Abstract
Depressive rumination – a central characteristic of Major Depressive Disorder (MDD) – is a maladaptive emotion regulation strategy that prolongs sad mood and depressive episodes. Considerable research demonstrates the emotional and behavioral consequences of depressive rumination, yet few studies investigate its effect on neuroendocrine functioning. The current study examined the effect of an emotion regulation manipulation on the trajectory of cortisol concentrations among individuals with MDD and healthy controls (CTL). Sadness was induced via forced failure. Participants then were randomly assigned to a depressive rumination or distraction emotion regulation induction. MDDs in the rumination condition exhibited less cortisol decline compared to MDDs in the distraction condition and compared to CTLs in either condition. Findings suggest that depressive rumination alters the trajectory of cortisol secretion in MDD and may prolong cortisol production. Results thereby provide important insights into the interaction of biological and psychological factors through which distress contributes to MDD.
Keywords: cortisol, HPA axis, depression, rumination, emotion regulation, experimental
A central feature of Major Depressive Disorder (MDD) is the tendency to respond to sadness with rumination, a maladaptive emotion regulation strategy that has been shown to predict the duration and severity of depressive episodes (McLaughlin & Nolen-Hoeksema, 2011; Nolen-Hoeksema, Wisco, & Lyubomirsky, 2008). The response styles theory (Nolen-Hoeksema et al., 2008) defines depressive rumination as a method of processing negative events by repetitively focusing on feelings of distress as well as the potential antecedents or repercussions of these feelings. A substantial body of research has demonstrated negative behavioral and emotional consequences of depressive rumination. Compared to more adaptive emotion regulation strategies, such as distraction, ruminative responses to sad mood diminish problem solving, increase engagement in maladaptive behaviors, and hinder recovery from negative events (Lyubomirsky & Tkach, 2004; Nolen-Hoeksema et al., 2008). Most notably, experimental research has shown that when individuals are in a sad mood state, rumination leads to more self-reported sadness compared to distraction (Feldner, Leen-Feldner, Zvolensky, & Lejuez, 2006). Depressive rumination, therefore, is believed to directly contribute to the pervasive low mood associated with depressive episodes (Morrow and Nolen-Hoeksema, 1990). In contrast to the considerable research examining the emotional and behavioral effects of depressive rumination, relatively little is understood about the consequences of depressive rumination on physical health, and in particular, on neuroendocrine functioning.
Recent theories posit that the maladaptive consequences of some forms of repetitive thought, including stressor-focused and depressive rumination, extend beyond emotional wellbeing to physical wellbeing (Brosschot, Gerin, & Thayer, 2006; see review by Watkins, 2008). Specifically, the continual processing or contemplation of a depressing or stressful event is predicted to alter individuals’ biological functioning. The neuroendocrine system plays a primary role in our body’s biological functioning (Patchev & Patchev, 2006). A central component of the neuroendocrine system is the hypothalamic-pituitary-adrenal (HPA) axis, a primary index of which is the hormone cortisol. Whereas moderate cortisol fluctuation facilitates adaptive responses to environmental changes, excess cortisol production – often stemming from chronic HPA axis activity – can be detrimental (Dedovic, Duchesne, Andrews, Engert, & Pruessner, 2009; Gold, Drevets, & Charney, 2002; Sephton & Speigel, 2003). Prolonged cortisol secretion leads to neurotoxicity in areas of the brain responsible for regulating emotions and coping effectively with distress (McEwen, 2006). Excessive cortisol secretion also has been shown to increase risk for medical conditions, including cancer, diabetes, and arthritis (McEwen, 1998), making it critical to understand factors associated with greater cortisol secretion.
Initial work in nonclinical populations has provided evidence for a connection between stressor-focused rumination and cortisol elevations (see review by Zoccola & Dickerson, 2012). Extending these findings to a sample of depressed adolescence, Stewart and colleagues (Stewart, Mazurka, Bond, Wynne-Edwards, & Harkness, 2013) found that trait depressive rumination was associated with elevated cortisol levels during the recovery period, whereas the tendency to use more adaptive emotion regulation strategies (e.g., distraction/problem solving) was associated with faster cortisol decline. The one study to use an experimental manipulation exposed participants to a sad mood induction and then randomly assigned them to a depressive rumination or distraction condition (Kuehner, Huffziger, & Liebsch, 2009). Results showed less cortisol decline in the rumination condition among students with high versus low depression symptoms. The effect of experimentally induced depressive rumination on cortisol levels, however, has never been examined within a clinically depressed sample.
The current study aimed to extend past research by examining the effects of induced depressive rumination versus distraction on cortisol secretion in clinically depressed and healthy control participants. Participants were exposed to a forced-failure paradigm, which was designed to place them in a sad mood state prior to the emotion regulation induction (Hammen, 2004). Participants then were randomly assigned to the depressive rumination or distraction condition. Salivary cortisol was measured when participants entered the lab, and during forced failure, emotion regulation, and post-emotion regulation periods. Overall, we expected cortisol levels to decline across the experiment as participants habituated to the stress of coming into the laboratory (Marceau, Dorn, & Susman, 2012). However, we expected depressive rumination to interrupt this cortisol decline. Specifically, we predicted that both depressed and healthy control participants in the depressive rumination condition would demonstrate less cortisol decline compared to individuals in the distraction condition. In addition, we expected that the effects of depressive rumination would be stronger in the group with clinical depression.
