Abstract
The prohormone Chromogranin A (CHGA) is ubiquitously found in vesicles of adrenal chromaffin cells and adrenergic neurons and it is processed to the hypotensive hormone peptide catestatin (CST). Both CHGA and CST regulate blood pressure and cardiac function. This study addresses their role in cardiac electrical activity. We have generated two genomically “humanized” transgenic mouse strains (HumCHGA31 and HumCHGA19) with varied CHGA expression and ability to rescue the Chga−/− phenotype (hypertensive, hyperadrenergic with dilated cardiomyopathy). The normotensive HumCHGA31 mice express CHGA at levels comparable to wild-type. In contrast, the hypertensive HumCHGA19 mice have low levels of CHGA. EKG recordings revealed that the QT interval, R-amplitude and QRS time-voltage integral are markedly longer in HumCHGA19 compared to wild-type and HumCHGA31 mice. These differences are accompanied by increased heart rate and QT variability, indicating that ventricular assault happens in a status of low levels of circulating CST.
Keywords: chromogranin A, catestain, genomically “humanized”CHGA mice, QT variability, cardiomyopathy index, ventricular power-frequency spectra
Introduction
The prohormone chromogranin A (CHGA) is a member of the granin family of proteins and is sequestered in granules of the adrenal medulla and postganglionic sympathetic axons along with catecholamines and calcium [1-3]. It plays a crucial role in biogenesis of the secretory granules itself, interacts with several proteins in the regulated secretory pathway and recruits cytosolic proteins to the membrane of granules [4,5]. The pro-protein CHGA is processed to generate several biologically active peptide fragments including the neuropeptide catestatin (CST). CST exerts sympatho-inhibitory effects by weakening nicotinic cholinergic pathway, thereby controlling blood pressure (BP) and heart rate (HR) [6]. CHGA has been reported to have significant dose-dependent myocardial contractile (inotropic), relaxing (lusitropic) and coronary (vasomotive) effects on the rat heart performance [7]. Administering CST in reperfusion reduces post-ischemic myocardial damages and dysfunction, suggesting a cardioprotective function for CST [8]. In humans the CHGA expression is heritable and elevated in essential hypertension [9,10]. Plasma concentration of CST is diminished both in patients with hypertension and their normotensive offspring at a genetic risk of developing the disease [11-13]. Heritability of CST has also been established in a genome-wide twin study with the hypertensive subjects displaying elevated CHGA coupled with diminished CST [14]. Thus CST levels in plasma may be an important “intermediate” phenotype in analysis of genetic risk for human hypertension. The Chga null mice lack CST and as a result have elevated circulating catecholamines and are hypertensive, with decreased baroreflex sensitivity [15]. Echocardiography measurements show cardiomyopathy and ventricular hypertrophy [16]. In sedated Chga−/− mice, the heart rate variability (HRV), an index of autonomic function is highly compromised and partially restored by CST replacement [17]. Meng et al. have observed elevated plasma CST post-acute myocardial infarction, leading to inhibition of catecholamine release and association with progressive ventricular remodeling [18].
Elevated circulating catecholamines elicit changes in ventricular electrical conduction time, as exemplified in diseases like idiopathic mitral valve prolapse [19]. A prolonged QT-interval observed in the electrocardiogram (EKG) profile signifies a delay in the ventricular repolarization phase, which renders the heart vulnerable to ischemic damage (IHD) or malignant arrhythmias such as torsade de pointes or ventricular tachycardia. QT interval prolongation has also been associated with lowered ventricular fibrillation threshold and occurrence of sudden cardiac death [20]. Ventricular instability in humans is often a feature of dilated (DCM) and hypertrophic cardiomyopathy.
We have previously described two genomically “humanized” CHGA mouse models, expressing sufficient (HumCHGA31, normotensive) vs. insufficient (HumCHGA19, untreated hypertensive) levels of human CHGA, that mimic the variability in human chromogranin A gene (CHGA) expression observed in the human population [21]. These transgenic models stably express the human CHGA gene in the mouse Chga knockout (Chga−/−) background. The variation in CHGA expression in these mice results in variable release of adreno-medullary catecholamines. The HumCHGA31 have circulating human CHGA and catecholamine levels comparable to wild-type (WT) mice. In contrast, the HumCHGA19 have 14-fold lower circulating human CHGA accompanied by elevated plasma catecholamine. Thus, the HumCHGA19 mice have elevated SBP and DBP [21].
We hypothesized that due to low levels of CHGA, the HumCHGA19 mice would have attenuated levels of the hypotensive CST peptide resulting in enhanced ventricular vulnerability. Our assumption was that both the ‘humanized’ CHGA mouse models with differential CHGA expression and ability to ‘rescue’ the hypertensive, hyper-adrenergic Chga null phenotype would reveal the involvement of CHGA in cardiac electrical activity. We measured the human CST levels in plasma of these mice and performed EKG to determine whether circulating CHGA and CST levels correlate with ventricular depolarization especially Bazett-corrected repolarization time (QTb) and atrio-ventricular conduction time (PQ) along with QTb/PQ changes (cardiomyopathy index as defined by Steare) [22]. The main finding of this study is that CHGA and therefore CST levels influence BP and cardiac electrical activity. Compared to HumCHGA31, the HumCHGA19 mice have low levels of plasma CHGA and CST, a scenario comparable to the hypertensive humans. HumCHGA19 mice display prolonged QTb, compressed RR cycle length variability, and increased QRSd coupled with decreased PQ resulting in compromised cardiac function, compared to HumCHGA31 with normal CST levels.
Materials and Methods
“Humanized” transgenic mouse models
The “humanized” mice were generated by bacterial artificial chromosome transgenesis as detailed earlier [21,16]. Both transgenic strains have the complete ~12 kb human CHGA transgene and the flanking native human chromosome 14 sequence. The founder Tg19CHGA+/−; Chga+/+ had ~ 42 kb upstream and ~19 kb downstream flanking sequence, whereas Tg31CHGA+/−; Chga+/+ had ~44 kb upstream and 155 kb downstream sequence. These mice were crossed with Chga−/− mice and subjected to brother-sister mating for ~ 10 generations to generate the transgenic lines lacking the mouse Chga alleles and having diploid copies of the transgene Tg19CHGA or Tg31CHGA. Thus, the WT mice express mouse Chga and both “humanized” strains express exclusively the human CHGA transgene. All three strains of mice have the identical mixed-background (50% C57BL/6: 50% 129/SvJ).
Animal husbandry
Mice were kept under pathogen-free conditions, maintained on a normal murine chow-diet, and allowed to have water ad libitum. Experiments were carried out in accordance with the Institutional Animal Care and Use for scientific purposes and with the guidelines adopted by the National Institutes of Health. All possible steps were taken to avoid animals’ suffering during the experiment. The three groups of mice (age: 28.4-29.1 weeks) of either sex were used in the study.
Measurement of blood pressure, plasma catestatin and heart weight: body weight ratio
The previously described noninvasive tail-cuff method was used to measure BP of all 3 strains of mice for 5 days [21]. SBP and DBP data were subjected to statistical one-way ANOVA with multiple-comparison post-hoc tests using SPSS Statistics software from IBM. The BP was also measured in HumCHGA31and WT mice using telemetric - DSI transmitters. Telemetry signals relayed the data to a signal processor (DataQuest A.R.T. Gold, version 2.3; Data Sciences International) connected to a desktop personal computer (Hewlett-Packard, Portland, OR). After the implantation surgery, the animals were rested for 10 days for normalization of the diurnal pattern of BP, before recording BP in these conscious mice. The data obtained from both the procedures were compared by student t-test.
