Abstract
Objective
This study sought to determine the frequency of possible cardiopulmonary drug-drug interactions among pregnant women who received intrapartum magnesium sulfate (MgSO4).
Methods
Pregnant women admitted to an Intermountain Healthcare facility between January 2009 and October 2011 were studied if they received one or more doses of MgSO4. Concomitant medications were electronically queried from an electronic health records system. Adverse events were identified using administrative discharge codes. The frequency of cardiopulmonary drug-drug interactions was compared among women who did, and did not, receive aminoglycoside antibiotics, antacids / laxatives, calcium channel blockers, corticosteroids, diuretics, neuromuscular blocking agents, and vitamin D analogs, all of which are contraindicated for patients receiving MgSO4.
Results
Overall, 683 women received intrapartum MgSO4 during the study period. A total of 219 MgSO4 potentially interacting drugs were identified among 155 (23%) unique patients. The most commonly identified potentially interacting agents included calcium channel blockers (26%), diuretics (25%), and antacids / laxatives (19%). Longer hospital stays were significantly associated with increasing numbers of MgSO4 interacting drugs (P<0.001). Three of 53 (6%) women who received furosemide experienced a cardiac arrest, compared to 0 of 618 (0%) women who did not receive furosemide (Fisher’s Exact Test P<0.001).
Conclusion
Intrapartum administration of drugs that interact with MgSO4 is common and associated with prolonged hospital stays and potentially cardiopulmonary drug-drug interactions. Caution is warranted when prescribing MgSO4 in combination with known interacting medications.
Keywords: acute respiratory failure, adverse drug reactions, adverse events, cardiac arrest, drug-drug interactions
INTRODUCTION
Magnesium sulfate (MgSO4) is currently used in pregnancy for prevention and treatment of pre-eclampsia and eclampsia, and more recently, for fetal neuroprophylaxis prior to preterm birth. While a Cochrane Review of women at risk of preterm birth who received MgSO4 demonstrated a neuroprotective effect for the fetus,1 the United States Food and Drug Administration (FDA) recently revised MgSO4 prescription drug labels to indicate that there is “positive evidence of human fetal risk when the drug is used during pregnancy.”2 This change was prompted following a review of the published literature in which 18 cases of skeletal abnormalities were reported among neonates exposed to MgSO4 for a mean duration of 10 weeks.3–6 The recently updated FDA guidelines now caution that MgSO4 should not be used for more than 7 days to prevent pre-term labor.2 Although MgSO4 has been used historically to prevent pre-term labor,7 several randomized controlled trials have now confirmed that it is ineffective as a tocolytic.8–11
Many adverse events can result from MgSO4 administration. One of the most serious is the neuromuscular blockade which can be observed on a continuum from diminished deep tendon reflexes to somnolence to flaccid paralysis.12 The diaphragm and other respiratory muscles may also be affected and cause respiratory compromise.12 It has long been recognized that MgSO4 administration alters maternal calcium metabolism when used alone or when used concurrently with calcium-channel blockers such as nifedipine.13 Magnesium also acts as a calcium channel antagonist, and therefore, can cause bradycardia, hypotension, electrocardiogram changes (i.e., P–Q interval prolongation and widened QRS complex), and at very high levels, cardiac arrest.14 Hypocalcemia can also result from MgSO4 therapy by inhibition of parathyroid hormone.15 The potential also exists for severe and/or prolonged respiratory depression which can result from the concomitant use of aminoglycoside antibiotics and other neuromuscular blocking agents.16,17 Many other drugs and drug classes have been identified that interact with MgSO4, which can result in an increased pharmacological effect or increased risk of magnesium toxicity, both of which increase the potential for adverse events.18
The extent to which pregnant women receiving MgSO4 are simultaneously exposed to potentially-interacting drugs is unknown. In this study, the prescribing patterns of concomitant drugs were evaluated along with the frequency with which pregnant women were exposed to MgSO4 and one or more potentially-interacting drugs. As a secondary objective, the frequency of cardiopulmonary drug-drug interactions and their impact upon clinical outcomes were examined.