Methods and Materials
Participants
Adults 18 to 60 years of age were recruited via newspaper advertisements and Internet postings. Inclusion and exclusion criteria were determined via an in-person Structured Clinical Interview for DSM-IV (SCID; First, Gibbon, & Williams, 1996). Three clinical psychology graduate students and one post-doctoral fellow completed the SCIDs. All interviewers completed more than 20 hours of training in videotapes, live observation, written tests, and group supervision in addition to the formal coursework required by the doctoral program. Inter-rater disagreements or queries were discussed via a biweekly SCID meeting. Inter-rater reliability was excellent, κ = 1.00. Two groups were included: those who met criteria for current MDD and those who did not meet criteria for any past or current Axis I disorder (Control; CTL). Participants were excluded due to severe head trauma, learning disabilities, bipolar disorder, psychotic symptoms, alcohol or substance abuse within the past six months, or health conditions known to interfere with hypothalamic-pituitary-adrenal (HPA) axis activity (including pregnancy and endocrine disorders, per Kudielka, Hellhammer, & Wust, 2009). After excluding one extreme outlier (CTL), whose initial cortisol value was more than 10 SDs greater than the mean, there were 46 participants in the MDD group and 51 in the CTL group.
At the time of testing, 16 MDD participants were on medication(s), including psychotropic medication (15) and oral contraceptives (1). Percent of depressed participants who were on medication did not differ across emotion regulation condition, χ2(1, N=46) = 1.53, p = .22. In addition, 5 CTL participants were on medication(s) at the time of testing, including oral contraceptives (4) and blood pressure medication (1). Percent of control participants who were on medication did not differ across emotion regulation condition, χ2(1, N=51) = 0.18, p = .67. Within the MDD group, 35 participants met criteria for a comorbid anxiety disorder. Percent with comorbidity did not differ across emotion regulation condition, χ2(1, N=46) = 1.80, p = .18
Forced-Failure Paradigm
Three forced-failure tasks were used to induce mild sadness. The first was a 15-minute facial identification task with false feedback indicating that the participant performed poorly (Tran, Siemer, & Joormann, 2011). Participants were asked to identify the emotional expression (happy, sad, angry) depicted in subliminally presented facial expressions. Participants repeatedly received feedback that they were performing poorly relative to others who had already completed the task, and the experimenter urged participants to try harder. The second task was a 5-minute anagram task, in which approximately 30% of the anagrams were unsolvable (van Randenborgh, Hüffmeier, LeMoult, & Joormann, 2010). Participants were given 5 minutes to solve as many anagrams as possible but were allowed only 30 seconds to solve each anagram. The third was a serial subtraction task (Kirschbaum, Pirke, & Hellhammer, 1993). Participants were given 5 minutes to count backward aloud from 2,083 to zero in 13-step sequences as quickly and accurately as possible. If an error was made, the experimenter would say “error, start again at 2,083.” No participant reached zero in the time allotted.
Emotion Regulation (ER) Induction
Participants were randomly assigned to either a depressive rumination or distraction condition, adapted from the frequently used emotion regulation (ER) induction procedure developed by Nolen-Hoeksema and Morrow (1993). This ER manipulation was selected given its use in prior studies on depressive rumination (see review by Lyubomirsky & Tkach, 2004), its use when examining the relation between depressive rumination and cortisol in a student sample (Kuehner et al., 2009), and its consistency with Nolen-Hoeksema and colleague’s definition of depressive rumination (Nolen-Hoeksema, 1991; Nolen-Hoeksema et al., 2008). Regardless of condition, participants viewed seven prompts one-at-a-time on the computer screen. They were asked to think and write about each prompt for two minutes. The prompts differed by condition. Depressive rumination prompts focused participants’ attention on thoughts that were emotion or self focused (e.g., “why things turn out the way they do for you.”). Distraction prompts focus participants’ attention on thoughts that were unrelated to the self (e.g., “the layout of a mall you have been to.”). Participants’ written statements were later coded by two independent raters who were blind to group and condition. Rumination score ratings, which were based on Hilt and Pollak (2013), were made on a 5-point Likert scale ranging from 1 (Not at all ruminating) to 5 (Completely ruminating), ICC = .84.
Measures
Sadness ratings
Self-reported sadness was assessed at 10 points: upon entering the lab, following a five-minute nature video, during the forced-failure paradigm, after the forced-failure paradigm, immediately after the ER induction, and five times during the nature video. Participants utilized an 11-point Likert-scale ranging from 0 (not at all) to 10 (very much). To test the specific effects of the forced-failure and ER-induction, we focused our analyses on assessments made following the nature video, during the forced-failure paradigm, and immediately after the ER induction. The general pattern of findings does not differ based on whether all 10 samples are used, with the three-way time by group by condition interaction remaining significant at Order 4, F(1, 91) = 5.85, p= .02, η2 = .06.
Questionnaires
Participants completed the Beck Depression Inventory-II (BDI; Beck, Steer, & Brown, 1996), a 21-item measure assessing depressive symptom severity (α = .97). Additionally, participants completed the Ruminative Responses Scale of the Response Style Questionnaire (RRS; Nolen-Hoeksema & Morrow, 1991), a 22-item self-report questionnaire assessing individual differences in the tendency to ruminate when sad (α = .99).
Cortisol Collection and Assay
Cortisol was extracted from saliva collected using salivette swabs (Sarstedt, Numbrecht, Germany). Samples were stored at −20°C until shipped to a laboratory for cortisol assay. Samples were centrifuged at 3000rpm for 5 minutes to produce a clear supernatant of low viscosity. Using a commercially available immunoassay with chemiluminescence detection, 50µL were removed for cortisol analysis. The lower detection limit of this assay was 0.43nmol/L. Intra- and interassay coefficients of variation were below 8% for low (3nmol/L) and high (25nmol/L) cortisol levels.
Procedure
The experiment was approved by the Institutional Review Board at the University of Miami, and all experiments were performed in accordance with ascribed guidelines and regulations. In-person SCIDs were conducted by trained graduate students. Participants who met inclusion and exclusion criteria were invited to return for the main study session. We gave participants verbal and written instructions to refrain from eating, drinking other than water, using nicotine, brushing their teeth, and exercising for 2 hours prior to the main study session.