Plasma samples were collected from “humanized” mice euthanized by deep anesthesia in isoflurane and stored at −70°C until CST was assayed using the CST (human specific) EIA kit from Phoenix Pharmaceutical Inc. (California, U.S.A.) according to the manufacturer's instructions.
To evaluate cardiac hypertrophy morphometrically, hearts were excised from the euthanized mice, weighed and used to determine the ratio of heart weight (mg): body weight (g) [23,24].
Electrophysiology
EKG recordings were carried out in conscious mice at an ambient temperature of 20-22°C between the hours of 10:00–15:00 to minimize circadian influences. EKG complexes were sampled from recordings with clearly defined onset and termination signals. Surface EKG was performed using stainless steel micro needles (Grass instruments, Quincy, MA) strapped to the limbs of the mouse. The mouse was transferred to a conventional laboratory murine cage in which it could move freely. Experiments were carried out in a noise-free environment with ambient light. All moving artifacts in the EKG signals were filtered out during analysis. Lead II EKG signals were sampled at a rate of 2 kHz and digitized with a 16-bit analog-to-digital converter (Powerlab 8-30, AD instruments). Signal data were stored directly onto a hard disk for post-processing. The mice were allowed to become accustomed to the recording environment for ~10 min prior to storing of the EKG data for analysis. Only data from continuous recording of 20-30 EKG signals was used for analysis. The RR intervals in the EKG were extracted using a detection algorithm based on thresholding. All extreme (<70 ms and >300 ms) RR intervals were excluded from analysis. Atrial and ventricular wave-complexes were analyzed with a scanning speed of 500 mm/s. EKG data were analyzed from quasi-stationary conditions of RR and QT time-series, necessary for variability analysis from a standpoint of signal-processing. The QRS duration was measured from the onset of the Q wave to the peak negative deflection of the S wave (this is in contrast to return of S-wave to the isoelectric baseline in human designated as QRS duration). QT interval durations and other wave complexes were determined from signal averaged of considerable number of lead II EKG signal (SAEKG) or manually evaluated. EKG waves are defined as the positions where the signal derivative changes its sign. Each QT interval was measured from the beginning of the QRS complex up to the end point of the T-wave, defined as the intersection of the steepest slope of the descending T wave and the isoelectric line. Original QT values were corrected (QTb) using the Bazett formula [25]. In humans, prolonged corrected QT is defined as a QTb interval >440 ms [20]. Typically, the EKG parameters in mice are 7-8 fold different compared to humans. For example, the heart rate in humans is 70-80 beats/min and in mice: 550 beats/min, the QRS duration in humans is 0.12-0.2 sec and in mice 10-15 ms [26,27]. Therefore, we considered a QTb value >65 ms as prolonged QTb in mice. Influence of HR on QTb was analyzed statistically. QRS wave data were subjected to power-frequency analysis following sampling at FFT256 with 50% overlap and a Welch-correction filter. Variability in QT intervals is recognized as an assessment of temporal myocardial repolarization lability. Therefore, in all strains of mice we evaluated beat-to-beat ventricular repolarization variability quantified by the QT variability index (QTVI), according to Berger et al. [28].
Statistical Analysis
Data were evaluated in Instat software (Graphpad, San Diego, CA) and subjected to one-way ANOVA analysis, following normality and Kolmogorov-Smirnov tests for Gaussian data distribution. These data were reported as mean differences among the test groups and p-values generated after application of Mann–Whitney U-test, Kruskal–Wallis test or Welch-corrected test. Relationships between variables were evaluated with Spearman or Pearson rank correlation tests. Post hoc multiple-comparison procedure of Tukey-Krammer honestly significant difference (HSD) test was used to analyze the difference between strain means, if applicable. When testing the effects of CHGA on multiple correlated traits, we estimated the false discovery rate (FDR), to minimize false negative results while maximizing true positive results, using the Excel calculator of FDR from a distribution of pvalues,at http://www.rowett.ac.uk/~gwh/fdr.html.
Results
Blood pressure and circulating CST in “humanized” CHGA mice
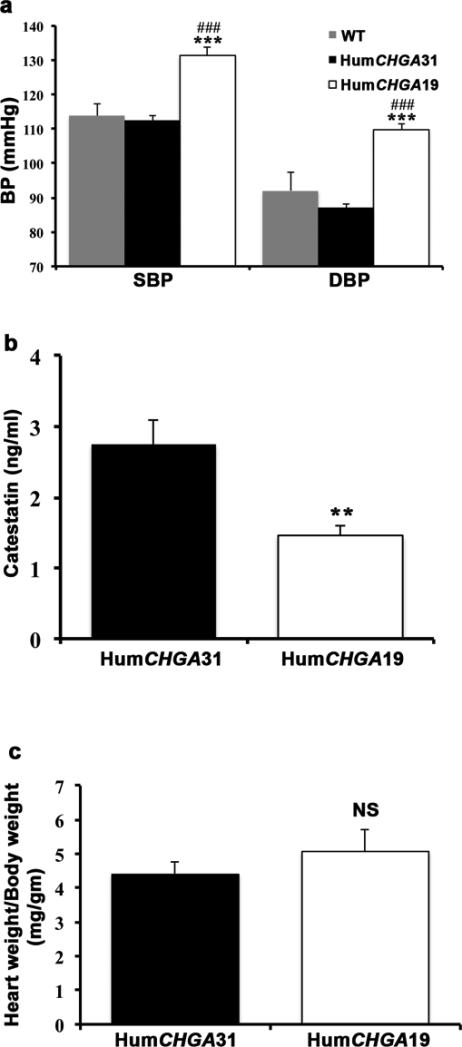
The HumCHGA19 mice used in this study displayed higher SBP (~17-19 mmHg) and DBP (~17-22 mmHg) compared to HumCHGA31and WT mice (Fig. 1a), consistent with our previously published results [21]. The BP was measured by tail-cuff method in mice, prior to EKG recordings. Comparison using statistical student t-test of BP data obtained by both indirect tail-cuff and direct telemetric measurements for WT (SBP: p=0.984; DBP: p=0.163) and HumCHGA31 (SBP: p=0.119; DBP p=0.278) mice confirmed that results from both methods were similar. Hypertensive patients’ display attenuated levels of CST [11-13]. Therefore a competitive enzyme immunoassay was employed to detect the circulating levels of human CST and/or related peptides in both the strains of “humanized”CHGA mice. The hypertensive HumCHGA19 mice had diminished CST (0.5 fold) compared to HumCHGA31mice (Fig. 1b). The heart weight (mg)/body weight (g) ratio of the HumCHGA19 mice was not significantly (p= 0.078) higher from that of HumCHGA31 mice, suggesting that cardiac hypertrophy is not an overriding attribute in differences between the strains (Fig. 1c; supplementary Fig. 1). Therefore, to assess the implications of CHGA and CST expression levels in cardiac electrical activity, EKG was performed on these mice.