MATERIALS AND METHODS
Ethics approval
Ethical approval was granted for this retrospective chart review study. This study was reviewed and granted a waiver of informed consent by the University of Utah and Intermountain Healthcare Institutional Review Boards.
Study design
Pregnant women who were hospitalized and received intravenous MgSO4 at an Intermountain facility from January of 2009 through October 2011 were identified. Patients receiving one or more doses of MgSO4 were included in this study. Demographics, concomitant medications given ± 48 hours from MgSO4 administration, and clinical outcomes were obtained electronically by querying Intermountain’s electronic data warehouse system.
Magnesium sulfate dosing
Standardized MgSO4 administration protocols are used at Intermountain facilities. Typically, a standard loading dose of 4–6 grams was infused over 15–30 minutes, followed by 1–2 grams/hour administered as a continuous infusion. This standardized dosing regimen may differ slightly between the 17 institutions studied and whether treatment was for preeclampsia/eclampsia and/or fetal neuroprophylaxis. MgSO4 administration was not targeted to achieve a specific therapeutic range; however, patients were monitored for signs and symptoms of toxicity.19
Assessment of cardiopulmonary drug-drug interactions
The adverse effects of MgSO4 have primarily been attributed to its action as a smooth muscle relaxant.20 At high serum magnesium levels (>15 mEq/L) cardiac arrest and acute respiratory failure have been reported.21 Cardiopulmonary adverse events were identified in this population of pregnant women using International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9) discharge diagnosis codes. Patients with cardiac arrest and acute respiratory failure were initially identified using ICD-9 codes (427.5 and 511.81, respectively) and their medical records were then manually reviewed to confirm the diagnosis.
Statistical methods
Descriptive statistics were used to calculate the number of MgSO4 drug-drug interactions. Drug interactions classified as ‘major’ or ‘moderate’ according to a clinical significance classification algorithm featured on Drugs.com were evaluated in this study.18 This classification scheme incorporates data from Micromedex™, Cerner Multum™, and Wolters Kluwer™ and was current as of 18 June 2013.
A generalized logistic regression model with a categorical number / extent-of-interactions term was used to evaluate the influence of MgSO4 drug-drug interactions upon total hospital length of stay. Pairwise t-tests were used to assess the significance of the drug-drug interaction upon the mean hospital length of stay. Categorical variables were compared using the χ2 test or Fisher’s exact test, as appropriate. All statistical analyses were performed in Stata 11.2 (StataCorp, College Station, TX) and R 3.0.1 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Demographic and clinical characteristics
Overall, 683 women received intravenous MgSO4 over the study period. Of these, 130 (19%) received MgSO4 for the treatment of mild or not-otherwise-specified pre-eclampsia. Severe pre-eclampsia was documented as the primary indication for treatment in 341 (50%) cases and eclampsia in 8 (1%) cases. There were 267 (39%) diagnosed with early-onset delivery. Diagnosis of both pre-eclampsia / eclampsia and early-onset delivery was common and occurred in 210 (31%) of these cases. The mean age of these women was 27.4 (standard deviation (SD) ± 5.6) years (Table 1). Most women had singleton pregnancies (96%) and delivered at a mean gestational age of 35.5 (± 3.2) weeks. Newborn weights ranged from 680 to 5520 (mean 2546) grams.
Table 1.
Demographic and clinical characteristics of the 683 women who received intrapartum magnesium sulfate and their newborn infants.
