Abstract
Introduction
Androgen deprivation therapy (ADT) is associated with significant bone loss and an increase in fracture risk among prostate cancer survivors (PCS). We investigated whether impact + resistance training could stop ADT-related declines in bone mineral density (BMD) among PCS on ADT.
Methods
We randomized 51 PCS (mean age: 70.2 yrs) currently prescribed ADT to participate in one year of impact + resistance training (Prevent Osteoporosis with Impact + Resistance; POWIR) or in an exercise placebo program of stretching exercise (FLEX). Outcomes were proximal femur (total hip, femoral neck, and greater trochanter) and spine (L1-L4) BMD (g/cm2) and bone turnover markers (serum osteocalcin (ng/ml) and urinary deoxypyrodinoline cross-links (Dpd; nmol/mmol Cr)).
Results
Retention in the one-year study was 84% and median attendance to supervised classes was 84% in POWIR and 74% in FLEX. No study-related injuries were reported. There were no significant differences between groups for average L1-L4 BMD or for BMD at any hip site. When examining individual vertebrae, POWIR has a significant effect on preservation of BMD (−0.4%) at the L4 vertebrae compared to losses (−3.1%) in FLEX, (p=0.03).
Conclusion
Impact + resistance training was a safe and acceptable form of exercise for older PCS on ADT. Among our limited sample, POWIR did not appear to have a clinically meaningful effect on hip or spine BMD, but some evidence of skeletal adaptation to resistance + impact training in an androgen-deprived state was apparent. Future studies need to be conducted on a larger sample of patients and should consider modifications to POWIR that could further enhance loading across the spine and at the hip to preserve BMD at these clinically relevant sites.
Keywords: osteoporosis, fracture, physical activity, weight training, cancer survivor, androgen deprivation therapy
INTRODUCTION
Most men (93%) diagnosed with prostate cancer will be alive 15 years later and many even longer making efforts to manage treatment-related side effects imperative (1). Androgen deprivation therapy (ADT) may be prescribed to prostate cancer survivors (PCS) for a biochemical recurrence, as adjuvant therapy along with radiation, or prior to surgery for high-risk localized prostate cancer (8). Bone loss is a common and serious consequence of ADT with rapid declines in hip and spine bone mineral density (BMD) in the first year of ADT ranging from 2% to 8% (6, 10, 26, 33) and annual rates of loss over time averaging 4.6% (14). These rates of bone loss surpass the 0.5%-1.0% loss attributable to aging alone and even the 2%-4% associated with menopause in women (23). Low BMD is a significant risk factor for fracture and retrospective studies report significantly elevated relative risks of fracture associated with ADT ranging from 1.21 (95% CI: 1.09-1.34) (31) to 1.37 (95% CI: 1.2-1.57) (29). Fractures carry considerable morbidity in all persons and in PCS fractures are associated with shorter survival times compared with survivors who do not experience a fracture (24).
Exercise may provide a non-pharmacologic strategy for managing bone loss from ADT (7) and has extra-skeletal benefits; however, no empirically-derived exercise guidelines to manage skeletal health in men on ADT exist. The ideal exercise during ADT for prostate cancer may be impact and resistance training because this combination provides an optimal loading strategy to target the clinically relevant skeletal sites of the hip and spine (20, 39). Recently, we reported that a program of resistance + impact exercise increased BMD at the hip and stopped BMD loss at the spine in prematurely menopausal breast cancer survivors (40) and preserved hip and spine BMD in older (>50 years) breast cancer survivors (36,37). There are few trials evaluating the effect of any type of exercise on BMD in healthy adult or older adult men (20) and it is unclear whether or not PCS on ADT can tolerate the types and levels of exercise training shown to have skeletal benefits in women with or without cancer. The purpose of the present study was to determine whether POWIR (Prevent Osteoporosis With Impact + Resistance) could prevent bone loss and reduce bone turnover in PCS on ADT.
METHODS
Design
We conducted a 12-month single-blind randomized controlled trial comparing two parallel groups: 1) progressive, moderate-intensity resistance + impact training (POWIR) and 2) a control program of flexibility training (FLEX). Primary outcomes were measured at baseline, 6 and 12 months. Testing and exercise training took place at Oregon Health & Science University (OHSU).
