection causes less lamina disruption than infection with the wild-type virus (Bjerke et al., 2003; Reynolds et al., 2004; Simpson-Holley et al., 2005). This can be explained by failure of UL34-null virus to recruit PKCs at the NE and possibly other kinases that phosphorylate lamina (Leach and Roller, 2010; Park and Baines, 2006). In comparison to HSV-1(F), infection with US3-null or US3-kinase dead mutant viruses leads to exacerbated lamina disorganization (Bjerke and Roller, 2006). While the mechanisms of this observation are unknown, pUS3 might directly or indirectly increase the activity of cellular/viral factors that mediate lamina disruption. Thus, severity of predicted lamina disruption in UL34-null, HSV-1(F) and US3-null viruses correlates with degree of NE breakdown, suggesting that disruption of lamina organization might help destabilize the NE, facilitating breakdown. This is consistent with experiments with pharmacological inhibitors that indicated that cellular factors that regulate the cell-cycle might be important for the NE breakdown induced by PrV mutants (Grimm et al., 2012). Specifically, CDK1 is an important contributor to disruption of the lamina during mitosis, and the CDK inhibitor Roscovitine inhibited the NE breakdown induced by ΔUL31Pass and ΔUL34Pass (Grimm et al., 2012). US3 deletion may, however, have multiple effects on NEBD, since it also phosphorylates pUL31, and this phosphorylation is required for efficient de-envelopment fusion or virus release to the cytoplasm (Mou et al., 2009). If the effect of US3-mediated pUL31 phosphorylation is to enhance de-envelopment fusion, however, it is not clear how failure to phosphorylate pUL31 might enhance NEBD.
The inhibition of NE breakdown seen in the double gB/gH deletion infection suggests a role for membrane fusion. gB and gH promote fusion of primary virions with the ONM (11). This function is redundant, since single deletions have a much smaller effect (for gB) or no effect (for gH). Furthermore, the function of gB in de-envelopment is dependent upon residues important for its intrinsic membrane fusion activity, and on its phosphorylation by US3 (Wisner et al., 2009; Wright et al., 2009). In Tor1a−/− MEFs the fusion-promoting activity of gB and gH could be misregulated, leading to abnormal fusion events at the NE. This is consistent with the apparent vesicularization of the nuclear envelope that accompanied breakdown. While we cannot attribute this misregulation to loss of torsin function, the cell-type dependence of NE breakdown suggests that some cellular factors play a critical regulatory role in regulation of membrane fusion at the nuclear envelope during HSV infection.
The dependence on gB and gH function for NEBD seen here contrasts with a recent report showing that single deletions of gB or gH do not inhibit the NEBD induced by PrV variants ΔUL31Pass and ΔUL34Pass (Schulz et al., 2013). This parallels a difference in gB and gH function in de-envelopment fusion during capsid nuclear egress – double deletions of gB and gH do not inhibit de-envelopment fusion in PrV, but do inhibit in HSV infections (Farnsworth et al., 2007; Klupp et al., 2008). This need not indicate a fundamental difference in nuclear envelope fusion between the two viruses. The double gB/gH deletion in HSV is only moderately impaired for replication suggesting that there is an alternative, less efficient de-envelopment mechanism in play. It has been recently reported that uninfected cells have an envelopment-de-envelopment nuclear egress mechanism for RNP complexes (Speese et al., 2012). It is possible that herpesviruses use both virus-specific and hijacked cellular processes for membrane fusion at the NE with different viruses using these alternative mechanisms to different degrees.
NE breakdown and virus replication
In comparison to Tor1a+/+, about two thirds fewer capsids accumulated in the nucleus of TA MEFs (Table 1), which might entirely account for lower virus production in Tor1a−/− MEFs. Accumulation of viral products seemed to be unaffected by in the absence of TA (Fig. 2), suggesting a defect in capsid stability and/or formation. The relationship between NE integrity and capsid production is not clear. One would expect that mixing the nuclear and cytoplasmic content would have a negative effect on capsid accumulation for two reasons. First, presence of cytoplasmic factor(s) inside the nucleus might inhibit capsid assembly and/or stability and second, diffusion of nuclear products might results in less efficient capsid formation.