| Maternal characteristics at the time of delivery | Mean (± SD) |
|
| |
| Age (yrs) | 27.4 (± 5.6) |
| Height (cm) | 165.2 (± 17.6) |
| Pre-pregnancy weight (kg) | 74.5 (± 20.6) |
| Current weight (kg) | 88.5 (± 20.5) |
| Estimated weight gain/loss during pregnancy (kg) | 13.9 (± 8.1) |
| Estimated body mass index (BMI) at time of delivery | 32.6 (± 7.5) |
|
| |
| Maternal race / ethnicity | Number (%) |
|
| |
| American Indian / Alaskan Native | 9 (1%) |
| Asian | 12 (2%) |
| Black | 8 (1%) |
| Hispanic | 108 (16%) |
| Pacific Islander | 16 (2%) |
| White | 517 (76%) |
| Other | 4 (1%) |
| Unknown / Not reported | 9 (1%) |
|
| |
| Maternal diagnoses * | Number (%) |
|
| |
| Mild pre-eclampsia / not otherwise specified | 130 (19%) |
| Severe pre-eclampsia | 341 (50%) |
| Eclampsia | 8 (1%) |
| Early-onset delivery | 267 (39%) |
| Early-onset delivery plus Pre-eclampsia / eclampsia | 210 (31%) |
|
| |
| Monitoring of magnesium serum concentrations | Number (%) |
|
| |
| Mothers with one concentration measured | 673 (99%) |
| Median (range; IQR) concentrations measured/patient | 2 (1–18; IQR 1–3) |
| Mean ± SD of 1st concentrations measured | 5.0 ± 1.9 mg/dL |
|
| |
| Newborn characteristics | |
|
| |
| Gestational age at delivery, weeks | 35.5 (± 3.2) |
| Birthweight, grams | 2,546 (± 792) |
Sums to more than 100% due to patients with multiple overlapping diagnoses (e.g., pre-eclampsia and early-onset delivery).
Interacting medications
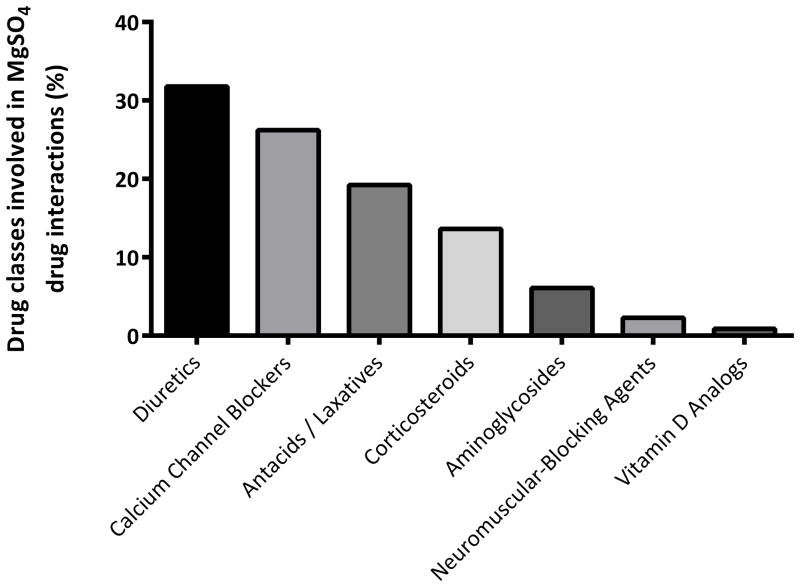
A total of 219 MgSO4 drug-drug interactions were identified among this cohort of pregnant women. At least one MgSO4 drug-drug interaction was detected for 155 (23%) unique patients (Table 2). As shown in Figure 2, the majority of MgSO4 drug interactions occurred among women who were concomitantly prescribed calcium channel blockers (e.g., nifedipine), diuretics (e.g., furosemide), and antacids / laxatives (e.g., magnesium hydroxide). A minority (2%) received the aminoglycoside agent gentamicin, which has been reported to potentiate the neuromuscular blocking activity of magnesium (Table 3).17
Table 2.
