Abstract
Background
Acute allograft dysfunction (AAD) is an important cause of morbidity among heart transplant recipients. The role of donor specific antibodies (DSA) in AAD, with the increasing use of Single Antigen Bead (SAB) assays that have improved the ability to detect DSA, remains unclear.
Methods
We retrospectively reviewed 329 heart transplant recipients followed at our institution. AAD was defined as an acute decline in left ventricular ejection fraction to <50% and a decrement of ≥10% compared to baseline in the absence of cellular rejection. AAD patients were compared with matched 30 heart transplant controls.
Results
There were 10 (3%) patients with AAD, 4 (40%) had DSA detectable by SAB assay compared to 16 (53%) controls (p=0.43). Peak DSA mean fluorescent intensity levels (MFI) were significantly higher at baseline (class I and class II) in AAD compared to controls. DSA MFI values increased at the time of AAD and returned to baseline values in follow-up for these AAD patients (p<0.05), but remained unchanged over time for controls. Six (60%) patients and 1 (3%) control had antibody-mediated rejection (AMR) by endomyocardial biopsy (p<0.01). There were 4 (40%) AAD patients with no DSA or AMR.
Conclusions
AAD after heart transplant is a heterogeneous process characterized by: 1) AMR and DSA, 2) AMR but no DSA, and 3) No AMR or DSA. The presence of DSA is not associated with AAD but quantity assessed by MFI levels may play a role.
Keywords: heart transplant, allograft dysfunction, donor specific antibodies, antibody mediated rejection
INTRODUCTION
Acute allograft dysfunction (AAD) is an important cause of morbidity and mortality among heart transplant recipients.(1) Acute cellular rejection (ACR) is generally recognized as the most common cause of AAD,(2) although other commonly described causes include antibody mediated rejection (AMR) and coronary allograft vasculopathy (CAV).(3) A significant proportion of patients may also develop AAD from unexplained mechanisms.(3) Despite the importance of this complication, there remains significant uncertainty regarding the risk factors for its development and its prognosis.
Anti-human leukocyte antigen (HLA) antibodies have been implicated in the pathogenesis of AAD however their role in AAD is unclear for two major reasons. First, until the advent of solid phase assays, older techniques to detect anti-HLA antibodies had limited diagnostic application and utility.(4) Second, anti-HLA antibodies have not sequentially been measured in patients with AAD and have not been systematically compared to controls, limiting the diagnostic interpretation of their detection in prior studies. The development of solid phase assays has resulted in improved sensitivity and specificity for detecting HLA mediated immune mechanisms of allograft dysfunction among heart transplant recipients.
Solid phase assays, in particular Single Antigen Bead (SAB) assays, have demonstrated high sensitivity not only for detecting but also for quantifying levels of circulating donor specific anti-HLA antibodies (DSA). The detection of AMR, an important cause of AAD, has also been improved by the ability to stain for the presence of C4d deposition on endothelial tissue following endomyocardial biopsy (EMB).(5) The purpose of this study was to; 1) assess the role of DSA in patients with AAD from a large cohort of heart transplant recipients, 2) to define their presence in the context of newer histologic techniques of assessing AMR to elucidate the pathophysiology of AAD in the absence of ACR.
RESULTS
Baseline Patient Characteristics
AAD was observed in 10 (3%) patients during the study period. Table 1 shows clinical and echocardiographic data at diagnosis for AAD patients and matched controls. The mean age of AAD patients was 53±13 years and 4 (40%) were female. Ten percent of AAD patients and 13% of controls received dual organ transplant, all of whom received heart-kidney transplant. No patients or controls had a prior history of ACR grade ≥2. As expected, echocardiography demonstrated significant left ventricular (LV) dilation and reduced ejection fraction (EF) for AAD patients compared with controls however LV wall thickness was not significantly different. LV mass but not mass index was significantly higher in AAD patients (Table 1).
Table 1.
Clinical, transplant, echocardiographic and immunosuppression characteristics of acute allograft dysfunction patients and heart transplant recipient controls at the time of diagnosis and control matching
| Variable | AAD Patients (N=10) | Controls (N=30) | p-value |
|---|---|---|---|
| Clinical and Transplant Variable | |||
| Age (years) | 53 ± 13 | 56 ± 14 | 0.24 |
| Female | 4 (40%) | 12 (40%) | 1.0 |
| Body mass index (kg/m2) | 26.4 ± 8.5 | 28.7 ± 9.2 | 0.10 |
| Heart rate (BPM) | 103 ± 16 | 87 ± 10 | <0.01 |
| Systolic blood pressure (mmHg) | 123 ± 17 | 121 ± 12 | 0.49 |
| Diastolic blood pressure (mmHg) | 75 ± 9 | 73 ± 8 | 0.68 |
| Age at transplantation (years) | 51 ± 6 | 53 ± 8 | 0.13 |
| Donor age | 35 ± 15 | 27 ± 20 | 0.04 |
| Ischemic time (minutes) | 186 ± 62 | 171 ± 73 | 0.38 |
| Heart failure etiology | 0.01 | ||
| Ischemic cardiomyopathy | 4 (40%) | 8 (27%) | |
| Dilated cardiomyopathy | 5 (50%) | 13 (43%) | |
| Hypertrophic cardiomyopathy | 1 (10%) | 2 (7%) | |
| Restrictive cardiomyopathy | 0 | 7 (7%) | |
| Congenital heart disease | 0 | 5 (16%) | |
| Dual organ transplant* | 1 (10%) | 4 (13%) | 0.35 |
| LVAD before transplant | 1 (10%) | 5 (16%) | 0.11 |
| Echocardiographic Variables | |||
| LV ejection fraction (%) | 34 ± 12 | 63 ± 8 | <0.01 |
| LV septal thickness (mm) | 11 ± 2 | 11 ± 3 | 0.76 |
| LV posterior wall thickness (mm) | 11 ± 2 | 11 ± 2 | 0.82 |
| LV end-diastolic diameter (mm) | 49 ± 7 | 46 ± 6 | 0.03 |
| LV end-systolic diameter (mm) | 41 ± 9 | 29 ± 6 | 0.01 |
| LV mass (g) | 200 ± 26 | 177 ± 18 | 0.02 |
| LV mass index (g/m2) | 101 ± 17 | 93 ± 12 | 0.12 |
| Immunosuppression Variables | |||
| Calcineurin / mTOR inhibitor | 10 (100%) | 30 (100%) | 1.0 |
| Cyclosporine | 7 (70%) | 18 (60%) | 0.19 |
| Mean dose (mg) | 325 ± 189 | 287 ± 184 | 0.02 |
| Mean trough level (ng/mL) | 213 ± 207 | 181 ± 99 | 0.04 |
| Tacrolimus | 0 | 6 (20%) | <0.01 |
| Mean dose (mg) | - | 4.6 ± 3.4 | |
| Mean trough level (ng/mL) | - | 10.3 ± 3.5 | |
| Sirolimus | 4 (40%) | 6 (20%) | 0.06 |
| Mean dose (mg) | 3.0 ± 0.6 | 2.6 ± 1.0 | 0.05 |
| Mean trough level (ng/mL) | 16.0 ± 8.9 | 10.0 ± 5.1 | 0.03 |
| Antimetabolite | 9 (90%) | 28 (93%) | 0.87 |
| Azathioprine | 3 (30%) | 9 (30%) | 1.0 |
| Mean dose (mg) | 108 ± 68 | 128 ± 63 | 0.17 |
| Mycophenolate mofetil | 6 (60%) | 19 (63%) | 0.74 |
| Mean dose (mg) | 2083 ± 585 | 1974 ± 539 | 0.02 |
| Prednisone | 7 (70%) | 22 (73%) | 0.56 |
| Mean dose (mg/kg/day) | 0.14 ± 0.07 | 0.15 ± 0.06 | 0.78 |
Data are presented as mean ±standard deviation for continuous data or as frequency (percentage) for categorical data.
