Abstract
Objective
To evaluate age-related differences in inflammation biomarkers during the first 72 hours of hospitalization for sepsis.
Methods
This was a secondary analysis of a prospective observational cohort of adult patients (n=855) from ten, urban, academic emergency departments with confirmed infection and two or more systemic inflammatory response syndrome criteria. We analyzed six inflammation-related biomarkers—chemokine (CC-motif) ligand-23 (CCL-23); C-reactive protein (CRP); interleukin-1 receptor antagonist (IL-1ra); neutrophil gelatinase-associated lipocalin (NGAL); peptidoglycan recognition protein (PGRP); and tumor necrosis factor receptor-1a (TNFR-1a)—measured at presentation and 3, 6, 12, 24, 48, or 72 hours later.
Results
The median age was 56 (IQR 43–72) years and sepsis severity was 38% sepsis, 16% severe sepsis without shock, and 46% septic shock; the overall 30-day mortality was 12%. Older age was associated with higher sepsis severity: 41% of subjects aged 18–34 years had severe sepsis or septic shock compared to 71% for those aged ≥65 years (p<0.001). In longitudinal models adjusting for demographics, co-morbidities, and infection source, older age was associated with higher baseline values for CCL-23, IL-1ra, NGAL, and TNFR-1a (all p<0.05). However, older adults had higher mean values during the entire 72-hour period only for NGAL and TNFR-1a, and higher final 72-hour values only for TNFR-1a. Adjustment or stratification by sepsis severity did not change the age-inflammation associations.
Conclusion
While older adults had higher levels of inflammation at presentation and an increased incidence of severe sepsis and septic shock, these age-related differences in inflammation largely resolved during the first 72 hours of hospitalization.
Keywords: sepsis, infection, biomarkers, severity, geriatrics, critical care, emergency medicine
INTRODUCTION
Severe sepsis is a syndrome of infection-related acute organ dysfunction that hospitalizes 750,000 annually, resulting in 215,000 deaths and an estimated $16.7 billion in direct medical costs in the US (1). While adults and children of all ages are susceptible to severe sepsis, older adults (age ≥65 years) account for over half of emergency department (ED) visits and hospitalizations, and the incidence and case-fatality rates of severe sepsis both rise dramatically with age (2–5).
Inflammation is a key contributor to both the prevention and pathophysiology of severe sepsis. While inflammation plays an important role in normal host defense, locally beneficial host responses become detrimental during uncontrolled systemic activation (6–8). Altered regulation of inflammation is postulated to be a central explanation as to why older adults with acute infection have exponentially higher risk of developing severe sepsis than younger adults (8–11). Indeed, a hallmark of sepsis syndromes is the altered initiation of pro-inflammatory responses at the onset of infection and anti-inflammatory responses during resolution (12, 13).
Serum biomarkers of inflammation provide the ability to both prognosticate severity of sepsis (14) and evaluate the timing and trajectory of the inflammation during the acute infection. However, few studies longitudinally report age-related differences in inflammatory biomarkers, as most studies have been cross-sectional at variable times during their hospital course. Existing data are contradictory on whether older adults have greater or suppressed acute inflammation and whether this contributes to the poorer outcomes of severe sepsis in older adults (7, 8, 15, 16). In this study, we analyzed six novel biomarkers of inflammation measured serially for 72 hours as part of a large, multicenter longitudinal cohort of emergency department (ED) patients admitted for sepsis (14). The primary objective was to evaluate age-related differences in these inflammation biomarkers during first 72 hours of hospital presentation.
MATERIALS AND METHODS
Study Design and Participants
This study was a secondary analysis of a prospective, multicenter, observational cohort performed in ten academic centers in the United States over an 18-month period. This cohort was comprised of a convenience sample of ED patients hospitalized for suspected sepsis based on the following inclusion criteria: 1) ED patients age ≥18 years; 2) presumptive source of infection suspected by the treating clinician or a serum lactate level >2.5 mmol/L; and 3) at least two of the four criteria for systemic inflammatory response syndrome: a) temperature >38°C or <36°C; b) respirations >20 breaths/min or partial pressure of carbon dioxide <32 mm Hg; c) heart rate >90 beats/minute; d) white blood cell count >12,000 cells/mL or <4,000 cells/mL or greater than 10% immature forms. The exclusion criteria were pregnancy, do-not-resuscitate status, or cardiac arrest.
The original purpose of this investigation was to identify a panel of biomarkers to predict risk of organ dysfunction, shock, and death and has been previously reported (14). The study was approved by the Institutional Review Board for Human Research at each participating center and included consent for secondary analysis of the data collected. In the present study, we performed a secondary analysis to evaluate age-related differences of the inflammation-related biomarkers.
Data Collection
Details of the data collection for the overall cohort of 1,038 subjects are described previously (14). Briefly, after obtaining written informed consent, pertinent demographic data and comorbid conditions were collected along with whole blood that were centrifuged and cryopreserved within 1 hour of collection. During the subsequent 72-hour period from enrollment, we collected serial vital signs, whole blood samples, the results from available laboratory testing, and the presumed source of infection. Although all subjects had suspected sepsis at enrollment, for this analysis we excluded 166 subjects for whom infection was thought unlikely based on the hospital course (e.g., alternate diagnosis determined and treatment for infection discontinued). Additionally, 17 subjects were removed from the analyses due to not having biomarker data at any of our time points (time = 0, 3, 6, 12, 24, 48, or 72 hours). Thus all 855 patients in our analysis had sepsis (infection plus systemic inflammatory response syndrome), and contributed biomarker data for at least one time-point (see Table, Digital Content 1 for availability of each biomarker by study time-point). We further classified sepsis as severe sepsis based on presence of organ dysfunction or septic shock based on hypotension after fluid resuscitation using a classification scheme adapted from the American College of Chest Physicians/Society for Critical Care Medicine consensus criteria (17). These classifications were based on the worst sepsis severity during the first 72 hours after enrollment.