Frequency of identified drug interactions among pregnant women receiving magnesium sulfate (MgSO4).
| Total number of patients receiving MgSO4 | 683 (100%) |
|
| |
| Patients with one or more drug-drug interaction(s) | 155 (23%) |
|
| |
| MODERATE* (Moderately Significant) Interaction | 142 (21%) |
|
| |
| 1 Interacting drug | 105 (15%) |
| 2 Interacting drugs | 25 (4%) |
| 3 Interacting drugs | 10 (1%) |
| 4 Interacting drugs | 1 (<1%) |
| 5 Interacting drugs | 1 (<1%) |
|
| |
| MAJOR* (Highly Significant) Interaction | 13 (2%) |
|
| |
| 1 MAJOR interacting drug | 4 (1%) |
| 1 MAJOR and 1 MODERATE interacting drug | 6 (1%) |
| 1 MAJOR and 2 MODERATE interacting drugs | 3 (<1%) |
Moderate and major drug-drug interactions were classified according to the drug interaction classification featured on Drugs.com.18 Data sources include Micromedex™, Cerner Multum™, Wolters Kluwer™ and others.
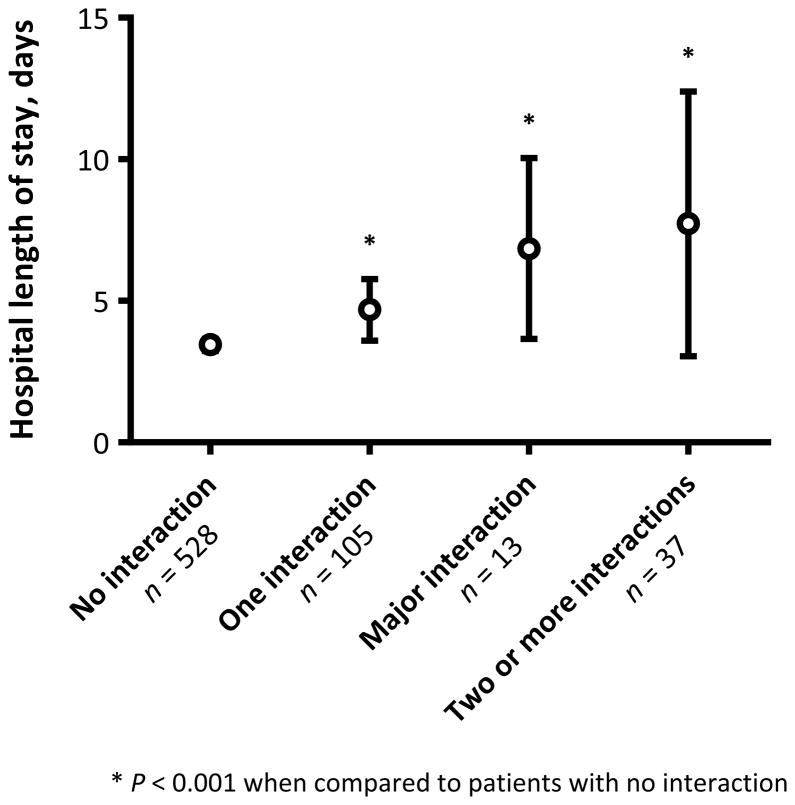
Figure 2.
Total hospital length of stay among women who received intrapartum magnesium sulfate (MgSO4) by the number and type of identified drug-drug interactions. White circles represent mean values and black bars indicate 95% confidence intervals. Major drug-drug interactions were classified according to the drug interaction classification scheme featured on Drugs.com.18
Table 3.
Classification and potential risks associated with interacting drugs.
| Drug Class | Classification of Drug Interaction* | Potential Risk |
|---|---|---|
| Aminoglycoside antibiotics | Major | Possess neuromuscular blocking activity; Potential additive pharmacological effect |
| Antacids / laxatives | Moderate | May increase the risk of magnesium toxicity |
| Calcium channel blockers | Moderate | May result in hypotension and neuromuscular blockade |
| Corticosteroids | Moderate | Potential for significant loss of electrolytes; Potentiates the risk of hypokalemia |
| Diuretics | Moderate | May potentiate the pharmacological effects of diuretics |
| Neuromuscular-blocking agents | Moderate | Magnesium salts may enhance the effects of neuromuscular-blocking agents |
| Vitamin D analogs | Moderate | Increases the risk of hypermagnesemia; Potential additive pharmacological effect |
Moderate and major drug-drug interactions were classified according to the drug interaction classification featured on Drugs.com.18 Data sources include Micromedex™, Cerner Multum™, Wolters Kluwer™ and others.