All AAD patients and controls who underwent dual organ transplant received a heart-kidney transplant. AAD-acute allograft dysfunction, BPM-beats per minute, LV-left ventricle, LVAD-left ventricular assist device, mTOR-mammalian target of rapamycin.
Baseline Immunosuppression Therapy
All patients were maintained on a standard immunosuppression regimen with a calcineurin inhibitor or sirolimus at the time of AAD diagnosis (Table 1). Only 1 patient had a subtherapeutic drug level at AAD diagnosis (trough cyclosporine level 73 ng/mL). Mean cyclosporine and sirolimus dose and trough levels were actually higher for AAD patients than controls. By comparison, more controls were taking tacrolimus (20%) for immunosuppression than AAD patients (0%) and more AAD patients were on sirolimus (40% versus 20%).
Occurrence of Acute Allograft Dysfunction
The mean duration between transplant and AAD diagnosis was 4.0±4.8 years (range 35 days to 12.7 years) (Table 1). Four (40%) patients developed early AAD, defined as occurring ≤1 year after transplant (mean 137±119 days, range 35 to 308 days). Among the remainder of AAD patients, the mean time between transplant and AAD was 6.5±5.0 years (range 1.1 to 12.7 years). Five (50%) AAD patients presented with clinical signs or symptoms of heart failure or cardiogenic shock, while 5 (50%) presented with mild and non-specific symptoms (predominantly fatigue). The mean LV EF at baseline (mean 11±8 months prior to AAD diagnosis) was 58±5%, and at the time of diagnosis was 34±12% (see supplemental digital content [SDC] figure 1).
Baseline Immune Surveillance
All patients underwent cytotoxic crossmatch and panel reactive antibodies (PRA) screening at the time of transplant (Table 2). No AAD patients or controls had a positive cytotoxic crossmatch or positive frozen lymphocyte antibody panel PRA. Of the 6 AAD patients who underwent T-cell and B-cell flow cytotoxic crossmatch (FXM) at transplant, 1 (17%) had a positive T-cell (mean channel shift [MCS] 93) and 1 (17%) had a positive B-cell (MCS 166) FXM, and both of these patients developed early AAD (at 35 and 85 days after transplant, respectively). No controls who had FXM performed had a positive T-cell or B-cell FXM. Two (20%) AAD patients (1 positive for class I DSA and 1 positive for class II DSA) and 2 (7%) controls (both positive for class I DSA) had a positive virtual crossmatch (VXM), indicating an increased presence of DSA at baseline in patients with AAD (Table 2).
Table 2.
Pretransplant crossmatch results and time of diagnosis Single Antigen Bead assay results of acute allograft dysfunction patients and heart transplant recipient controls
| Variable | AAD Patients (N=10) | Controls (N=30) | p-value |
|---|---|---|---|
| Pretransplant Crossmatch | |||
| Cytotoxic crossmatch positive | 0 | 0 | 1.0 |
| FLAP PRA positive (>10%) | 0 | 0 | 1.0 |
| FXM performed | 6 (60%) | 11 (37%) | 0.02 |
| T-cell FXM positive | 1 (17%) | 0 | |
| B-cell FXM positive | 1 (17%) | 0 | |
| Virtual crossmatch positive* | 2 (20%) | 2 (7%) | 0.04 |
| Anti-HLA class I DSA | 1 (10%) | 2 (7%) | |
| Anti-HLA class II DSA | 1 (10%) | 0 | |
| Anti-HLA class I and II DSA | 0 | 0 | |
| At AAD Diagnosis or Control Matching† | |||
| Positive DSA | 4 (40%) | 16 (53%) | 0.43 |
| Anti-HLA class I DSA | 3 (30%) | 2 (7%) | 0.02 |
| Mean peak class I (MFI) | 1526 ± 2904 | 307 ± 1174 | 0.01 |
| Mean cumulative class I (MFI) | 3056 ± 6108 | 378 ± 1440 | 0.01 |
| Anti-HLA class II DSA | 3 (30%) | 15 (50%) | 0.05 |
| Mean peak class II (MFI) | 2229 ± 4538 | 1467 ± 3215 | 0.02 |
| Mean cumulative class II (MFI) | 6886 ± 17401 | 2476 ± 6434 | <0.01 |
| Anti-HLA class I and II DSA | 2 (20%) | 1 (3%) | 0.03 |
Positive virtual crossmatch=MFI>2000.
Results compared at the time of diagnosis for AAD patients and at the time of matching for controls. Data are presented as mean ±standard deviation for continuous data or as frequency (percentage) for categorical data. AAD-acute allograft dysfunction, DSA-donor specific antibodies, FLAP=frozen lymphocyte antibody panel, FXM-flow cytometric crossmatch, HLA-human leukocyte antigen, MFI-mean fluorescence intensity, PRA-panel of reactive antibodies,
Donor Specific Antibodies by SAB Assay
SAB assays to detect the presence of circulating class I and II DSA were performed on all AAD patients at diagnosis and compared with routine surveillance SAB testing of controls at a matched time period after transplant (Table 2). DSA were detected in 4 (40%) AAD patients, including 1 (10%) with class I DSA alone, 1 (10%) with class II DSA alone, and 2 (20%) with both class I and II DSA. In comparison, DSA were detected in 16 (53%) controls (p=0.43 compared with AAD patients), including 1 (3%) with class I DSA alone, 14 (47%) with class II DSA alone, and 1 (3%) with both class I and class II DSA. Class I DSA were detected in a higher proportion of AAD patients (30%) than controls (7%) (p=0.02). Mean peak and cumulative class I DSA levels were significantly higher in AAD patients than controls (p=0.01 and p=0.01, respectively). Class II DSA were detected in a lower proportion of AAD patients (30%) than controls (50%) with borderline significance (p=0.05). Mean peak and cumulative class II DSA levels were significantly higher for AAD patients than controls (p=0.02 and p<0.01, respectively). There was a significantly higher proportion of AAD patients with both class I and class II DSA detected compared with controls (20% versus 3%, p=0.03).