Biomarker Selection
All biomarker measurements were independently performed in a single laboratory (Biosite, San Diego, CA). An initial exploratory screening of ~150 biomarkers from roughly the first 250 patients was performed to identify a focused list of biomarkers to be tested in the entire cohort. Nine biomarkers were selected based on the performance and feasibility to afford the greatest potential for a point-of-care biomarker panel (the purpose of the original study). For this analysis, we further narrowed this list to six of the biomarkers related to inflammation as follows: chemokine (CC-motif) ligand-23 (CCL-23); C-reactive protein (CRP); interleukin-1 receptor antagonist (IL-1ra); neutrophil gelatinase-associated lipocalin (NGAL); peptidoglycan recognition protein (PGRP); and tumor necrosis factor receptor 1a (TNFR-1a). These biomarkers were measured using standard immunoassay techniques involving the use of recombinant murine antibodies, as previously described (14). We did not include the three biomarkers from the original study that were not related to inflammation (Brain natriuretic peptic, D-dimer, and Protein C).
Statistical analysis
We present descriptive data as proportions, means (standard deviations), and medians (interquartile ranges). For the primary analysis, we evaluated the association age-related differences in inflammation biomarker values over the 72-hour study period. Age was stratified into five evenly distributed categories (18–34, 35–49, 50–64, 65–79, and ≥80 years). The distribution for each of the six biomarkers was evaluated, and each was natural log transformed to normalize the data.
To be able to model the longitudinal data with the flexibility to incorporate the changes in trajectories, we used a backward-selection-type strategy, first fitting knots at each time-point of data collection (hours 3, 12, 24, and 48). We removed knots where there was not a significant change in trajectory for the biomarkers globally. For each age group, we modeled our final piecewise-linear function with knots at 24 and 48 hours, resulting in a model which both showed the changes over time well and also provides for sensible times of data collection for clinicians. Maximum likelihood estimation for incomplete repeated measures was used for these repeated measure data. This approach enabled us to include all available data in the analyses and lower the assumptions about randomness of missing data.
Using mixed longitudinal regression, we modeled the biomarker values for each age group, adjusting for fixed co-variates (sex, race/ethnicity, co-morbid conditions, and source of infection). Because we hypothesized that inflammation mediates the association between age and sepsis severity, we did not include sepsis severity as a co-variate in the primary analysis. However, we did do a sensitivity analysis including sepsis severity to evaluate the extent to which sepsis severity confounded the association between age and inflammation. To obtain a more complete evaluation of these associations, we also evaluated the prevalence of each sepsis severity classification by age group, and modeled the association between sepsis severity and inflammatory biomarker values during the 72-hour time period.
The biomarker longitudinal trajectories were summarized by calculating the mean values over the course of the 72-hour time period. The association between age and biomarker levels for the overall mean, at baseline and at 72 hours was tested by fitting a linear regression between the model-estimated biomarker level (adjusted for all covariates) and age. For these tests, standard statistical conventions were used (e.g. 2-tailed tests where p-values <0.05 were considered statistically significant). Transformed values were exponentiated for interpretation and comparison in the actual units of the biomarker. We performed statistical analyses using SAS 9.3 (Cary, NC, USA).
RESULTS
Baseline Characteristics and Clinical Outcomes
Table 1 displays baseline characteristics of the 855 study subjects analyzed in this study. The median age was 56 years (inter-quartile range 43–72) and 49% of subjects were racial/ethnic minorities. The most common co-morbidities were hypertension (47%) and diabetes mellitus (29%) and the most common sources of infection were pulmonary (35%) and genitourinary (18%). During the first 72 hours, 62% of subjects were classified as severe sepsis or septic shock. Vital status was known at day 30 for 695 (81%) subjects, of which the mortality rate was 12%.
Table 1.