Participants
Men were recruited through the Oregon State Cancer Registry, clinician referral, support groups, community events, and study advertisements. Interested men were screened for following eligibility criteria: histologic evidence of prostate cancer, currently receiving ADT, not currently receiving chemotherapy, no evidence of bone metastases in the hip or spine, BMD T-score ≥ −2.5, no prescribed bone-active medications other than ADT (e.g., bisphosphonates), no regular participation in ≥30 minutes of moderate-vigorous resistance training ≥2 times per week. Medical clearance was obtained from potential participants’ physicians prior to enrollment. The OHSU Institutional Review Board approved the study and informed consent was obtained from each participant prior to study enrollment.
Power analysis
The PASS 2000 program(13) was used to conduct a power analysis based on a 2 × 3 mixed-design analysis of variance using initial and change values for primary outcome measures from our prior work (36). With an n=25 participants per group, we would have power >.90 to detect a 2% difference in BMD change over 12 months between groups with α<0.05. To protect against 15% attrition (30,36) we aimed to enroll and randomize 30 participants per group.
Study Interventions
Participants in both groups were prescribed an exercise program consisting of two, one-hour supervised classes and one home-based session per week for 12 months. The resistance plus impact intervention (POWIR) for this study was developed in our prior interventions in people without cancer (32,39) and subsequently tested in breast cancer survivors (36, 40). POWIR complied with the American College of Sports Medicine (ACSM) recommendations for preserving bone health in adults by using resistance and/or impact exercise at moderate to high bone loading forces (17,18). Resistance exercise consisted of free weights to apply resistance - dumbbells for upper body, weighted vests (loaded as a percent of body weight) for lower body and a barbell for one combined upper + lower body exercise. Resistance exercises employed in POWIR specifically utilized musculature with attachments directly on the skeletal sites of interest (21,39) and included wall-sits, 90-degree squats, bent-knee deadlifts, lunges, 1-arm row, chest press, lateral raise and push-ups. Impact exercise consisted of two-footed jumps from the ground to a target height 1” from the floor with a bent-knee landing, performed with weighted vests on and in sets of 10 jumps. Training volume and progression of resistance and impact exercises were based on our previous studies (39) (Table 1). Exercises at home were similar as in class sessions but performed without weighted vests and replacing weights with resistance bands.
Participants in the FLEX performed a series of whole body stretching and relaxation exercises in a seated or lying position. Exercises were chosen to minimize weight-bearing forces so that little stimulus to the musculoskeletal system was applied and energy expenditure was minimal.
Procedures
Trained technicians, blinded to group assignment, administered tests at baseline, 6 and 12 months. Randomization was stratified by length of ADT use (>1 year or ≤1 year) and current aerobic activity (≥ vs < 90 min/week) following the completion of baseline testing.
Measures
BMD (g/cm2) of the proximal femur (total hip, greater trochanter, femoral neck) and lumbar spine (L1-L4) was assessed by dual energy x-ray absorptiometry (DXA; Hologic QDR Discovery Wi; software version 12.0). Due to the potential for age-related calcification of soft tissues (e.g., aortic calcification) to introduce artifacts on DXA scans that obscure accurate measurement of spine BMD in older persons, we followed the International Society of Clinical Densitometery guidelines to exclude affected vertebra from analyses across all time points for a particular subject. In two cases a vertebra was excluded from analysis. Repeating analyses after removing these cases did not change statistical outcomes. Our in-house coefficients of variation for DXA measured BMD using a small subsample of older men and women (n=9) are as follows: L1=2.4%, L2=1.6%, L3=2.4%, L4=1.4%, L1-L4= 0.9%, total hip = 1.1%; femoral neck=1.1%; greater trochanter = 1.2%.
Bone turnover was assessed by serum osteocalcin (ng/ml), a byproduct of bone formation and urinary deoxypyrodinoline cross-links (nmol/l), a byproduct of bone degradation. Blood and urine samples were collected in the morning after 12-hour fast and stored at −70°C for analysis. Analyses were conducted by ELISA using commercial kits (Diagnostic Systems Laboratory, Inc.) with inter-assay CV’s of 5.7% and 6.2% and intra-assay CV’s of 8% and 4.8% for deoxypyridinoline and osteocalcin, respectively.