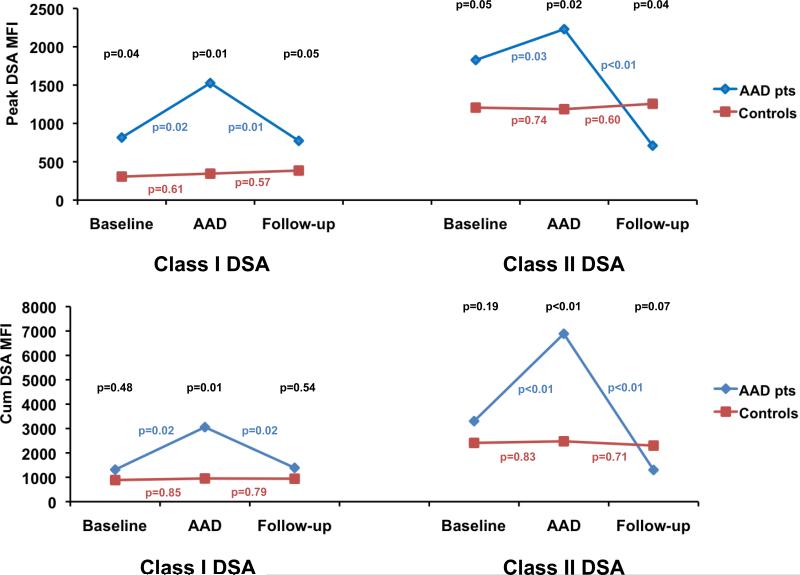
Serial mean peak and cumulative class I and class II DSA mean fluorescence intensity (MFI) levels for AAD patients and controls are presented in Figure 1. DSA at all time-points with the exception of follow-up class II DSA levels were higher for AAD patients than controls. For AAD patients, both class I and class II mean peak and cumulative DSA MFI values rose at the time of AAD diagnosis in comparison with baseline levels, and then subsequently fell to baseline or lower in follow-up, while MFI values did not significantly change over time for controls (Figure 1).
Figure 1. Serial donor specific antibody levels in acute allograft dysfunction patients and controls.
Mean peak (top) and cumulative (bottom) anti-HLA class I (left) and class II (right) donor specific antibody (DSA) MFI levels of acute allograft dysfunction (AAD) patients (blue) and control heart transplant recipients (red) at baseline, AAD diagnosis (or time of matching for controls) and follow-up. P-values above the curves (in black) compare DSA levels between AAD patients and controls at each matched time point. P-values adjacent to the curves compare serial DSA levels between time points for AAD patients and controls, respectively. HLA-human leukocyte antigen, MFI-mean fluorescence intensity level.
Endomyocardial Biopsy and Coronary Angiography Findings
EMB was performed on all patients at the time of AAD diagnosis, and compared with routine surveillance EMB results of controls at a matched time period after transplant (Table 3). Six (60%) AAD patients and 1 (3%) control showed evidence of AMR on EMB (p<0.01). At baseline EMB (mean 14±7 months prior to AAD diagnosis), no AAD patients were AMR positive on EMB. ACR was demonstrated by EMB for 3 (30%) of AAD patients and 6 (20%) controls (p=0.22), all of whom had grade 1R rejection (Table 3). Calculated transplant rejection score (TRS) was not significantly different between AAD patients and controls (Table 3).
Table 3.
Endomyocardial biopsy and coronary angiography results of acute allograft dysfunction patients and heart transplant recipient controls
| Variable | AAD Patients (N=10) | Controls (N=30) | p-value |
|---|---|---|---|
| Endomyocardial biopsy | |||
| C4d stain positive | 6 (60%) | 1 (3%) | <0.01 |
| CD68 stain positive | 2 (20%) | 1 (3%) | 0.02 |
| Macrophage infiltration | 3 (30%) | 0 | 0.04 |
| Endothelial swelling | 2 (20%) | 0 | 0.07 |
| AMR grade* | <0.01 | ||
| 0 | 4 (40%) | 29 (97%) | |
| 1 | 6 (60%) | 1 (3%) | |
| ACR grade* | 0.22 | ||
| 0R | 7 (70%) | 24 (80%) | |
| 1R | 3 (30%) | 6 (20%) | |
| 2R | 0 | 0 | |
| 3R | 0 | 0 | |
| Transplant rejection score | 0.4 ± 0.2 | 0.3 ± 0.2 | 0.41 |
| Coronary angiography | |||
| Normal coronary arteries | 4 (40%) | 12 (40%) | 0.75 |
| Coronary atherosclerosis* | |||
| Mild | 6 (60%) | 16 (53%) | 0.55 |
| Moderate | 0 | 2 (7%) | 0.19 |
| Severe | 0 | 0 | 0.33 |
| Intravascular ultrasound performed | 2 (20%) | 4 (13%) | 0.65 |
| †Significant CAV | 0 | 2 (7%) | 0.36 |
According to International Society for Heart and Lung Transplantation criteria.
Coronary stenosis of ≥70% and/or distal pruning of secondary side branches. Data are presented as mean ±standard deviation for continuous data or as frequency (percentage) for categorical data. AAD-acute allograft dysfunction, ACR-acute cellular rejection, AMR-antibody mediated rejection, CAV-coronary allograft vasculopathy.
SDC Table 1 shows EMB findings for AAD patients in conjunction with SAB assay results at baseline, AAD diagnosis and follow-up. Three distinct categories of AAD patients are demonstrated. The first group is 4 (40%) patients with AMR on EMB and circulating DSA at baseline and/or AAD diagnosis. Within this group are two patients with no detectable DSA prior to AAD who developed de-novo or new DSA at the time of AAD, including one patient who had new class I DSA (which resolved following treatment) and another patient who had new class II DSA (which persisted following treatment). Of the other two patients with detectable DSA at baseline, both developed new DSA at the time of AAD diagnosis, including one patient who developed new class I and II DSA which resolved after treatment, and another who developed only new class II DSA at AAD which resolved after treatment (as did some class I and II DSA detected at baseline). The second group is 2 (20%) patients with AMR on EMB but no detectable circulating DSA, which could be explained by adsorption of antibodies to the transplanted organ. The third group is 4 (40%) patients who had no evidence of either AMR on EMB or had any detectable DSA, suggestive of a mechanism other than an anti-HLA antibody mediated cause of AAD. The occurrence of coronary atherosclerosis and CAV were not significantly different between AAD patients and controls (Table 3). Of the 3 AAD patients with class II DSA, all 3 had evidence of CAV on coronary angiography, while a similarly high proportion of controls with class II DSA had CAV (13/15, 87%). Clinical, echocardiographic, immunosuppression, immune surveillance, EMB and coronary angiography data for each of the three categories of AAD patients are presented in Tables 4, 5 and 6, respectively.