The main study session was scheduled between 12pm and 6pm to minimize the effects of diurnal fluctuation in cortisol levels. The session consisted of a 5-minute nature video, 30-minute forced-failure paradigm, 16-minute ER induction, and 35-minute post-ER period. During the post-ER period participants watched a nature video. Timing of saliva samples was selected to identify changes in cortisol levels as a result of the ER manipulation. Participants provided 11 cortisol samples: immediately upon entering the lab (enter lab), after the five-minute nature video, in the middle of the forced failure induction, at the end of the forced-failure period (30 minutes after failure onset; post-failure), in the middle of the ER induction, at the end of the ER induction and every 7 minutes thereafter (post-ER 1–6). Lastly, participants completed the questionnaires. Due to missing data or duplicate information, samples 2, 3, and 5 were not included in the final analyses.
Statistical Analyses
Demographic data, affect ratings, and baseline differences between diagnostic group (MDD, CTL) and ER condition (rumination, distraction) were examined in SPSS 20.0 (SPSS Inc., USA) using analyses of variance (ANOVAs) and chi-square tests. Cortisol data were analyzed using multilevel modeling; a series of growth models were conducted with hierarchical linear modeling software (HLM), Version 6.01 (Raudenbush, Bryk, & Congdon, 2004). Multilevel modeling is ideal for analyzing nested data. Multilevel growth models allow researchers to partition the variance into two levels: Level 1 (within individual) represents intraindividual variability in scores at different measurement occasions, and Level 2 (between individuals) represents the variability between individuals’ scores. HLM was specifically selected because it allows the examination of variably spaced measurement occasions or observations that are unevenly spaced over time (Hrushka et al., 2005). In our models, the exact time of cortisol collection was allowed to vary by individual, thereby providing a more precise estimation of time for each participant (Singer & Willet, 2003). Diagnostic group, ER condition, and group × condition were examined as predictors at Level 2.
Results
Participant Characteristics
Participant characteristics are presented in Table 1. There were no significant differences in age across group, F(1, 93) = 0.19, p = .67, η2 = .002 or condition, F(1, 93) = 0.01, p = .93, η2 = .00001, and the group by condition interaction was not significant, F(1, 93) = 2.75, p = .11,η2 = .03. There was also no significant difference in ethnicity across group, χ2(1, N=96) = 0.53, p = .47, or condition, χ2(1, N=96) = 0.04, p = .83. Although there was evidence of differences in proportion who were female across group, χ2(1, N=97) = 3.67, p = .06, and condition, χ2(1, N=97) = 3.71, p = .05, the proportion female did not differ across ER condition within the MDD group, χ2(1, N=46) = 0.81, p = .37. There were, however, slightly fewer females in the rumination condition than the distraction condition within the CTL group, χ2(1, N=51) = 3.36, p = .07. Thus, gender was included as a covariate when testing the main study hypotheses. As expected, the MDD group obtained significantly higher BDI scores than the CTL group, F(1, 93) = 235.54, p < .001, η2 = .72; however, there was no significant main effect of condition, F(1, 93) = 1.19, p = .28, η2 = .01, or group by condition interaction, F(1, 93) = 0.15, p = .70, η2 = .002. In addition, although the MDD group obtained significantly higher RRS scores than the CTL group, F(1, 92) = 325.11, p < .001, η2 = .78, there was no significant main effect of condition, F(1, 92) = 0.24, p = .63, η2 = .003, or group by condition interaction, F(1, 92) = 0.03, p = .87, η = .0003.1 As expected, the MDD group reported significantly higher baseline sadness scores than the CTL group, F(1, 91) = 45.68, p < .001, η2 = .33; however, sadness scores did not significantly differ by ER condition, F(1, 91) = 0.32, p = .57, η2 = .004, and the group by condition interaction was not significant, F(1, 91) = 0.42, p = .52, η2 = .01.2 Cortisol levels when participants entered the laboratory did not significantly differ by group, F(1, 93) = 0.78, p = .38, η2 = .01, or condition, F(1, 93) = .001, p = .98, η2 = .00001, and the group by condition interaction was not significant, F(1, 93) = 1.81, p = .18, η2 = .02.
Table 1.
Participant Demographics
| CTL (N= 51) |
MDD (N= 46) |
|||
|---|---|---|---|---|
| Variable | Rumination | Distraction | Rumination | Distraction |
| Age, M (SD) | 34.27 (11.59) | 38.48 (11.54) | 39.30 (12.25) | 35.52 (12.08) |
| Sex (female:male) | 7:19 | 13:12 | 12:11 | 15:8 |
| Caucasian, % | 40 | 44 | 35 | 32 |
| BDI, M (SD) | 2.81 (4.65) | 4.21 (5.18) | 28.71 (11.66) | 31.23 (11.39) |
| RRS, M (SD) | 30.84 (10.16) | 31.48 (8.76) | 66.22 (8.72) | 67.52 (10.98) |
| Rumination Score, M (SD) | 10.50 (3.50) | 7.21 (0.41) | 17.35 (5.06) | 8.87 (4.42) |
Emotion Regulation Check
Participants’ written responses during the ER induction were coded to determine the amount they were ruminating (See Table 1; Rumination Score). There was a significant main effect of group, F(1, 92) = 30.57, η2 = .25, and condition, F(1, 92) = 58.48, η2 = .39, ps < .001. In addition, the group by condition interaction was significant, F(1, 92) = 11.36, p = .001, η2 = .11. Importantly, individuals assigned to the rumination condition ruminated significantly more than those assigned to the distraction condition in both the CTL, t(48) = 4.57, and MDD groups, t(44) = 6.05, ps < .001. Although between-group differences were not found within the distraction condition, t(45) = 1.83, p = .07, MDDs in the rumination condition ruminated significantly more than CTLs in the rumination condition, t(47) = 5.56, p < .001.
