Abstract
Purpose
There are few data examining cardiovascular physiology throughout a marathon. This study was devised to characterize electrocardiographic activity continuously throughout a marathon.
Methods
Cardiac activity was recorded from 19 subjects wearing a Holter monitor during a marathon. The 19 subjects (14 men and 5 women) were aged 39 ± 16 years (mean ± SD) and completed a marathon in 4:32:16 ± 1:23:35. Heart rate (HR), heart rate variability (HRV), T-wave amplitude, T-wave amplitude variability, and T-wave alternans (TWA) were evaluated continuously throughout the marathon.
Results
Averaged across all subjects, HRV, T-wave amplitude variability, and TWA increased throughout the marathon. Increased variability in T-wave amplitude occurred in 86% of subjects, characterized by complex oscillatory patterns and TWA. Three minutes after the marathon, HR was elevated and HRV was suppressed relative to the pre-marathon state.
Conclusion
HRV and T-wave amplitude variability, especially in the form of TWA, increase throughout a marathon. Increasing TWA as a marathon progresses likely represents a physiologic process as no arrhythmias or cardiac events were observed.
Keywords: variability, t-wave, heart rate, marathon, running
Introduction
Over the last two decades, the popularity of amateur marathons has risen dramatically. Nearly 500,000 individuals crossed the finish line of a marathon in 2012 (Lamppa 2013). Long-distance running is known to provide excellent health benefits and is associated with lower disability and increased life expectancy (Chakravarty et al. 2008; Sarna et al. 1993). However, there is some concern that marathon running may be dangerous to the heart.
For example, sudden cardiac arrest (SCA) represents the leading cause of death during a marathon (Webner et al. 2012). At least 40 people (1.01 per 100,000) suffered a SCA during a marathon in the last ten years with a mortality rate of 0.63 per 100,000 (Kim et al. 2012). While SCA during a marathon remains rare, the reported incidence of SCA in marathoners likely underestimates the true incidence as it was tabulated from media, Internet searches, and surveys.
SCA occurs more frequently during the last four (Webner et al. 2012) to 6.2 miles (Kim et al. 2012) of a marathon. It remains unclear whether cardiac instability increases towards the end of a marathon because the cardiovascular response throughout the marathon remains unknown and understudied. The present study characterized electrocardiogram (ECG) changes in individuals as they completed a marathon.
Methods
Subjects and Protocol
Written informed consent was obtained from all subjects and the Institutional Review Board of the University of Pittsburgh approved all activities. Ten participants were screened at each of three events: the 2012 Pittsburgh marathon, the 2013 Galveston marathon, and the 2013 Seabrook Lucky Trail marathon. All participants in each marathon were invited to participate using flyers and informational emails. Subjects were selected to participate in the study based on their ability to wear the Holter monitor throughout the marathon, and their willingness to have their blood drawn before and after the race. Subjects were selected on a first-come, first-serve basis. The number of subjects in each race was limited by the number of Holter monitors available. An effort was made to recruit subjects of both sexes and of a variety of ages and running abilities to most accurately reflect the general population.
In the 1-3 hour period prior to the start of a marathon, five-lead Holter monitors (Nasiff, Central Square, New York) were secured to subjects using tape, benzoin, and often compression shirts. Each Holter monitor collected 3 channels of data. For channel 1, the negative lead was applied to the center of the manubrium at the top of the sternum and the positive lead was applied to the left mid-clavicular line at the level of the 6th rib in V3 position. For channel 2, the negative lead was applied at the center of the manubrium at the top of the sternum and the positive lead was applied to the left anterior axillary line at the level of the 6th rib in V5 position. Channel 3 was generated using a negative lead placed 1 inch to the right of the xiphoid process on the rib, just below V1 position at the bottom of the rib cage. The positive lead was placed at the left anterior axillary line at the level of the 6th rib in V5 position.
For each subject, the channel with the best quality of data and least amount of noise or interference was selected for further analysis. Channel 1 was selected for analysis in 13 subjects. Channel 2 was selected for 7 subjects and Channel 3 was selected for 1 subject.
Electrocardiographic (ECG) data were recorded at a sampling rate of 256 Hz before, during, and after the marathon. All data from Holter monitors were recorded directly onto an external data card. After the data were imported using Cardiocard software (Nasiff, Central Square, New York), they were smoothed using a 5-point moving average and detrended across the entire recording and again over each QRST complex using customized software (MATLAB, Mathworks, Natick, MA). All data were visually inspected to confirm adequate quality for analysis and to ensure that premature ventricular contractions (PVC) were excluded from analysis. Each heartbeat was defined as the peak of each R wave.
Fourteen subjects (A in Table 1) also wore GT3X+ accelerometers (ActiLife, Pensacola, Florida) which monitored their activity continuously at 100 Hz throughout the marathon. Before and after the marathon, 5-10mL of blood was obtained from each subject to acquire basic metabolic profiles (BMP), creatine kinase (CK) levels, and troponin levels. Before the marathon, the subjects' body fat was estimated using skin fold calipers (Lange, Ann Arbor, MI) applied at the chest, abdomen, and thigh for men and triceps, supra-iliac, and thigh for women. Measurements were taken three times at each site. The average skinfold thickness at each site was used to estimate body fat.
Table 1.