Table 4.
Clinical, transplant, echocardiographic and immunosuppression characteristics of acute allograft dysfunction patients grouped by antibody mediated rejection and donor specific antibody status
| Variable | AMR+ DSA+ (N=4) | AMR+ DSA- (N=2) | AMR- DSA- (N=4) |
|---|---|---|---|
| Clinical and Transplant Variable | |||
| Age (years) | 52 ± 14 | 55 ± 9 | 51 ± 12 |
| Female | 1 (25%) | 1 (50%) | 2 (50%) |
| Body mass index (kg/m2) | 26.9 ± 5.2 | 27.3 ± 9.8 | 25.4 ± 6.1 |
| Heart rate (BPM) | 110 ± 19 | 98 ± 11 | 104 ± 17 |
| Systolic blood pressure (mmHg) | 125 ± 15 | 122 ± 19 | 124 ± 18 |
| Diastolic blood pressure (mmHg) | 74 ± 9 | 70 ± 8 | 77 ± 6 |
| Age at transplantation (years) | 50 ± 7 | 48 ± 8 | 52 ± 11 |
| Donor age | 31 ± 16 | 34 ± 11 | 36 ± 15 |
| Heart failure etiology | |||
| Ischemic cardiomyopathy | 1 (25%) | 0 | 3 (75%) |
| Dilated cardiomyopathy | 3 (75%) | 2 (100%) | 0 |
| Hypertrophic cardiomyopathy | 0 | 0 | 1 (25%) |
| Dual organ transplant | 1 (25%) | 0 | 0 |
| LVAD before transplant | 0 | 1 (50%) | 0 |
| Echocardiographic Variables | |||
| LV ejection fraction (%) | 33 ± 11 | 30 ± 14 | 35 ± 10 |
| LV septal thickness (mm) | 12 ± 3 | 10 ± 2 | 10 ± 2 |
| LV posterior wall thickness (mm) | 11 ± 1 | 11 ± 3 | 12 ± 2 |
| LV end-diastolic diameter (mm) | 51 ± 9 | 49 ± 6 | 48 ± 6 |
| LV end-systolic diameter (mm) | 40 ± 10 | 40 ± 11 | 42 ± 9 |
| LV mass (g) | 212 ± 31 | 190 ± 17 | 206 ± 15 |
| LV mass index (g/m2) | 105 ± 16 | 101 ± 18 | 99 ± 15 |
| Immunosuppression Variables | |||
| Calcineurin / mTOR inhibitor | 4 (100%) | 2 (100%) | 4 (100%) |
| Cyclosporine | 4 (100%) | 0 | 3 (75%) |
| Mean dose (mg) | 379 ± 205 | 304 ± 173 | 289 ± 169 |
| Mean trough level (ng/mL) | 219 ± 197 | 243 ± 152 | 184 ± 227 |
| Tacrolimus | 0 | 0 | 0 |
| Mean dose (mg) | - | - | - |
| Mean trough level (ng/mL) | - | - | - |
| Sirolimus | 1 (25%) | 2 (100%) | 1 (25%) |
| Mean dose (mg) | 3.2 ± 0.7 | 3.1 ± 0.6 | 2.7 ± 0.6 |
| Mean trough level (ng/mL) | 16.7 ± 9.2 | 15.2 ± 6.1 | 15.9 ± 8.1 |
| Antimetabolite | 4 (100%) | 2 (100%) | 3 (75%) |
| Azathioprine | 2 (50%) | 0 | 1 (25%) |
| Mean dose (mg) | 129 ± 88 | - | 77 ± 59 |
| Mycophenolate mofetil | 2 (50%) | 2 (100%) | 2 (50%) |
| Mean dose (mg) | 2299 ± 673 | 1689 ± 429 | 1982 ± 559 |
| Prednisone | 2 (50%) | 1 (50%) | 4 (100%) |
| Mean dose (mg/kg/day) | 0.13 ± 0.06 | 0.16 ± 0.03 | 0.16 ± 0.09 |
Data are presented as mean ±standard deviation for continuous data or as frequency (percentage) for categorical data. AMR-antibody mediated rejection, BPM-beats per minute, DSA-donor specific antibody, LV-left ventricle, LVAD-left ventricular assist device, mTOR-mammalian target of rapamycin.
Table 5.
Pretransplant crossmatch results and time of diagnosis Single Antigen Bead assay results of acute allograft dysfunction patients grouped by antibody mediated rejection and donor specific antibody status
| Variable | AMR+ DSA+ (N=4) | AMR+ DSA- (N=2) | AMR- DSA-(N=4) |
|---|---|---|---|
| Pretransplant Crossmatch | |||
| Cytotoxic crossmatch positive | 0 | 0 | 0 |
| FLAP PRA positive (>10%) | 0 | 0 | 0 |
| FXM performed | 3 (75%) | 1 (50%) | 2 (50%) |
| T-cell FXM positive | 1 (25%) | 0 | 0 |
| B-cell FXM positive | 0 | 1 (50%) | 0 |
| Virtual crossmatch positive* | 2 (50%) | 0 | 0 |
| Anti-HLA class I DSA | 1 (25%) | 0 | 0 |
| Anti-HLA class II DSA | 1 (25%) | 0 | 0 |
| Anti-HLA class I and II DSA | 0 | 0 | 0 |
Positive virtual crossmatch=MFI±2000. Data are presented as mean ±standard deviation for continuous data or as frequency (percentage) for categorical data. AAD-acute allograft dysfunction, AMR-antibody mediated rejection, DSA-donor specific antibodies, FLAP=frozen lymphocyte antibody panel, FXM-flow cytometric crossmatch, HLA-human leukocyte antigen, PRA-panel of reactive antibodies,
Table 6.
Endomyocardial biopsy and coronary angiography results of acute allograft dysfunction patients grouped by antibody mediated rejection and donor specific antibody status
| Variable | AMR+ DSA+ (N=4) | AMR+ DSA- (N=2) | AMR- DSA- (N=4) |
|---|---|---|---|
| Endomyocardial biopsy | |||
| C4d stain positive | 4 (100%) | 2 (100%) | 0 |
| CD68 stain positive | 2 (50%) | 0 | 0 |
| Macrophage infiltration | 2 (50%) | 1 (50%) | 0 |
| Endothelial swelling | 1 (25%) | 1 (50%) | 0 |
| AMR grade* | |||
| 0 | 0 | 0 | 4 (100%) |
| 1 | 4 (100%) | 2 (100%) | 0 |
| ACR grade* | |||
| 0R | 2 (50%) | 1 (50%) | 4 (100%) |
| 1R | 2 (50%) | 1 (50%) | 0 |
| 2R | 0 | 0 | 0 |
| 3R | 0 | 0 | 0 |
| Transplant rejection score | 0.4 ± 0.2 | 0.4 ± 0.2 | 0.3 ± 0.2 |
| Coronary angiography | |||
| Normal coronary arteries | 2 (50%) | 1 (50%) | 1 (25%) |
| Coronary atherosclerosis* | |||
| Mild | 2 (50%) | 1 (50%) | 3 (75%) |
| Moderate | 0 | 0 | 0 |
| Severe | 0 | 0 | 0 |
| Intravascular ultrasound performed | 1 (25%) | 0 | 1 (25%) |
| †Significant CAV | 0 | 0 | 0 |
According to International Society for Heart and Lung Transplantation criteria.