Baseline Characteristics of 855 Study Subjects
| Characteristic | n | % |
|---|---|---|
| Age, years | ||
| 18–34 | 104 | 12% |
| 35–49 | 209 | 24% |
| 50–64 | 231 | 27% |
| 65–79 | 199 | 23% |
| ≥80 | 112 | 13% |
| Female Sex | 444 | 52% |
| Race/Ethnicity | ||
| Non-Hispanic white | 432 | 51% |
| Non-Hispanic black | 333 | 39% |
| Hispanic | 67 | 7.8% |
| Other | 23 | 2.7% |
| Co-Morbid Conditions | ||
| Hypertension | 403 | 47% |
| Diabetes mellitus | 244 | 29% |
| Cardiovascular disease | 215 | 25% |
| Prior stroke | 85 | 10% |
| Immunocompromised state | 149 | 17% |
| Cirrhosis | 27 | 3.2% |
| Renal insufficiency | 133 | 16% |
| Source of Infection | ||
| Pulmonary | 296 | 35% |
| Genitourinary | 154 | 18% |
| Skin/soft tissue | 57 | 6.7% |
| Catheter-related | 42 | 4.9% |
| Abdominal | 36 | 4.2% |
| Other | 270 | 32% |
| Sepsis Group (worst syndrome over first 72 hours) | ||
| Sepsis | 323 | 38% |
| Severe sepsis | 138 | 16% |
| Septic shock | 394 | 46% |
| Mortality at Day 30 | ||
| Died | 84 | 9.8% |
| Alive | 611 | 71% |
| Unknown | 160 | 19% |
|
| ||
| Biomarkers Values | Mean (SD) | Median (IQR) |
| Chemokine (CC-motif) ligand-23, ng/mL | 3.5 (3.4) | 2.5 (1.4–4.5) |
| C-reactive protein, μg/mL | 89 (61) | 74 (39–139) |
| Interleukin-1 receptor antagonist, pg/mL | 2613 (4455) | 780 (320–2398) |
| Neutrophil gelatinase-associated lipocalin, ng/mL | 293 (345) | 156 (61–382) |
| Peptidoglycan recognition protein, ng/mL | 132 (162) | 69 (44–154) |
| Tumor necrosis factor receptor 1a, ng/mL | 19 (25) | 10 (5.6–20) |
Association of Older Age and Inflammation Biomarkers with Sepsis Severity
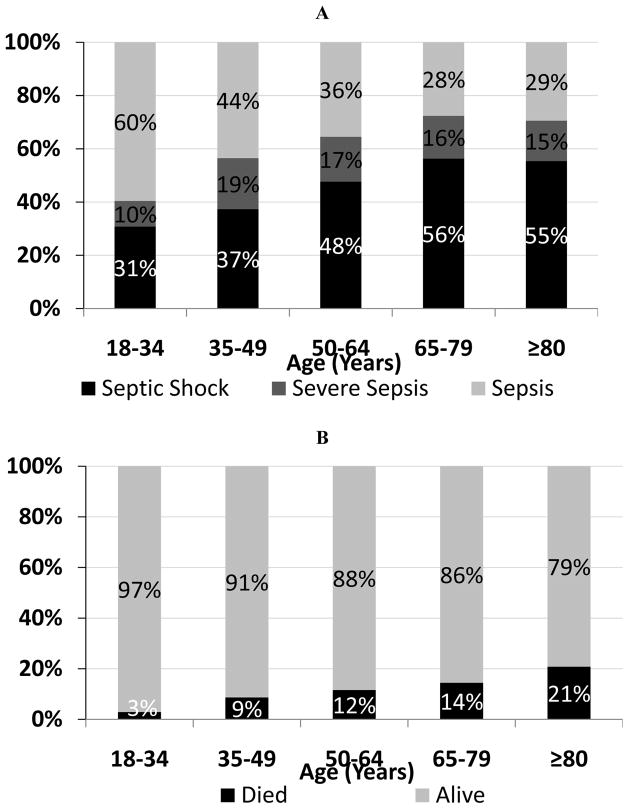
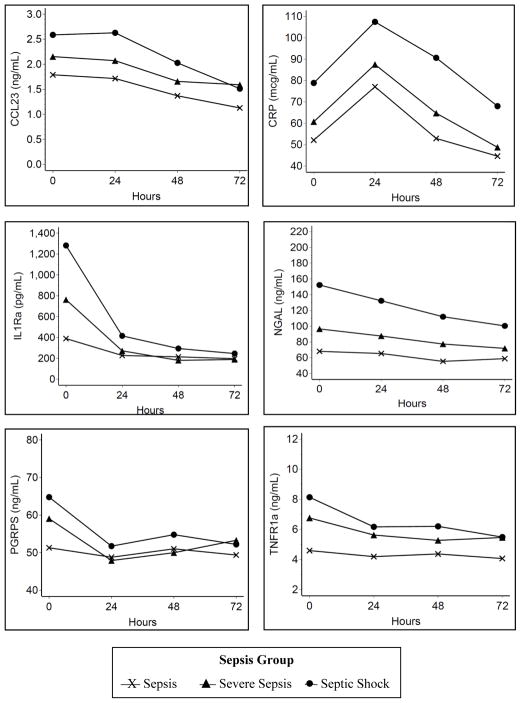
The association of age with sepsis severity and 30-day mortality is presented in Figure 1. As anticipated, sepsis severity was higher in patients with older age in our cohort with 41% of subjects aged 18–34 years with severe sepsis or septic shock compared to 71% for those aged ≥65 years (p<0.001). In addition, older age was associated with higher 30-day mortality, ranging from 2.9% in subjects aged 18–34 years compared to 21% for those aged ≥80 years (p<0.001). Greater sepsis severity was associated with higher baseline values for each of the six inflammatory biomarkers (Figure 2; all p<0.05). In addition, septic shock was associated with a greater overall mean value during the first 72 hours for five of the six biomarkers (p<0.05 for CCL23, CRP, IL-1ra, NGAL, and TNFR-1a). These results confirm that both older age and higher acute inflammation was associated greater sepsis severity.
Figure 1. Association of age with sepsis severity (A) and 30-day mortality (B).
Figure 2. Longitudinal association between sepsis group and biomarkers.