Fig. 1. The BP of genomically “humanized” CHGA mice correlated with circulating CST levels.
The “humanized” transgenic mice express the human CHGA gene and their BP was measured along with that of the WT mice. In these “humanized” CHGA mice, circulating levels of the human CST was also measured. (a) Elevated BP in HumCHGA19 mice: The SBP was significantly different between groups of mice as determined by one-way ANOVA with multiple-comparison post-hoc tests (p< 0.0001). Specifically, HumCHGA19 had higher SBP compared to WT (### p= 0.0004) and HumCHGA31 (*** p= 0.00005). DBP also varied significantly in the HumCHGA19 mice compared to HumCHGA31 and WT mice (p< 0.0001). In HumCHGA19 mice the DBP was elevated compared to both WT and HumCHGA31 (###, *** p= 0.00001). (b) The plasma concentration of the hypotensive CST peptide is inversely correlated with BP: Circulating CST was measured in the mouse plasma using a competitive ELISA that quantitates human CST and thus expression of the transgene in the “humanized CHGA mouse models. The HumCHGA31 mice exhibit normal BP, had almost twice as much CST as the hypertensive HumCHGA19 mice (** p< 0.007). (c) Heart weight (mg): body weight (g) ratio is not significantly different between both the transgenic strains
Atrio-ventricular conduction (PQ interval)
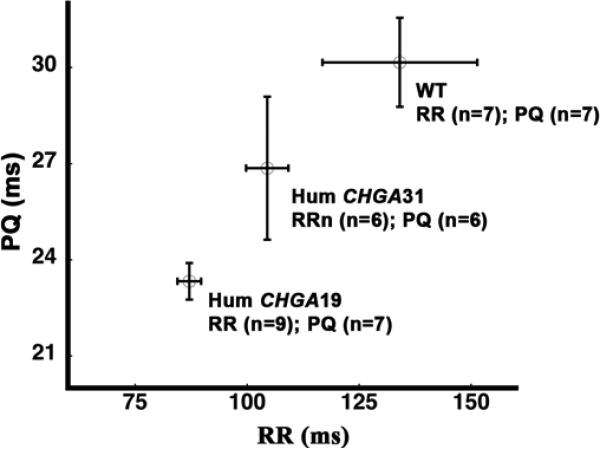
The atrio-ventricular conduction time (PQ) is an estimate of AV node function that measures the duration for the electrical impulse to travel from the SA node through the AV node to the ventricles. At rest, PQ generally decreased with decreased sinus cycle length (RR). However at a given RR interval, inter-subject variability was observed in the PQ interval in all 3 groups (Fig. 2). Inter-subject variability is a function of the subject's pre-existent vagal and sympathetic tone. PQ conduction times and their association with sinus cycle length data are presented in Table 1. The PQ values in HumCHGA19 were lower compared to WT and HumCHGA31 mice. The sinus cycle lengths were not significantly different between HumCHGA31 and WT. However, these differences were significant between HumCHGA19 vs. WT and between HumCHGA19 vs. HumCHGA31. The cardiac parameters measured in this study (Table 1) agree with those observed in WT mice by Xing et al.[29].
Fig. 2. Dependence of atrio-ventricular conduction time (PQ) on sinus cycle length (RR).
The EKG profiles of the WT and ‘humanized’ transgenic HumCHGA19 and HumCHGA31 mice revealed dependence of PQ conduction time on RR. Statistical analysis did not reveal any significant differences (p> 0.05) in the PQ/RR relationship among the three groups. The number of mice used in the analysis is indicated by “n”
Table 1.
Atrio-ventricular conduction time (PQ) and its dependence on sinus cycle length (RR)
| WT | HumCHGA31 | HumCHGA19 | ANOVA (F and p values) | |
|---|---|---|---|---|
| n | 7 | 5 | 7 | -- |
| PQ (ms) | 30.16 ± 1.39 | 26.86 ± 2.23 | 23.33 ± 0.57 | F= 39.08 p< 0.001 (FDR p<0.001) |
| PQCI (ms) | 27.0 - 33.31 | 21.33 - 32.41 | 22.04 - 24.63 | -- |
| n | 7 | 6 | 9 | -- |
| RR (ms) | 134.09 ± 17.28 | 104.50 ± 4.73 | 87.09 ± 2.64 | F= 42.39 p<0.001 (FDR p<0.001) |
| RRCI (ms) | 94.93 - 173.24 | 93.40 - 115.60 | 81.35 - 92.83 | -- |
The atrio-ventricular conduction values (PQ) in HumCHGA19 mice were significantly different from WT (p± 0.001) and HumCHGA31 (p± 0.01) mice. Sinus cycle lengths (RR) did not significantly differ between HumCHGA31 and WT (p± 0.05). However the RR values following a Welch-correction factor (PQCI and RRCI), varied significantly between HumCHGA19 vs. WT (p± 0.02 ± 0.01) and between HumCHGA 9 vs. HumCHGA31 (p± 0.005). The PQ and RR values are represented as mean values ± standard deviation. The range in PQCI and RRCI values are shown. The number of experimental animals is represented in row ‘n’. Shown in the last column are the F and p values of the data analyzed by one-way analysis of variance (ANOVA), followed by the Bonferroni post hoc test when the p value was < 0.05, to measure the difference between groups. FDR: False Discovery Rate.
The percentage of the atrio-ventricular conduction time to the sinus cycle length (PQ/RR) was found to be around 25% in the awake WT mice and was totally unaffected by transgenesis (both HumCHGA19 and HumCHGA31). A slope of PQ to RR: tan θ ≈ 45° in Fig. 2, suggests that mice in each of the studied group make an effort to optimize energy efficiency via adequate systolic and diastolic volume with increased or decreased sinus rhythm. Therefore, there are no apparent physiological defects in the atrio-ventricular conduction among the tested mice. An inverse relationship of PQ conduction to RR in human has been described earlier [30] and seems to hold true for mice investigated in the present study. PQ conduction time data for WT mice in this study resemble the earlier report of mice intracardiac electrogram [31].
Non-parametric Kruskal-Wallis with Dunn's multiple comparison test, revealed a significant difference in heart rates (HR=[1/RR]) amongst WT and HumCHGA31vs. HumCHGA19 (Table 1). Analysis of our preliminary (unpublished) experimental data also revealed that all time domain parameters of HRV, e.g. SDNN, RMSSD and NN6 values were significantly lower in HumCHGA19 than that of WT and HumCHGA31.