Coronary stenosis of ≥70% and/or distal pruning of secondary side branches. Data are presented as mean ±standard deviation for continuous data or as frequency (percentage) for categorical data. AAD-acute allograft dysfunction, ACR-acute cellular rejection, AMR-antibody mediated rejection, CAV-coronary allograft vasculopathy, DSA-donor specific antibody.
Management and Outcomes
At our institution all patients presenting with AAD in the absence of histologic evidence for ACR are initially treated for a presumed diagnosis of AMR and receive plasmapheresis and intravenous methylprednisolone. Plasmapheresis is discontinued if EMB is negative for AMR and serial SAB testing is negative for DSA. In this series 3 (30%) patients also received rituximab and 3 (30%) received high-dose intravenous immunoglobulin. No patients received hemofiltration after transplant in this study, and none received plasmapheresis prior to transplant. The median follow-up duration after AAD was 19±12 months (range 2 to 37 months), and for control recipients was 21±6 months (range 12 to 41 months) (p=0.25). All AAD patients demonstrated improvement in LV EF (SDC figure 1). The mean LV EF one year following AAD was 47±13%, and at the most recent follow-up was 55±8%. No patients had recurrence of AAD in the follow-up period. Only 1 (10%) AAD patient died in the follow-up period of sepsis secondary to small bowel perforation. By comparison, 2 (7%) control recipients died in the follow-up period p=0.64), one of intracranial hemorrhage and one of progressive renal failure.
DISCUSSION
This study provides important insights into the role of circulating anti-HLA DSA detected by SAB assay and AMR utilizing C4d staining in heart transplant recipients presenting with AAD not due to ACR. The principle findings are that patients with AAD are a heterogeneous group belonging to 3 major categories: 1) patients with AMR by EMB and circulating DSA, 2) patients with AMR but no DSA, and 3) patients with neither AMR nor DSA. Although the prevalence of DSA in AAD patients was similar to asymptomatic controls, patients in the first group had relatively higher levels of DSA both prior to and at the time of AAD diagnosis. DSA levels rose at the time of AAD diagnosis and subsequently fell following treatment, returning to prior levels or lower. In controls, DSA levels remained relatively constant over time, suggesting that not just the presence of circulating DSA but also the quantity as assessed by MFI levels plays a significant role in the pathogenesis of AAD. These findings highlight the importance of serial quantification of DSA using SAB assays. A greater proportion of AAD patients in the first group had class I DSA than class II compared to controls, suggesting that class I DSA may be more likely to induce AAD in the absence of significant ACR. Supporting this further is that while both AAD patients and controls developed new DSA since the time of transplant, controls were more likely to develop new class II DSA while AAD patients were more likely to develop new class I DSA. Lastly, the third group of AAD patients with neither AMR nor DSA represents a substantial proportion who may have developed allograft dysfunction mediated by humoral or immune factors other than an HLA-mediated phenomenon. Even though AAD resolved with augmented immunosuppression therapy in all cases, suggesting an immunologic role in pathogenesis, our study demonstrates that the mechanism behind AAD in a significant proportion of affected heart transplant recipients remains poorly understood.
Our study attempts to clarify the challenges associated with determining the etiology of AAD. The diagnosis of AMR is often made by default in many patients presenting with AAD in the absence of ACR on EMB.(3) The use of modern techniques such as C4d staining have improved the diagnosis of AMR, which previously relied upon less sensitive EMB findings such as immunoglobulin deposition by immunofluorescence and endothelial swelling on EMB. Detection of DSA can aid in the diagnosis of AMR.(6) Tan et al found a good correlation between the presence of C4d deposition and DSA in patients with allograft dysfunction in a cohort of 330 heart transplant recipients.(7) However, their study also found a significant proportion of patients with positive C4d staining and circulating DSA who had no evidence of allograft dysfunction. DSA MFI levels were not reported in their study. Another study found biopsy evidence of AMR in 21 asymptomatic patients without evidence of LV dysfunction, although SAB assays for detecting DSA were not performed.(8) The finding of either C4d deposition and/or circulating DSA in stable asymptomatic patients complicates our understanding of the mechanism of AAD, and in fact there are no specific histologic findings that have been found to be predictive of hemodynamic derangements in transplant recipients.(9,10) Furthermore, the high sensitivity of SAB assays may lead to the detection of DSA which are non-complement activating and not clinically relevant, while phenomenon such as the prozone effect may lead to false negatives. A recent study in pediatric and adult heart transplant recipients suggested that a novel SAB assay that can detect C1-q binding anti-HLA antibodies correlated better with complement-dependent cytotoxic crossmatch results than standard SAB-IgG assays, as well as the subsequent development of acute and persistent AMR, while being less susceptible to prozone effect.(11) Further research is needed to study serial changes in SAB C1-q testing results in patients with AAD.
This is the first study to evaluate patients with AAD by serial DSA measurement using SAB assays and comparing these with asymptomatic control heart transplant recipients without allograft dysfunction. Our study found that DSA are present in both AAD patients and controls in similar proportions. Important differences between these groups were higher DSA levels at baseline and rising DSA levels at diagnosis among AAD patients, a greater proportion of whom also had class I DSA compared with controls. Previous studies have also suggested that class I DSA may play a greater role in the development of allograft dysfunction than class II.(12) And while both AAD patients and controls in our study developed de-novo DSA after transplant in comparable proportions, controls were far more likely to develop new class II DSA. Toplisky et al analyzed the relation of preformed DSA measured by SAB assay and CAV in heart transplant recipients, and found that the presence of preformed class II DSA were associated with an increased risk for accelerated CAV as detected by 3-dimensional volumetric intravascular ultrasound.(13) Although serial post-transplant DSA levels were not reported in their study, these results taken together with our findings shed further light on the pathologic roles of class I and II DSA.