Demographic, clinical history and chronic medical conditions using the Charlson Comorbidity Index (5) were obtained by self-report at baseline. Changes in physical activity and diet over the study were measured with the Community Health Activity Model Program for Seniors (CHAMPS) physical activity questionnaire (34) and the 2005 Block Food Frequency Questionnaire at each testing visit (4).
Statistical Analysis
Data were initially analyzed using an intent-to-treat (ITT) approach via Hierarchical Linear Modeling (HLM; HLM 6.08 software) keeping each participant within his originally assigned group and regardless of missing data. We also evaluated intervention effects only in participants who completed the study using separate 2 (group) x 3 (time) mixed-design analysis of covariance (MD-ANCOVA). Age and time since diagnosis were included as covariates in all analyses because either might influence tolerance or adaptability to training. HLM was repeated to examine potential effect modification by ADT dosing regimen (continuous versus intermittent) or age. Since ours was a preliminary study we did not adjust p values for multiple comparisons in order to reduce the likelihood of false negatives.
RESULTS
Over the two-year recruitment period, 467 men expressed interest in the study, with 51 enrolled in the trial and randomized to POWIR (n=29) or FLEX (n=22) (Fig 1). Due to limited recruitment time we were unable to enroll the target sample to account for planned 15% attrition, leading to unbalanced sample sizes from block randomization. On average, participants were in their mid to upper 70’s, overweight, and had normal skeletal health (T score ≥ −1.0) (Table 2). Roughly a quarter or fewer men had metastatic disease and a low proportion of participants had received chemotherapy. Participants were an average of 6 years past diagnosis and on ADT for just over 2-3 years. Intervention groups were not different at baseline on any demographic characteristic, physical activity levels, energy and calcium intake, or outcome measure. Physical activity levels and energy and calcium intake did not change differently between groups over time. One participant in FLEX began taking a bisphosphonate during the study, thus analyses were repeated removing his data.
Figure 1.
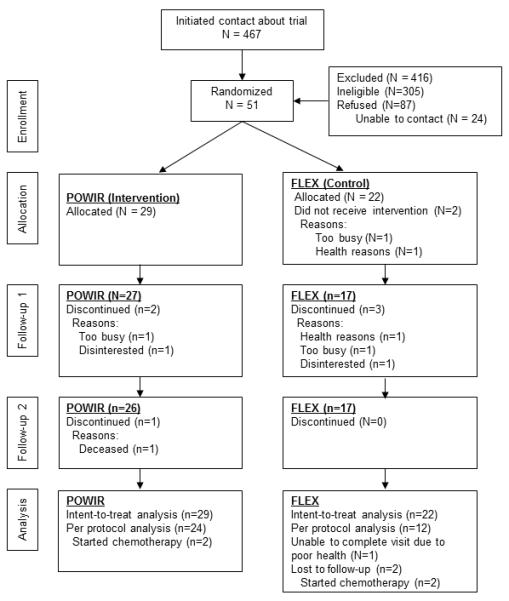
Header: Participant flow throughout the trial
Retention of men in the yearlong study was 84% and slightly better within POWIR (90%) than FLEX (77%). Reasons for withdrawing from the study were similar in nature and in proportion between study groups (Fig 1). Men who withdrew from the program were more likely to have received chemotherapy compared to men who remained in the study (p=0.02). The median attendance for supervised-only and home-only attendance was 84%, and 43%, respectively for POWIR and 74% and 51%, for FLEX. Final intensity for POWIR was just shy of the planned progression, stopping at 13% of body weight and 8-12 RM by month 9. The final prescribed intensity for jumps was achieved by month 9, but jump number was held at 50 because participants tolerated this volume of impact exercise well and prior literature suggested that 50 jumps were sufficient for skeletal adaptation (15). There were no injuries or adverse events associated with participation in either intervention. Two participants in POWIR performed a modified version of the program for >6 months due to knee and shoulder discomfort from higher workloads. Data analyses were repeated excluding these men and results were unchanged.