Manipulation Check
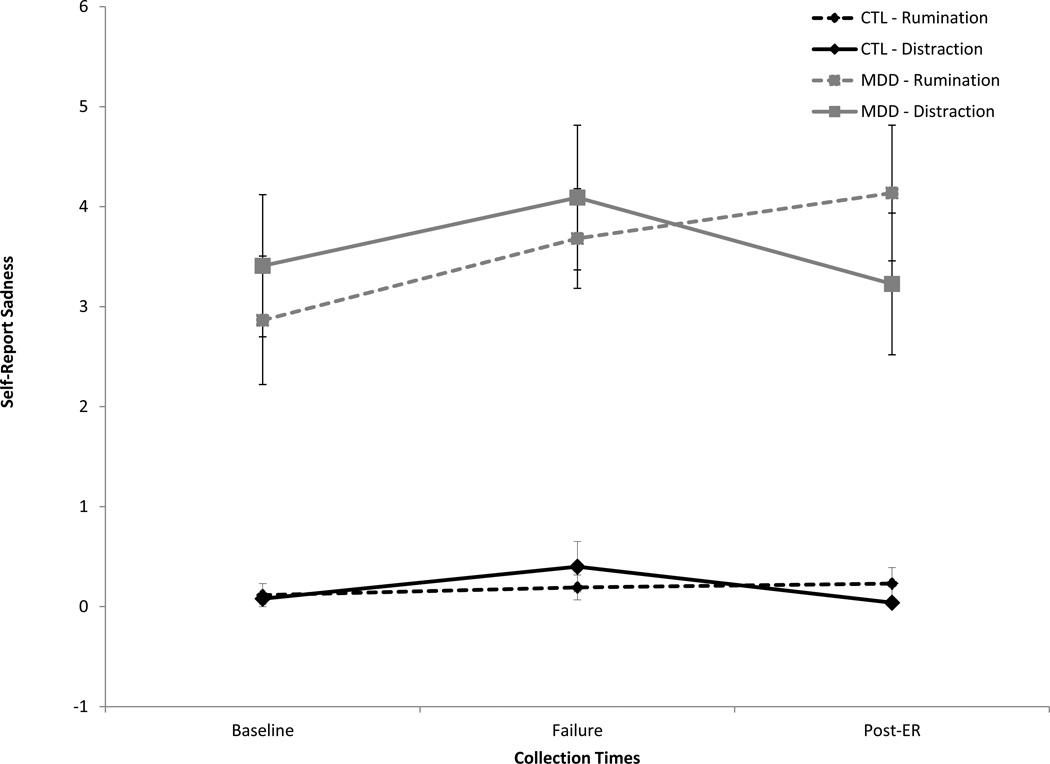
See Figure 1 for sadness ratings. The repeated-measures ANOVA on sadness scores revealed a main effect of group, F(1, 91) = 69.63, p < .001, η2 = .43, indicating higher self-reported sadness in the MDD versus CTL group. There was also a main effect of time, F(2, 182) = 4.17, p = .02, η2 = .04, which was qualified by a time by ER condition interaction, F(2, 182) = 4.22, p = .02, η2 = .04. No other main or interaction effects were significant, Fs < 2.05, η2 < .03, ps > .05.
Figure 1.
Self-reported sadness by group (MDD vs. CTL) and condition (rumination vs. distraction).
Follow-up tests examining the change in sadness from baseline to failure revealed a significant increase in sadness, t(94) = 3.25, p = .002, which did not significantly differ between the rumination and distraction conditions, t(93) = 0.26, p = .80. However, the change in sadness from failure to post-ER differed by condition, t(93) = 2.35, p= .02. Whereas sadness ratings significantly decreased in the distraction group, t(46) = 2.44, p = .02, sadness ratings did not significantly change in the rumination group, t(47) = 0.91, p = .373
Effects of ER Condition on Salivary Cortisol
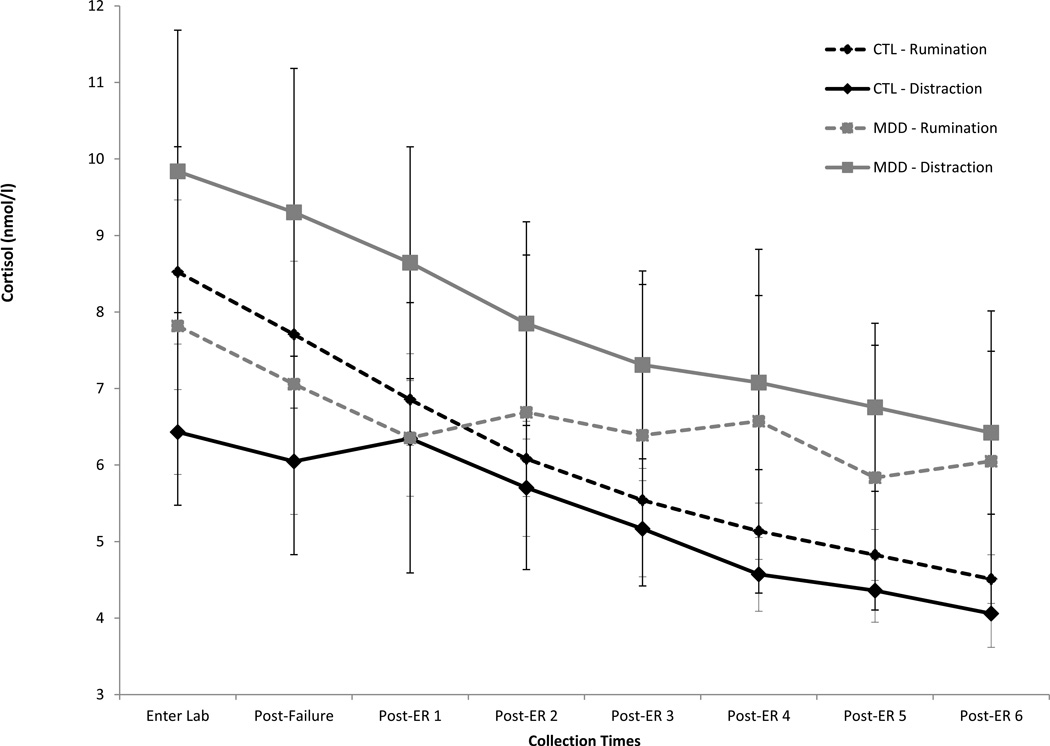
See Figure 2 for cortisol data. In line with our study design and previous research (Taylor et al., 2006), a two-rate piecewise linear growth model (Raudenbush & Bryk, 2002) was used to simultaneously model linear changes in cortisol before and after the ER induction. The Level 1 function was as follows:
Cortisol= π0j + π1j(pre-ER)+ π2j(post-ER)+ eij
Where π0j represents cortisol level when participant j entered the laboratory, π1j represents the slope of cortisol prior to the ER induction for participant j, and π2j represents the slope of cortisol after the ER induction for participant j. Table 2 provides the average intercept, pre-ER, and post-ER coefficients, each of which had sufficient random effects variance, ps < .001. Whereas cortisol level did not change prior to the ER induction, there was a significant decline in cortisol level after the ER induction.