Although loss of UL34 expression resulted in diminished NEBD in Tor1a−/− MEFs, about 40% of nuclei still showed evidence of breakdown (Figure 4). This NE breakdown did not significantly enhance replication of UL34-null virus (Figure 6). If nuclear breakdown were completely sufficient to suppress a nuclear egress defect, we would have expected recovery of replication close to what is seen for wild-type virus on the Tor1a−/− MEFs. That we did not observe this result suggests that, at least for HSV, simply allowing nucleocapsids access to the cytoplasm is insufficient to overcome loss of the normal primary envelopment pathway. This may indicate that the UL34-dependent nuclear egress pathway accomplishes more than simple transit of nucleocapsids to the cytoplasm and may represent a maturation step. This contrasts strongly with the normal replication phenotype of ΔUL31Pass and ΔUL34Pass PrV (Klupp et al., 2011). While it is possible that differences between HSV and PrV are at the root of this, it is also possible that adaptations in the PrV mutants in addition to those that induce NE breakdown may contribute strongly to their growth efficiency.
Highlights.
We show that wild-type HSV can induce breakdown of the nuclear envelope in a specific cell system.
The viral fusion proteins gB and gH are required for induction of nuclear envelope breakdown.
Nuclear envelope breakdown cannot compensate for deletion of the HSV UL34 gene.
Acknowledgments
The authors wish to thank Jean Ross and the University of Iowa Central Microscopy Research Facility for excellent technical assistance in TEM. This work was supported by NIH grants R21 AI099597 to R.J.R., R01 NS077730 to W. D. and RO1 EY018755 to D. J.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bjerke SL, Cowan JM, Kerr JK, Reynolds AE, Baines JD, Roller RJ. Effects of charged cluster mutations on the function of herpes simplex virus type 1 UL34 protein. J Virol. 2003;77:7601–7610. doi: 10.1128/JVI.77.13.7601-7610.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjerke SL, Roller R. Roles for herpes simplex type 1 UL34 and US3 proteins in disrupting the nuclear lamina during herpes simplex virus type 1 egress. Virology. 2006;347(2):261–76. 261–276. doi: 10.1016/j.virol.2005.11.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdette A, Churchill P, Caldwell G, Caldwell K. The early-onset torsion dystonia-associated protein, torsinA, displays molecular chaperone activity in vitro. Cell Stress and Chaperones. 2010;15:605–617. doi: 10.1007/s12192-010-0173-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke B, Stewart CL. The nuclear lamins: flexibility in function. Nat Rev Mol Cell Biol. 2013;14:13–24. doi: 10.1038/nrm3488. [DOI] [PubMed] [Google Scholar]
- Caldwell GA, Cao S, Sexton EG, Gelwix CC, Bevel JP, Caldwell KA. Suppression of polyglutamine-induced protein aggregation in Caenorhabditis elegans by torsin proteins. Human Molecular Genetics. 2003;12:307–319. doi: 10.1093/hmg/ddg027. [DOI] [PubMed] [Google Scholar]
- Callan AC, Bunning S, Jones OT, High S, Swanton E. Biosynthesis of the dystonia-associated AAA+ ATPase torsinA at the endoplasmic reticulum. Biochem J. 2007;401:607–612. doi: 10.1042/BJ20061313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camozzi D, Pignatelli S, Valvo C, Lattanzi G, Capanni C, Dal Monte P, Landini MP. Remodelling of the nuclear lamina during human cytomegalovirus infection: role of the viral proteins pUL50 and pUL53. J Gen Virol. 2008;89:731–740. doi: 10.1099/vir.0.83377-0. [DOI] [PubMed] [Google Scholar]
- Chang YEVS, Krug C, Sears PW, Roizman AEB. The null mutant of the U(L)31 gene of herpes simplex virus 1: construction and phenotype in infected cells. Journal of Virology. 1997;71:8307–8315. doi: 10.1128/jvi.71.11.8307-8315.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen XP, Hu XH, Wu SH, Zhang YW, Xiao B, Shang HF. RNA interference-mediated inhibition of wild-type Torsin A expression increases apoptosis caused by oxidative stress in cultured cells. Neurochem Res. 2010;8 doi: 10.1007/s11064-010-0177-4. [DOI] [PubMed] [Google Scholar]