Using the ITT approach in the full sample, there were no significant differences over time between POWIR and FLEX groups for L1-L4 BMD or for BMD at any hip site. Since ours was the first controlled study to examine whether the skeleton would respond to loading exercise in a testosterone and estrogen deplete state, we also explored differences between POWIR and FLEX on individual lumbar vertebrae. In the full ITT sample, there were significant differences over time between groups at the L4 site (Coefficient on slope of time=0.018, SE=.008, t(46)=2.3, p=0.03), but not at other vertebrae. L4 BMD was preserved in the POWIR group (−0.4%) compared to loss (−3.1%) in controls (Fig 2). There was no effect modification of ADT dosing regimen or age on any BMD or bone turnover measure. Similarly, further adjusting analyses for length of time on ADT did not change statistical outcomes. Among the smaller sample of men completing the study, differences at the L4 site were larger but not significant, likely due to reduced statistical power (Table 3). Changes in serum osteocalcin did not significantly differ between groups. Changes in deoxypyrodinoline were not different between groups in the ITT analysis, but among completers FLEX significantly decreased compared to increases in POWIR (Table 3). When repeating analyses excluding data from the participant who started bisphosphonate treatment group differences disappeared (p=0.10).
Figure 2.
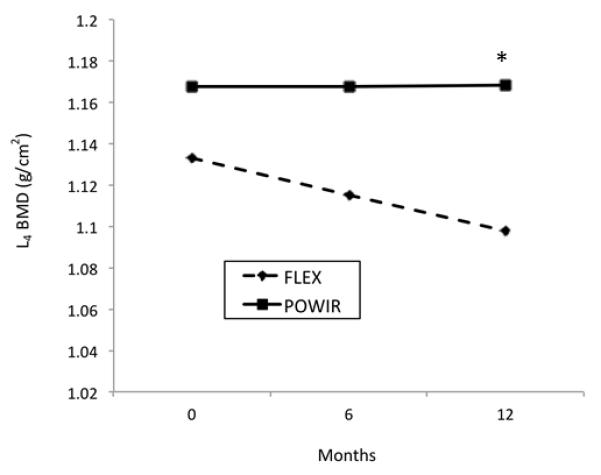
Header: Pattern of changes in L4 spine BMD (g/cm2) in FLEX and POWIR across the 12-month intervention for all participants (N=51) using estimated marginal means for the full sample.
Footer: * significant difference between slopes, p=0.03
DISCUSSION
Among our sample of PCS on ADT, a combined impact + resistance exercise (i.e., POWIR) program showed some evidence for preservation of BMD in the lumbar spine. POWIR appeared unable to prevent declines at the hip compared to a program of low intensity stretching and had no effect on bone turnover. POWIR was a moderate-vigorous intensity training program and was well tolerated by the majority of older men, with only two participants requiring a modification of their training program (e.g., reduced weight and alternate exercises). Supervised POWIR classes were well attended across the year (84%), but home-program attendance was only half as good (43%).
Ours is the first controlled trial to report the effects of exercise on bone health in PCS on ADT. Only one other study has reported an effect of exercise on BMD in men on ADT, a single-group study of a 20-week machine-based resistance training program in men on ADT which did not change whole body or hip BMD. Spine BMD was not reported in that study (9). In fact, few trials of exercise to improve BMD in healthy men have been reported. Out of six published controlled trials of exercise on bone health in men without cancer, only one 12-month trial of resistance + impact loading in older men (mean age 60.7 yrs) (19) reported a significant BMD change at the hip. In that study, the impact loading program consisted of 90-180 jumps from varying heights and directions three times per week, which was a higher dose of impact loading than we prescribed in POWIR. Since ours was the first study to employ impact loading in PCS on ADT, a treatment that frequently causes muscle weakness, frailty and incontinence, we cautiously prescribed the minimum dose of jumps shown to improve hip BMD in adult women (2,3) and slowly progressed intensity with added weight. The majority of men tolerated the jumps well and reached the target of 50 jumps plus 10% body weight. Researchers designing future trials should consider further increasing the number of jumps and cautiously varying direction of loading to deliver a greater stimulus to the hip. The older age of our PCS and their ADT use may have also contributed to our null findings at the hip, similar to our findings in earlier studies of breast cancer survivors, that early estrogen deprivation and older age each independently blunted the effect of POWIR on hip BMD (37,40).