Each subject is listed in the temporal order in which they were recruited. Subj # indicates each subject's number. *denotes a subject with pre-marathon, marathon, and post-marathon data. ** denotes one of the 2 additional subjects with pre-marathon data and partial marathon data. A denotes subjects who wore actigraphs. Race time indicates the number of seconds each subject took to complete the marathon. Subjects 1-10 ran the 2012 Pittsburgh marathon, subjects 11-18 ran the 2013 Galveston marathon, and subjects 19-28 ran the 2013 Seabrook Lucky trail marathon.
| Subj # | Race time (s) | M/F | Age | Body Fat (%) |
BMI |
|---|---|---|---|---|---|
| 1*A | 15241 | M | 52 | 19.75 | 23.41 |
| 2*A | 18461 | F | 51 | 22.3 | 21.76 |
| 3*A | 18870 | M | 30 | 11.8 | 23.09 |
| 4* | 10810 | M | 25 | 3.75 | 22.43 |
| 5* | 17774 | F | 29 | 23.7 | 21.46 |
| 6*A | 19194 | M | 35 | 13.7 | 22.24 |
| 7* | 10921 | M | 25 | 10.2 | 22.67 |
| 8 | 22626 | F | 25 | 25 | 20.55 |
| 9*A | 16216 | M | 58 | 19 | 25.59 |
| 10* | 16874 | M | 28 | 12 | 20.93 |
| 11 | 19844 | F | 58 | 17.57 | 20.6 |
| 12*A | 15689 | M | 62 | 19.45 | 23.9 |
| 13*A | 20901 | M | 66 | 19.3 | 23.8 |
| 14*A | 19541 | F | 37 | 29.1 | 24.7 |
| 15*A | 17600 | M | 28 | 15.9 | 26.6 |
| 16* | 16655 | M | 47 | 18.75 | 26.4 |
| 17 | 17563 | F | 48 | 22.1 | 24.1 |
| 18* | 18530 | F | 54 | - | - |
| 19*A | 15903 | F | 30 | 19 | 20.8 |
| 20*A | 13409 | M | 34 | 7.6 | 23.1 |
| 21 | 20265 | M | 66 | 22.8 | 23.8 |
| 22*A | 24911 | M | 50 | 25 | 35.2 |
| 23 | 23682 | F | 42 | 29.9 | 26.6 |
| 25*A | 19212 | M | 48 | 19.4 | 23.4 |
| 26** | 13484 | M | 42 | 13.3 | 23.1 |
| 27 | 23732 | F | 47 | 27.6 | 23 |
| 28** | 17788 | F | 55 | 14.8 | 19.3 |
Definitions
This study focused on specific measures of cardiac activity throughout a marathon. The primary measures of cardiac activity included: heart rate (HR), HRV, T-wave amplitude, T-wave amplitude variability, and T-wave alternans (TWA). Heart rate variability was calculated as the root mean squared of the difference between subsequent R-R intervals (RMSSD), one measure of HRV (Task Force 1996). T-wave amplitude was defined as the height of the T-wave at its maximum point (mV) normalized by the magnitude of the corresponding R-wave (mV), to control for variations in total signal amplitude. T-wave amplitude variability was defined as the standard deviation of the normalized T-wave amplitude. TWA, one form of T-wave variability, was defined as the average difference in normalized maximal amplitude between consecutive T-waves (Nearing and Verrier 2002a). T-wave amplitude, T-wave amplitude variability, and TWA are expressed in arbitrary units as they were normalized by the corresponding R-wave. HR, HRV, T-wave amplitude, T-wave amplitude variability, and TWA were all calculated using customized software (MATLAB, Mathworks, Natick, MA).
Holter Analyses: Elapsed Time Analysis and Proportion of race Analysis
HR, HRV, T-wave amplitude, T-wave amplitude variability, and TWA changes during the marathon were quantified using two separate analyses, termed an “elapsed time” analysis and a “proportion of race” analysis. Both the elapsed time and proportion of race analyses evaluated HR, HRV, TWA, and T-wave variability throughout the marathon; however, the elapsed time analysis examined changes in cardiac activity as a function of the total duration of exercise while the proportion of race analysis determined changes in cardiac activity relative to the proportion of the marathon completed.
In the elapsed time analysis, non-overlapping 30-minute bins of data for each subject were normalized by the first 30-minutes of exercising data for the same subject to account for variability in measures of cardiac activity between individuals (Martinmaki et al. 2006). These normalized values were analyzed using linear regression to evaluate how cardiac activity changed throughout the marathon in relation to total duration of exercise.
Prior studies report that the incidence of cardiac arrest increases towards the end of a marathon, irrespective of finishing time (Kim et al. 2012; Webner et al. 2012). It was therefore important to determine how cardiac activity changed as a function of the proportion of the marathon completed in addition to the total duration of exercise. The proportion of race analysis was implemented such that cardiac activity across all runners at equivalent points in the marathon could be compared, irrespective of each runner's pace. This method divided each subject's continuous ECG data into four equal proportions of time such that each proportion represented one-quarter of the total race time for each subject. Using this approach, each proportion included data from 19 subjects and were compared using Friedman tests to control for inter-subject variability with Dunn's post-test comparisons because the data were not normally distributed. In order for a change to be considered a marathon-related effect on cardiac activity, the p value from both the elapsed time and proportion of race analysis was required to be < 0.05.
Correlations between marathon-related changes in each measure of cardiac activity with race time were performed using Spearman correlation. For these analyses, the degree of change in each measure of cardiac activity was defined as the percent change in each measure between the last and first 30-minute bin in the elapsed time analysis.
Pre-marathon recordings included three minutes of data recorded 17 +/- 11 minutes (mean +/- SD) before the start of the marathon, while post-marathon recordings included three minutes of data that began three minutes after completion of the marathon. Wilcoxon matched pairs tests were used to compare each measure of cardiac activity pre- versus post-marathon. Means ± SD were reported unless otherwise specified. All statistical analyses were performed using Prism (Graphpad, La Jolla, CA).
Activity Analysis
Acceleration data from 13 subjects was imported into ActiLife software directly from each GT3X+ device (ActiLife, Pensacola, Florida). These data were used to quantify changes in acceleration throughout the marathon in both the elapsed time and proportion of race analysis using Prism (Graphpad, La Jolla, CA).
Blood Analyses
Blood was drawn from a subset of subjects between 1 and 21 days prior to the marathon and again immediately after the marathon. Whole-blood BMP (i-STAT, Abbott, Princeton, New Jersey), CK (ECPK-100, BioAssay Systems, Hayward, CA) and troponin-I levels (BQ015C kits, GenTaur, San Jose, CA, respectively) were compared before and after the marathon using Wilcoxon matched pairs tests (Prism, Graphpad, La Jolla, CA).