- Cohen S, Marr AK, Garcin P, Panté N. Nuclear Envelope Disruption Involving Host Caspases Plays a Role in the Parvovirus Replication Cycle. Journal of Virology. 2011;85:4863–4874. doi: 10.1128/JVI.01999-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Noronha CMC, Sherman MP, Lin HW, Cavrois MV, Moir RD, Goldman RD, Greene WC. Dynamic Disruptions in Nuclear Envelope Architecture and Integrity Induced by HIV-1 Vpr. Science. 2001;294:1105–1108. doi: 10.1126/science.1063957. [DOI] [PubMed] [Google Scholar]
- De Vos WH, Houben F, Kamps M, Malhas A, Verheyen F, Cox J, Manders EMM, Verstraeten VLRM, van Steensel MAM, Marcelis CLM, van den Wijngaard A, Vaux DJ, Ramaekers FCS, Broers JLV. Repetitive disruptions of the nuclear envelope invoke temporary loss of cellular compartmentalization in laminopathies. Human Molecular Genetics. 2011;20:4175–4186. doi: 10.1093/hmg/ddr344. [DOI] [PubMed] [Google Scholar]
- Ejercito PM, Kieff ED, Roizman B. Characteristics of herpes simplex virus strains differing in their effect on social behavior of infected cells. Journal of General Virology. 1968;2:357–364. doi: 10.1099/0022-1317-2-3-357. [DOI] [PubMed] [Google Scholar]
- Farnsworth A, Wisner TW, Webb M, Roller R, Cohen G, Eisenberg R, Johnson DC. Herpes simplex virus glycoproteins gB and gH function in fusion between the virion envelope and the outer nuclear membrane. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:10187–10192. doi: 10.1073/pnas.0703790104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs W, Klupp BG, Granzow H, Osterrieder N, Mettenleiter TC. The interacting UL31 and UL34 gene products of pseudorabies virus are involved in egress from the host-cell nucleus and represent components of primary enveloped but not mature virions. J Virol. 2002;76:364–378. doi: 10.1128/JVI.76.1.364-378.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Alegre P, Bode N, Davidson BL, Paulson HL. Silencing Primary Dystonia: Lentiviral-Mediated RNA Interference Therapy for DYT1 Dystonia. The Journal of Neuroscience. 2005;25:10502–10509. doi: 10.1523/JNEUROSCI.3016-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Alegre P, Paulson HL. Aberrant Cellular Behavior of Mutant TorsinA Implicates Nuclear Envelope Dysfunction in DYT1 Dystonia. The Journal of Neuroscience. 2004;24:2593–2601. doi: 10.1523/JNEUROSCI.4461-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodchild RE, Dauer WT. The AAA+ protein torsinA interacts with a conserved domain present in LAP1 and a novel ER protein. J Cell Biol. 2005;168:855–862. doi: 10.1083/jcb.200411026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodchild RE, Kim CE, Dauer WT. Loss of the dystonia-associated protein torsinA selectively disrupts the neuronal nuclear envelope. Neuron. 2005;48:923–932. doi: 10.1016/j.neuron.2005.11.010. [DOI] [PubMed] [Google Scholar]
- Granata A, Schiavo G, Warner TT. TorsinA and dystonia: from nuclear envelope to synapse. Journal of Neurochemistry. 2009;109:1596–1609. doi: 10.1111/j.1471-4159.2009.06095.x. [DOI] [PubMed] [Google Scholar]
- Granata A, Warner TT. The role of torsinA in dystonia. European Journal of Neurology. 2010;17:81–87. doi: 10.1111/j.1468-1331.2010.03057.x. [DOI] [PubMed] [Google Scholar]
- Grimm KS, Klupp BG, Granzow H, Müller FM, Fuchs W, Mettenleiter TC. Analysis of Viral and Cellular Factors Influencing Herpesvirus-Induced Nuclear Envelope Breakdown. Journal of Virology. 2012;86:6512–6521. doi: 10.1128/JVI.00068-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamirally S, Kamil JP, Ndassa-Colday YM, Lin AJ, Jahng WJ, Baek MC, Noton S, Silva LA, Simpson-Holley M, Knipe DM, Golan DE, Marto JA, Coen DM. Viral mimicry of Cdc2/cyclin-dependent kinase 1 mediates disruption of nuclear lamina during human cytomegalovirus nuclear egress. PLoS Pathogens. 2009;5:e1000275. doi: 10.1371/journal.ppat.1000275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewett J, Ziefer P, Bergeron D, Naismith T, Boston H, Slater D, Wilbur J, Schuback D, Kamm C, Smith N, Camp S, Ozelius LJ, Ramesh V, Hanson PI, Breakefield XO. TorsinA in PC12 cells: Localization in the endoplasmic reticulum and response to stress. Journal of Neuroscience Research. 2003;72:158–168. doi: 10.1002/jnr.10567. [DOI] [PubMed] [Google Scholar]