In this study, POWIR showed some evidence of preventing bone loss in the spine, a potentially important finding. Our results were in contrast with those of Kukuljan (19) suggesting that POWIR exercises that loaded the lower back (e.g., deadlift, 1-arm row, push-ups) are key components for improving BMD, which would be consistent with our previous work showing the site specific effect of resistance exercises at the spine (38). POWIR effects on spine BMD were only significant at the L4 vertebra, though changes in BMD for the average of the L1-L4 region trended in the hypothesized directions. L4 is the most load-bearing of the four measured lumbar vertebra used during sitting and standing and thus added loads by performing upper body exercises in a standing position and when rising from a squat position (i.e., chair stands and bent-knee deadlifts) may have reached the threshold for an osteogenic effect. Since a trend was apparent for preventing BMD declines across lumbar vertebra it is possible that higher loads, a longer duration of loading or incorporation of more lateral loads to increase sheer forces at the upper lumbar regions could enhance the effect of POWIR at the spine.
A strength of this study is that it was the first to target loss of BMD in PCS on ADT – a group of cancer survivors at known risk for developing osteoporosis and fractures—(7,35) using an empirically tested osteogenic exercise program known to stop bone loss in women with or without cancer (37,38,39). The study was strengthened in comparison to the few prior studies of BMD in men by including an exercise placebo group rather than usual care to protect against unequal attrition, contamination by increased physical activity in controls, and risks associated with inactivity. Importantly, our program was well accepted and tolerated by these older PCS as evidenced by strong retention and compliance rates for POWIR that were similar to those reported in other controlled trials of supervised resistance exercise that were far shorter in duration than ours (3 months (27) to 6 months (28)).
Limitations of the study include, the modest sample size, low compliance to home-based training, and inclusion of aerobically active men in the sample. We did not exclude men who were aerobically active from participating because impact and resistance exercise load the skeleton in distinctly different ways than aerobic exercise and are superior for osteogenesis (16,21,22). Similar physical activity levels and the lack of change over time suggest little influence of aerobic activity on our findings. However, it is possible that participation in aerobic exercise could contribute to the normal T-scores in our sample, which are atypical of older men, and may have created a ceiling effect similar to that which we have observed in our prior work in women (40). Given the normal BMD T-scores, engagement in aerobic exercise and motivation to participate in a 12 month-supervised intervention, our results may not fully generalize to the broader population of older PCS on ADT who may be frailer, less active, and less motivated. Despite extending our recruitment period beyond the originally planned 9 months, we were unable to recruit our planned sample size. The sample size in our study was similar to or greater than that for other center-based exercise trials in men on ADT (11,12), but had insufficient representation of ADT types and doses to fully explore any potential moderating influence of treatment on skeletal adaptations. The lower sample size likely contributed to our failure to detect significant differences between groups for L1-L4 or hip BMD despite observable trends (Fig 2). However, the p-value for the ITT analysis of L4 BMD was not marginal, lowering the risk of a Type I error. Home-based exercise day to increase the weekly dose of exercise without increasing the participant burden in the study, but attendance to home-training was low. Possibly the men preferred exercising with a group rather than alone, which could be further explored in a future trial.
The results of our trial suggest that resistance + impact training, a combined training mode known for its osteogenic capacity, is well-tolerated and acceptable among PCS on ADT and shows preliminary efficacy for reducing bone loss at the spine. Our exercise program failed to affect the hip, probably due to an inadequate volume of loading, thus greater attention to how to best effectively load this skeletal site of high clinical relevance is critical for future trials. Participants attended sessions for 12 months without adverse events and the potential benefits of exercise on BMD and other health outcomes may be identified with more intense exercises in larger samples. A 1%-2% increase in BMD translates to a 7%-14% decrease in fracture risk (16), thus further refining the POWIR program to maximally load both clinically relevant skeletal sites could have future implications for fracture prevention. Given the paucity of controlled trials examining the skeletal benefits of exercise for PCS, who are likely to be at risk of fracture, our trial provides a starting basis for future trials.