Results
Twenty-eight subjects provided consent for the study (Table 1). One subject failed to start the marathon. Data from six other subjects were excluded due to noise during the recording, data card malfunction, or Holter malfunction. Data from the remaining 21 individuals (* in Table 1) were analyzed before and during the marathon. In two of these 21 subjects (** in Table 1), data collection was interrupted towards the end of the marathon due to mechanical failure. Partial data (61% and 41% of their total marathon running time, respectively) from these two subjects were used in the elapsed time analysis alone. ECG data from the remaining 19 subjects were used in both the elapsed time and proportion of race analyses. After removing noise, 84-100% of data from each subject was used for analysis. Nine subjects completed the 2012 Pittsburgh marathon, 6 completed the 2013 Galveston marathon, and 4 completed the 2013 Seabrook Lucky Trail marathon. The two subjects with partial marathon data completed the 2013 Seabrook marathon. Sample data from one subject is depicted in Figure 1, demonstrating ECG recordings that were acquired before, during, and after the marathon.
Fig. 1.
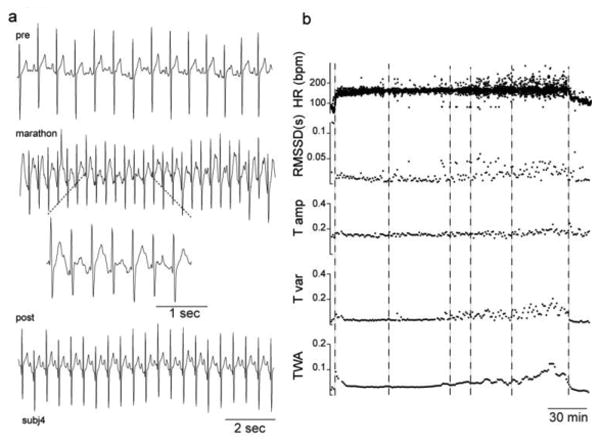
Sample data from one subject. a) Detrended ECG data recorded from the Holter before (pre), during (marathon), and after the marathon (post.) The dotted lines denote an expanded view of the ECG data. b) Heart rate (HR) in beats per minute (bpm), HRV (RMSSD) in seconds (s), and T-wave amplitude (T amp), T-wave variability (T var), and T-wave alternans (TWA) in arbitrary units from the same subject as Figure a are plotted as a function of time instantaneously (HR) or in 1-minute intervals (all others). Box and whisker plots represent range, median, and interquartiles. Vertical dashed lines indicate the start, the 10-kilometer marker, the half-marathon marker, the 15-mile marker, the 20-mile marker, and the finish line.
Subjects' performance in the race
The 19 subjects comprised a group of 14 men and 5 women aged 39 ± 16 (mean ± SD) who completed a marathon in 4:32:16 ± 1:23:35. The average body fat of this cohort was 16 ± 7% and BMI was 23 ± 6. When including the two additional subjects with partial marathon data, the group was comprised of 15 men and 6 women aged 42 ± 13 who completed a marathon in 4:44:06 ± 54:06. The average body fat was 17 ± 6% and BMI was 24 ± 3. Due to limited supply, only 13 subjects wore actigraphs (A in Table 1). Averaged across all runners, acceleration decreased during the marathon by both the elapsed time analysis (p = 0.0007, slope = -.06) and the proportion of race method (p = 0.0035).
Heart rate did not change throughout the marathon, but was elevated after the marathon
Averaged across all subjects, HR decreased with time throughout the marathon (Figure 2a) by the elapsed time analysis (p = 0.04, slope = -0.02; Figure 2b), but not by the proportion of race analysis (p = 0.07; Figure 2c). Therefore, there was no robust change in HR. When examining individual subjects, HR decreased during the marathon in 10 subjects and increased during the marathon in 11 subjects. A positive correlation was evident between marathon-related changes in HR and race time (p = 0.02, R = -0.5), such that faster runners tended to exhibit an increase in HR as the marathon progressed. Three minutes after completion of the marathon, HR was elevated relative to HR just prior to the marathon (p = 0.0001; Figure 2d).
Fig. 2.
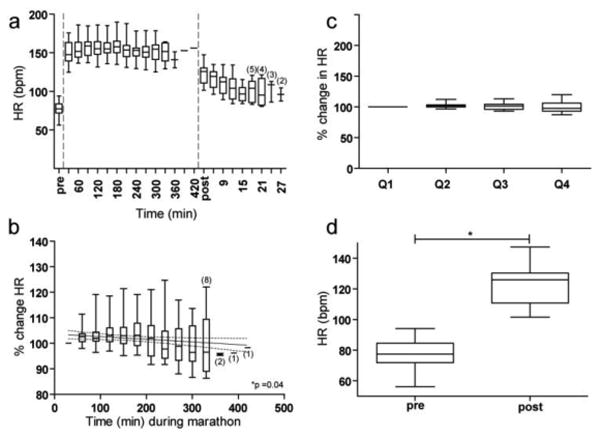
Changes in HR (bpm) before, during, and after the marathon. Box and whisker plots represent range, median, and interquartiles. a) HR before, during, and after the marathon is plotted continuously. Before and after the marathon, each plot represents the average HR over 3 minutes. During the marathon, each plot represents the average HR over 30 minutes. Small numbers in parentheses denote the number of subjects included if the sample size ≤ 5. b) The change in HR over time during the marathon in 30-minute non-overlapping intervals relative to the first 30 minutes of the marathon (first horizontal dash). The solid line indicates the best fit regression line and the dotted lines represent the 95% confidence interval. Small numbers in parentheses denote the number of subjects included in each average if the sample size was ≤ 8. c) The change in HR during each proportion of the marathon relative to the first proportion of the marathon (first horizontal dash.) d) HR pre- versus post-marathon.