Sepsis Group
 Sepsis
Sepsis
 Severe Sepsis
Severe Sepsis
 Septic Shock
Septic Shock
Model adjusted for age, sex, race/ethnicity, co-morbid conditions, and source of infection
Older Age and Inflammation Biomarkers
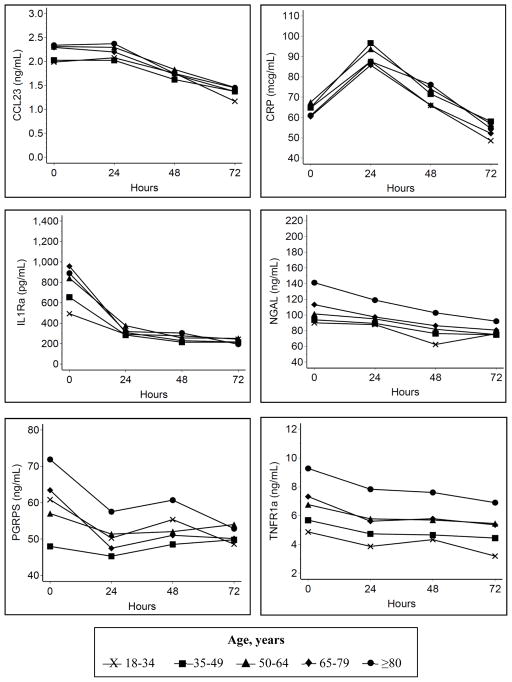
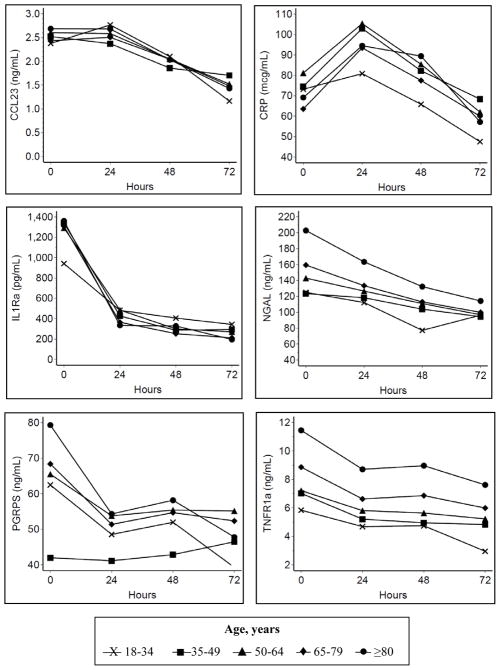
Having confirmed these findings to validate the data characteristics based on well-accepted associations, our focus then turned to the association between older age and these biomarkers of acute inflammation (Figure 3 and Table 2). Older age was associated with higher baseline values for CCL-23, IL-1ra, NGAL, and TNFR-1a (all p<0.05). By hour 72, biomarker values were similar across all groups, except for TNFR-1a, which remained higher with older age. The overall mean during the first 72 hours were similar across all age groups for CCL-23, CRP, IL-1ra, and PGRP (all p>0.05) and was higher with older age only for NGAL and TNFR-1a. The fixed effects of other baseline co-variates in this primary longitudinal model are provided in Supplemental Digital Content 2 (Table showing additional estimates for the model).
Figure 3. Longitudinal association between age and biomarkers.
Age, years
 18–34
18–34
 35–49
35–49
 50–64
50–64
 65–79
65–79
 ≥80
≥80
Model adjusted for sex, race/ethnicity, co-morbid conditions, and source of infection
Table 2.
Adjusted biomarker estimates of baseline values and change from baseline during the first 72 hours of hospitalization
| Biomarkers | Age, years Estimates (95%CI) | p-values for trend | ||||
|---|---|---|---|---|---|---|
| 18–34 | 35–49 | 50–64 | 65–79 | ≥80 | ||
| Baseline | ||||||
| CCL23, ng/mL | 2.0 (1.6–2.4) | 2.0 (1.7–2.4) | 2.3 (2.0–2.7) | 2.3 (1.9–2.7) | 2.3 (1.9–2.8) | 0.04 |
| CRP, μg/mL | 88 (74–101) | 94 (82–105) | 94 (83–105) | 87 (75–99) | 79 (65–93) | 0.24 |
| IL-1ra, pg/mL | 493 (363–670) | 655 (511–839) | 841 (661–1071) | 958 (741–1238) | 889 (658–1201) | 0.03 |
| NGAL, ng/mL | 90 (73–111) | 94 (79–112) | 101 (85–121) | 113 (94–136) | 141 (114–173) | 0.02 |
| PGRP, ng/mL | 61 (49–76) | 48 (40–58) | 57 (48–68) | 63 (52–77) | 72 (58–90) | 0.22 |
| TNFR-1a, ng/mL | 4.9 (4.1–5.8) | 5.7 (4.9–6.6) | 6.8 (5.8–7.9) | 7.3 (6.2–8.6) | 9.3 (7.7–11) | 0.004 |
| 72 Hours | ||||||
| CCL23, ng/mL | 1.2 (0.9–1.5) | 1.4 (1.1–1.7) | 1.5 (1.2–1.7) | 1.4 (1.1–1.6) | 1.4 (1.2–1.8) | 0.13 |
| CRP, μg/mL | 73 (52–94) | 79 (65–93) | 79 (66–91) | 75 (61–88) | 72 (57–87) | 0.66 |
| IL-1ra, pg/mL | 243 (164–362) | 213 (163–279) | 252 (197–322) | 216 (167–279) | 194 (145–260) | 0.23 |
| NGAL, ng/mL | 76 (59–99) | 75 (62–91) | 75 (63–90) | 81 (67–98) | 92 (74–114) | 0.10 |
| PGRP, ng/mL | 49 (37–64) | 50 (41–61) | 54 (45–65) | 50 (41–61) | 53 (42–66) | 0.27 |
| TNFR-1a, ng/mL | 3.2 (2.5–4.1) | 4.4 (3.7–5.3) | 5.5 (4.6–6.4) | 5.4 (4.5–6.4) | 6.9 (5.7–8.4) | 0.009 |
| Overall 72-hour mean | ||||||
| CCL23, ng/mL | 1.8 (1.5–2.2) | 1.8 (1.5–2.1) | 2.0 (1.7–2.3) | 1.9 (1.6–2.2) | 2.0 (1.6–2.4) | 0.11 |
| CRP, μg/mL | 69 (55–86) | 75 (63–90) | 76 (63–90) | 68 (57–82) | 73 (59–90) | 0.93 |
| IL-1ra, pg/mL | 303 (229–401) | 283 (227–353) | 354 (286–437) | 318 (254–397) | 342 (267–439) | 0.28 |
| NGAL, ng/mL | 77 (62–95) | 83 (70–99) | 88 (74–104) | 93 (78–111) | 112 (92–136) | 0.02 |
| PGRP, ng/mL | 53 (43–66) | 48 (40–57) | 53 (45–63) | 52 (43–62) | 60 (49–73) | 0.30 |