Adverse drug-drug interactions
A cardiopulmonary adverse event occurred among 13 of 155 (8%) women who received one or more MgSO4 interacting drugs. None of the 531 women who did not receive an interacting drug experienced a cardiopulmonary adverse event (Fisher’s Exact test P<0.001). Twelve of the 13 (92%) cardiopulmonary adverse events were documented among pregnant women who simultaneously received a diuretic agent. The most commonly identified diuretic was furosemide, which accounted for 11 (85%) of the cardiopulmonary adverse drug-drug interactions. Three of 53 (6%) women who received furosemide with MgSO4 experienced a cardiac arrest as compared to 0 of 618 who did not receive furosemide (Fisher’s Exact test P<0.001). Additionally, 9 (17%) furosemide-treated women developed acute respiratory failure as compared to 2 of 618 (0.3%) women who did not receive furosemide (Fisher’s Exact Test P<0.001).
Clinical outcomes
Women who were prescribed one or more potentially MgSO4 interacting drugs delivered earlier (mean gestational age 35 vs. 36 weeks; P=0.01) and gave birth to newborns with lower birth weights (mean 2400 vs. 2587 grams; P=0.01). Additionally, maternal hospital stays were prolonged among women who had a MgSO4 drug-drug interaction identified (Figure 2). Among women who received intrapartum MgSO4 without the concomitant administration of a potentially interacting medication the mean hospital length of stay was 3.4 (± 2.5) days, as compared to 4.7 (± 5.6) days among women who were prescribed one potentially interacting drug and 7.8 (± 14.0) days among women who received two or more potentially interacting drugs (P<0.001 for all).
COMMENT
Pregnant women are frequently prescribed MgSO4 with one or more concomitant medications that are known to interact and have the potential to result in toxicity.22 The most commonly prescribed classes of MgSO4 interacting drugs included diuretics, calcium channel blockers, and antacids / laxatives. Women who received a MgSO4 interacting drug had longer hospital stays and a lower gestational age at delivery. Concomitant administration of the diuretic agent furosemide was associated with higher rates of cardiac arrest and acute respiratory failure.
Acute or chronic hypertensive disorders affect up to 8% of pregnant women in the United States.23 Complications of pre-eclampsia, including severe hypertension requiring pharmacotherapy and major fluid imbalances that cause cerebral or pulmonary edema, typically occur within the first two weeks after delivery.24 Orally administered diuretics, such as furosemide, are thought to decrease the need for antihypertensive therapies, reduce the incidence of side effects, and shorten hospital stays.25 However, laxatives may potentiate the pharmacological effects of diuretics.26 MgSO4 can cause significant fluid and electrolyte loss, the effects of which may be additive to those of diuretics.27 Metabolic imbalances – including the depletion of sodium, potassium, magnesium, and zinc – may occur and have been associated with heart failure and cardiac arrhythmias.28,29 In this study, it was found that the concomitant administration of MgSO4 and furosemide was associated with an increased risk of cardiopulmonary adverse events, which may have occurred as a consequence of profound fluid loss and electrolyte imbalances.
Other commonly identified MgSO4 interacting drugs included calcium channel blockers and antacids / laxatives. Calcium channel blockers are routinely used to treat pregnancy-induced hypertension; however, guidelines from the National Heart Lung and Blood Institute advise clinicians to avoid concomitantly administering calcium channel blockers and MgSO4 on the basis of two case reports that described two cases of severe maternal hypotension and two cases of transient neuromuscular blockade.30–32 In this study, nearly 1 in 10 pregnant women simultaneously received MgSO4 and a calcium channel blocker and two (4%) experienced a cardiopulmonary drug-drug interaction. Several case reports have described iatrogenic magnesium overdoses resulting in cardiac conduction delays, asystole, apnea, and coma following the concomitant administration of MgSO4 and another magnesium-containing antacid / laxative.33,34 Although pregnancy results in a physiologic increase in renal function,35 more than 1 in 20 pregnant women in this study contemporaneously received MgSO4 and another magnesium-containing antacid / laxative. In this population, close clinical monitoring for signs and symptoms of magnesium toxicity is warranted.
