Abstract
Widespread deposition of TAR DNA-binding protein of 43 kDa (TDP-43), a major protein inclusion commonly found in frontotemporal lobar degeneration (FTLD) and amyotrophic lateral sclerosis (ALS) can also be seen in a subset of cases with Alzheimer’s disease (AD). Some of these AD cases have TDP-43 immunoreactivity in basal ganglia (BG) and substantia nigra (SN), regions that when affected can be associated with parkinsonian signs or symptoms, or even features suggestive of frontotemporal dementia. Here, we examined the presence of clinical features of FTLD, parkinsonian signs and symptoms, and BG atrophy on MRI, in 51 pathologically confirmed AD cases (Braak neurofibrillary tangle stage IV–VI) with widespread TDP-43 deposition, with and without BG and SN involvement. All 51 cases had presented with progressive cognitive impairment with prominent memory deficits. None of the patients demonstrated early behavioral disinhibition, apathy, loss of empathy, stereotyped behavior, hyperorality, and/or executive deficits. Furthermore, TDP-43 deposition in BG or SN had no significant association with tremor (p = 0.80), rigidity (p = 0.19), bradykinesia (p = 0.19), and gait/postural instability (p = 0.39). Volumes of the BG structures were not associated with TDP-43 deposition in the BG. The present study demonstrates that TDP-43 deposition in pathologically confirmed AD cases is not associated with a clinical manifestation suggestive of FTLD, or parkinsonian features.
Keywords: TDP-43, Alzheimer’s disease, Frontotemporal dementia, Parkinsonism
Introduction
The TAR DNA-binding protein of 43 kDa (TDP-43) is a major protein inclusion commonly found in frontotemporal lobar degeneration (FTLD) and amyotrophic lateral sclerosis (ALS) [1]. However, TDP-43 can also be seen in other neurodegenerative diseases, including Alzheimer’s disease (AD) [2]. TDP-43 is present in 19–57 % of AD cases [3]. We and others have previously demonstrated that AD cases can have coexisting widespread TDP-43 pathology affecting the amygdala, hippocampus, entorhinal cortex, and temporal, frontal and parietal neocortices [4–6]. Our recent study showed that TDP-43 deposition in AD occurs in a stereotypic fashion over five distinctive stages [3]. The accumulation of TDP-43 is first noted in the amygdala (stage I) followed by entorhinal cortex and subiculum (stage II). The TDP-43 deposition then spreads to hippocampal dentate gyrus and occipitotemporal cortex (stage III), and eventually involves temporal (stage IV) and frontal neocortices and basal ganglia (stage V). Interestingly, a similar pattern of widespread TDP-43 immunore-activity involving frontotemporal cortex and basal ganglia is seen in FTLD [7]. Therefore, the presence of TDP-43 in AD, particularly stages IV and V, could indicate concomitant FTLD. If this were the case, we would expect that features suggestive of FTLD should be present in those AD cases with widespread TDP-43. Here, we assessed for the presence of clinical features suggestive of FTLD, in a large cohort of AD cases with widespread TDP-43 deposition. Given that some of the cases with widespread TDP-43 also had TDP-43 deposited in the basal ganglia (BG) and substantia nigra (SN), we also determined whether TDP-43 deposition in the BG and SN are associated with parkinsonian features, and whether TDP-43 deposition in the BG is associated with BG atrophy on MRI.
Methods
One hundred and ninety-five cases with a pathological diagnosis of intermediate-high probability AD [8] (Braak neurofibrillary tangle stage IV–VI) and TDP-43 immunoreactivity were reviewed to identify cases in which TDP-43 deposition was widespread as in FTLD and affected immunoreactivity in amygdala, entorhinal cortex, dentate granule cells of the hippocampus, occipitotemporal cortex and inferior temporal or frontal cortex (Fig. 1). Therefore, only TDP-43 stages IV and V cases were included. Cases in which TDP-43 immunoreactivity was not widespread and hence focal or limited (stages I–III) were excluded from further analysis. Fifty-one of the 195 AD cases with TDP-43 met criteria and had widespread deposition, as shown in the Fig. 1. All 51 cases were reviewed via light microscopy using a multi-headed microscope, by two investigators (DWD, KAJ), to determine whether TDP-43 deposition was also present in BG or SN (Fig. 1).
Fig. 1.