Ventricular depolarization time (QRS duration)
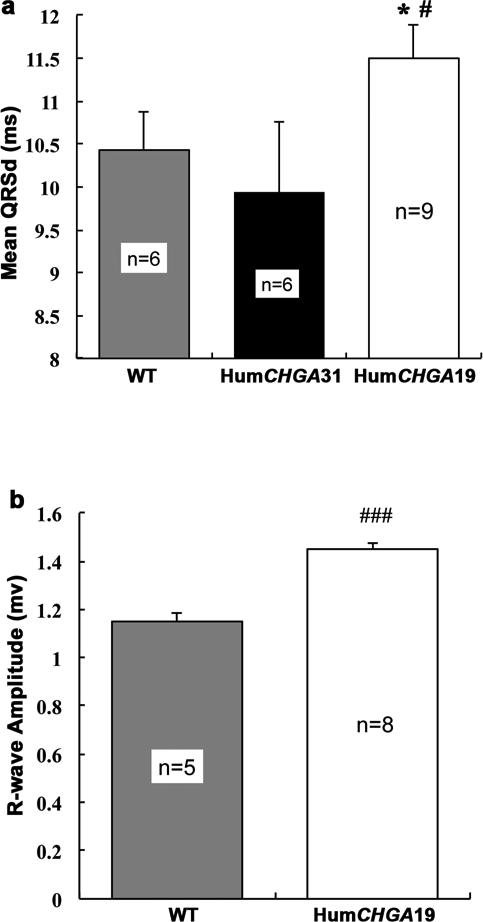
Measurements of ventricular depolarization time and R-wave amplitude are shown in Figs. 3a and 3b, respectively. QRS duration (QRSd) of ventricular complex was larger in the HumCHGA19 group and differed significantly from the QRSd of WT and the HumCHGA31 mice. In summary, the values of QRSd are HumCHGA19 > WT > HumCHGA31. In HumCHGA19 along with an increase in QRSd, R-wave amplitude in QRS complex also increased (Fig. 3b). However, no correlation was observed between QRSd and heart weight/body weight ratios for both strains of mice (HumCHGA31 r= -0.19, two-tailed p= 0.76; and the HumCHGA19 r= -0.22, p= 0.72).
Fig. 3. EKG characteristics of the QRS wave duration and amplitude.
The hypertensive HumCHGA19 mice displayed prolonged QRSd and increase in R-wave voltage. (a) QRS-wave duration in Lead II EKG: The HumCHGA19 mice show significantly longer ventricular depolarization duration (QRSd) compared to WT and HumCHGA31mice (#, * p< 0.05). The QRSd in the HumCHGA19 group (CI: 10.62 – 12.38 ms) differed significantly from the WT mice QRSd (CI: 9.37 - 11.49 ms) and from the HumCHGA31 (CI: 8.03 - 11.84 ms). The QRSd did not differ significantly between HumCHGA31 and WT groups (p= 0.28). No correlation was observed between heart weight (mg): body weight (g) ratio and QRSd for either transgenic strains. (b) R-wave amplitude of QRS-wave in Lead II EKG: The HumCHGA19 mice had a marked increase in mean R-wave amplitude (1.45 mv) compared with WT mice (1.15 mV) (### p< 0.001)
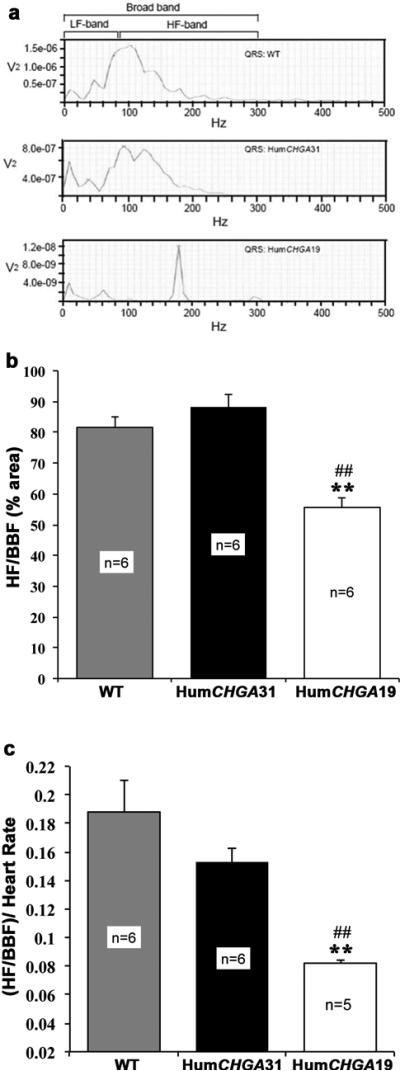
QRS-wave data were also segmented into low (LF) and high frequency (HF) spectral band for possible differentiation of the ventricular power structure among the studied groups. Frequency range was selected on the basis of earlier experiments in mice [32]. However, we observed massive power loss occurred in all experimental groups and reached almost null power at frequencies >300 Hz. Therefore, we chose 0-80 Hz as LF band, 80-300 Hz as HF band and 0-300Hz in which 98% power resides, as broadband frequency (BBF) spectral band. A representation of QRS-wave power-frequency profile is presented in Fig. 4a. A conspicuous finding was that in the HumCHGA19 group, HF peak power of QRS-wave spectrum narrowly centered around 150-200 Hz, whereas, HF peak power in the HumCHGA31 and WT mice showed a distinct leftward shift toward LF (<150 Hz). However, in the HumCHGA31 and WT mice the frequency span, over which peak power in the HF range occurred, was broader and not as narrow as observed in HumCHGA19 group. The QRS spectra were analyzed for all the 3 strains of mice and their characteristics are presented in Fig. 4b. Statistical analysis via non-parametric ANOVA of the HF area in the QRS spectra showed that the average % of time spent in the HF in the BBF decreased, as the RR decreased. Markedly so it was evidenced in the HumCHGA19 mice (Fig. 4c). In summary, percentage area of HF/BBF: HumCHGA19 < WT < HumCHGA31 and for area dependence on heart rate [(HF/BBF)/HR]: HumCHGA19 < HumCHGA31<WT.
Fig. 4. The HF-band power segment of the BBF in the QRS-wave is reduced in Hum CHGA 19 mice.
The broadband frequency area (BBF) of the QRS wave segments were further analyzed. The BBF comprises of the sympathetic LF (low frequency) and the parasympathetic HF (high frequency) areas, indicative of efferent activity at the sinus node. (a) An illustration of power-frequency of QRS-wave plot: These plots were derived from actual experiments. Note in HumCHGA19 mice the frequency shift of peak power in the HF range along with a decrease in total power across the frequency range. (b) The QRS-wave segmented into average % of time spent in HF: Percentage of HF (80-300 Hz) area in the total BBF in the QRS-wave was significantly reduced in HumCHGA19 mice compared to WT and HumCHGA31 mice (##, ** p< 0.01). Analyzed data of the QRS spectra had the following characteristics: HumCHGA19- BBF area: 0.52, 0-80 Hz: 0.21, 80-300 Hz: 0.31 (HF: 59.61% of BBF), HumCHGA31- BBF area: 2.038, 0-80 Hz: 0.53, 80-300 Hz: 1.50 (HF: 73.60% of BBF) and WT- BBF area: 2.00, 0-80 Hz: 0.49, 80-300 Hz: 1.51 (75.5% of BBF). The area values are in arbitrary unit (au). (c) Influence of HR on the HF (80-300 Hz) part of BBF (0-300 Hz) spectral area: Bar graph shows that the fraction of HF area in the BBF of HumCHGA19 significantly lowered as compared to mice in other groups, as the sinus cycle length decreases (##, ** p< 0.01)
Ventricular repolarization duration (QT variability index)
Coupling of heart rate (1/RR) and ventricular repolarization time (QT) in humans has been extensively described in the literature, albeit not without dispute. Therefore, we examined the association between these variables in WT mice, using the Bazett-correction of QT (QTb). The variables were statistically linear and not significantly deviated (p>0.52) from the linearity (Pearson correlation coefficient, r= 0.89). Having confirmed the validity of the Bazett formula in this series of experiments, the Bazett-correction (QTb) factor [QTb= (QTu/RR1/2)] was applied to other mouse models as well. A graphic representation of the QTb values is shown in Fig. 5. WT and HumCHGA31 mice maintained a significant positive linear relation of QTb and HR. In contrast HumCHGA19 QTb almost failed to follow changes in HR and the relationship of these variables became non-significant (p>0.58). Heart rate and QTu exhibited beat-to-beat variability among the studied groups and was apparent in the calculated values of coefficient of variation (cv: standard deviation/sample means).