Supplementary Material
Acknowledgements
This study was funded by a grant from the Lance Armstrong Foundation Grant to Dr. Winters-Stone. We thank the Oregon State Cancer Registry for their recruitment assistance; Thera-band, Inc. for providing resistance bands for home exercise programs; and our research assistants, exercise trainers and participants.
The results of the present study do not constitute endorsement by ACSM
Funding source: Lance Armstrong Foundation ClinicalTrials.gov NCT0060686
Footnotes
Conflict of interest The authors declare that they have no competing financial interests in relation to the work described in this manuscript.
The authors have no disclosures to report
REFERENCES
- 1.American Cancer Society Web Site . Cancer Facts & Figures. American Cancer Society; Atlanta: [Cited 2013 Oct 1]. 2013. Available from: http://www.cancer.org/research/cancerfactsfigures/cancerfactsfigures/cancer-facts-figures-2013. [Google Scholar]
- 2.Bassey E, Ramsdale S. Increase in femoral bone mineral density in young women following high impact exercise. Osteoporos Int. 1994;4:72–5. doi: 10.1007/BF01623226. [DOI] [PubMed] [Google Scholar]
- 3.Bassey E, Rothwell M, Littlewood J, Pye D. Pre- and postmenopausal women have different bone mineral density responses to the same high-impact exercise. J Bone Miner Res. 1998;13(12):1805–13. doi: 10.1359/jbmr.1998.13.12.1805. [DOI] [PubMed] [Google Scholar]
- 4.Binkley N, Bilezikian JP, Kendler DL, Leib ES, Lewiecki EM, Petak SM. Summary of the International Society For Clinical Densitometry 2005 Position Development Conference. J Bone Miner Res. 2007;22(5):643–5. doi: 10.1359/jbmr.070204. [DOI] [PubMed] [Google Scholar]
- 5.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 6.Diamond TH, Higano CS, Smith MR, Guise TA, Singer FR. Osteoporosis in men with prostate carcinoma receiving androgen-deprivation therapy: recommendations for diagnosis and therapies. Cancer. 2004;100(5):892–9. doi: 10.1002/cncr.20056. [DOI] [PubMed] [Google Scholar]
- 7.Eastham JA. Bone health in men receiving androgen deprivation therapy for prostate cancer. J Urol. 2007;177(1):17–24. doi: 10.1016/j.juro.2006.08.089. [DOI] [PubMed] [Google Scholar]
- 8.Freedland SJ, Moul JW. Prostate specific antigen recurrence after definitive therapy. J Urol. 2007;177(6):1985–91. doi: 10.1016/j.juro.2007.01.137. [DOI] [PubMed] [Google Scholar]
- 9.Galvao DA, Nosaka K, Taaffe DR, Spry N, Kristjanson LJ, McGuigan MR. Resistance training and reduction of treatment side effects in prostate cancer patients. Med Sci Sports Exerc. 2006;38(12):2045–52. doi: 10.1249/01.mss.0000233803.48691.8b. [DOI] [PubMed] [Google Scholar]
- 10.Galvao DA, Spry NA, Taaffe DR, Newton RU, Stanley J, Shannon T, et al. Changes in muscle, fat and bone mass after 36 weeks of maximal androgen blockade for prostate cancer. Br J Urol Int. 2008;102(1):44–7. doi: 10.1111/j.1464-410X.2008.07539.x. [DOI] [PubMed] [Google Scholar]
- 11.Galvao DA, Taaffe DR, Spry N, Joseph D, Newton RU. Combined resistance and aerobic exercise program reverses muscle loss in men undergoing androgen suppression therapy for prostate cancer without bone metastases: A randomized controlled trial. J Clin Oncol. 2010;28(2):340–7. doi: 10.1200/JCO.2009.23.2488. [DOI] [PubMed] [Google Scholar]
- 12.Galvao DA, Taaffe DR, Spry N, Newton RU. Exercise can prevent and even reverse adverse effects of androgen suppression treatment in men with prostate cancer. Prostate Cancer Prost Dis. 2007;10(4):340–6. doi: 10.1038/sj.pcan.4500975. [DOI] [PubMed] [Google Scholar]