Figure 2.
Cortisol levels by group (MDD vs. CTL) and condition (rumination vs. distraction).
Table 2.
Hierarchical linear modeling of salivary cortisol
| Predictors | Coefficient | SE | t-value | p-value |
|---|---|---|---|---|
| Level 1 | ||||
| Intercept | 8.13 | 0.76 | 10.72 | < .001 |
| Pre-ER (linear) | −0.01 | 0.01 | 1.14 | 0.256 |
| Post-ER (linear) | −0.04 | 0.01 | 6.19 | < .001 |
| Level 2 | ||||
| Intercept | 6.03 | 1.80 | 3.36 | 0.001 |
| Sex | 3.40 | 1.52 | 2.24 | 0.028 |
| Group | 0.17 | 2.09 | 0.08 | 0.936 |
| Condition | −1.23 | 2.05 | 0.60 | 0.548 |
| Group × Condition | 3.70 | 2.93 | 1.26 | 0.210 |
| Pre-ER (linear) | −0.03 | 0.02 | 1.25 | 0.214 |
| Sex | 0.01 | 0.02 | 0.78 | 0.436 |
| Group | −0.001 | 0.03 | 0.04 | 0.967 |
| Condition | 0.02 | 0.02 | 0.96 | 0.338 |
| Group × Condition | −0.01 | 0.04 | 0.20 | 0.839 |
| Post-ER (linear) | −0.03 | 0.01 | 2.37 | 0.020 |
| Sex | −0.02 | 0.01 | 2.12 | 0.036 |
| Group | 0.03 | 0.02 | 1.93 | 0.057 |
| Condition | 0.01 | 0.02 | 0.64 | 0.522 |
| Group × Condition | −0.04 | 0.02 | 2.00 | 0.048 |
At Level 2 we tested the effects of diagnostic group, ER condition, and the group-by-condition interaction predicting Level 1 parameters. Diagnostic group was dummy coded as 0 (CTL) and 1 (MDD). ER condition was dummy coded as 0 (rumination) and 1 (distraction). Sex (also dummy coded with 0 [female] and 1 [male]) was included in all analyses given evidence of differences between groups and conditions in percent female.4 We specified the following models at Level 2:
Intercept: π0j = β00 + β01(sex)+ β02(group)+ β03(condition)+ β04 (group × condition)+ r0
Slope Pre-ER: π1j = β10 + β11(sex)+ β12(group)+ β13(condition)+ β14(group × condition)+ r1
Slope Post-ER: π2j = β20 + β21(sex)+ β22(group)+ β23(condition)+ β24(group × condition)+ r2
Coefficient estimates and significance tests can be found in Table 2. Cortisol levels when participants entered the lab were influenced by sex, with males displaying higher cortisol levels than females. Cortisol levels when participants entered the lab did not differ by group or condition, and the group by condition interaction was not significant. As expected, change in cortisol prior to the ER induction was not influenced by sex, group, or condition, and the group by condition interaction was not significant. Change in cortisol after the ER induction, however, differed by sex, with males displaying steeper cortisol decline than females. There was a marginally significant main effect of group, suggesting flatter cortisol decline in the MDD versus CTL group, and this was moderated by the expected group × condition interaction.
Additional HLM analyses were run to follow-up on the group × condition interaction predicting change in cortisol after the ER induction. Within the CTL group, cortisol levels declined post-ER in both the rumination, β = −0.04, t(48) = 2.74, p = .01, and distraction conditions, β = −0.02, t(48) = 2.24, p = .03. Cortisol decline did not differ between the rumination and distraction conditions, β = 0.01, t(48) = 0.87, p = .39. In contrast, within the MDD group, cortisol levels declined post-ER for those in the distraction condition, β = −0.03, t(43) = 2.34, p = .02, but cortisol levels failed to decline for MDDs in the rumination condition, β = 0.00, t(43) = 0.18, p = .86. Moreover, MDDs in the rumination condition experienced less cortisol decline compared to MDDs in the distraction condition, β = −0.04, t(43) = 1.99, p = .05, and compared to CTLs in either condition, β = 0.03, t(71) = 2.40, p = .02.