Heart rate variability increased during the marathon, but was suppressed after the marathon
Averaged across all subjects, HRV increased during the marathon (Figure 3a) by the elapsed time analysis (p < 0.0001, slope = 0.34; Figure 3b) and the proportion of race analysis (p = 0.046; Figure 3c). Twelve subjects exhibited an increase in HRV during the marathon while 9 subjects exhibited a decrease in HRV. There was no relationship between marathon-related changes in HRV and total race time. Three minutes after completion of the marathon, HRV was decreased relative to HRV just prior to the marathon (p = 0.001; Figure 3d).
Fig. 3.
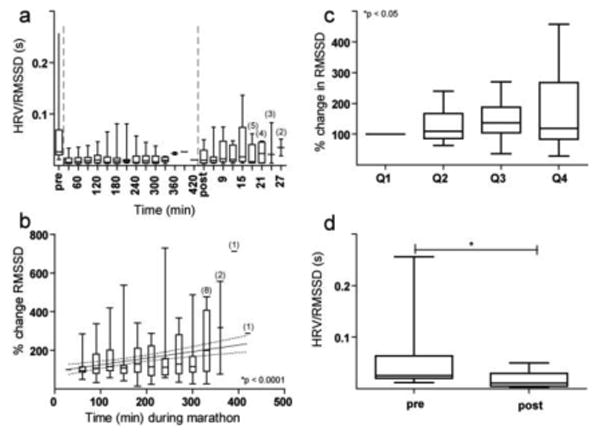
Changes in HRV using RMSSD (seconds) before, during, and after the marathon. a) HRV before, during, and after the marathon are plotted continuously. b) The change in HRV over time during the marathon in 30-minute non-overlapping intervals. c) The change in HRV during each proportion of the marathon. d) HRV pre- versus post-marathon. Unless otherwise stated, the conformities of Figure 3 are identical to that of Figure 2.
T-wave amplitude variability increased during the marathon, but normalized after the marathon
Averaged across all subjects, T-wave amplitude (Figure 4b) increased by the elapsed time analysis (p < 0.0001, slope =0.09; Figure 4d) in 17 of 21 subjects, but did not change by the proportion of race analysis (Figure 4f). There was no relationship between marathon-related changes in T-wave amplitude and race time. Three minutes after completion of the marathon, T-wave amplitude was no different from pre-marathon values (p = 0.35; Figure 4h).
Fig. 4.
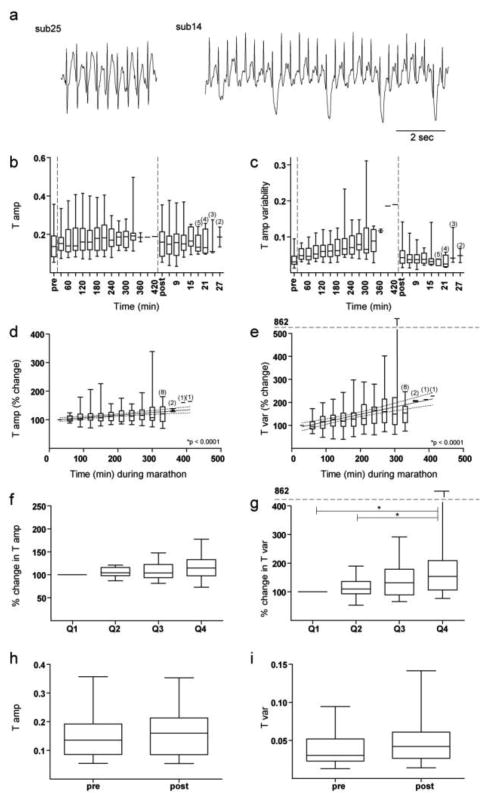
Changes in T-waves before, during, and after the marathon. a) Examples of ECG tracings from 2 subjects showing variability in T-waves during the marathon. b) T-wave amplitude before, during, and after the marathon are plotted continuously in arbitrary units. c) T-wave amplitude variability before, during, and after the marathon are plotted continuously in arbitrary units. d) The change in T-wave amplitude over time during the marathon in 30-minute non-overlapping intervals. e) The change in T-wave amplitude variability over time during the marathon in 30-minute non-overlapping intervals. f) The change in T-wave amplitude during each proportion of the marathon. g) The change in T-wave amplitude variability during each proportion of the marathon. h) T-wave amplitude pre- versus post-marathon in arbitrary units. i) T-wave amplitude variability pre- versus post-marathon in arbitrary units. Unless otherwise stated, all conformities of Figure 4 are identical to that of Figure 2.
In contrast, T-wave amplitude variability increased dramatically throughout the marathon (Figure 4a,c) by both the elapsed time analysis (p < 0.0001, slope = 0.32, Figure 4e) and the proportion of race analysis (p=0.0025; Figure 4g). T-wave amplitude variability increased during the marathon in 18 of 21 subjects. There was no relationship between marathon-related changes in T-wave amplitude variability and race time. T-wave amplitude variability was similar both before the marathon and three minutes after the marathon (p = 0.08; Figure 4i).
Evidence of T-wave alternans increased during the marathon, but normalized after the marathon
Increases in T-wave amplitude variability during the marathon included complex oscillations and TWA (Figures 1, 5a, 5b). TWA increased markedly throughout the marathon by both the elapsed time (p < 0.0001, slope = 0.33; Figure 5c) and the proportion of race analysis (p = 0.0002; See Figures 5d). TWA increased in 18 of 21 subjects. There was no relationship between the magnitude of change in TWA and race time. TWA was similar both before the marathon and three minutes after the marathon (p = 0.07; Figure 5e).
Fig. 5.