- Hewett JW, Tannous B, Niland BP, Nery FC, Zeng J, Li Y, Breakefield XO. Mutant torsinA interferes with protein processing through the secretory pathway in DYT1 dystonia cells. Proceedings of the National Academy of Sciences. 2007;104:7271–7276. doi: 10.1073/pnas.0701185104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson DC, Baines JD. Herpesviruses remodel host membranes for virus egress. Nat Rev Micro. 2011;9:382–394. doi: 10.1038/nrmicro2559. [DOI] [PubMed] [Google Scholar]
- Jungwirth M, Dear ML, Brown P, Holbrook K, Goodchild R. Relative tissue expression of homologous torsinB correlates with the neuronal specific importance of DYT1 dystonia-associated torsinA. Hum Mol Genet. 2010;19:888–900. doi: 10.1093/hmg/ddp557. [DOI] [PubMed] [Google Scholar]
- Kim CE, Perez A, Perkins G, Ellisman MH, Dauer WT. A molecular mechanism underlying the neural-specific defect in torsinA mutant mice. Proceedings of the National Academy of Sciences. 2010;107:9861–9866. doi: 10.1073/pnas.0912877107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klupp B, Altenschmidt J, Granzow H, Fuchs W, Mettenleiter TC. Glycoproteins required for entry are not necessary for egress of pseudorabies virus. J Virol. 2008;82:6299–6309. doi: 10.1128/JVI.00386-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klupp BG, Granzow H, Fuchs W, Keil GM, Finke S, Mettenleiter TC. Vesicle formation from the nuclear membrane is induced by coexpression of two conserved herpesvirus proteins. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:7241–7246. doi: 10.1073/pnas.0701757104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klupp BG, Granzow H, Mettenleiter TC. Primary envelopment of pseudorabies virus at the nuclear membrane requires the UL34 gene product. J Virol. 2000;74:10063–10073. doi: 10.1128/jvi.74.21.10063-10073.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klupp BG, Granzow H, Mettenleiter TC. Effect of the pseudorabies virus US3 protein on nuclear membrane localization of the UL34 protein and virus egress from the nucleus. J Gen Virol. 2001;82:2363–2371. doi: 10.1099/0022-1317-82-10-2363. [DOI] [PubMed] [Google Scholar]
- Klupp BG, Granzow H, Mettenleiter TC. Nuclear Envelope Breakdown Can Substitute for Primary Envelopment-Mediated Nuclear Egress of Herpesviruses. Journal of Virology. 2011;85:8285–8292. doi: 10.1128/JVI.00741-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kustedjo K, Bracey MH, Cravatt BF. Torsin A and its torsion dystonia-associated mutant forms are lumenal glycoproteins that exhibit distinct subcellular localizations. J Biol Chem. 2000;275:27933–27939. doi: 10.1074/jbc.M910025199. [DOI] [PubMed] [Google Scholar]
- Leach N, Bjerke SL, Christensen DK, Bouchard JM, Mou F, Park R, Baines J, Haraguchi T, Roller RJ. Emerin is hyperphosphorylated and redistributed in herpes simplex virus type 1-infected cells in a manner dependent on both UL34 and US3. J Virol. 2007;81:10792–10803. doi: 10.1128/JVI.00196-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach NR, Roller RJ. Significance of host cell kinases in herpes simplex virus type 1 egress and lamin-associated protein disassembly from the nuclear lamina. Virology. 2010;406:127–137. doi: 10.1016/j.virol.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang L, Tanaka M, Kawaguchi Y, Baines JD. Cell lines that support replication of a novel herpes simplex virus 1 UL31 deletion mutant can properly target UL34 protein to the nuclear rim in the absence of UL31. Virology. 2004;329:68–76. doi: 10.1016/j.virol.2004.07.030. [DOI] [PubMed] [Google Scholar]
- Maric M, Shao J, Ryan RJ, Wong C-S, Gonzalez-Alegre P, Roller RJ. A functional role for torsinA in herpes simplex virus type 1 nuclear egress. J Virol. 2011;85:9667–9779. doi: 10.1128/JVI.05314-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean PJ, Kawamata H, Shariff S, Hewett J, Sharma N, Ueda K, Breakefield XO, Hyman BT. TorsinA and heat shock proteins act as molecular chaperones: suppression of α-synuclein aggregation. Journal of Neurochemistry. 2002;83:846–854. doi: 10.1046/j.1471-4159.2002.01190.x. [DOI] [PubMed] [Google Scholar]
- Milbradt J, Auerochs S, Marschall M. Cytomegaloviral proteins pUL50 and pUL53 are associated with the nuclear lamina and interact with cellular protein kinase C. J Gen Virol. 2007;88:2642–2650. doi: 10.1099/vir.0.82924-0. [DOI] [PubMed] [Google Scholar]