Discussion
This study is the first to examine the effect of a depressive rumination versus distraction manipulation on salivary cortisol in clinically depressed and healthy control participants. Results showed that the ER manipulation affected the trajectory of depressed participants’ cortisol levels. Prior to the ER induction, cortisol production did not differ by group or condition. Following the ER induction, however, cortisol decline differed by group and condition. MDDs in the rumination condition exhibited less cortisol decline compared to MDDs in the distraction condition and compared to CTLs in either ER condition. In fact, cortisol levels significantly declined for MDDs in the distraction condition, CTLs in the depressive rumination condition, and CTLs in the distraction condition, whereas cortisol levels did not significantly decline for MDDs in the depressive rumination condition.
Although authors have posited that forms of repetitive thought, such as stressor-focused rumination, change salivary cortisol secretion (Brosschot et al., 2006; Watkins, 2008), the effects of depressive rumination on salivary cortisol had never been experimentally investigated in a clinically depressed sample. The majority of past research linking rumination and cortisol has been correlational (e.g., Zoccola, Dickerson, & Zaldivar, 2008), making it difficult to determine whether rumination leads to higher cortisol levels or whether higher cortisol levels lead to rumination. This study demonstrates, for the first time, that experimentally induced depressive rumination alters cortisol decline in MDD. Our findings are in line with an experimental study using a non-clinical sample, in which undergraduates were exposed to a sad mood induction and then randomly assigned to ruminate on their sad mood, distract themselves from it, or mindfully self-focus (e.g., Kuehner et al., 2009). Similar to results from the current study, participants reporting high depression symptoms who were assigned to the depressive rumination condition showed less cortisol decline. Such findings lend support to perseverative cognition models (Brosschot et al., 2006; Watkins, 2008), which suggest that repetitive thought processes – such as depressive rumination – have consequences for both emotional and physical wellbeing.
Results from the current study advance our understanding of MDD in several ways. For one, results have direct relevance for the stress sensitization/kindling model of depression (Hammen, 2005; Post, 1992). This model posits that neurobiological changes during depressive episodes lead to increasing interdependence between negative events and depression. Past research indicates that such neurobiological change can come from chronic cortisol activation, which increases atrophy of brain regions such as the prefrontal cortex and hippocampus, and in turn hinders one’s ability to regulate emotional responses to negative events (Gold, Drevents, & Charney, 2002; McEwen, 1998). With this in mind, our findings suggest that depressive rumination increases the chance that depressed individuals will experience neurobiological changes that sensitize them to future negative events, thereby increasing their chance of experiencing recurrent depressive episodes.
In addition, knowledge elucidated from this study has important implications for our understanding of health and disease in MDD. A diagnosis of depression places individuals at increased risk for poor health outcomes, including faster progression of illness, immunosuppression, and increased risk of cardiac events (e.g., Kiecolt-Glaser, McGuire, Robles, & Glaser, 2002). Past research has demonstrated that chronic cortisol hypersecretion has substantial effects on cardiovascular health and immune functioning (McEwen, 1998; McEwen, 2008). Results from the current study suggest that depressive rumination may contribute to the cortisol hypersecretion that places depressed individuals at increased risk for health difficulties. This possibility has important implications for clinical interventions for individuals with MDD and comorbid health conditions. For example, interventions that target depressive rumination may be an important addition to behavioral medicine.
Interestingly, healthy controls in the current study did not differ in their cortisol decline based on whether they were assigned to ruminate or distract. Although unexpected, this is in line with a study by Young and Nolen-Hoeksema (2001), in which high and low trait ruminators did not differ in their cortisol response to stress. The authors attributed their null results to a lack of depressive rumination in their non-clinical sample, as determined by a retrospective analysis of participants’ thought samples. A similar explanation might apply to the current study given that our manipulation check suggested that the depressive rumination induction was less effective for CTLs than MDDs. However, there is evidence supporting the effectiveness of our emotion regulation induction in both the CTL and MDD groups: In both groups, participants exhibited more rumination when in the depressive rumination compared to distraction condition. In addition, depressive rumination prolonged self-reported sadness in both the CTL and MDD group. Thus, another explanation for our findings might be considered. It is possible that depressive rumination did not affect CTLs’ cortisol levels due to effective functioning of their HPA axis. An important component of HPA axis functioning is the ability to down-regulate cortisol production when optimal cortisol levels have been reached. This is accomplished via negative feedback loops; receptors on the hypothalamus, pituitary, and hippocampus identify elevated levels of cortisol and signal the HPA axis to decrease cortisol production (McEwen, 2006). The chronic cortisol elevation often associated with MDD can damage the sensitivity of glucocorticoid receptors, and thus contribute to difficulty down regulating cortisol production (Burke, Davis, Otte, & Mohr, 2005; McEwen, 2008). In contrast, healthy functioning of negative feedback loops helps avoid excess cortisol production and may have protected CTLs against the effects of depressive rumination. Although additional research is needed to explore this possibility, it is in line with results from Kuehner et al. (2009) showing that participants who reported low depressive symptoms did not differ in their cortisol decline based on whether they were in the depressive rumination or distraction condition.
Several limitations of the current study should be mentioned. For one, many participants with depression also met criteria for a comorbid psychiatric diagnosis. However, participants were randomly assigned to either the rumination or distraction condition and there were no systematic differences between the two conditions. Second, several participants were taking medications. Although the sample size in the current study prevented us from examining the effects of specific medication classes on change in cortisol, the percent of individuals on medication did not significantly differ between the rumination and distraction conditions in either group. In addition, the current study did not include an assessment of baseline cortisol. Given that the first cortisol sample was taken almost immediately after participants arrived in the laboratory and there is typically a 10 to 20 minute lag in cortisol response (Dickerson & Kemeny, 2004), there would not have been enough time for cortisol levels to reach to baseline. Given that the goal of the current study was to examine change in cortisol as a result of the ER induction, we structured the study procedure to maximize the amount of time and number of cortisol samples after the ER induction. As a result, however, we are unable to answer questions related to baseline cortisol or changes from baseline in this sample. The lack of baseline cortisol sample may explain why we did not see a change in cortisol from when participants entered the laboratory to the end of the forced failure induction. Any potential decline in cortisol due to participants’ habituating to the laboratory may have been offset by potential increases in cortisol due to the forced failure induction. Future research might examine this question more closely.