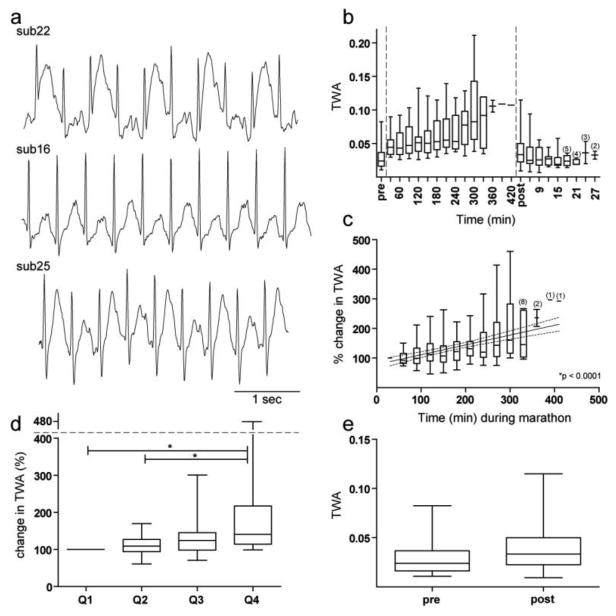
Changes in TWA before, during, and after the marathon. a) Examples of ECG tracings from 3 separate subjects showing evidence of TWA during the marathon. b) TWA before, during, and after the marathon are plotted continuously in arbitrary units. c) The change in TWA over time during the marathon in 30-minute non-overlapping intervals. d) The change in TWA during each proportion of the marathon. e) TWA pre- versus post-marathon in arbitrary units. Unless otherwise stated, all conformities of Figure 5 are identical to that of Figure 2.
Marathon-related changes in BMP, CK, and troponin
Sodium (140.1 ± 2.14 mEq/L pre; 139.7 ± 3.3 post), potassium (4.2 ± 0.4 mEq/L pre; 4.3 ± 0.4 post), calcium (1.2 ± 0.06 mg/dl pre and post), hematocrit (43.4 ± 3.8% pre; 42.7 ± 5.4 post), and hemoglobin (14.8 ± 1.3 gm/dl pre; 14.5 ± 1.8 post) did not differ pre- versus post-marathon. No subject's sodium was < 132 or > 145 and no subject's potassium was < 3.5 or > 5 either before or after the marathon. Troponin-I was undetectable pre- and post-marathon.
Creatinine (Cr) increased from 0.96 ± 0.2 mg/dl before the marathon to 1.5 ± 0.4 after the marathon (p < 0.0001). Blood urea nitrogen (BUN) also increased from 16.8 ± 5.4 mg/dl before the marathon to 20.8 ± 5.5 after the marathon (p = 0.005). The post-marathon BUN:Cr ratio was ≤ 10 in 2 subjects with Cr levels of 1.7 mg/dl and 2.6. CK was increased from 30 ± 36 U/L before the marathon to 110 ± 70 after the marathon (p < 0.0001). One subject's post-marathon CK value reached 281. Post-marathon CK levels were not correlated with marathon-related changes in HR, HRV, T-wave amplitude, T-wave amplitude variability, or TWA.
Discussion
This is the first study to examine continuous ECG data during a marathon. Nearly all prior studies examining the cardiac effects of marathon completion evaluated subjects pre- and post-marathon. We found that HRV, T-wave amplitude variability, and T WA increased throughout a marathon. Relative to pre-marathon values, post-marathon HR increased and HRV decreased, while T-wave amplitude variability and TWA remained unchanged. There was no evidence of acute myocyte damage following the marathon.
Marathon-related changes in T-wave amplitude variability and T-wave alternans
T-wave amplitude variability and T WA increased throughout the marathon in nearly all participants (examples in Figures 1a,4a, and 5a). TWA is thought to be caused by a spatial dispersion of repolarization that can occur following ischemia to a focal region of the heart (Chinushi et al. 1998) or by temporal dispersion of repolarization that occurs with rapid alterations of calcium and ion cycling at higher HR (Kavesh et al. 1998; Pastore et al. 1999; Schwartz and Malliani 1975). Increasing TWA throughout the marathon was not related to changes in HR, as HR remained unchanged throughout the marathon. Furthermore, post-marathon HR was elevated relative to pre-marathon HR, while TWA returned to baseline. Increasing metabolic demands throughout the marathon could increase temporal dispersion of repolarization by altering ion cycling (Verrier and Malik 2013).
The clinical implications of increasing TWA throughout a marathon remain unclear. Abnormalities in repolarization and calcium cycling are linked to arrhythmogenesis (Verrier and Malik 2013), and TWA is associated with an increased risk of ventricular arrhythmias (Adam et al. 1984; Raeder et al. 1992). Spatial and temporal dispersions of repolarization are thought to cause arrhythmias by preventing depolarization in a specific group of myocytes or by inducing conduction block and fibrillation (Narayan 2006). In response to ischemia, TWA evolves into higher order oscillations in T-wave amplitude that can culminate in ventricular fibrillation (Nearing and Verrier 2002b), visibly similar to the oscillatory nature of T-wave amplitude variability observed in this study (Figure 1a).
Exercise stress test-induced TWA reportedly predicts the inducibility of sustained ventricular tachycardia or ventricular fibrillation (Estes et al. 1997) and is associated with cardiac mortality and sudden cardiac death (Verrier et al. 2011). A greater magnitude of TWA is also associated with a higher risk of developing ventricular tachycardia (Kavesh et al. 1998) and ischemia-induced ventricular fibrillation, independent of HR (Nearing et al. 1996; Nearing et al. 1994; Nieminen et al. 2011). Thus, it would seem plausible that increasing TWA as a marathon progresses might heighten the risk of arrhythmias. However, nearly all subjects in this study exhibited marathon-related increases in TWA without developing an arrhythmia or cardiac event. Furthermore, only ∼14% of SCAs during a marathon are thought to be attributable to arrhythmias (Kim et al. 2012). Thus, increased TWA as a marathon progresses most likely represents a physiologic response to prolonged exercise . However, it is difficult to ignore the previously established association between TWA and arrhythmias. As subjects in this study were not screened for pre-existing cardiac pathology, future studies would be necessary to delineate the physiologic implications of marathon-related changes in TWA in separate clinical populations.