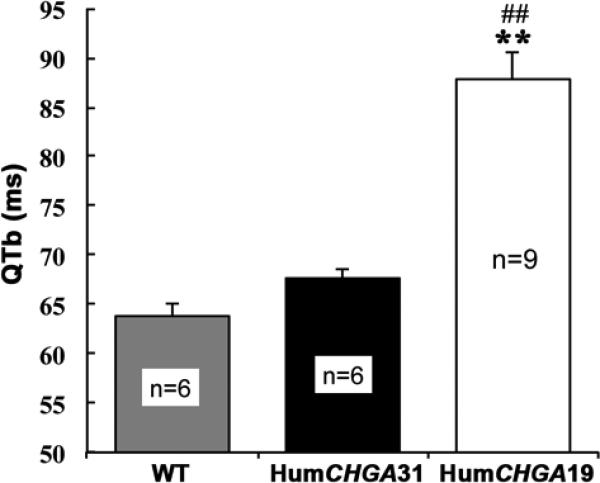
Fig. 5. Bazett-corrected QTb in mice.
Note the significantly (##, ** p< 0.01) prolonged QTb (index of myocardial repolarization status) in HumCHGA19 compared to WT and HumCHGA31 mice
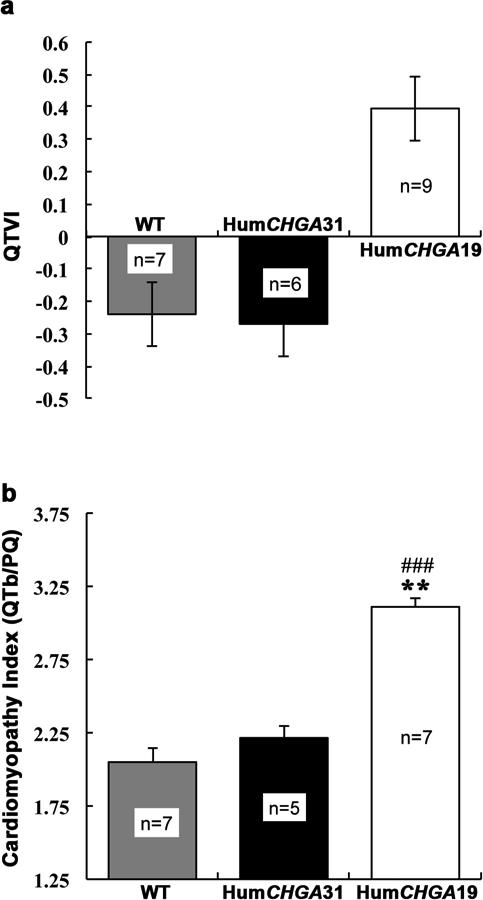
QTVI is an indicator of cardiac repolarization lability and cardiac sympathetic function. The QTVI was significantly greater in HumCHGA19 than in either HumCHGA31 or WT mice as shown in Fig. 6a. Respective cv values in HumCHGA31 and WT for HR were 0.097 and 0.304, and for QTb 0.067 and 0.137. In sharp contrast, the HumCHGA19 mice had an extremely constrained HR cv value of 0.081and for QTu a value of 0.112, without any discernible relation with instantaneous HR. Differences in QTVI among the mice were also reflected in the parameters of QTb/PQ. Particularly the mean PQ duration of 23 ms was distinctly lower in the HumCHGA19 group, a reduction of over 23% and 15% from the values obtained in the WT and HumCHGA31 respectively. Similarly, corrected QT (QTb) value was markedly higher in HumCHGA19, an increase of 38% and 28% compared to WT and HumCHGA31 respectively, and as a result, the magnitude of QTb/PQ (cardiomyopathy index) increased significantly in HumCHGA19 (Fig. 6b). Again, the QTb/PQ ratio is not significantly correlated with heart weight: body weight ratio (HumCHGA31 r= -0.36, p= 0.55; HumCHGA19 r= -0.83, p= 0.08).
Fig. 6. Influence of CHGA gene expression on log10 QT variability index (QTVI) and cardiomyopathy index (QTb/PQ).
From “humanized” and WT conscious mice, EKG signal was acquired in Lead II configuration at a sampling rate of 2 kHz (a) Elevated QTVI in HumCHGA19 mice: Compared to HumCHGA31 and WT mice, HumCHGA19 have augmented beat-to-beat fluctuations in QT interval that are larger than normal and uncoupled from variations in HR. (b) Cardiomyopathy index (QTb/PQ): Significant increase in the QTb/PQ ratio in HumCHGA19 was observed as compared to WT (### p< 0.001) and in the HumCHGA31 mice (** p< 0.01), suggesting electrophysiological parallel of increased myopathy of cardiac muscles. However the correlation between the hypertensive HumCHGA19 strain's heart size and increased QTb/PQ was not significant (p= 0.082).
Discussion
The main findings of this study are that CHGA and thus CST expression level is involved in fine-tuning of ventricular repolarization during QT interval. The BP responses obtained in mice with varying CHGA dosage and the data presented here, suggest a critical role for sympathetic stimulation and catecholamine levels for supporting QT prolongation [33,21]. The effect of sympathetic blockage on the QT: RR ratio has been demonstrated by pharmacological autonomic blockade experiments of Extramiana et al. [34]. In humans, QTb greater than >480 ms especially >500 ms, has been reported to cause sudden cardiac death. Theoretically, in mice this should correspond to a QTb value of >60 ms. In HumCHGA19 mice QTb values > 60 ms was routinely observed, suggesting that these mice are susceptible to ventricular arrhythmia. In HumCHGA19 mice, a dysfunction in cardiac performance occurs because the QT coefficients of variation (cv) values are higher and the RR cycle length variability fairly compressed, compared to HumCHGA31 (Table 2).
Table 2.
Uncorrected QT (QTu) interval and Sinus cycle length (RR)
| WT | HumCHGA31 | HumCHGA19 | ANOVA (F and p values) | |
|---|---|---|---|---|
| n | 6 | 6 | 9 | -- |
| RR interval (ms) | 140.44 ± 6.21 | 104.78 ± 1.55 | 87.09 ± 0.93 | F= 437.925 p< 0.001 (FDR p<0.001) |
| QTu interval (ms) | 23.26 ± 0.24 | 21.62 ± 0.23 | 24.07 ± 0.39 | -- |
| RR (cv) | 0.283 | 0.093 | 0.083 | -- |
| QTu (cv) | 0.067 | 0.066 | 0.126 | F= 110.55 p< 0.001 (FDR p<0.001) |
The values of coefficient of variation (cv) of RR and uncorrected QT interval are calculated (100 × std dev/ average value). In HumCHGA19 mice the QT (cv) is higher and fairly compressed in variability in the RR cycle length, compared to HumCHGA31 and WT mice. ‘n’ indicates the number of experimental mice in each group. The last column shows the F and p values of the one-way ANOVA. FDR: False Discovery Rate.