Despite these limitations, the current study provides important information about how rumination affects cortisol production in MDD. In doing so, this study advances integrated emotional-cognitive-biological models of MDD (Watkins, 2008; Hammen, 2005; Post, 1992). Moreover, keeping in mind the consequences of prolonged cortisol levels on cardiovascular health and immune functioning (McEwen, 1998), rumination may be a key factor that places depressed individuals at increased risk for poor health outcomes, such as increased risk of a cardiac event and faster progression of illness. Thus, identifying rumination as a mechanism underlying excess cortisol production in MDD may have important implications for understanding not only the maintenance and recurrence of depressive episodes but also the comorbid physical health conditions associated with depression (see Watkins, 2008, for a review).
Highlights.
-
▪
Examined the effect of a depressive rumination versus distraction induction on cortisol decline
-
▪
Depressive rumination altered cortisol production in group with Major Depressive Disorder (MDD)
-
▪
Compared to distraction, depressive rumination hindered both emotional and cortisol decline
-
▪
Findings advance integrative emotional-biological-cognitive models of depression
Acknowledgements
This research was partially supported by a grant from the National Institute of Mental Health [F31MH086246].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
One CTL participant (rumination) did not provide ethnicity data or complete the RRS.
Sadness ratings could not be obtained from two MDD participants (one rumination and one distraction).
Exploratory analyses conducted on a negative affect composite score (sad, angry, tense, anxious, irritated, upset, and nervous) revealed an increase in negative affect during the forced-failure induction, tpaired(95) = 4.84, p < .001, and a decrease in negative affect following the forced-failure induction, tpaired(95) = 2.62, p < .02. The increase in negative affect during the forced-failure induction was greater in the MDD versus CTL group, t(94) = 2.49, p < .02. No other main or interactive effects were significant.
We also examined whether gender moderated the effect of group, condition, or the group by condition interaction. There was no evidence that gender significantly interacted with any of the predictor variables to influence cortisol levels when participants’ entered the lab (π0j), slope prior to the ER induction (π1j) or slope following the ER induction (π2j), all ps > .05.
References
- Armbruster D, Mueller A, Strobel A, Lesch K, Brocke B, Kirschbaum C. Children under stress—COMT genotype and stressful life events predict cortisol increase in an acute social stress paradigm. International Journal of Neuropsychopharmacology. 2012;15(9):1229–1239. doi: 10.1017/S1461145711001763. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- Brosschot JF, Gerin W, Thayer JF. The perseverative cognition hypothesis: A review of worry, prolonged stress-related physiological activation, and health. Journal of Psychosomatic Research. 2006;60(2):113–124. doi: 10.1016/j.jpsychores.2005.06.074. [DOI] [PubMed] [Google Scholar]
- Burke HM, Davis MC, Otte C, Mohr DC. Depression and cortisol responses to psychological stress: a meta-analysis. Psychoneuroendocrinology. 2005;30:846–856. doi: 10.1016/j.psyneuen.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Cohen S, Schwartz JE, Epel E, Kirschbaum C, Sidney S, Seeman T. Socioeconomic status, race, and diurnal cortisol decline in the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Psychosomatic Medicine. 2006;68:41–50. doi: 10.1097/01.psy.0000195967.51768.ea. [DOI] [PubMed] [Google Scholar]
- Dedovic K, Duchesne A, Andrews J, Engert V, Pruessner JC. The brain and the stress axis: the neural correlates of cortisol regulation in response to stress. Neuroimage. 2009;47(3):864–871. doi: 10.1016/j.neuroimage.2009.05.074. [DOI] [PubMed] [Google Scholar]
- Feldner MT, Leen-Feldner EW, Zvolensky MJ, Lejuez CW. Examining the association between rumination, negative affectivity, and negative affect induced by a paced auditory serial addition task. Journal of behavior therapy and experimental psychiatry. 2006;37(3):171–187. doi: 10.1016/j.jbtep.2005.06.002. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM–IV Axis I Disorders—Clinician Version (SCID–CV) Washington, DC: American Psychiatric Press; 1996. [Google Scholar]
- Gold PW, Drevets WC, Charney DS. New insights into the role of cortisol and the glucocorticoid receptor in severe depression. Biological Psychiatry. 2002;52:381–385. doi: 10.1016/s0006-3223(02)01480-4. [DOI] [PubMed] [Google Scholar]
- Hammen C. Stress and depression. Annual Review Clinical Psychology. 2005;1:293–319. doi: 10.1146/annurev.clinpsy.1.102803.143938. [DOI] [PubMed] [Google Scholar]
- Hilt LM, Pollak SD. Characterizing the ruminative process in young adolescents. Journal of Clinical Child and Adolescent Psychology. 2013;42:519–530. doi: 10.1080/15374416.2013.764825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, McGuire L, Robles TF, Glaser R. Psychoneuroimmunology: psychological influences on immune function and health. Journal of consulting and clinical psychology. 2002;70:537. doi: 10.1037//0022-006x.70.3.537. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke K-M, Hellhammer DH. The 'Trier Social Stress Test'- a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28:76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Hellhammer DH, Wust S. Why do we respond so differently? Reviewing determinants of human salivary cortisol responses to challenge. Psychoneuroendocrinology. 2009;34:2–18. doi: 10.1016/j.psyneuen.2008.10.004. [DOI] [PubMed] [Google Scholar]