Marathon-related changes in HR and HRV
The finding that HRV increased throughout the marathon was surprising, as previous work has shown that HRV is suppressed during exercise and that HRV decreases with increasing oxygen consumption during exercise (Perini et al. 2000). Increasing HRV is unlikely attributable to changes in HR, because HR remained unchanged, or to an increasing frequency of PVCs, because PVCs were excluded from analysis. Atrial arrhythmias could increase HRV as the marathon progresses; however, no arrhythmias were observed in any of the subjects. Vagally-mediated HRV diminishes when an individual is exercising at greater than 50-60% of maximal performance (Tulppo et al. 1998; Tulppo et al. 1996; Yamamoto et al. 1991). Throughout the marathon, a progressive decrease in vagally-mediated HRV in conjunction with an increase in sympathetic drive may increase HRV throughout the marathon.
Relative to the beginning of the marathon, HRV decreased after the marathon while HR increased. An elevation in nocturnal resting HR and a suppression of nocturnal resting HRV have been previously reported after completion of a marathon, and are thought to reflect parasympathetic withdrawal with delayed sympathetic de-activation (Hynynen et al.). The imbalance generated by suppressed vagal activity and increased sympathetic activity (Malik and Camm 1993) has the potential to induce electrical instability, generating a pro-arrhythmic state. Indeed, low resting HRV represents a strong and independent predictor of mortality and arrhythmic complications following acute myocardial infarction (Kleiger et al. 1987) and is thought to reflect cardiac instability (Tsuji et al. 1996). Suppressed HRV after a marathon could suggest increased cardiac instability after a marathon. Indeed, endurance athletes have a greater incidence of ventricular arrhythmias (Abdulla and Nielsen 2009; Heidbuchel et al. 2012) than the general population.
Marathon-related changes in blood markers of cardiac damage
In an effort to identify whether marathon-related changes in cardiac activity might be associated with acute cardiac stress or damage, we measured marathon-related changes in troponin and CK. Cardiac troponin did not increase after the marathon. This finding is consistent with some studies (Lucia et al. 1999; Smith et al. 2004) while others report that cardiac troponin levels are elevated to ≥ 0.03 ng/ml in ∼40% of people immediately post-marathon (Fortescue et al. 2007; Neilan et al. 2006).
Total CK levels increased after the marathon. In previous studies, CK and CK-MB, were found to increase 4-8 hours after a marathon (Lucia et al. 1999) and 24 hours post-marathon (Siegel et al. 1997). This finding is thought to reflect primarily skeletal muscle damage, as individuals with elevated post-marathon CK and CK-MB levels were asymptomatic and free of cardiac events one year later (Siegel et al. 1997) with no evidence of myocardial necrosis on cardiac MRI (Mousavi et al. 2009). Importantly, there were no correlations between post-marathon CK and marathon-related changes in cardiac activity.
Limitations and Potential Confounds
Increasing variability in T-wave amplitude during the marathon might be attributable to increasingly poor contact of the electrodes to the body or variations in respiratory cycle. The extensive taping methods and compression shirts employed in this study maximally optimized the quality of ECG signal obtained. Any variability in electrode contact would affect the amplitude of both the R-wave and T-wave. Therefore, the T-wave amplitudes were normalized by corresponding R-wave amplitudes. Of note, the post-marathon recordings were performed without altering the electrode contacts, yet T-wave amplitude variability returned to pre-marathon values within three minutes following the marathon. Above all, alternations in electrode contact would be unlikely to explain an increase in the difference between consecutive heart beats' T-wave amplitudes, measured as TWA.
While great efforts were made to ensure that electrode contacts remained stable and data were visually inspected to ensure that the quality of recordings remained uniform throughout the marathon, exercise-related changes in electrode contacts may represent one minor source of artifact. The respiratory cycle could also influence the changes in HRV and T-wave amplitude variability that we observed.
Other limitations of this study included the small sample size and heterogeneity of the population. We did not attempt to exclude any individual based on age or gender in order to obtain a dataset most representative of typical marathon participants. Indeed, the average finish time of 4:32:16 ± 1:23:35 was similar to the average finish time of all marathon runners: 4:28:48 (Lamppa 2013). Roughly 10% of our subjects qualified for the Boston Marathon, which is similar to the 10.4% of all marathoners who qualify in a given year (Hamilton 2013). While this cohort was representative of the general population of marathon participants, there were too few subjects to divide our data into further subgroups based on age, gender, or training duration and type. Future studies would be necessary to determine whether individual measures of cardiac activity would be differentially influenced by age, gender, training, or pre-existing cardiac disease.
Conclusions
Heart rate variability, T-wave amplitude variability, and TWA increased throughout a marathon. Increasing TWA as a marathon progresses likely represents a physiologic process as no arrhythmias or cardiac events were observed. The implications of these changes in different clinical populations remain an important topic of future study in an effort to improve the safety of endurance sport.
Acknowledgments
This study was funded by the Department of Emergency Medicine at the University of Pittsburgh Medical Center, the Pittsburgh Emergency Medicine Foundation, and the Medical Scientist Training Program at the University of Pittsburgh School of Medicine. We would also like to thank Patrice Matamoros for her help with the Pittsburgh marathon, Robby Sabban for his help with the Seabrook Lucky Trail marathon, and Jana Landry, and Kim Bachmeier for their help with the Galveston marathon. We would also like to thank Stacy Gerstel, Priya Khorana, and Lindsey Russo for technical assistance, and especially thank each of the subjects for volunteering their time to participate in this study.
Funding for this work from: Department of Emergency Medicine, University of Pittsburgh Medical Center, Pittsburgh, PA, and a post-doctoral fellowship from the University of Pittsburgh Medical Center, Pittsburgh, PA.
Abbreviations
- SCA
sudden cardiac arrest
- HR
heart rate
- HRV
heart rate variability
- TWA
t-wave alternans
- ECG
electrocardiogram
- CK
creatine kinase
- RMSSD
root mean squared of the successive differences
- BMP
Basic metabolic profile
- Cr
Creatinine
- PVC
Premature ventricular contraction
Footnotes
The authors have no conflict of interest to disclose.