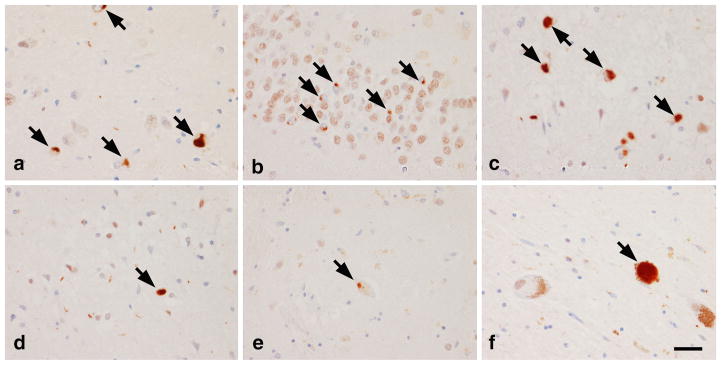
TDP-43 deposition was widespread in all cases, involving amygdala (a), hippocampal dentate fascia (b), entorhinal cortex (c), and inferior temporal cortex (d). In a subset of these cases, TDP-43 immunoreactivity could also be identified in basal ganglia (e) and substantia nigra (f). Bar 30 μm, arrows neuronal cytoplasmic inclusions
All 51 cases had been evaluated at Mayo Clinic, Rochester, MN, by a board certified behavioral neurologist. All were prospectively enrolled and followed longitudinally in our Alzheimer’s Disease Research Center between 1996 and 2012, and had come to autopsy. The medical records, including initial and subsequent clinical assessments, comprehensive neurologic exam by expert behavioral neurologists, and neuropsychometric assessments, were reviewed for the presence of clinical features suggestive of FTLD, in accordance with recently proposed diagnostic criteria [9]. The following clinical features were assessed: early behavioral disinhibition, apathy, loss of empathy, stereotyped behavior, hyperorality, and/or executive deficits. In addition, the presence of parkinsonian features, including resting tremor, cogwheel rigidity, limb bradykinesia, and gait/postural instability were assessed.
Of the 51 cases, 38 had undergone a volumetric MRI during life using a standardized protocol. If more than one MRI was available, then the MRI closest to death was selected for analysis. All images underwent pre-processing correction for gradient non-linearity and intensity nonuniformity. Atlas-based parcellation using SPM5 and the automated anatomical labeling (AAL) atlas were used to calculate volumes of the basal ganglia (caudate nucleus, putamen and globus pallidus). Total intracranial volume (TIV) was calculated by propagating a template-drawn TIV mask to subject space using SPM5. All regional volumes were scaled by TIV to correct for head size.
Chi square tests were performed to compare clinical features across subjects with and without BG or SN TDP-43 deposition. Mann–Whitney U tests were used to compare basal ganglia volumes across both groups. All statistical analysis was performed utilizing JMP software, version 9.0 (SAS Institute Inc, Cary, NC). A p value<0.05 was considered statistically significant.
This study was approved by the Mayo Clinic IRB. Informed consent was obtained from both the patients and their family members or significant others.
Results
The participants included 32 women and 19 men with median age at death of 88.2 years (interquartile range 83.4–92.6). All 51 subjects had presented with progressive cognitive impairment with prominent memory deficits that were evident on clinical assessment and neuropsychometric studies. Based on clinical presentation, all participants were diagnosed with Alzheimer’s dementia. None of the individuals presented with behavioral disinhibition (i.e. socially inappropriate behavior, impulsivity, loss of manner), apathy, loss of empathy, stereotyped behavior, hyperorality, and/or executive deficits. None of the participants received a presenting diagnosis of frontotemporal dementia. Fourteen subjects developed behavioral dyscontrol, such as hypersexuality, socially embarrassing behavior, and aggression, at later stages of the disease. One exhibited apathy 5 years after initial diagnosis, and two developed significant aphasia. However, none received a clinical diagnosis of frontotemporal dementia prior to death. None had a positive family history of FTLD or of amyotrophic lateral sclerosis. The distribution of TDP-43 deposition did not differ between the participants with and without late behavioral symptoms or language deficits.
Of the 51 participants, 25 showed TDP-43 deposition in BG or SN. Eleven showed TDP-43 deposition in both the BG and SN, while 13 showed TDP-43 deposition in SN only and one showed TDP-43 deposition in BG only. The group of 25 subjects with BG or SN deposition did not differ from the individuals without BG or SN deposition in age at death (88.4, 83.6–91.7 vs 88.1, 83.6–93.4, p = 0.30) or female gender (64 % vs. 62 %, p = 0.86). The group of 25 participants with BG or SN deposition had no significant association with parkinsonian features, including tremor (p = 0.80), rigidity (p = 0.19), bradykinesia (p = 0.19), and gait/postural instability (p = 0.39). Volumes of the caudate nucleus, putamen and globus pallidus did not differ between the participants with TDP-43 deposition in the BG versus those without deposition in the BG (Table 1).