The interpretation of this study’s findings is subject to several limitations. First, we have reported an association between several drugs that are known to interact with MgSO4 and their impact upon clinical outcomes, however due to the observational retrospective design of this study we cannot determine causality nor control for concurrent illness or disease severity. Therefore, we cannot rule out that the clinical differences described herein are not secondary to drug-drug interactions. Clinical caution is therefore warranted when considering the use of these drugs concurrently.36 Second, the use of ICD-9 discharge codes may lead to misclassification of adverse drug-drug interactions; however, medical records were manually reviewed for each of the patients who had an ICD-9 code for cardiac arrest or acute respiratory failure and they were found to feature 100% agreement. Furthermore, in a separate control cohort of 299 women who received intrapartum furosemide alone over the same timeframe, none experienced a cardiac arrest or an episode of acute respiratory failure. Lastly, it was not possible to associate number of maternal MgSO4 doses with the development of adverse events, although this warrants future study.
The concomitant administration of MgSO4 and other drugs known to result in adverse drug interactions is common among pregnant women. The number of MgSO4 drug-drug interactions was associated with both longer hospital stays and lower gestational age at delivery. Moreover, several women experienced cardiac arrest and acute respiratory failure. MgSO4 drug-drug interactions occurred most frequently among patients receiving concomitant diuretics, calcium channel blockers, and antacids / laxatives. All obstetric care providers should be aware of these cardiopulmonary drug-drug interactions and consider prescribing non-interacting medications when alternative therapies are available.
Figure 1.
Drug classes identified in magnesium sulfate (MgSO4) drug-drug interactions among hospitalized pregnant women.
Acknowledgments
Funding Sources: Dr. Clark was funded in part by NIH grant K23 HD061910 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development. Dr. Bonkowsky was funded in part by NIH grant DP2 MH100008 from the National Institute of Mental Health and from the Primary Children’s Medical Center Foundation.
Footnotes
Conflict of Interest Statement: The authors report no conflict of interest. There was no involvement by study sponsors, in: (1) study design; (2) the collection, analysis, and interpretation of data; (3) the writing of the report; and (4) the decision to submit the paper for publication.
References
- 1.Doyle LW, Crowther CA, Middleton P, et al. Magnesium sulphate for women at risk of preterm birth for neuroprotection of the fetus. Cochrane Database Syst Rev. 2009:CD004661. doi: 10.1002/14651858.CD004661.pub2. [DOI] [PubMed] [Google Scholar]
- 2.U.S. Food and drug administration. Fda recommends against prolonged use of magnesium sulfate to stop pre-term labor due to bone changes in exposed babies. [Accessed 26 june 2013];Drug safety communications. at: Http://www.Fda.Gov/downloads/drugs/drugsafety/ucm353335.Pdf.