- Morris JB, Hofemeister H, O’Hare P. Herpes simplex virus infection induces phosphorylation and delocalization of emerin, a key inner nuclear membrane protein. J Virol. 2007;81:4429–4437. doi: 10.1128/JVI.02354-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mou F, Forest T, Baines JD. Us3 of Herpes Simplex type 1 Encodes a Promiscuous Protein Kinase That Phosphorylates and Alters Localization of Lamin A/C in Infected Cells. J Virol. 2007;81:6459–6470. doi: 10.1128/JVI.00380-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mou F, Wills E, Baines JD. Phosphorylation of the U(L)31 protein of herpes simplex virus 1 by the U(S)3-encoded kinase regulates localization of the nuclear envelopment complex and egress of nucleocapsids. J Virol. 2009;83:5181–5191. doi: 10.1128/JVI.00090-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muranyi W, Haas J, Wagner M, Krohne G, Koszinowski UH. Cytomegalovirus recruitment of cellular kinases to dissolve the nuclear lamina. Science. 2002;297:854–857. doi: 10.1126/science.1071506. [DOI] [PubMed] [Google Scholar]
- Naismith TV, Dalal S, Hanson PI. Interaction of torsinA with its major binding partners is impaired by the dystonia-associated DeltaGAG deletion. J Biol Chem. 2009;284:27866–27874. doi: 10.1074/jbc.M109.020164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naismith TV, Heuser JE, Breakefield XO, Hanson PI. TorsinA in the nuclear envelope. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:7612–7617. doi: 10.1073/pnas.0308760101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nery FC, Zeng J, Niland BP, Hewett J, Farley J, Irimia D, Li Y, Wiche G, Sonnenberg A, Breakefield XO. TorsinA binds the KASH domain of nesprins and participates in linkage between nuclear envelope and cytoskeleton. J Cell Sci. 2008;121:3476–3486. doi: 10.1242/jcs.029454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozelius LJ, Hewett JW, Page CE, Bressman SB, Kramer PL, Shalish C, de Leon D, Brin MF, Raymond D, Corey DP, Fahn S, Risch NJ, Buckler AJ, Gusella JF, Breakefield XO. The early-onset torsion dystonia gene (DYT1) encodes an ATP-binding protein. Nature Genetics. 1997;17:40–48. doi: 10.1038/ng0997-40. [DOI] [PubMed] [Google Scholar]
- Park R, Baines J. Herpes simplex virus type 1 infection induces activation and recruitment of protein kinase C to the nuclear membrane and increased phosphorylation of lamin B. Journal of Virology. 2006;80:494–504. doi: 10.1128/JVI.80.1.494-504.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds AE, Liang L, Baines JD. Conformational changes in the nuclear lamina induced by herpes simplex virus type 1 require genes UL31 and UL34. J Virol. 2004;78:5564–5575. doi: 10.1128/JVI.78.11.5564-5575.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds AE, Ryckman BJ, Baines JD, Zhou Y, Liang L, Roller RJ. UL31 and UL34 proteins of herpes simplex virus type 1 form a complex that accumulates at the nuclear rim and is required for envelopment of nucleocapsids. J Virol. 2001;75:8803–8817. doi: 10.1128/JVI.75.18.8803-8817.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds AE, Wills EG, Roller RJ, Ryckman BJ, Baines JD. Ultrastructural localization of the herpes simplex virus type 1 UL31, UL34, and US3 proteins suggests specific roles in primary envelopment and egress of nucleocapsids. J Virol. 2002;76:8939–8952. doi: 10.1128/JVI.76.17.8939-8952.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roller RJ. Nuclear Egress of Herpesviruses. Virologica Sinica. 2008;23:406–415. [Google Scholar]