Circulating DSA have been considered to play a significant role in the development of allograft dysfunction, however despite using a sensitive solid phase assay our study found a significant proportion of AAD patients (60%) had no detectable DSA. This may suggest that non-HLA mediated phenomenon such as antibodies to vimentin, other cardiac proteins such as myosin, or non-HLA endothelial antigens may play an important role in the development of AAD.(14,15) Minor antigens, non-HLA antigens and self-antigens are becoming increasingly recognized as a difficult to detect cause for graft dysfunction for multiple solid organs including heart transplants. This effect may be exacerbated by the interplay between allo- and autoimmunity in some recipients.(16) Another possible explanation for the group with AAD and AMR without DSA is the absorption of DSA or non-HLA antibodies on to the endothelial surface of the allograft, or the formation of immune complexes of anti-HLA antibodies with soluble circulating HLA antigens. (4,17,18) The detection and clinical significance of such antibodies or complexes remain poorly understood and an important area of further research. Non-immunologic mediated mechanisms of AAD such as medication toxicity, graft fibrosis, arrhythmogenic mechanisms and idiopathic dysfunction warrant consideration in the setting of AAD when EMB and DSA testing are negative. Our analysis was limited by the exclusion of patients with ACR grade ≥2R because performance of SAB assay for DSA detection in this setting was not routinely performed at our institution. This precluded assessment of the contribution of DSA in AAD patients with ACR. The role of preformed anti-HLA antibodies has been reported to be associated with both elevated risk of ACR and morbidity and mortality in transplant recipients.(19-21) Pre-transplant DSA levels in patients who subsequently develop ACR has been described by Gandhi et al, who found that an MFI >1500 was associated with an increased risk of acute rejection.(22) Furthermore, while EMB has been previously shown to be sensitive for detecting significant rejection,(23) ACR may have been missed in some AAD patients included in this analysis due to the limitations of myocardial sampling. This may have obscured the findings of this study specific to the objective of determining the role of DSA in AAD without ACR.
Conclusion
This study identifies three groups of heart transplant recipients who present with AAD in the absence of cellular rejection. The first is patients with AMR by EMB who also have circulating anti-HLA DSA detected by SAB assay. The second is AAD patients with AMR by EMB but no circulating DSA. The third group is patients with no AMR and no circulating DSA before, during or after AAD diagnosis, suggesting involvement of non-HLA antibodies or other pathologic mechanisms in AAD. Irrespective of presentation, all patients in this series survived their AAD event with improvement of LV EF with immune modulating therapy. Further study is required to identify triggers of AMR including the role of quantifying DSA especially after transplant and to evaluate non-HLA mediated mechanisms of AAD, which may facilitate better strategies for risk assessment and prevention.
METHODS
Study Population
This was a single-center, observational cohort study. The patient records of 329 consecutive adult heart transplant recipients followed at Mayo Clinic (Rochester Minnesota) between June 2006 and February 2010 were retrospectively analyzed. Clinical data were collected from patient medical records. See SDC for our institution's transplant immunosuppression regimen. Total TRS was calculated for each patient as previously described.(24) There were 19 (6%) patients excluded for a diagnosis of ACR, defined as International Society for Heart and Lung Transplantation (ISHLT) ≥grade 2R as determined by EMB.(25) All patients provided written informed consent permitting access to their medical records for research purposes. The final study population comprised 310 patients. This study was approved by the Mayo Clinic Institutional Review Board.
Surveillance Post Transplantation
In addition to being performed for clinical symptoms or signs suggestive of rejection, routine EMB was performed weekly for the first 6 weeks after transplant beginning 1 week after completion of induction therapy, then every 2 weeks between 6 weeks and 3 months, monthly between 3 and 6 months, and then every 3 months until the end of the second year. EMB was performed using standard technique.(26,27) The diagnosis of AMR was made based on ISHLT criteria,(25,28) including assessment of C4d staining of endothelial tissue (5,6) and histologic features such as macrophage infiltration and endothelial swelling following EMB.(29,30) Assessment for C4d was performed using both paraffin imunohistochemical staining (31) and immunofluorescence staining (12) in all samples. C4d staining was routinely performed on all surveillance EMBs as well as at the time of AAD presentation. Patients underwent comprehensive transthoracic echocardiography according to standard American Society of Echocardiography (ASE) criteria (32) at hospital discharge after transplant, at the time of each EMB between discharge and 2 years, and annually thereafter. Patients underwent invasive coronary angiography 3 months after transplant to screen for CAV, and then annually thereafter. All patients with AAD underwent coronary angiography upon presentation or soon after. CAV was graded according to standard ISHLT criteria.(33) Significant CAV was defined as coronary stenosis of ≥70% and/or distal pruning of secondary side branches.
Diagnosis of Acute Allograft Dysfunction
Patients underwent echocardiography at presentation with suspected AAD. AAD was defined as an acute reduction in LV EF to <50%, and by a ≥10% decrement in comparison with the most recent prior echocardiogram. ASE guidelines define an abnormal LV EF as <55%,(32) therefore a conservative cut-off value of LV EF <50% was chosen to account for any variability of this measurement by echocardiography.
Complement-Dependent Cytotoxic and Flow Cytotoxic Crossmatch
All patients underwent measurement of panel PRA prior to transplant with CDC anti-human globulin (AHG) assay using a 56-well commercial T-lymphocyte frozen cell tray (Gentak Inc., Liberty North Carolina). A positive reaction was defined as >50% cytotoxicity. All patients were tested using a T-cell AHG-CDC crossmatch assay, and those who underwent transplant after 2006 were subject to T-cell and B-cell FXM. A positive FXM was defined as a MCS greater than 52 and 106 for T-cells and B-cells, respectively, as previously described.(34) FXM was performed retrospectively at our institution within 24 hours after transplant.
Solid Phase Assays
Pre-transplant serum was screened for circulating anti-HLA antibodies using a panel of multiple color-coded microspheres, each coated with a purified single HLA class I or class II antigen (LABScreen Single Antigen Beads, One Lambda) on a LABScan 100 flow analyzer (Luminex Corporation, Austin Texas).(22) Results are expressed as the MFI for each anti-HLA antibody detected (corresponding to the strength of the antibody reaction) with the aid of an analysis program (HLA Fusion, version 1.2.1b, Lamba One). In addition, screening for DSA was performed at the time of transplant, at 1, 3 and 6 months post-transplant as part of routine surveillance, and then annually thereafter. DSA screening was also performed at the time AAD diagnosis and in follow-up after treatment completion. The most recent surveillance SAB assay prior to AAD diagnosis was considered baseline, and was compared with AAD diagnosis and follow-up studies.