- 13.Hintze J. PASS 6.0 Power Analysis and Sample Size for Windows. NCSS; Keysville, Utah: 1996. [Google Scholar]
- 14.Israeli RS, Ryan CW, Jung LL. Managing bone loss in men With locally advanced prostate cancer receiving androgen deprivation therapy. J Urol. 2008;179(2):414–23. doi: 10.1016/j.juro.2007.09.028. [DOI] [PubMed] [Google Scholar]
- 15.Kato T, T T, Yamashita T, Hatanaka Y, Honda A, Umemura Y. Effect of low-repetition jump training on bone mineral density in young women. J Appl Physiol. 2006;100(3):839–43. doi: 10.1152/japplphysiol.00666.2005. [DOI] [PubMed] [Google Scholar]
- 16.Kelley G, Kelley K, Kohrt W. Effects of ground and joint reaction force exercise on lumbar spine and femoral neck bone mineral density in postmenopausal women: a meta-analysis of randomized controlled trials. BMC Musculosk Dis. 2012;13(1):177. doi: 10.1186/1471-2474-13-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kohrt WM, Bloomfield SA, Little KD, Nelson ME, Yingling VR. American College of Sports Medicine Position Stand: physical activity and bone health. Med Sci Sports Exerc. 2004;36(11):1985–96. doi: 10.1249/01.mss.0000142662.21767.58. [DOI] [PubMed] [Google Scholar]
- 18.Kraemer WJ, Adams K, Cafarelli E, Dudley GA, Dooly C, Feigenbaum MS, et al. American College of Sports Medicine position stand. Progression models in resistance training for healthy adults. Med Sci Sports Exerc. 2002;34(2):364–80. doi: 10.1097/00005768-200202000-00027. [DOI] [PubMed] [Google Scholar]
- 19.Kukuljan S, Nowson CA, Bass SL, Sanders K, Nicholson GC, Seibel MJ, et al. Effects of a multi-component exercise program and calcium–vitamin-D3-fortified milk on bone mineral density in older men: a randomised controlled trial. Osteoporos Int. 2009;20(7):1241–51. doi: 10.1007/s00198-008-0776-y. [DOI] [PubMed] [Google Scholar]
- 20.Marques EA, Mota J, Carvalho J. Exercise effects on bone mineral density in older adults: a meta-analysis of randomized controlled trials. Age. 2012;34(6):1493–515. doi: 10.1007/s11357-011-9311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martyn-St James M, Carroll S. A meta-analysis of impact exercise on postmenopausal bone loss: the case for mixed loading exercise programmes. Br J Sports Med. 2009;43(12):898–908. doi: 10.1136/bjsm.2008.052704. [DOI] [PubMed] [Google Scholar]
- 22.Martyn-St James M, Carroll S. Meta-analysis of walking for preservation of bone mineral density in postmenopausal women. Bone. 2008;43(3):521–31. doi: 10.1016/j.bone.2008.05.012. [DOI] [PubMed] [Google Scholar]
- 23.North American Menopause Society Management of osteoporosis in postmenopausal women: 2010 position statement of The North American Menopause Society. Menopause. 2010;17(1):25–54. doi: 10.1097/gme.0b013e3181c617e6. [DOI] [PubMed] [Google Scholar]
- 24.Oefelein MGRV, Conrad W, Resnick MI. Skeletal fractures negatively correlate with overall survival in men with prostate cancer. J Urol. 2002;168(3):1005–7. doi: 10.1016/S0022-5347(05)64561-2. [DOI] [PubMed] [Google Scholar]
- 25.Raudenbush SW, Bryk AS, Congdon R. Hierarchical linear and nonlinear modeling. 2nd Ed SSI; Lincolnwood, IL: 2004. Conceptual and statistical background for hierarchical generalized linear modeline; pp. 102–3. [Google Scholar]
- 26.Ryan CW, Huo D, Demers LM, Beer TM, Lacerna LV. Zoledronic acid initiated during the first year of androgen deprivation therapy increases bone mineral density in patients with prostate cancer. J Urol. 2006;176(3):972–8. doi: 10.1016/j.juro.2006.04.078. [DOI] [PubMed] [Google Scholar]