- Roller RJ, Bjerke SL, Haugo AC, Hanson S. Analysis of a charge cluster mutation of herpes simplex virus type 1 UL34 and its extragenic suppressor suggests a novel interaction between pUL34 and pUL31 that Is necessary for membrane curvature around capsids. J Virol. 2010;84:3921–3934. doi: 10.1128/JVI.01638-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roller RJ, Haugo AC, Kopping NJ. Intragenic and extragenic suppression of a mutation in herpes simplex virus-1 UL34 that affects both nuclear envelope targeting and membrane budding. J Virol. 2011;85:11615–11625. doi: 10.1128/JVI.05730-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roller RJ, Zhou Y, Schnetzer R, Ferguson J, DeSalvo D. Herpes simplex virus type 1 UL34 gene product is required for viral envelopment. Journal of Virology. 2000;74:117–129. doi: 10.1128/jvi.74.1.117-129.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryckman BJ, Roller RJ. Herpes simplex virus type 1 primary envelopment: UL34 protein modification and the US3-UL34 catalytic relationship. J Virol. 2004;78 doi: 10.1128/JVI.78.1.399-412.2004. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz KS, Klupp BG, Granzow H, Mettenleiter TC. Glycoproteins gB and gH Are Required for Syncytium Formation but Not for Herpesvirus-Induced Nuclear Envelope Breakdown. J Virol. 2013;87:9733–9741. doi: 10.1128/JVI.01401-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher D, Tischer BK, Trapp S, Osterrieder N. The protein encoded by the US3 orthologue of Marek’s disease virus is required for efficient de-envelopment of perinuclear virions and involved in actin stress fiber breakdown. J Virol. 2005;79:3987–3997. doi: 10.1128/JVI.79.7.3987-3997.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott ES, O’Hare P. Fate of the inner nuclear membrane protein lamin B receptor and nuclear lamins in herpes simplex virus type 1 infection. J Virol. 2001;75:1818–1830. doi: 10.1128/JVI.75.18.8818-8830.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson-Holley M, Colgrove RC, Nalepa G, Harper JW, Knipe DM. Identification and Functional Evaluation of Cellular and Viral Factors Involved in the Alteration of Nuclear Architecture during Herpes Simplex Virus 1 Infection. J Virol. 2005;79:12840–12851. doi: 10.1128/JVI.79.20.12840-12851.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson-Holly M, Baines J, Roller R, Knipe D. Herpes simplex virus 1 UL31 and UL34 promote the late maturation of viral replication compartments to the nuclear periphery. J Virol. 2004;78:5591–5600. doi: 10.1128/JVI.78.11.5591-5600.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speese Sean D, Ashley J, Jokhi V, Nunnari J, Barria R, Li Y, Ataman B, Koon A, Chang YT, Li Q, Moore Melissa J, Budnik V. Nuclear Envelope Budding Enables Large Ribonucleoprotein Particle Export during Synaptic Wnt Signaling. Cell. 2012;149:832–846. doi: 10.1016/j.cell.2012.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vander Heyden AB, Naismith TV, Snapp EL, Hanson PI. Static retention of the lumenal monotopic membrane protein torsinA in the endoplasmic reticulum. The EMBO journal. 2011;30:3217–3231. doi: 10.1038/emboj.2011.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas JD, Hatch EM, Anderson DJ, Hetzer MW. Transient nuclear envelope rupturing during interphase in human cancer cells. Nucleus. 2012;3:88–100. doi: 10.4161/nucl.18954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White SR, Lauring B. AAA+ ATPases: Achieving Diversity of Function with Conserved Machinery. Traffic. 2007;8:1657–1667. doi: 10.1111/j.1600-0854.2007.00642.x. [DOI] [PubMed] [Google Scholar]
- Wisner TW, Wright CC, Kato A, Kawaguchi Y, Mou F, Baines J, Roller R, Johnson DC. Herpesvirus gB-induced fusion between the virion envelope and outer nuclear membrane during virus egress is regulated by a viral kinase. J Virol. 2009 doi: 10.1128/JVI.01462-08. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright CC, Wisner TW, Hannah BP, Eisenberg RJ, Cohen GH, Johnson DC. Fusion between Perinuclear Virions and the Outer Nuclear Membrane Requires the Fusogenic Activity of Herpes Simplex Virus gB. J Virol. 2009;83:11847–11856. doi: 10.1128/JVI.01397-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang K, Baines JD. Domain within Herpes Simplex Virus 1 Scaffold Proteins Required for Interaction with Portal Protein in Infected Cells and Incorporation of the Portal Vertex into Capsids. Journal of Virology. 2008;82:5021–5030. doi: 10.1128/JVI.00150-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye GJ, Roizman B. The essential protein encoded by the UL31 gene of herpes simplex virus 1 depends for its stability on the presence of UL34 protein. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:11002–11007. doi: 10.1073/pnas.97.20.11002. [DOI] [PMC free article] [PubMed] [Google Scholar]