- Kuehner C, Huffziger S, Liebsch K. Rumination, distraction and mindful self-focus: effects on mood, dysfunctional attitudes and the cortisol stress response. Psychological Medicine. 2009;39:219–228. doi: 10.1017/S0033291708003553. [DOI] [PubMed] [Google Scholar]
- Lyubomirsky S, Caldwell ND, Nolen-Hoeksema S. Effects of ruminative and distracting responses to depressed mood on retrieval of autobiographical memories. Journal of Personality and Social Psychology. 1998;75:166–177. doi: 10.1037//0022-3514.75.1.166. [DOI] [PubMed] [Google Scholar]
- Lyubomirsky S, Tkach C. The consequences of dysphoric rumination. In: Papageorgiou C, Wells A, editors. Depressive Rumination: Nature, Theory, and Treatment of Negative Thinking in Depression. Chichester, England: John Wiley & Sons; 2004. pp. 21–41. [Google Scholar]
- Marceau K, Dorn LD, Susman EJ. Stress and puberty-related hormone reactivity, negative emotionality, and parent-adolescent relationships. Psychoneuroendocrinology. 2012;37(8):1286–1298. doi: 10.1016/j.psyneuen.2012.01.001. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Protective and damaging effects of stress mediators. New England Journal of Medicine. 1998;338:171–179. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Stress, adaptation, and disease: Allostasis and allostatic load. Annals of the New York Academy of Sciences. 2006;840:33–44. doi: 10.1111/j.1749-6632.1998.tb09546.x. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Central effects of stress hormones in health and disease: Understanding the protective and damaging effects of stress and stress mediators. European journal of pharmacology. 2008;583(2):174–185. doi: 10.1016/j.ejphar.2007.11.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin K, Nolen-Hoeksema S. The role of rumination in promoting and preventing depression in adolescent girls. In: Strauman T, Costanzo P, Garber J, Abramson LY, editors. Depression in Adolescent Girls. New York: Guilford; 2011. pp. 112–129. [Google Scholar]
- Morrow J, Nolen-Hoeksema S. Effects of responses to depression on the remediation of depressive affect. Journal of Personality and Social Psychology. 1990;58:519–527. doi: 10.1037//0022-3514.58.3.519. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S, Morrow J. A prospective study of depression and posttraumatic stress symptoms after a natural disaster: the 1989 Loma Prieta Earthquake. Journal of personality and social psychology. 1991;61(1):115. doi: 10.1037//0022-3514.61.1.115. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S, Morrow J. Effects of rumination and distraction on naturally occurring depressed mood. Cognition and Emotion. 1993;7:561–570. [Google Scholar]
- Nolen-Hoeksema S, Wisco BE, Lyubomirsky S. Rethinking rumination. Perspectives on Psychological Science. 2008;3:400–424. doi: 10.1111/j.1745-6924.2008.00088.x. [DOI] [PubMed] [Google Scholar]
- Patchev VK, Patchev AV. Experimental models of stress. Dialogues in Clinical Neuroscience. 2006;8:417–432. doi: 10.31887/DCNS.2006.8.4/vpatchev. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post RM. Transduction of Psychosocial Stress Into the Neurobiology. American Journal of Psychiatry. 1992;149:999–1010. doi: 10.1176/ajp.149.8.999. [DOI] [PubMed] [Google Scholar]
- Schroder KE, Carey MP, Vanable PA. Methodological challenges in research on sexual risk behavior: I. Item content, scaling, and data analytical options. Annals of Behavioral Medicine. 2003;26(2):76–103. doi: 10.1207/s15324796abm2602_02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sephton SE, Speigel D. Circadian disruption in cancer: A neuroendocrine-immune pathway from stress to disease. Brain, Behavior, and Immunity. 2003;17:321–328. doi: 10.1016/s0889-1591(03)00078-3. [DOI] [PubMed] [Google Scholar]
- Stewart JG, Mazurka R, Bond L, Wynne-Edwards KE, Harkness KL. Rumination and impaired cortisol recovery following a social stressor in adolescent depression. Journal of Abnormal Child Psychology. doi: 10.1007/s10802-013-9740-1. (in press). [DOI] [PubMed] [Google Scholar]
- Tran TB, Siemer M, Joormann J. Implicit interpretation biases affect emotional vulnerability: A training study. Cognition and Emotion. 2011;25:546–558. doi: 10.1080/02699931.2010.532393. [DOI] [PubMed] [Google Scholar]
- van Randenborgh A, Hüffmeier J, LeMoult J, Joormann J. Letting go of unmet goals: Does self-focused rumination impair goal disengagement? Motivation and Emotion. 2010;34(4):325–332. [Google Scholar]
- Watkins ER. Constructive and unconstructive repetitive thought. Psychological Bulletin. 2008;134:163–206. doi: 10.1037/0033-2909.134.2.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon KL, Joormann J. Stress reactivity in social anxiety disorder with and without comorbid depression. Journal of Abnormal Psychology. 2012;121:250–255. doi: 10.1037/a0025079. [DOI] [PubMed] [Google Scholar]
- Young EA, Nolen-Hoeksema S. Effect of ruminations on the saliva cortisol response to a social stressor. Psychoneuroendocrinology. 2001;26:319–329. doi: 10.1016/s0306-4530(00)00059-7. [DOI] [PubMed] [Google Scholar]
- Zoccola PM, Dickerson SS. Assessing the relationship between rumination and cortisol: A review. Journal of Psychosomatic Research. 2012;73:1–9. doi: 10.1016/j.jpsychores.2012.03.007. [DOI] [PubMed] [Google Scholar]
- Zoccola PM, Dickerson SS, Zaldivar FP. Rumination and cortisol responses to laboratory stressors. Psychosomatic Medicine. 2008;70:661–667. doi: 10.1097/PSY.0b013e31817bbc77. [DOI] [PubMed] [Google Scholar]