In humans, the prolongation of QRS complex duration is regularly listed among electrocardiographic signs of left ventricular hypertrophy (LVH). QRSd has also been found to correlate with left ventricular mass [35]. Dunn et al. also reported progressively increased QRSd with progressive left ventricular hypertrophy in SHR rats [36]. These findings are in line with the observations of this study, in which prolonged QRSd is an unique feature in HumCHGA19 mice and can be considered as a possible corollary to anatomical LVH. Earlier echocardiographic data of Chga−/− mice also indicated LVH [16]. The increase in R-wave voltage observed in HumCHGA19 may tentatively be attributed to hypertrophic status of the ventricle. This is also similar to “LIFE” clinical study that shows longer QRS and QT interval measures in the hypertensive population [37]. However, the HumCHGA31 and HumCHGA19 strains do not differ significantly in their heart weight:body weight ratios, and these ratios do not correlate with QRSd values or the cardiomyopathy index (QTb/PQ). Therefore cardiac hypertrophy is not an dominant trait differences between strains. VanderBrink et al. found that HF band area of QRS depolarization wave in mice was eighteen-fold over that of human (55% vs 3%) and postulated that HF area might be positively related to the HR [31]. This interpretation is based on known HR differences across species (man vs mice). Although, this interpretation may have validity, it is apparently invalid in case of compensatory or most likely pathological sinus tachycardia, as shown in our experiments, in which sinus tachycardia in HumCHGA19 in effect significantly reduced HF area. Therefore, we counter the notion that HF QRS band power may somehow be positively related to HR under all circumstances. Our observation of reduced relative HF-band power in HumCHGA19 is more synchronous with the published results of Bhargava et al. who evidenced in cardiac patients attenuation of HF-QRS power, with arrhythmogenicity[38].
It appears that the change in QTVI for HumCHGA19 was contributed mainly by the reduction in HR variance (84% over WT), whereas restitution toward normalcy of QTVI in HumCHGA31 was primarily achieved via a big reduction in QTb variance (>73% over WT). In HumCHGA19 mice QT duration was prolonged, QTb /PQ increased and PQ conduction time decreased. These are indices of cardiomyopathy as proposed by Comi et al. in patients with Duchenne and Becker muscular dystrophy [39]. Shorter PQ interval and prolonged QTb interval with tachycardia may be the manifestation of relative sympatheticotonia. Moreover, changes in QRS HF characteristics in HumCHGA19 suggest possible existence of ventricular enlargement, as suggested by Schlegel [40]. It is concluded that previously reported hypertension in HumCHGA19 alters autonomic modulation of their HR, ventricular depolarization and repolarization profiles [21]. Clinically, heightened level of CHGA has been found in patients with CHF, MI and LVH with consequent QT abnormality [41,42]. However, this investigation proves that reduced CHGA/CST can also lead to QT prolongation and electrocardiographic features of LVH, suggesting that non-optimal (both low and high CHGA) can be responsible for the EKG characteristics mentioned. To this aspect, future strategy should focus on reducing hypertension using specific non-competitive nicotinic inhibitory agent such as CST natural variants [43]. Since optimal expression of CHGA by HumCHGA31, ameliorates QT abnormality observed in HumCHGA19, the conjecture that CHGA expression level plays a significant role is firmly established. The fate of QT status may depend on the proportional contribution by both CST fragment and full length CHGA. The CST region knockout mouse model could further reveal the intricacies involved in the seemingly paradoxical events. Observed EKG changes in HumCHGA19 mice may just lower the threshold of ventricular susceptibility, increasing cardiovascular risk. Zhang et al. observed an unique relationship between CHGA level and survival rate in patients [44]. Their data indicate higher survival rate in patients with low CHGA in plasma. Whether such an observation is a direct link and applies to HumCHGA19 mice is a mere gesture.
Experimental limitations: The relation of the QT interval and HR is modified by a number of physiologic processes. The mechanism causing the change in HR may variably influence ventricular repolarization. Determination of the onset and the end of cardiac phenomena based on the partial view given by a single lead (in this case Lead II) is intrinsically limited, as discussed by Malik and therefore, may limit a robust interpretation quantitatively, without affecting the qualitative judgment [45]. However it is not known if QT abnormality in HumCHGA19 is mediated by the autonomic influences predominantly or by the non-autonomic pathway, e.g. exaggerated fractional shortening and consequent heavy ejection fraction loss. Also, the ionic current behavior for repolarization in mice is somewhat different from that of human. Therefore, drawing parallel between these species can be brought into question. We hypothesize that triggering of QTb prolongation at the low end of CHGA expression spectrum is most likely caused by autonomic dysfunction. However, the evidence in Chga−/− would substantially strengthen the argument albeit speculative in absence of direct evidence [15]. The findings of the present study demonstrate that BP increases observed in our earlier investigation affects ventricular repolarization status, along with changes in the depolarization properties [21]. In the face of elevated catecholamine secretion, finely regulated by the proportional contributions of circulating CHGA and catecholamine release inhibitory fragment, CST, QTb is prolonged. Future studies will involve delineating the mechanism by which the action potential duration at the level of ventricular myocytes is required to strengthen arguments concerning QT prolongation. Thus, our “humanized”CHGA mice are unique models to address the role of CHGA and CST in human cardiac electrical activity.
Supplementary Material
Acknowledgments
This study was supported by grants from the National Institutes of Health -K01DK069613 and R01 HL108629 to Vaingankar, S.M. We would like to thank Professors O'Connor, D. T., Ana Pajor, Mahata, S. K. and Ahmad, H.R. for their critical input.