Sensitization and SAB MFI Level
Recipient SAB testing collected within 24 hours prior to transplant was compared with potential donor HLA typing for a pre-transplant VXM. Our institution's laboratory has found a good correlation between DSA levels and FXM results, namely that the FXM is positive when the DSA MFI is >2000.(22) Thus for this study a DSA MFI >2000 was used to define anti-HLA antibody sensitization for a positive VXM. See SDC for additional details regarding HLA typing. For serial DSA measurements in relation to post-transplant baseline, AAD diagnosis and follow-up levels, the presence of all DSA with a MFI ≥300 were reported. In our laboratory MFI levels <300 are not reported because they are considered beyond the sensitivity of the assay.(13,22)
Statistical Analysis
Data are presented as the mean±standard deviation for continuous data or as frequency and percentage for categorical data. Each patient who developed AAD was compared to a selected sample of three control heart transplant recipients with no history of allograft dysfunction, who were matched with AAD patients by age, gender, donor-recipient gender, ischemic time (±90 minutes) and duration since transplant. Data acquired at the time of diagnosis for AAD patients was compared with routine surveillance testing data for controls performed at the time closest to the post-transplant date of diagnosis for the matched AAD patient. Serial SAB assay results were also compared between groups at baseline (defined as the most recent SAB prior to diagnosis for AAD patients or prior to matching for controls) and in follow-up (the next most recent SAB after treatment for AAD patients or after matching for controls). Comparisons between AAD patients and controls were performed using the t-test or Wilcoxon rank sum test as indicated for continuous variables and Fischer's exact test for categorical variables. The Wilcoxon sign rank test or paired t-test was used to test changes in DSA levels within groups at different time points as indicated. All p-values were 2-sided and a value of ≤0.05 was considered statistically significant. Statistical analyses were performed using JMP version 9.0 statistical software (SAS Institute Inc., Cary, North Carolina).
Supplementary Material
Acknowledgments
None
Funding Support: This publication was made possible by CTSA Grant Number UL1 TR000135 (NLP) from the National Center for Advancing Translational Sciences (NCATS).
ABBREVIATIONS
- AAD
acute allograft dysfunction
- ACR
acute cellular rejection
- AHG
anti-human globulin
- AMR
antibody mediated rejection
- ASE
American Society of Echocardiography
- CAV
cardiac allograft vasculopathy
- CDC
complement-dependent cytotoxic assay
- DSA
donor specific antibodies
- EF
ejection fraction
- EMB
endomyocardial biopsy
- FXM
flow cytotoxic crossmatch
- HLA
human leukocyte antigen
- ISHLT
International Society for Heart and Lung Transplantation
- LV
left ventricle
- MCS
mean channel shift
- MFI
mean fluorescence intensity
- PRA
panel of reactive antibodies
- SAB
single antigen bead
- TRS
transplant rejection score
- VXM
virtual crossmatch
Footnotes
1. Nowell M. Fine, MD Contribution: Research design, manuscript preparation, research performance, data analysis Address: Mayo Clinic, 200 First Street SW, Rochester Minnesota USA, 55901 nowellfine@gmail.com Funding support: None, Conflicts of Interest: None
2. Richard C. Daly, MD Contribution: Research design, manuscript preparation Address: Mayo Clinic, 200 First Street SW, Rochester Minnesota USA, 55901 daly.richard@mayo.edu Funding support: None, Conflicts of Interest: None
3. Nisha Shankar, MD Contribution: Research design, manuscript preparation, research performance Address: Mayo Clinic, 200 First Street SW, Rochester Minnesota USA, 55901 dr.nisha.shankar@gmail.com Funding support: None, Conflicts of Interest: None
4. Soon J. Park, MD, MS Contribution: Research design, manuscript preparation Address: Mayo Clinic, 200 First Street SW, Rochester Minnesota USA, 55901 park.soon@mayo.edu Funding support: None, Conflicts of Interest: None
5. Sudhir S. Kushwaha, MD Contribution: Research design, manuscript preparation Address: Mayo Clinic, 200 First Street SW, Rochester Minnesota USA, 55901 kushwaha.sudhir@mayo.edu Funding support: None, Conflicts of Interest: None
6. Manish J. Gandhi, MD Contribution: Research design, manuscript preparation, analytical tools Address: Mayo Clinic, 200 First Street SW, Rochester Minnesota USA, 55901 gandhi.manish@mayo.edu Funding support: None, Conflicts of Interest: None
7. Naveen L. Pereira, MD (corresponding author) Contribution: Research design, manuscript preparation, research performance, data analysis Address: Mayo Clinic, 200 First Street SW, Rochester Minnesota USA, 55901 Pereira.naveen@mayo.edu Funding Support: This publication was made possible by CTSA Grant Number UL1 TR000135 from the National Center for Advancing Translational Sciences (NCATS). Conflicts of Interest: None
Disclosures: None
REFERENCES
- 1.Mills RM, Naftel DC, Kirklin JK, et al. Heart transplant rejection with hemodynamic compromise: a multiinstitutional study of the role of endomyocardial cellular infiltrate. Cardiac Transplant Research Database. J Heart Lung Transplant. 1997;16:813–21. [PubMed] [Google Scholar]
- 2.Follansbee WP, Kiernan JM, Curtiss EI, Zerbe TR, Mock C, Kormos RL. Changes in left ventricular systolic function that accompany rejection of the transplanted heart: a serial radionuclide assessment of fifty-three consecutive cases. Am Heart J. 1991;121:548–56. doi: 10.1016/0002-8703(91)90725-w. [DOI] [PubMed] [Google Scholar]
- 3.Shahzad K, Aziz QA, Leva JP, et al. New-onset graft dysfunction after heart transplantation--incidence and mechanism-related outcomes. J Heart Lung Transplant. 2011;30:194–203. doi: 10.1016/j.healun.2010.08.026. [DOI] [PubMed] [Google Scholar]
- 4.Uber WE, Self SE, Van Bakel AB, Pereira NL. Acute antibody-mediated rejection following heart transplantation. Am J Transplant. 2007;7:2064–74. doi: 10.1111/j.1600-6143.2007.01900.x. [DOI] [PubMed] [Google Scholar]
- 5.Behr TM, Feucht HE, Richter K, et al. Detection of humoral rejection in human cardiac allografts by assessing the capillary deposition of complement fragment C4d in endomyocardial biopsies. J Heart Lung Transplant. 1999;18:904–12. doi: 10.1016/s1053-2498(99)00043-1. [DOI] [PubMed] [Google Scholar]
- 6.Reed EF, Demetris AJ, Hammond E, et al. Acute antibody-mediated rejection of cardiac transplants. J Heart Lung Transplant. 2006;25:153–9. doi: 10.1016/j.healun.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 7.Tan CD, Sokos GG, Pidwell DJ, et al. Correlation of donor-specific antibodies, complement and its regulators with graft dysfunction in cardiac antibody-mediated rejection. Am J Transplant. 2009;9:2075–84. doi: 10.1111/j.1600-6143.2009.02748.x. [DOI] [PubMed] [Google Scholar]