- 27.Segal RJ, Reid RD, Courneya KS, Malone SC, Parliament MB, Scott CG, et al. Resistance exercise in men receiving androgen deprivation therapy for prostate cancer. J Clin Oncol. 2003;21(9):1653–9. doi: 10.1200/JCO.2003.09.534. [DOI] [PubMed] [Google Scholar]
- 28.Segal RJ, Reid RD, Courneya KS, Sigal RJ, Kenny GP, Prud’Homme DG, et al. Randomized controlled trial of resistance or aerobic exercise in men receiving radiation therapy for prostate cancer. J Clin Oncol. 2009;27(3):344–51. doi: 10.1200/JCO.2007.15.4963. [DOI] [PubMed] [Google Scholar]
- 29.Shahinian VB, Kuo Y-F, Freeman JL, Goodwin JS. Risk of fracture after androgen deprivation for prostate cancer. New Engl J Med. 2005;352(2):154–64. doi: 10.1056/NEJMoa041943. [DOI] [PubMed] [Google Scholar]
- 30.Shaw JM, Snow CM. Weighted vest exercise improves indices of fall risk in older women. J Gerontol. 1998;53:M53–8. doi: 10.1093/gerona/53a.1.m53. [DOI] [PubMed] [Google Scholar]
- 31.Smith MR, Lee WC, Brandman J, Wang Q, Botteman M, Pashos CL. Gonadotropin-releasing hormone agonists and fracture risk: A claims-based cohort study of men with nonmetastatic prostate cancer. J Clin Oncol. 2005;23(31):7897–903. doi: 10.1200/JCO.2004.00.6908. [DOI] [PubMed] [Google Scholar]
- 32.Snow CM, Shaw JM, Winters KM, Witzke KA. Long-term exercise using weighted vests prevents hip bone loss in postmenopausal women. J Gerontol A Biol Sci Med Sci. 2000;55(9):M489–91. doi: 10.1093/gerona/55.9.m489. [DOI] [PubMed] [Google Scholar]
- 33.Spry NA, Galvão DA, Davies R, La Bianca S, Joseph D, Davidson A, et al. Long-term effects of intermittent androgen suppression on testosterone recovery and bone mineral density: results of a 33-month observational study. Br J Urol Int. 2009;104(6):806–12. doi: 10.1111/j.1464-410X.2009.08458.x. [DOI] [PubMed] [Google Scholar]
- 34.Stewart A, Mills K, King A, Haskell W, Gillis D, Ritter P. CHAMPS physical activity questionnaire for older adults: outcomes for interventions. Med Sci Sports Exerc. 2001;33(7):1126–41. doi: 10.1097/00005768-200107000-00010. [DOI] [PubMed] [Google Scholar]
- 35.VanderWalde A, Hurria A. Aging and osteoporosis in breast and prostate cancer. Cancer. 2011;61(3):139–56. doi: 10.3322/caac.20103. [DOI] [PubMed] [Google Scholar]
- 36.Winters-Stone K, Dobek J, Nail L, Bennett JA, Naik A, Schwartz A. Strength training stops bone loss and builds muscle in postmenopausal breast cancer survivors: a randomized controlled trial. Breast Cancer Res Treat. 2011;27(2):447–56. doi: 10.1007/s10549-011-1444-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Winters-Stone K, Leo M, Schwartz A. Exercise effects on hip bone mineral density in older, postmenopausal breast cancer survivors are age dependent. Arch Osteoporos. 2012;7(1-2):301–6. doi: 10.1007/s11657-012-0071-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Winters-Stone K, Snow C. Site-specific response of bone to exercise in premenopausal women. Bone. 2006;39(6):1203–9. doi: 10.1016/j.bone.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 39.Winters-Stone KM, Dobek J, Nail LM, Bennett JA, Leo MC, Torgrimson-Ojerio B, et al. Impact + resistance training improves bone health and body composition in prematurely menopausal breast cancer survivors: a randomized controlled trial. Osteoporos Int. 2013;24(5):1637–46. doi: 10.1007/s00198-012-2143-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Winters KM, Snow CM. Initial values predict musculoskeletal response to exercise in premenopausal women. Med Sci Sports Exerc. 2003;35(10):1691–1696. doi: 10.1249/01.MSS.0000089338.66054.A5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