Abbreviations and Acronyms
- au
arbitrary unit
- BP
blood pressure
- Chga
mouse chromogranin A gene
- CHGA
human chromogranin A gene
- CHGA
chromogranin A protein
- CI
statistical confidence interval
- CST
catestatin
- cv
coefficient of variation = [standard deviation/sample mean]
- DBP
diastolic BP
- DCM
dilated cardiomyopathy
- EKG
electrocardiography
- HR
heart rate
- HumCHGA19
genomically “humanized” transgenic mice Tg19CHGA+/+; Chga−/−
- HumCHGA31
genomically “humanized” transgenic mice Tg31CHGA+/+; Chga−/−
- HRV
heart rate variability
- PQ
atrio-ventricular conduction time
- QRSd
duration of QRS wave complex
- QTb
Bazett-corrected QT interval
- QTb/PQ
cardiomyopathy index
- QTu
uncorrected QT interval
- QTVI
QT variability index
- RR
sinus cycle length
- SBP
systolic BP
- WT
wild-type mice
Footnotes
Disclosures
The authors declare no conflicts of interest
References
- 1.Taupenot L, Harper KL, O'Connor DT. The chromogranin-secretogranin family. N Engl J Med. 2003;348:1134–1149. doi: 10.1056/NEJMra021405. [DOI] [PubMed] [Google Scholar]
- 2.Helle KB. The chromogranins. Historical perspectives. Adv Exp Med Biol. 2000;482:3–20. doi: 10.1007/0-306-46837-9_1. [DOI] [PubMed] [Google Scholar]
- 3.Iacangelo AL, Eiden LE. Chromogranin A: current status as a precursor for bioactive peptides and a granulogenic/sorting factor in the regulated secretory pathway. Regul Pept. 1995;58:65–88. doi: 10.1016/0167-0115(95)00069-n. [DOI] [PubMed] [Google Scholar]
- 4.Mahapatra NR, Taupenot L, Courel M, Mahata SK, O'Connor DT. The trans-Golgi proteins SCLIP and SCG10 interact with chromogranin A to regulate neuroendocrine secretion. Biochemistry. 2008;47:7167–7178. doi: 10.1021/bi7019996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elias S, Delestre C, Ory S, Marais S, Courel M, Vazquez-Martinez R, Bernard S, Coquet L, Malagon MM, Driouich A, Chan P, Gasman S, Anouar Y, Montero-Hadjadje M. Chromogranin A induces the biogenesis of granules with calcium- and actin-dependent dynamics and exocytosis in constitutively secreting cells. Endocrinology. 2012;153:4444–4456. doi: 10.1210/en.2012-1436. [DOI] [PubMed] [Google Scholar]
- 6.Mahapatra NR, Mahata M, Mahata SK, O'Connor DT. The chromogranin A fragment catestatin: specificity, potency and mechanism to inhibit exocytotic secretion of multiple catecholamine storage vesicle co-transmitters. J Hypertens. 2006;24:895–904. doi: 10.1097/01.hjh.0000222760.99852.e0. [DOI] [PubMed] [Google Scholar]
- 7.Pasqua T, Corti A, Gentile S, Pochini L, Bianco M, Metz-Boutigue MH, Cerra MC, Tota B, Angelone T. Full-length human chromogranin-A cardioactivity: myocardial, coronary, and stimulus-induced processing evidence in normotensive and hypertensive male rat hearts. Endocrinology. 2013;154:3353–3365. doi: 10.1210/en.2012-2210. [DOI] [PubMed] [Google Scholar]
- 8.Penna C, Alloatti G, Gallo MP, Cerra MC, Levi R, Tullio F, Bassino E, Dolgetta S, Mahata SK, Tota B, Pagliaro P. Catestatin improves post-ischemic left ventricular function and decreases ischemia/reperfusion injury in heart. Cell Mol Neurobiol. 2010;30:1171–1179. doi: 10.1007/s10571-010-9598-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O'Connor DT, Takiyyuddin MA, Printz MP, Dinh TQ, Barbosa JA, Rozansky DJ, Mahata SK, Wu H, Kennedy BP, Ziegler MG, Wright FA, Schlager G, Parmer RJ. Catecholamine storage vesicle protein expression in genetic hypertension. Blood Press. 1999;8:285–295. doi: 10.1080/080370599439508. [DOI] [PubMed] [Google Scholar]
- 10.Takiyyuddin MA, Parmer RJ, Kailasam MT, Cervenka JH, Kennedy B, Ziegler MG, Lin MC, Li J, Grim CE, Wright FA, O'Connor DT. Chromogranin A in human hypertension. Influence of heredity. Hypertension. 1995;26:213–220. doi: 10.1161/01.hyp.26.1.213. [DOI] [PubMed] [Google Scholar]
- 11.Kennedy BP, Mahata SK, O'Connor DT, Ziegler MG. Mechanism of cardiovascular actions of the chromogranin A fragment catestatin in vivo. Peptides. 1998;19:1241–1248. doi: 10.1016/s0196-9781(98)00086-2. [DOI] [PubMed] [Google Scholar]
- 12.Mahata SK, Mahata M, Livsey Taylor CV, Taupenot L, Parmer RJ, O'Connor DT. The novel catecholamine release-inhibitory peptide catestatin (chromogranin A344-364). Properties and function. Adv Exp Med Biol. 2000;482:263–277. doi: 10.1007/0-306-46837-9_21. [DOI] [PubMed] [Google Scholar]
- 13.O'Connor DT, Kailasam MT, Kennedy BP, Ziegler MG, Yanaihara N, Parmer RJ. Early decline in the catecholamine release-inhibitory peptide catestatin in humans at genetic risk of hypertension. J Hypertens. 2002;20:1335–1345. doi: 10.1097/00004872-200207000-00020. [DOI] [PubMed] [Google Scholar]
- 14.O'Connor DT, Zhu G, Rao F, Taupenot L, Fung MM, Das M, Mahata SK, Mahata M, Wang L, Zhang K, Greenwood TA, Shih PA, Cockburn MG, Ziegler MG, Stridsberg M, Martin NG, Whitfield JB. Heritability and genome-wide linkage in US and australian twins identify novel genomic regions controlling chromogranin a: implications for secretion and blood pressure. Circulation. 2008;118:247–257. doi: 10.1161/CIRCULATIONAHA.107.709105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gayen JR, Gu Y, O'Connor DT, Mahata SK. Global disturbances in autonomic function yield cardiovascular instability and hypertension in the chromogranin a null mouse. Endocrinology. 2009;150:5027–5035. doi: 10.1210/en.2009-0429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mahapatra NR, O'Connor DT, Vaingankar SM, Hikim AP, Mahata M, Ray S, Staite E, Wu H, Gu Y, Dalton N, Kennedy BP, Ziegler MG, Ross J, Mahata SK. Hypertension from targeted ablation of chromogranin A can be rescued by the human ortholog. J Clin Invest. 2005;115:1942–1952. doi: 10.1172/JCI24354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dev NB, Gayen JR, O'Connor DT, Mahata SK. Chromogranin A and the Autonomic System: Decomposition of Heart Rate Variability and Rescue by Its Catestatin Fragment. Endocrinology. 2010;151:2760–2768. doi: 10.1210/en.2009-1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meng L, Wang J, Ding W, Han P, Yang Y, Qi L, Zhang B. Plasma catestatin level in patients with acute myocardial infarction and its correlation with ventricular remodelling. Postgrad Med J. 2013;89:193–196. doi: 10.1136/postgradmedj-2012-131060. [DOI] [PubMed] [Google Scholar]
- 19.Puddu PE, Pasternac A, Tubau JF, Krol R, Farley L, Champlain JD. QT interval prolongation and increased plasma catecholamine levels in patients with mitral valve prolapse. Am Heart J. 1983;105:422–428. doi: 10.1016/0002-8703(83)90359-9. [DOI] [PubMed] [Google Scholar]