- 3.Yokoyama K, Takahashi N, Yada Y, et al. Prolonged maternal magnesium administration and bone metabolism in neonates. Early human development. 2010;86:187–91. doi: 10.1016/j.earlhumdev.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 4.Wedig KE, Kogan J, Schorry EK, et al. Skeletal demineralization and fractures caused by fetal magnesium toxicity. Journal of perinatology: official journal of the California Perinatal Association. 2006;26:371–4. doi: 10.1038/sj.jp.7211508. [DOI] [PubMed] [Google Scholar]
- 5.Malaeb SN, Rassi AI, Haddad MC, et al. Bone mineralization in newborns whose mothers received magnesium sulphate for tocolysis of premature labour. Pediatric radiology. 2004;34:384–6. doi: 10.1007/s00247-004-1148-1. [DOI] [PubMed] [Google Scholar]
- 6.Kaplan W, Haymond MW, McKay S, et al. Osteopenic effects of mgso4 in multiple pregnancies. Journal of pediatric endocrinology & metabolism: JPEM. 2006;19:1225–30. doi: 10.1515/jpem.2006.19.10.1225. [DOI] [PubMed] [Google Scholar]
- 7.Besinger RE, Niebyl JR. The safety and efficacy of tocolytic agents for the treatment of preterm labor. Obstetrical & gynecological survey. 1990;45:415–40. doi: 10.1097/00006254-199007000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Cotton DB, Strassner HT, Hill LM, et al. Comparison of magnesium sulfate, terbutaline and a placebo for inhibition of preterm labor. A randomized study. The Journal of reproductive medicine. 1984;29:92–7. [PubMed] [Google Scholar]
- 9.Haghighi L. Prevention of preterm delivery: Nifedipine or magnesium sulfate. International journal of gynaecology and obstetrics: the official organ of the International Federation of Gynaecology and Obstetrics. 1999;66:297–8. doi: 10.1016/s0020-7292(99)00095-8. [DOI] [PubMed] [Google Scholar]
- 10.Morales WJ, Madhav H. Efficacy and safety of indomethacin compared with magnesium sulfate in the management of preterm labor: A randomized study. American journal of obstetrics and gynecology. 1993;169:97–102. doi: 10.1016/0002-9378(93)90138-9. [DOI] [PubMed] [Google Scholar]
- 11.Wilkins IA, Lynch L, Mehalek KE, et al. Efficacy and side effects of magnesium sulfate and ritodrine as tocolytic agents. American journal of obstetrics and gynecology. 1988;159:685–9. doi: 10.1016/s0002-9378(88)80035-8. [DOI] [PubMed] [Google Scholar]
- 12.Krendel DA. Hypermagnesemia and neuromuscular transmission. Semin Neurol. 1990;10:42–5. doi: 10.1055/s-2008-1041252. [DOI] [PubMed] [Google Scholar]
- 13.Cruikshank DP, Pitkin RM, Reynolds WA, et al. Effects of magnesium sulfate treatment on perinatal calcium metabolism. I. Maternal and fetal responses. American journal of obstetrics and gynecology. 1979;134:243–9. doi: 10.1016/s0002-9378(16)33027-7. [DOI] [PubMed] [Google Scholar]
- 14.Agus ZS, Morad M. Modulation of cardiac ion channels by magnesium. Annu Rev Physiol. 1991;53:299–307. doi: 10.1146/annurev.ph.53.030191.001503. [DOI] [PubMed] [Google Scholar]
- 15.Cholst IN, Steinberg SF, Tropper PJ, et al. The influence of hypermagnesemia on serum calcium and parathyroid hormone levels in human subjects. N Engl J Med. 1984;310:1221–5. doi: 10.1056/NEJM198405103101904. [DOI] [PubMed] [Google Scholar]
- 16.Pittinger C, Adamson R. Antibiotic blockade of neuromuscular function. Annual review of pharmacology. 1972;12:169–84. doi: 10.1146/annurev.pa.12.040172.001125. [DOI] [PubMed] [Google Scholar]
- 17.L’Hommedieu CS, Nicholas D, Armes DA, et al. Potentiation of magnesium sulfate--induced neuromuscular weakness by gentamicin, tobramycin, and amikacin. The Journal of pediatrics. 1983;102:629–31. doi: 10.1016/s0022-3476(83)80209-1. [DOI] [PubMed] [Google Scholar]
- 18.Drugs.com. Magnesium sulfate drug interactions. [Accessed 26 June 2013];Drug Information Online. at www.drugs.com/drug-interactions/magnesium-sulfate-index.html.