| TNFR-1a, ng/mL | 4.0 (3.3–4.9) | 4.8 (4.1–5.6) | 5.9 (5.0–6.8) | 5.9 (5.0–6.9) | 7.8 (6.5–9.3) | 0.01 |
Abbreviations: CCL-23, chemokine (CC-motif) ligand-23; CRP, C-reactive protein; IL-1ra, interleukin-1 receptor antagonist; NGAL, neutrophil gelatinase-associated lipocalin; PGRP, peptidoglycan recognition protein; TNFR-1a, tumor necrosis factor receptor 1a
Model adjusted for sex, race/ethnicity, co-morbid conditions, and source of infection
Sensitivity Analyses
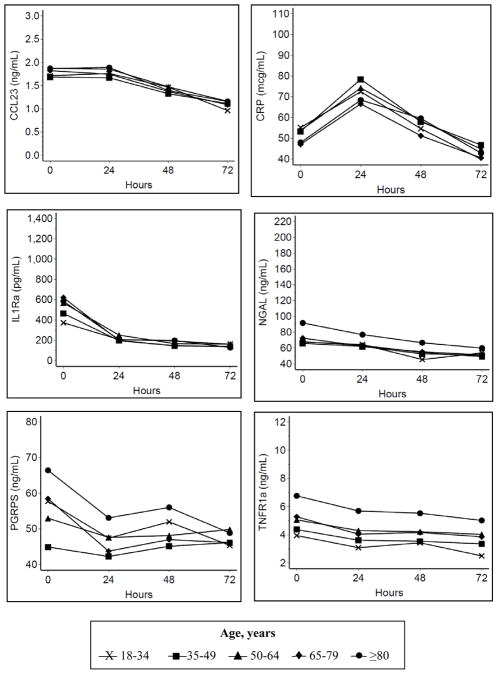
We performed additional sensitivity analyses to evaluate the potential for confounding by severity. Because we hypothesized that inflammation mediates the association between age and sepsis severity, sepsis severity was not included as a covariate in the primary analysis. In one sensitivity analysis (Figure 4), sepsis severity was added as a covariate and the relative associations between age and biomarker values were similar to the primary analysis (Figure 3), although the overall magnitudes of the adjusted biomarker values were lower. In addition, when we performed a pre-specified subgroup analysis for the highest sepsis severity (septic shock), the relative associations between age and biomarker values were also similar to the primary analysis, although, as expected, the overall magnitudes of the adjusted biomarker values were higher (Figure 5).
Figure 4. Longitudinal association between age and biomarkers, adjusted for sepsis severity.
Age, years
 18–34
18–34
 35–49
35–49
 50–64
50–64
 65–79
65–79
 ≥80
≥80
Model adjusted for sex, race/ethnicity, co-morbid conditions, source of infection, and sepsis severity
Figure 5. Longitudinal association between age and biomarkers (limited to septic shock).
Age, years
 18–34
18–34
 35–49
35–49
 50–64
50–64
 65–79
65–79
 ≥80
≥80
Model adjusted for sex, race/ethnicity, co-morbid conditions, and source of infection
DISCUSSION
To our knowledge, this is the largest study of age-related differences in inflammation biomarkers measured longitudinally during the acute care phase of sepsis. Our results indicate that older adults have higher levels of inflammation at presentation for sepsis, as measured by CCL-23, IL-1ra, NGAL, and TNFR-1a, even after adjusting for sepsis severity. However, while older adults had an increased incidence of severe sepsis and septic shock, older adults have a similar acute trajectory for most, but not all, inflammatory biomarkers measured during the first 72 hours of hospitalization. Only TNFR-1a remained associated with older age at 72 hours, and differences while statistically significant, were relatively small in magnitude. These results have important implications on understanding the role of acute inflammation in the higher sepsis severity and worse long-term outcomes observed with older age.
Prior studies strongly support an association between acute inflammation and sepsis outcomes. For example, higher inflammation during the acute phase of sepsis has been associated with mortality in epidemiological studies and animal models of sepsis (18–23). In addition, persistent sepsis-associated inflammation at hospital discharge is associated with increased long-term mortality (24). Our results also confirm a strong association between the measured inflammatory biomarkers and sepsis severity, at baseline and during the first 72 hours.