- 20.Algra A, Tijssen JG, Roelandt JR, Pool J, Lubsen J. QTc prolongation measured by standard 12-lead electrocardiography is an independent risk factor for sudden death due to cardiac arrest. Circulation. 1991;83:188–194. doi: 10.1161/01.cir.83.6.1888. [DOI] [PubMed] [Google Scholar]
- 21.Vaingankar SM, Li Y, Corti A, Biswas N, Gayen J, O'Connor DT, Mahata SK. Long human CHGA flanking chromosome 14 sequence required for optimal BAC transgenic “rescue” of disease phenotypes in the mouse Chga knockout. Physiol Genomics. 2010;41:91–101. doi: 10.1152/physiolgenomics.00086.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Steare SE, Dubowitz V, Benatar A. Subclinical cardiomyopathy in Becker muscular dystrophy. Br Heart J. 1992;68:304–308. doi: 10.1136/hrt.68.9.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mir SA, Chatterjee A, Mitra A, Pathak K, Mahata SK, Sarkar S. Inhibition of signal transducer and activator of transcription 3 (STAT3) attenuates interleukin-6 (IL-6)-induced collagen synthesis and resultant hypertrophy in rat heart. The Journal of biological chemistry. 2012;287:2666–2677. doi: 10.1074/jbc.M111.246173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sen S, Tarazi RC, Khairallah PA, Bumpus FM. Cardiac hypertrophy in spontaneously hypertensive rats. Circulation research. 1974;35:775–781. doi: 10.1161/01.res.35.5.775. [DOI] [PubMed] [Google Scholar]
- 25.Bazett HC. An analysis of time relations of electrocardiogram. Heart. 1920;7:53–70. [Google Scholar]
- 26.Decher N, Wemhoner K, Rinne S, Netter MF, Zuzarte M, Aller MI, Kaufmann SG, Li XT, Meuth SG, Daut J, Sachse FB, Maier SK. Knock-out of the potassium channel TASK-1 leads to a prolonged QT interval and a disturbed QRS complex. Cell Physiol Biochem. 2011;28:77–86. doi: 10.1159/000331715. [DOI] [PubMed] [Google Scholar]
- 27.Mitchell GF, Jeron A, Koren G. Measurement of heart rate and Q-T interval in the conscious mouse. The American journal of physiology. 1998;274:H747–751. doi: 10.1152/ajpheart.1998.274.3.H747. [DOI] [PubMed] [Google Scholar]
- 28.Berger RD, Kasper EK, Baughman KL, Marban E, Calkins H, Tomaselli GF. Beat-to-Beat QT Interval Variability. Circulation. 1997;96:1557–1565. doi: 10.1161/01.cir.96.5.1557. [DOI] [PubMed] [Google Scholar]
- 29.Xing S, Tsaih SW, Yuan R, Svenson KL, Jorgenson LM, So M, Paigen BJ, Korstanje R. Genetic influence on electrocardiogram time intervals and heart rate in aging mice. Am J Physiol Heart Circ Physiol. 2009;296:H1907–1913. doi: 10.1152/ajpheart.00681.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Atterhog JH, Loogna E. PR interval in relation to heart rate during exercise and the influence of posture and autonomic tone. J Electrocardiol. 1977;10:331–336. doi: 10.1016/s0022-0736(77)80005-8. [DOI] [PubMed] [Google Scholar]
- 31.VanderBrink BA, Link MS, Aronovitz MJ, Saba S, Sloa SB, Homoud MK, Estes NAM. Assessment of Atrioventricular Nodal Physiology in the mouse. J Interv Card Electrophysiol. 1999;3:207–212. doi: 10.1023/a:1009842105146. [DOI] [PubMed] [Google Scholar]
- 32.Wang XJ, Ai HB. Studies on the relativity of power spectrum of QRS complex with heart rate, duration of QRS and Vp-p of QRS. Shandog J Biomed Eng. 1999;18:32–38. [Google Scholar]
- 33.Vaingankar SM, Li Y, Biswas N, Gayen J, Choksi S, Rao F, Ziegler MG, Mahata SK, O'Connor DT. Effects of chromogranin A deficiency and excess in vivo: biphasic blood pressure and catecholamine responses. J Hypertens. 2010;28:817–825. doi: 10.1097/HJH.0b013e328336ed3e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Extramiana F, Tavernier R, Maison-Blanche P, Neyroud N, Jordaens L, Leenhardt A, Coumel P. Ventricular repolarization and Holter monitoring. Effect of sympathetic blockage on the QT/RR ratio. Arch Mal Coeur Vaiss. 2000;93:1277–1283. [PubMed] [Google Scholar]
- 35.Holt JH, Barnard ACL, Lynn MS. A study of the human heart as a multiple dipole electrical source. II. Diagnosis and quantification of left ventricular hypertrophy. Circulation. 1969;40:697–710. doi: 10.1161/01.cir.40.5.697. [DOI] [PubMed] [Google Scholar]
- 36.Dunn FG, Pfeffer MA, Frolich ED. ECG alterations with progressive left ventricular hypertrophy in spontaneous hypertension. Clin Exp Hypertens. 1978;1:67–86. doi: 10.3109/10641967809068596. [DOI] [PubMed] [Google Scholar]
- 37.Oikarinen L, Nieminen MS, Viitasalo M, Toivonen L, Wachtell K, Papademetriou V, Jern S, Dahlöf B, Devereux RB, Okin PM. Relation of QT interval and QT dispersion to echocardiographic left ventricular hypertrophy and geometric pattern in hypertensive patients. The LIFE study. The Losartan Intervention For Endpoint Reduction. J Hypertens. 2001;19:1883–1891. doi: 10.1097/00004872-200110000-00025. [DOI] [PubMed] [Google Scholar]
- 38.Bhargava V, Goldberger A. Myocardial infarction diminishes both low and high frequency QRS potentials: power spectrum analysis of lead II. J Electrocardiol. 1981;14:57–60. doi: 10.1016/s0022-0736(81)80029-5. [DOI] [PubMed] [Google Scholar]
- 39.Comi LI, Nigro G, Politano L, Petretta VR. The cardiomyopathy of Duchenne/Becker consultands. Int J Cardiol. 1992;34:297–305. doi: 10.1016/0167-5273(92)90028-2. [DOI] [PubMed] [Google Scholar]
- 40.Schlegel TT KW, DePalma JL, Feiveson AH, Wilson JS, Rahman MA, Bungo MW. Real-time 12-lead high-frequency QRS electrocardiography for enhanced detection of myocardial ischemia and coronary artery disease. Mayo Clin Proc. 2004;79:339–350. doi: 10.4065/79.3.339. [DOI] [PubMed] [Google Scholar]
- 41.Ceconi C, Ferrari R, Bachetti T, Opasic C, Volterrani M, Colombo B, Parrinello G, Corti A. Chromogranin A in heart failure; a novel neurohumoral factor and a predictor for mortality. Eur Heart J. 2002;23:967–974. doi: 10.1053/euhj.2001.2977. [DOI] [PubMed] [Google Scholar]
- 42.Omland T, Dickstein K, Syversen U. Association between plasma chromogranin A concentration and long-term mortality after myocardial infarction. Am J Med. 2003;114:25–30. doi: 10.1016/s0002-9343(02)01425-0. [DOI] [PubMed] [Google Scholar]
- 43.Mahata SK, Mahata M, Fung MM, O'Connor DT. Catestatin: A multifunctional peptide from chromogranin A. Regul Pept. 2010;165:52–62. doi: 10.1016/j.regpep.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang D, Lavaux T, Voegeli AC, Lavigne T, Castelain V, Meyer N, Sapi R, Aunis D, Boutigue MHM, Schneider F. Prognostic Value of Chromogranin A at Admission In Critically Ill Patients: A Cohort Study in a Medical Intensive Care Unit. Clin Chem. 2008;54:1947–1503. doi: 10.1373/clinchem.2007.102442. [DOI] [PubMed] [Google Scholar]
- 45.Malik M. Errors and misconceptions in ECG measurement used for the detection of drug induced QT-interval prolongation. J Electrocardiol. 2004;37:25–33. doi: 10.1016/j.jelectrocard.2004.08.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.