Table 1.
Basal ganglia volumes measured on MRI in AD cases with widespread TDP-43 with and without TDP-43 deposition in the basal ganglia
| No TDP-43 deposition in basal ganglia (n = 39) | TDP-43 deposition in basal ganglia (n = 12) | p value | |
|---|---|---|---|
| Caudate volumea | 0.406 (0.364–0.445) | 0.367 (0.309–0.444) | 0.27 |
| Putamen volumea | 0.426 (0.402–0.454) | 0.433 (0.352–0.454) | 0.55 |
| Pallidum volumea | 0.043 (0.026–0.052) | 0.041 (0.037–0.057) | 0.67 |
Volumes are expressed as a percentage of total intracranial volume. Data shown as median (interquartile range)
Discussion
The present study demonstrates that TDP-43 deposition in pathologically confirmed AD is not associated with the clinical manifestation of FTLD or with Parkinsonism.
Previous studies of TDP-43 in AD have not reported clinical features of FTLD [10, 11]. However, those studies were limited since they included cases with any regional TDP-43 deposition, i.e. the majority did not have widespread TDP-43 deposition typical of FTLD. The lack of clinical features of FTLD therefore suggests that TDP-43 deposition in AD does not simply represent a combination of typical FTLD and AD. It is unlikely that the AD pathology is completely masking any FTLD associated clinical features in all of our 51 participants. Furthermore, in two of our previous studies [11, 12], imaging analysis did not identify patterns of atrophy suggestive of typical FTLD. Although some of our participants later developed behavioral changes that are commonly associated with FTLD, these symptoms are not uncommonly observed in patients with late stages of AD [13], suggesting that these features are not likely related to the late presentation of FTLD in these subjects.
We also did not find any association between TDP-43 deposition in the BG or SN and Parkinsonism. This is interesting since Parkinsonism in any setting is associated with involvement of these two structures [14]. TDP-43 deposition in the BG and SN is therefore unlikely to be associated with any significant, concomitant neuronal loss in these two structures, in keeping with the fact that volumes of the BG structures were not significantly reduced in those who had TDP-43 deposition in the BG.
Interestingly, all participants in this study had presented with memory loss consistent with Alzheimer’s dementia. In fact, all participants were evaluated by an expert behavioral neurologist, and all were diagnosed as having Alzheimer’s dementia. There are two possible explanations for our findings. First, the presence of TDP-43 in AD is clinically silent, i.e. TDP-43 is not associated with any clinical manifestations. This has been shown not to be the case. While TDP-43 is not associated with FTLD clinical features, it is in fact associated with memory loss, as recently demonstrated [12]. We have previously compared TDP-43 positive AD subjects with TDP-43 negative AD subjects in regards to their cognition and pathological and neuroimaging features. Intriguingly, TDP-43 positive subjects scored worse on Mini-Mental State Examination, Clinical Dementia Rating Scale Sum of Boxes, the memory sub-score of the Dementia Rating Scale, and the Boston Naming Test than TDP-43 negative subjects, even after correcting for higher Braak stage, apolipoprotein ε4, Lewy bodies, and hippocampal sclerosis. Furthermore, TDP-43 positive AD subjects had greater medial temporal atrophy in comparison to TDP-43 negative subjects. These findings were also more prominent in patients with higher TDP-43 burden in the dentate cell layer of the hippocampus and in those with more widespread distribution of TDP-43. In accordance with our current findings, however, scores on Neuropsychiatric Inventory Questionnaire, a measure of behavioral impairment, did not differ between the two groups. Collectively, the results from our previous and current studies suggest that TDP-43 strongly influences features that are associated with AD, including memory loss and medial temporal atrophy, but not behavioral features that are associated with FTLD.