While older adults clearly have a higher incidence and severity of sepsis (2–5), the role of inflammation in driving these outcomes is unclear. Indeed, competing theories suggest that poorer sepsis outcomes in the elderly may be driven by increased or prolonged acute inflammation causing secondary host damage, or by enhanced immunosuppression leading to delayed recovery and secondary infections (7, 8). Explanations for higher acute inflammation in older patients include inefficient pathogen clearance causing prolonged stimulation of immune responses, greater predisposition for inflammation in immunosenescence, and limited physiologic reserve causing greater feedback for pro-inflammatory cytokine release. In experimental sepsis, older mice have greater acute inflammation, which directly correlates to higher mortality (25–27). However, immune responses in animal models of sepsis may correlate well to human responses (28). In human studies of healthy volunteers infused with lipopolysaccharide (LPS), older age was associated with prolonged inflammation, particularly for TNF-α and TNFR-1 (29). While providing additional information about healthy human subjects, these findings may not reflect immune responses in the frail older patients that are most predisposed to sepsis and associated adverse outcomes (2).
In a preliminary study of 22 patients hospitalized with pneumococcal infections, baseline and day 3 levels of pro-inflammatory cytokines were similar, but TNF-α and TNFR-1 were higher at day 7 in older patients, suggesting a delayed resolution of inflammation (30). Subsequently, in a large cohort study of patients hospitalized for community-acquired pneumonia. Kale and colleagues reported large age-related differences in 90-day mortality but similar inflammatory responses measured serially during the first 7 days of hospitalization (15). Data for patients with sepsis are limited to cross-sectional studies. For example, in a cohort of patients with septic shock, Marik and colleagues reported no age-related baseline differences in inflammatory biomarkers, except TNF-α, which was higher only in subjects ≥85 years old (16). However, these patients were enrolled later in the hospital course and in a more advanced stage of illness than those enrolled in the present study. Our results suggest that factors other than higher acute inflammation (e.g., inefficient clearance of pathogens) may primarily cause worse sepsis-related outcomes in older adults. Indeed, a recent study of peritoneal sepsis demonstrated that aged mice, compared to young mice, had higher mortality largely due to impaired innate immune responses and associated defective bacterial clearance despite similar pro-inflammatory cytokine responses (31).
Our study has several strengths and extends findings of prior studies in several ways. By enrolling patients early in their hospital course (i.e., in the ED), we were able to capture inflammation around the time of initiation of therapy. In addition, our cohort included a broad range of ages and sepsis severity. Contrary to some prior studies, we found that inflammation was elevated for several measured biomarkers at baseline. Potential explanations include 1): a higher baseline level of chronic inflammation observed in older adults (32); 2) presentation in a later stage of illness perhaps due to less specific signs of infection with aging (33); or 3) less regulated acute inflammation with immunosenescence (8, 11). During the first 72 hours, inflammatory biomarkers converged and age-related differences resolved, except for TNFR-1. These data indicate that interpretation of results from similar studies may depend on the biomarkers selected. Further, unlike prior studies (34), immunosuppression or decreased production of acute pro-inflammatory cytokines does not appear to be common during the first 72 hours of acute care in older septic patients.
Our results are consistent with prior studies that TNFR-1 appears to demonstrate the greatest age-related differences in infection- and sepsis-related inflammation. The reasons for this remain unknown, although TNFR-1 is increasingly recognized for its prognostic value in older adults across a variety of conditions (35–38). While TNF-α has been explored as a potential target in sepsis (39), therapy directed solely at TNF-α blockade has not been a successful therapeutic option (40). However, further study may shed light on the value of TNF and relative derivatives as a biomarker and the pathophysiological role in age-related susceptibility to sepsis.
Limitations
This study has several potential limitations. We relied on inflammatory biomarkers selected as part of the original study (14) and no stored samples were available for additional measurement of traditional (e.g., IL-6 or procalcitonin) or additional novel biomarkers. In addition, biomarkers were measured only during the first 72 hours; thus, we were unable to evaluate age-related differences over longer time intervals. Data were censored for potentially divergent reasons—improvement resulting in hospital discharge or deterioration resulting in death. However, we evaluated the available data for these subgroups and the trajectories were similar. We do not have data on pre-illness values of the biomarkers, nor was there standardization of timing of presentation relative to illness onset. For example, chronic inflammation might mediate the association between age and chronic diseases, such as coronary heart disease (41, 42). Thus, observed age-related differences in biomarker values at presentation for sepsis may reflect differences in chronic inflammation or time from illness onset to ED presentation. Finally, sepsis severity was both an outcome and a potential confounder of the age-inflammation association, which increases risk of confounding by severity. Sensitivity analyses that included sepsis severity as a covariate and stratified by sepsis severity did not materially change the results. However, further adjustment for overall illness severity, such as APACHE II scores, were not possible with available data and may lead to residual confounding.
Conclusions
Based on our assessment of six biomarkers of inflammation, in adjusted longitudinal models we found that older age was associated with greater inflammation at baseline; however, these differences resolved during the first 72 hours for all biomarkers except for TNFR-1a. We also found a strong association between age and both sepsis severity and 30-day mortality. Further characterization of sepsis in older patients is an important priority for the critical care community (43). As exuberant inflammation remains implicated in sepsis outcomes, we suggest that acute inflammation may not be the primary case of poorer outcomes in older patients with sepsis and that further study on the pathophysiology of higher age-related susceptibility to sepsis-related organ injury despite similar levels of inflammation. Attention should be focused on host factors that are global (frailty), organ-specific (chronic organ dysfunction), or immune-related (immunosenescence) and in the specific inflammatory biomarkers selected.
Supplementary Material
Acknowledgments
Source of Funding: The study was supported by Alere, Inc (formerly Biosite). Dr. Ginde was supported by NIH grant K23AG040708. Dr. Shapiro was supported NIH grant R01HL091757.