- 19.Lu JF, Nightingale CH. Magnesium sulfate in eclampsia and pre-eclampsia: Pharmacokinetic principles. Clinical pharmacokinetics. 2000;38:305–14. doi: 10.2165/00003088-200038040-00002. [DOI] [PubMed] [Google Scholar]
- 20.Pryde PG, Besinger RE, Gianopoulos JG, et al. Adverse and beneficial effects of tocolytic therapy. Seminars in perinatology. 2001;25:316–40. doi: 10.1053/sper.2001.27547. [DOI] [PubMed] [Google Scholar]
- 21.Paul AK. Essentials of anaesthesiology. New Delhi, India: Jaypee Brothers Medical Publishers Ltd; 2006. [Google Scholar]
- 22.Huang SM, Lesko LJ. Drug-drug, drug-dietary supplement, and drug-citrus fruit and other food interactions: What have we learned? Journal of clinical pharmacology. 2004;44:559–69. doi: 10.1177/0091270004265367. [DOI] [PubMed] [Google Scholar]
- 23.Roberts JM, Pearson G, Cutler J, et al. Summary of the nhlbi working group on research on hypertension during pregnancy. Hypertension. 2003;41:437–45. doi: 10.1161/01.HYP.0000054981.03589.E9. [DOI] [PubMed] [Google Scholar]
- 24.Magann EF, Martin JN., Jr Complicated postpartum preeclampsia-eclampsia. Obstetrics and gynecology clinics of North America. 1995;22:337–56. [PubMed] [Google Scholar]
- 25.Ascarelli MH, Johnson V, McCreary H, et al. Postpartum preeclampsia management with furosemide: A randomized clinical trial. Obstetrics and gynecology. 2005;105:29–33. doi: 10.1097/01.AOG.0000148270.53433.66. [DOI] [PubMed] [Google Scholar]
- 26.Fawcett WJ, Haxby EJ, Male DA. Magnesium: Physiology and pharmacology. British journal of anaesthesia. 1999;83:302–20. doi: 10.1093/bja/83.2.302. [DOI] [PubMed] [Google Scholar]
- 27.Rangan C. Diuretics, ipecac, and laxatives. In: Barceloux DG, editor. Medical toxicology of drug abuse. Hoboken, New Jersey: John Wiley & Sons, Inc; 2012. [Google Scholar]
- 28.Iseri LT, Freed J, Bures AR. Magnesium deficiency and cardiac disorders. The American journal of medicine. 1975;58:837–46. doi: 10.1016/0002-9343(75)90640-3. [DOI] [PubMed] [Google Scholar]
- 29.Duarte CG. Effects of ethacrynic acid and furosemide on urinary calcium, phosphate and magnesium. Metabolism: clinical and experimental. 1968;17:867–76. doi: 10.1016/0026-0495(68)90151-0. [DOI] [PubMed] [Google Scholar]
- 30.Report of the national high blood pressure education program working group on high blood pressure in pregnancy. American journal of obstetrics and gynecology. 2000;183:S1–S22. [PubMed] [Google Scholar]
- 31.Ben-Ami M, Giladi Y, Shalev E. The combination of magnesium sulphate and nifedipine: A cause of neuromuscular blockade. British journal of obstetrics and gynaecology. 1994;101:262–3. doi: 10.1111/j.1471-0528.1994.tb13126.x. [DOI] [PubMed] [Google Scholar]
- 32.Snyder SW, Cardwell MS. Neuromuscular blockade with magnesium sulfate and nifedipine. American journal of obstetrics and gynecology. 1989;161:35–6. doi: 10.1016/0002-9378(89)90226-3. [DOI] [PubMed] [Google Scholar]
- 33.Mordes JP, Wacker WEC. Excess magnesium. Pharmacol Rev. 1978;29:273–300. [PubMed] [Google Scholar]
- 34.McCubbin JH, Sibai BM, Abdella TN, Anderson GD. Cardiopulmonary arrest due to maternal hypermagnesaemia. Lancet. 1981;317:1058. doi: 10.1016/s0140-6736(81)92225-x. [DOI] [PubMed] [Google Scholar]
- 35.Maynard SE, Thadhani R. Pregnancy and the kidney. Journal of the American Society of Nephrology: JASN. 2009;20:14–22. doi: 10.1681/ASN.2008050493. [DOI] [PubMed] [Google Scholar]
- 36.Holland PW. Statistics and causal inference. Journal of the American Statistical Association. 1986;81:945–60. [Google Scholar]