In AD cases with coexisting TDP-43, the true effect of TDP-43 is likely being “masked” by the presence of other AD associated pathological changes, such as tau, that are also strongly associated with memory loss [15]. This does not necessarily mean that the TDP-43 associated memory loss is always eclipsed by the other AD pathology associated memory loss. In some instances, it is quite possible that the presenting memory loss is attributable to TDP-43, more so than the other AD pathologies. Given that some patients with AD pathology in the absence of TDP-43 pathology have normal cognitive function, and the differences in memory impairment between TDP-43 negative and TDP-43 positive subjects are more prominent at lower Braak stages [12], TDP-43 may be more “important” in the earlier stages of AD. More specifically, although TDP-43 has an effect at all stages, the effect of tau and other AD pathologies is so great at later stages that any effect of TDP-43 is relegated to no longer being significant [12]. On the other hand, the TDP-43 effect becomes more important at earlier stages when the influence of the other AD pathologies is less prominent. Further studies examining the roles of TDP-43 in AD progression will be crucial from a prognostic and therapeutic standpoint as this may provide a potential therapeutic target in the future.
Acknowledgments
We would like to acknowledge the Mayo Clinic Center for Translational Science Activities (CTSA) for statistical guidance. The study was funded by NIA grants R01 AG037491, R21 AG038736 and P50 AG016574.
Footnotes
Conflicts of interest On behalf of all authors, the corresponding author states that there is no conflict of interest.
Ethical standard This study has been approved by the Mayo Clinic Institutional Review Board and has therefore been performed in accordance with the standards laid down in the 1964 Declaration of Helsinki. All subjects consented for their data to be used for research.
Contributor Information
Youngsin Jung, Department of Neurology (Behavioral Neurology), Mayo Clinic, Rochester, MN, USA.
Dennis W. Dickson, Department of Neuroscience (Neuropathology), Mayo Clinic, Jacksonville, FL, USA
Melissa E. Murray, Department of Neuroscience (Neuropathology), Mayo Clinic, Jacksonville, FL, USA
Jennifer L. Whitwell, Department of Radiology, Mayo Clinic, Rochester, MN, USA
David S. Knopman, Department of Neurology (Behavioral Neurology), Mayo Clinic, Rochester, MN, USA
Bradley F. Boeve, Department of Neurology (Behavioral Neurology), Mayo Clinic, Rochester, MN, USA
Clifford R. Jack, Jr., Department of Radiology, Mayo Clinic, Rochester, MN, USA
Joseph E. Parisi, Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN, USA
Ronald C. Petersen, Department of Neurology (Behavioral Neurology), Mayo Clinic, Rochester, MN, USA
Keith A. Josephs, Email: josephs.keith@mayo.edu, Department of Neurology (Behavioral Neurology), Mayo Clinic, Rochester, MN, USA
References
- 1.Neumann MSD, Kwong LK, Truax AC, Micsenyi MC, Chou TT, Bruce J, Schuck T, Grossman M, Clark CM, McCluskey LF, Miller BL, Masliah E, Mackenzie IR, Feldman H, Feiden W, Kretzschmar HA, Trojanowski JQ, Lee VM. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314:130–133. doi: 10.1126/science.1134108. [DOI] [PubMed] [Google Scholar]
- 2.Amador-Ortiz C, Lin WL, Ahmed Z, Personett D, Davies P, Duara R, Graff-Radford NR, Hutton ML, Dickson DW. TDP-43 immunoreactivity in hippocampal sclerosis and Alzheimer’s disease. Ann Neurol. 2007;61:435–445. doi: 10.1002/ana.21154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Josephs KA, Murray ME, Whitwell JL, Parisi JE, Petrucelli L, Jack CR, Petersen RC, Dickson DW. Staging TDP-43 pathology in Alzheimer’s disease. Acta Neuropathol. 2014;127:441–450. doi: 10.1007/s00401-013-1211-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hu WT, Josephs KA, Knopman DS, Boeve BF, Dickson DW, Petersen RC, Parisi JE. Temporal lobar predominance of TDP-43 neuronal cytoplasmic inclusions in Alzheimer disease. Acta Neuropathol. 2008;116:215–220. doi: 10.1007/s00401-008-0400-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arai T, Mackenzie IR, Hasegawa M, Nonoka T, Niizato K, Tsuchiya K, Iritani S, Onaya M, Akiyama H. Phosphorylated TDP-43 in Alzheimer’s disease and dementia with Lewy bodies. Acta Neuropathol. 2009;117:125–136. doi: 10.1007/s00401-008-0480-1. [DOI] [PubMed] [Google Scholar]