References
- 1.Angus DC, Linde-Zwirble WT, Lidicker J, et al. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Ginde AA, Moss M, Shapiro NI, et al. Impact of older age and nursing home residence on clinical outcomes of US emergency department visits for severe sepsis. J Crit Care. 2013;28:606–611. doi: 10.1016/j.jcrc.2013.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kumar G, Kumar N, Taneja A, et al. Nationwide trends of severe sepsis in the 21st century (2000–2007) Chest. 2011;140:1223–1231. doi: 10.1378/chest.11-0352. [DOI] [PubMed] [Google Scholar]
- 4.Dombrovskiy VY, Martin AA, Sunderram J, et al. Rapid increase in hospitalization and mortality rates for severe sepsis in the United States: a trend analysis from 1993 to 2003. Crit Care Med. 2007;35:1244–1250. doi: 10.1097/01.CCM.0000261890.41311.E9. [DOI] [PubMed] [Google Scholar]
- 5.Martin GS, Mannino DM, Moss M. The effect of age on the development and outcome of adult sepsis. Crit Care Med. 2006;34:15–21. doi: 10.1097/01.ccm.0000194535.82812.ba. [DOI] [PubMed] [Google Scholar]
- 6.Seeley EJ, Matthay MA, Wolters PJ. Inflection points in sepsis biology: from local defense to systemic organ injury. Am J Physiol Lung Cell Mol Physiol. 2012;303:L355–L363. doi: 10.1152/ajplung.00069.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leentjens J, Kox M, van der Hoeven J, et al. Immunotherapy for the adjunctive treatment of sepsis: from immunosuppression to immunostimulation Time for a paradigm change? Am J Respir Crit Care Med. 2013;187:1287–1293. doi: 10.1164/rccm.201301-0036CP. [DOI] [PubMed] [Google Scholar]
- 8.Kale SS, Yende S. Effects of aging on inflammation and hemostasis through the continuum of critical illness. Aging Dis. 2011;2:501–511. [PMC free article] [PubMed] [Google Scholar]
- 9.Opal SM, Girard TD, Ely EW. The immunopathogenesis of sepsis in elderly patients. Clin Infect Dis. 2005;41:S504–S512. doi: 10.1086/432007. [DOI] [PubMed] [Google Scholar]
- 10.DeGaudio ER, Rinaldi S, Chelazzi C, et al. Pathophysiology of sepsis in the elderly: clinical impact and therapeutic considerations. Curr Drug Targets. 2009;10:60–70. doi: 10.2174/138945009787122879. [DOI] [PubMed] [Google Scholar]
- 11.Boyd AR, Orihuela CJ. Dysregulated inflammation as a risk factor for pneumonia in the elderly. Aging Dis. 2011;2:487–500. [PMC free article] [PubMed] [Google Scholar]
- 12.Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med. 2003;348:138–150. doi: 10.1056/NEJMra021333. [DOI] [PubMed] [Google Scholar]
- 13.Angus DC, van der Poll T. Severe sepsis and septic shock. N Engl J Med. 2013;369:840–851. doi: 10.1056/NEJMra1208623. [DOI] [PubMed] [Google Scholar]
- 14.Shapiro NI, Trzeciak S, Hollander JE, et al. A prospective multicenter derivation of a biomarker panel to assess risk of organ dysfunction, shock, and death in emergency department patients with suspected sepsis. Crit Care Med. 2009;37:96–104. doi: 10.1097/CCM.0b013e318192fd9d. [DOI] [PubMed] [Google Scholar]
- 15.Kale S, Yende S, Kong L, et al. The effects of age on inflammatory and coagulation-fibrinolysis response in patients hospitalized for pneumonia. PLoS One. 2010;5:e13852. doi: 10.1371/journal.pone.0013852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marik PE, Zaloga GP NORASEPT II Study Investigators. The effect of aging on circulating levels of proinflammatory cytokines during septic shock. J Am Geriatr Soc. 2001;49:5–9. doi: 10.1046/j.1532-5415.2001.49003.x. [DOI] [PubMed] [Google Scholar]
- 17.American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med. 1992;20:864–874. [PubMed] [Google Scholar]
- 18.Remick DG, Golgos GR, Siddiqui J, et al. Six at six: interleukin-6 measured 6 h after the initiation of sepsis predicts mortality over 3 days. Shock. 2002;17:463–467. doi: 10.1097/00024382-200206000-00004. [DOI] [PubMed] [Google Scholar]
- 19.Walley KR, Lukacs NW, Standiford TJ, et al. Balance of inflammatory cytokines related to severity and mortality of murine sepsis. Infect Immun. 1996;64:4733–4738. doi: 10.1128/iai.64.11.4733-4738.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Damas P, Ledoux D, Nys M, et al. Cytokine serum level during severe sepsis in human IL-6 as a marker of severity. Ann Surg. 1992;215:356–362. doi: 10.1097/00000658-199204000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oberholzer A, Souza SM, Tschoeke SK, et al. Plasma cytokine measurements augment prognostic scores as indicators of outcome in patients with severe sepsis. Shock. 2005;23:488–493. [PubMed] [Google Scholar]