References
- Abdulla J, Nielsen JR. Is the risk of atrial fibrillation higher in athletes than in the general population? A systematic review and meta-analysis. Europace. 2009;11:1156–1159. doi: 10.1093/europace/eup197. [DOI] [PubMed] [Google Scholar]
- Adam DR, Smith JM, Akselrod S, Nyberg S, Powell AO, Cohen RJ. Fluctuations in T-wave morphology and susceptibility to ventricular fibrillation. J Electrocardiol. 1984;17:209–218. doi: 10.1016/s0022-0736(84)80057-6. [DOI] [PubMed] [Google Scholar]
- Chakravarty EF, Hubert HB, Lingala VB, Fries JF. Reduced disability and mortality among aging runners: a 21-year longitudinal study. Arch Intern Med. 2008;168:1638–1646. doi: 10.1001/archinte.168.15.1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinushi M, Restivo M, Caref EB, El-Sherif N. Electrophysiological basis of arrhythmogenicity of QT/T alternans in the long-QT syndrome: tridimensional analysis of the kinetics of cardiac repolarization. Circ Res. 1998;83:614–628. doi: 10.1161/01.res.83.6.614. [DOI] [PubMed] [Google Scholar]
- Estes NA, 3rd, Michaud G, Zipes DP, El-Sherif N, Venditti FJ, Rosenbaum DS, Albrecht P, Wang PJ, Cohen RJ. Electrical alternans during rest and exercise as predictors of vulnerability to ventricular arrhythmias. Am J Cardiol. 1997;80:1314–1318. doi: 10.1016/s0002-9149(97)00694-2. [DOI] [PubMed] [Google Scholar]
- Fortescue EB, Shin AY, Greenes DS, Mannix RC, Agarwal S, Feldman BJ, Shah MI, Rifai N, Landzberg MJ, Newburger JW, Almond CS. Cardiac troponin increases among runners in the Boston Marathon. Ann Emerg Med. 2007;49:137–143. 143, e131. doi: 10.1016/j.annemergmed.2006.09.024. [DOI] [PubMed] [Google Scholar]
- Hamilton T. Life of a BQ Squeaker. Runner's World 2013 [Google Scholar]
- Heidbuchel H, Prior DL, La Gerche A. Ventricular arrhythmias associated with long-term endurance sports: what is the evidence? Br J Sports Med. 2012;46(suppl):i44–50. doi: 10.1136/bjsports-2012-091162. [DOI] [PubMed] [Google Scholar]
- Hynynen E, Vesterinen V, Rusko H, Nummela A. Effects of moderate and heavy endurance exercise on nocturnal HRV. Int J Sports Med. 2010;31:428–432. doi: 10.1055/s-0030-1249625. [DOI] [PubMed] [Google Scholar]
- Kavesh NG, Shorofsky SR, Sarang SE, Gold MR. Effect of heart rate on T wave alternans. J Cardiovasc Electrophysiol. 1998;9:703–708. doi: 10.1111/j.1540-8167.1998.tb00957.x. [DOI] [PubMed] [Google Scholar]
- Kim JH, Malhotra R, Chiampas G, d'Hemecourt P, Troyanos C, Cianca J, Smith RN, Wang TJ, Roberts WO, Thompson PD, Baggish AL. Cardiac arrest during long-distance running races. N Engl J Med. 2012;366:130–140. doi: 10.1056/NEJMoa1106468. [DOI] [PubMed] [Google Scholar]
- Kleiger RE, Miller JP, Bigger JT, Jr, Moss AJ. Decreased heart rate variability and its association with increased mortality after acute myocardial infarction. Am J Cardiol. 1987;59:256–262. doi: 10.1016/0002-9149(87)90795-8. [DOI] [PubMed] [Google Scholar]
- Lamppa R. 2013 Annual Marathon Report. Running USA (ed) 2013 [Google Scholar]
- Lucia A, Moran M, Perez M, Saborido A, Diaz E, Megias A, Chicharro JL. Short-term effects of marathon running in master runners: no evidence of myocardial injury. Int J Sports Med. 1999;20:482–486. doi: 10.1055/s-1999-8824. [DOI] [PubMed] [Google Scholar]
- Malik M, Camm AJ. Components of heart rate variability--what they really mean and what we really measure. Am J Cardiol. 1993;72:821–822. doi: 10.1016/0002-9149(93)91070-x. [DOI] [PubMed] [Google Scholar]
- Martinmaki K, Rusko H, Kooistra L, Kettunen J, Saalasti S. Intraindividual validation of heart rate variability indexes to measure vagal effects on hearts. Am J Physiol Heart Circ Physiol. 2006;290:H640–647. doi: 10.1152/ajpheart.00054.2005. [DOI] [PubMed] [Google Scholar]
- Mousavi N, Czarnecki A, Kumar K, Fallah-Rad N, Lytwyn M, Han SY, Francis A, Walker JR, Kirkpatrick ID, Neilan TG, Sharma S, Jassal DS. Relation of biomarkers and cardiac magnetic resonance imaging after marathon running. Am J Cardiol. 2009;103:1467–1472. doi: 10.1016/j.amjcard.2009.01.294. [DOI] [PubMed] [Google Scholar]
- Narayan SM. T-wave alternans and the susceptibility to ventricular arrhythmias. J Am Coll Cardiol. 2006;47:269–281. doi: 10.1016/j.jacc.2005.08.066. [DOI] [PubMed] [Google Scholar]
- Nearing BD, Hutter JJ, Verrier RL. Potent antifibrillatory effect of combined blockade of calcium channels and 5-HT2 receptors with nexopamil during myocardial ischemia and reperfusion in dogs: comparison to diltiazem. J Cardiovasc Pharmacol. 1996;27:777–787. doi: 10.1097/00005344-199606000-00003. [DOI] [PubMed] [Google Scholar]
- Nearing BD, Oesterle SN, Verrier RL. Quantification of ischaemia induced vulnerability by precordial T wave alternans analysis in dog and human. Cardiovasc Res. 1994;28:1440–1449. doi: 10.1093/cvr/28.9.1440. [DOI] [PubMed] [Google Scholar]