- 6.Higashi S, Iseki E, Yamamoto R, Minegishi M, Hino H, Fujisawa K, Togo T, Katsuse O, Uchikado H, Furukawa Y, Kosaka K, Arai H. Concurrence of TDP-43, tau and alpha-synuclein pathology in brains of Alzheimer’s disease and dementia with Lewy bodies. Brain Res. 2007;1184:284–294. doi: 10.1016/j.brainres.2007.09.048. [DOI] [PubMed] [Google Scholar]
- 7.Josephs KA, Stroh A, Dugger B, Dickson DW. Evaluation of subcortical pathology and clinical correlations in FTLD-U subtypes. Acta Neuropathol. 2009;118:349–358. doi: 10.1007/s00401-009-0547-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Working Group. Consensus recommendation for the postmortem diagnosis of Alzheimer’s disease. The National Institute on Aging, and Reagan Institute Working Group on Diagnostic Criteria for Neuropathologic Assessment of Alzheimer’s Disease. Neurobiol Aging. 1997;18(Supp1):S1–S2. [PubMed] [Google Scholar]
- 9.Rascovsky K, Hodges JR, Knopman D, Mendez MF, Kramer JH, Neuhaus J, van Swieten JC, Seelaar H, Dopper EG, Onyike CU, Hillis AE, Josephs KA, Boeve BF, Kertesz A, Seeley WW, Rankin KP, Johnson JK, Gorno-Tempini ML, Rosen H, Prioleau-Latham CE, Lee A, Kipps CM, Lillo P, Piguet O, Rohrer JD, Rossor MN, Warren JD, Fox NC, Galasko D, Salmon DP, Black SE, Mesulam M, Weintraub S, Dickerson BC, Diehl-Schmid J, Pasquier F, Deramecourt V, Lebert F, Pijnenburg Y, Chow TW, Manes F, Grafman J, Cappa SF, Freedman M, Grossman M, Miller BL. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. 2011;134:2456–2477. doi: 10.1093/brain/awr179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davidson YS, Raby S, Foulds PG, Robinson A, Thompson JC, Sikkink S, Yusuf I, Amin H, DuPlessis D, Troakes C, Al-Sarraj S, Sloan C, Esiri MM, Prasher VP, Allsop D, Neary D, Pickering-Brown SM, Snowden JS, Mann DM. TDP-43 pathological changes in early onset familial and sporadic Alzheimer’s disease, late onset Alzheimer’s disease and Down’s syndrome: association with age, hippocampal sclerosis and clinical phenotype. Acta Neuropathol. 2011;122:703–713. doi: 10.1007/s00401-011-0879-y. [DOI] [PubMed] [Google Scholar]
- 11.Josephs KA, Whitwell JL, Knopman DS, Hu WT, Stroh DA, Baker M, Rademakers R, Boeve BF, Parisi JE, Smith GE, Ivnik RJ, Petersen RC, Jack CRJ, Dickson DW. Abnormal TDP-43 immunoreactivity in AD modifies clinicopathologic and radiologic phenotype. Neurology. 2008;70:1850–1857. doi: 10.1212/01.wnl.0000304041.09418.b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Josephs KA, Whitwell JL, Weigand SD, Murray ME, Tosakulwong N, Liesinger AM, Petrucelli L, Senjem ML, Knopman DS, Boeve BF, Ivnik RJ, Smith GE, Jack CR, Parisi JE, Petersen RC, Dickson DW. TDP-43 is a key player in the clinical features associated with Alzheimer’s disease. Acta Neuropathol. 2014 doi: 10.1007/s00401-014-1269-z. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hart DJ, Craig D, Compton SA, Critchlow S, Kerrigan BM, Mcllroy SP, Passmore AP. A retrospective study of the behavioural and psychological symptoms of mid and late phase Alzheimer’s disease. Int J Geriatr Psychiatry. 2003;11:1037–1042. doi: 10.1002/gps.1013. [DOI] [PubMed] [Google Scholar]
- 14.DeLong MR. Primate models of movement disorders of basal ganglia origin. Trends Neurosci. 1990;13:281–285. doi: 10.1016/0166-2236(90)90110-v. [DOI] [PubMed] [Google Scholar]
- 15.Mitchell TW, Mufson EJ, Schneider JA, Cochran EJ, Nissanov J, Han LY, Bienias JL, Lee VM, Trojanowski JQ, Bennett DA, Arnold SE. Parahippocampal tau pathology in healthy aging, mild cognitive impairment, and early Alzheimer’s disease. Ann Neurol. 2002;51:182–189. doi: 10.1002/ana.10086. [DOI] [PubMed] [Google Scholar]