- 22.Bozza FA, Salluh JI, Japiassu AM, et al. Cytokine profiles as markers of disease severity in sepsis; a multiplex analysis. Crit Care. 2007;11:R49. doi: 10.1186/cc5783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kellum JA, Kong L, Fink MP, et al. Understanding the inflammatory cytokine response in pneumonia and sepsis: results of the Genetic and Inflammatory Markers of Sepsis (GenIMS) Study. Arch Intern Med. 2007;167:1655–1663. doi: 10.1001/archinte.167.15.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yende S, D’Angelo G, Mayr F, et al. Inflammatory markers at hospital discharge predict subsequent mortality after pneumonia and sepsis. Am J Respir Crit Care Med. 2008;177:1242–1247. doi: 10.1164/rccm.200712-1777OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tateda K, Matsumoto T, Miyazaki S, et al. Lipopolysaccharide-induced lethality and cytokine production in aged mice. Infect Immun. 1996;64:769–774. doi: 10.1128/iai.64.3.769-774.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Turnbull IR, Wlzorek JJ, Osborne D, et al. Effects of age on mortality and antibiotic efficacy in cecal ligation and puncture. Shock. 2003;19:310–313. doi: 10.1097/00024382-200304000-00003. [DOI] [PubMed] [Google Scholar]
- 27.Saito H, Sherwood ER, Varma TK, et al. Effects of aging on mortality, hypothermia, and cytokine induction in mice with endotoxemia or sepsis. Mech Ageing Dev. 2003;124:1047–1058. doi: 10.1016/j.mad.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 28.Seok J, Warren HS, Cuenca AG, et al. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc Natl Acad Sci USA. 2013;26:2507–2512. doi: 10.1073/pnas.1222878110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krabbe KS, Bruunsgaard H, Hansen CM, et al. Ageing is associated with a prolonged fever response in human endotoxemia. Clin Diagn Lab Immunol. 2001;8:333–338. doi: 10.1128/CDLI.8.2.333-338.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bruunsgaard H, Skinhoj P, Qvist J, et al. Elderly humans show prolonged in vivo inflammatory activity during pneumococcal infections. J Infect Dis. 1999;180:551–554. doi: 10.1086/314873. [DOI] [PubMed] [Google Scholar]
- 31.Nacionales DC, Gentile LF, Vanzant E, et al. Aged mice are unable to mount an effective myeloid response to sepsis. J Immunol. 2014;192:612–622. doi: 10.4049/jimmunol.1302109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vasto S, Candore G, Balistreri CR, et al. Inflammatory networks in ageing, age-related diseases and longevity. Mech Ageing Dev. 2007;128:83–91. doi: 10.1016/j.mad.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 33.Caterino JM. Evaluation and management of geriatric infections in the emergency department. Emerg Med Clin North Am. 2008;26:319–343. doi: 10.1016/j.emc.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 34.Gon Y, Hashimoto S, Hayashi S, et al. Lower serum concentrations of cytokines in elderly patients with pneumonia and impaired production of cytokines by peripheral blood monocytes in the elderly. Clin Exp Immunol. 1996;106:120–126. [PubMed] [Google Scholar]
- 35.Varadhan R, Yao W, Matteini A, et al. Simple biologically informed inflammatory index of two serum cytokines predicts 10 year all-cause mortality in older adults. J Gerontol A Biol Sci Med Sci. 2014;69:165–173. doi: 10.1093/gerona/glt023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Diniz BS, Teixeira AL, Ojopi EB, et al. Higher serum sTNFR1 level predicts conversion from mild cognitive impairment to Alzheimer’s disease. J Alzheimers Dis. 22:1305–1311. doi: 10.3233/JAD-2010-100921. [DOI] [PubMed] [Google Scholar]
- 37.Safranow K, Dziedziejko V, Rzeuski R, et al. Plasma concentration of TNF-alpha and its soluble receptors sTNFR1 and sTNFR2 in patients with coronary artery disease. Tissue Antigens. 2009;74:386–392. doi: 10.1111/j.1399-0039.2009.01332.x. [DOI] [PubMed] [Google Scholar]
- 38.Parsons PE, Matthay MA, Ware LB, et al. Elevated plasma levels of soluble TNF receptors are associated with morbidity and mortality in patients with acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2005;288:L426–L431. doi: 10.1152/ajplung.00302.2004. [DOI] [PubMed] [Google Scholar]
- 39.Cohen J, Carlet J. INTERSEPT. an international, multicenter, placebo controlled trial of monoclonal antibody to human tumor necrosis factor-alpha in patients with sepsis. International Sepsis Trial Study Group. Crit Care Med. 1996;24:1431–1440. doi: 10.1097/00003246-199609000-00002. [DOI] [PubMed] [Google Scholar]
- 40.Abraham E, Anzueto A, Gutierrez G, et al. Double-blind randomized controlled trial of monoclonal antibody to human tumour necrosis factor in treatment of septic shock. NORASEPTII Study Group. Lancet. 1998;351:929–933. [PubMed] [Google Scholar]
- 41.Sarwar N, Thompson AJ, Di Angelantonio E. Markers of inflammation and risk of coronary heart disease. Dis Markers. 2009;26:217–225. doi: 10.3233/DMA-2009-0646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Giovannini S, Onder G, Liperoti R, et al. Interleukin-6, C-reactive protein, and tumor necrosis factor-alpha as predicators of mortality in frail, community-living elderly individuals. J Am Geriatr Soc. 2011;59:1679–1685. doi: 10.1111/j.1532-5415.2011.03570.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Milbrandt EB, Eldadah B, Nayfield S, et al. Toward an integrated research agenda for critical illness in aging. Am J Respir Crit Care Med. 2010;182:995–1003. doi: 10.1164/rccm.200904-0630CP. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.