- Nearing BD, Verrier RL. Modified moving average analysis of T-wave alternans to predict ventricular fibrillation with high accuracy. J Appl Physiol. 2002a;92:541–549. doi: 10.1152/japplphysiol.00592.2001. 1985. [DOI] [PubMed] [Google Scholar]
- Nearing BD, Verrier RL. Progressive increases in complexity of T-wave oscillations herald ischemia-induced ventricular fibrillation. Circ Res. 2002b;91:727–732. doi: 10.1161/01.res.0000038887.17976.33. [DOI] [PubMed] [Google Scholar]
- Neilan TG, Januzzi JL, Lee-Lewandrowski E, Ton-Nu TT, Yoerger DM, Jassal DS, Lewandrowski KB, Siegel AJ, Marshall JE, Douglas PS, Lawlor D, Picard MH, Wood MJ. Myocardial injury and ventricular dysfunction related to training levels among nonelite participants in the Boston marathon. Circulation. 2006;114:2325–2333. doi: 10.1161/CIRCULATIONAHA.106.647461. [DOI] [PubMed] [Google Scholar]
- Nieminen T, Nanbu DY, Datti IP, Vaz GR, Tavares CA, Pegler JR, Nearing BD, Belardinelli L, Verrier RL. Antifibrillatory effect of ranolazine during severe coronary stenosis in the intact porcine model. Heart Rhythm. 2011;8:608–614. doi: 10.1016/j.hrthm.2010.11.029. [DOI] [PubMed] [Google Scholar]
- Pastore JM, Girouard SD, Laurita KR, Akar FG, Rosenbaum DS. Mechanism linking T-wave alternans to the genesis of cardiac fibrillation. Circulation. 1999;99:1385–1394. doi: 10.1161/01.cir.99.10.1385. [DOI] [PubMed] [Google Scholar]
- Perini R, Milesi S, Fisher NM, Pendergast DR, Veicsteinas A. Heart rate variability during dynamic exercise in elderly males and females. Eur J Appl Physiol. 2000;82:8–15. doi: 10.1007/s004210050645. [DOI] [PubMed] [Google Scholar]
- Raeder EA, Rosenbaum DS, Bhasin R, Cohen RJ. Alternating morphology of the QRST complex preceding sudden death. N Engl J Med. 1992;326:271–272. doi: 10.1056/NEJM199201233260414. [DOI] [PubMed] [Google Scholar]
- Sarna S, Sahi T, Koskenvuo M, Kaprio J. Increased life expectancy of world class male athletes. Med Sci Sports Exerc. 1993;25:237–244. [PubMed] [Google Scholar]
- Schwartz PJ, Malliani A. Electrical alternation of the T-wave: clinical and experimental evidence of its relationship with the sympathetic nervous system and with the long Q-T syndrome. Am Heart J. 1975;89:45–50. doi: 10.1016/0002-8703(75)90008-3. [DOI] [PubMed] [Google Scholar]
- Siegel AJ, Sholar M, Yang J, Dhanak E, Lewandrowski KB. Elevated serum cardiac markers in asymptomatic marathon runners after competition: is the myocardium stunned? Cardiology. 1997;88:487–491. doi: 10.1159/000177396. [DOI] [PubMed] [Google Scholar]
- Smith JE, Garbutt G, Lopes P, Pedoe DT. Effects of prolonged strenuous exercise (marathon running) on biochemical and haematological markers used in the investigation of patients in the emergency department. Br J Sports Med. 2004;38:292–294. doi: 10.1136/bjsm.2002.002873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Task Force of the European Society of Cardiology the North American Society of Pacing Electrophysiology. Heart rate variability: standards of measurement, physiological interpretation and clinical use. Circulation. 1996;93:1043–1065. [PubMed] [Google Scholar]
- Tsuji H, Larson MG, Venditti FJ, Jr, Manders ES, Evans JC, Feldman CL, Levy D. Impact of reduced heart rate variability on risk for cardiac events. The Framingham Heart Study. Circulation. 1996;94:2850–2855. doi: 10.1161/01.cir.94.11.2850. [DOI] [PubMed] [Google Scholar]
- Tulppo MP, Makikallio TH, Seppanen T, Laukkanen RT, Huikuri HV. Vagal modulation of heart rate during exercise: effects of age and physical fitness. Am J Physiol. 1998;274:H424–429. doi: 10.1152/ajpheart.1998.274.2.H424. [DOI] [PubMed] [Google Scholar]
- Tulppo MP, Makikallio TH, Takala TE, Seppanen T, Huikuri HV. Quantitative beat-to-beat analysis of heart rate dynamics during exercise. Am J Physiol. 1996;271:H244–252. doi: 10.1152/ajpheart.1996.271.1.H244. [DOI] [PubMed] [Google Scholar]
- Verrier RL, Klingenheben T, Malik M, El-Sherif N, Exner DV, Hohnloser SH, Ikeda T, Martinez JP, Narayan SM, Nieminen T, Rosenbaum DS. Microvolt T-wave alternans physiological basis, methods of measurement, and clinical utility--consensus guideline by International Society for Holter and Noninvasive Electrocardiology. J Am Coll Cardiol. 2011;58:1309–1324. doi: 10.1016/j.jacc.2011.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verrier RL, Malik M. Electrophysiology of T-wave alternans: Mechanisms and pharmacologic influences. J Electrocardiol. 2013 doi: 10.1016/j.jelectrocard.2013.07.003. [DOI] [PubMed] [Google Scholar]
- Webner D, DuPrey KM, Drezner JA, Cronholm P, Roberts WO. Sudden cardiac arrest and death in United States marathons. Med Sci Sports Exerc. 2012;44:1843–1845. doi: 10.1249/MSS.0b013e318258b59a. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Hughson RL, Peterson JC. Autonomic control of heart rate during exercise studied by heart rate variability spectral analysis. J Appl Physiol. 1991;71:1136–1142. doi: 10.1152/jappl.1991.71.3.1136. 1985. [DOI] [PubMed] [Google Scholar]