- 8.Wu GW, Kobashigawa JA, Fishbein MC, et al. Asymptomatic antibody-mediated rejection after heart transplantation predicts poor outcomes. J Heart Lung Transplant. 2009;28:417–22. doi: 10.1016/j.healun.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lones MA, Czer LS, Trento A, Harasty D, Miller JM, Fishbein MC. Clinical-pathologic features of humoral rejection in cardiac allografts: a study in 81 consecutive patients. J Heart Lung Transplant. 1995;14:151–62. [PubMed] [Google Scholar]
- 10.Michaels PJ, Espejo ML, Kobashigawa J, et al. Humoral rejection in cardiac transplantation: risk factors, hemodynamic consequences and relationship to transplant coronary artery disease. J Heart Lung Transplant. 2003;22:58–69. doi: 10.1016/s1053-2498(02)00472-2. [DOI] [PubMed] [Google Scholar]
- 11.Zeevi A, Lunz J, Feingold B, et al. Persistent strong anti-HLA antibody at high titer is complement binding and associated with increased risk of antibody-mediated rejection in heart transplant recipients. J Heart Lung Transplant. 2013;32:98–105. doi: 10.1016/j.healun.2012.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frank R, Molina MR, Wald JW, Goldberg LR, Kamoun M, Lal P. Correlation of circulating donor-specific anti-HLA antibodies and presence of C4d in endomyocardial biopsy with heart allograft outcomes: a single-center, retrospective study. J Heart Lung Transplant. 2013;32:410–7. doi: 10.1016/j.healun.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 13.Topilsky Y, Gandhi MJ, Hasin T, et al. Donor-specific antibodies to class II antigens are associated with accelerated cardiac allograft vasculopathy: a three-dimensional volumetric intravascular ultrasound study. Transplantation. 2013;95:389–96. doi: 10.1097/TP.0b013e318273878c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kobashigawa J, Mehra M, West L, et al. Report from a consensus conference on the sensitized patient awaiting heart transplantation. J Heart Lung Transplant. 2009;28:213–25. doi: 10.1016/j.healun.2008.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fredrich R, Toyoda M, Czer LS, et al. The clinical significance of antibodies to human vascular endothelial cells after cardiac transplantation. Transplantation. 1999;67:385–91. doi: 10.1097/00007890-199902150-00008. [DOI] [PubMed] [Google Scholar]
- 16.Angaswamy N, Tiriveedhi V, Sarma NJ, et al. Interplay between immune responses to HLA and non-HLA self-antigens in allograft rejection. Human immunology. 2013 doi: 10.1016/j.humimm.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rose ML. De novo production of antibodies after heart or lung transplantation should be regarded as an early warning system. J Heart Lung Transplant. 2004;23:385–95. doi: 10.1016/j.healun.2003.08.028. [DOI] [PubMed] [Google Scholar]
- 18.Suciu-Foca N, Reed E, Marboe C, et al. The role of anti-HLA antibodies in heart transplantation. Transplantation. 1991;51:716–24. doi: 10.1097/00007890-199103000-00033. [DOI] [PubMed] [Google Scholar]
- 19.Tambur AR, Bray RA, Takemoto SK, et al. Flow cytometric detection of HLA-specific antibodies as a predictor of heart allograft rejection. Transplantation. 2000;70:1055–9. doi: 10.1097/00007890-200010150-00011. [DOI] [PubMed] [Google Scholar]
- 20.Stastny P, Lavingia B, Fixler DE, Yancy CW, Ring WS. Antibodies against donor human leukocyte antigens and the outcome of cardiac allografts in adults and children. Transplantation. 2007;84:738–45. doi: 10.1097/01.tp.0000281918.51138.3f. [DOI] [PubMed] [Google Scholar]
- 21.Opelz G, Wujciak T. The influence of HLA compatibility on graft survival after heart transplantation. The Collaborative Transplant Study. The New England journal of medicine. 1994;330:816–9. doi: 10.1056/NEJM199403243301203. [DOI] [PubMed] [Google Scholar]
- 22.Gandhi MJ, DeGoey SR, Bundy K, et al. Effect of pretransplant human leukocyte antigen antibodies detected by solid-phase assay on heart transplant outcomes. Transplantation proceedings. 2011;43:3840–6. doi: 10.1016/j.transproceed.2011.08.077. [DOI] [PubMed] [Google Scholar]
- 23.Nakhleh RE, Jones J, Goswitz JJ, Anderson EA, Titus J. Correlation of endomyocardial biopsy findings with autopsy findings in human cardiac allografts. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 1992;11:479–85. [PubMed] [Google Scholar]
- 24.Raichlin E, Villarraga HR, Chandrasekaran K, et al. Cardiac allograft remodeling after heart transplantation is associated with increased graft vasculopathy and mortality. Am J Transplant. 2009;9:132–9. doi: 10.1111/j.1600-6143.2008.02474.x. [DOI] [PubMed] [Google Scholar]
- 25.Stewart S, Winters GL, Fishbein MC, et al. Revision of the 1990 working formulation for the standardization of nomenclature in the diagnosis of heart rejection. J Heart Lung Transplant. 2005;24:1710–20. doi: 10.1016/j.healun.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 26.Caves PK, Stinson EB, Billingham ME, Shumway NE. Serial transvenous biopsy of the transplanted human heart. Improved management of acute rejection episodes. Lancet. 1974;1:821–6. doi: 10.1016/s0140-6736(74)90480-2. [DOI] [PubMed] [Google Scholar]
- 27.Graham AF, Rider AK, Caves PK, et al. Acute rejection in the long-term cardiac transplant survivor. Clinical diagnosis, treatment and significance. Circulation. 1974;49:361–6. doi: 10.1161/01.cir.49.2.361. [DOI] [PubMed] [Google Scholar]
- 28.Kobashigawa J, Crespo-Leiro MG, Ensminger SM, et al. Report from a consensus conference on antibody-mediated rejection in heart transplantation. J Heart Lung Transplant. 2011;30:252–69. doi: 10.1016/j.healun.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hammond EH, Yowell RL, Nunoda S, et al. Vascular (humoral) rejection in heart transplantation: pathologic observations and clinical implications. J Heart Transplant. 1989;8:430–43. [PubMed] [Google Scholar]
- 30.Hammond EH, Yowell RL, Price GD, et al. Vascular rejection and its relationship to allograft coronary artery disease. J Heart Lung Transplant. 1992;11:S111–9. [PubMed] [Google Scholar]
- 31.Galambos C, Feingold B, Webber SA. Characterization of c4d immunostaining utilizing paraffin-embedded tissue of nonpresensitized pediatric heart transplant patients. Pediatric and developmental pathology : the official journal of the Society for Pediatric Pathology and the Paediatric Pathology Society. 2008;11:181–4. doi: 10.2350/07-04-0259.1. [DOI] [PubMed] [Google Scholar]
- 32.Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–63. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 33.Mehra MR, Crespo-Leiro MG, Dipchand A, et al. International Society for Heart and Lung Transplantation working formulation of a standardized nomenclature for cardiac allograft vasculopathy-2010. J Heart Lung Transplant. 2010;29:717–27. doi: 10.1016/j.healun.2010.05.017. [DOI] [PubMed] [Google Scholar]
- 34.Gloor JM, DeGoey S, Ploeger N, et al. Persistence of low levels of alloantibody after desensitization in crossmatch-positive living-donor kidney transplantation. Transplantation. 2004;78:221–7. doi: 10.1097/01.tp.0000128516.82593.47. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.