Abstract
Context
Family caregivers are a vital resource in the recovery of intensive care unit (ICU) survivors. Of concern, the stress associated with this role can negatively affect caregiver health. Fatigue, an important health indicator, has been identified as a predictor of various illnesses, greater use of health services, and early mortality. Examining the impact of fatigue on caregivers’ physical health can assist in identifying critical time points and potential targets for intervention.
Objectives
To describe self-reported fatigue in caregivers of ICU survivors from patients’ ICU admission to ≤ two weeks, two- and four-months post-ICU discharge.
Methods
Patient-caregiver pairs were enrolled from a medical ICU. Caregiver fatigue was measured using the Short-Form-36 Health Survey Vitality subscale (SF-36 Vitality). Caregiver psychobehavioral stress responses included depressive symptoms, burden, health risk behaviors, and sleep quality. Patient data included self-reported physical symptoms and disposition (home vs. institution).
Results
Forty seven patient-caregiver pairs were initially enrolled. Clinically significant fatigue (SF-36 Vitality ≤ 45) was reported by 43% to 53% of caregivers across the time points and these caregivers reported worse scores in measures of depressive symptoms, burden, health risk behaviors and sleep quality, and patients’ symptom burden. In 26 caregivers with data for all time points (55% of the total sample), SF-36 Vitality scores showed trends of improvement when the patient returned home and greater impairment when institutionalization continued.
Conclusion
In caregivers of ICU survivors, fatigue is common and potentially linked with poor psychobehavioral responses. Worsening fatigue was associated with greater symptom distress and long-term patient institutionalization.
Keywords: intensive care unit, family caregivers, caregiver health, fatigue, depressive symptoms, burden, long-term outcomes
Introduction
More than five million Americans are admitted to an intensive care unit (ICU) annually.1 Having a loved one in the ICU places profound demands on physical and mental energy in family caregivers. In patients who require prolonged mechanical ventilation, ICU survival often leads to a lengthy recovery because of the presence of cognitive and functional deficits.2 Some patients regain their former level of health, but many do not.2,3 Thus, caregiving demands may continue or escalate following ICU discharge, placing caregivers at risk for psychological symptoms.4–7
Research, mostly involving family caregivers of persons living with chronic conditions (e.g., dementia, cancer), has identified that the stress of caregiving can impact physical and mental health outcomes.8–11 Whereas caregiving in chronic conditions typically involves disease progression and steadily increasing demands, demands placed on caregivers of ICU survivors are not well defined. In the U.S., a majority of patients are transferred to a long-term care facility for rehabilitation following ICU discharge and, later, optimally to home.12 Prior to home discharge, patients may experience multiple transitions between care settings that are frequent and unpredictable.12 In a one-year cohort study, 103 patients who survived to hospital discharge following ICU admission had 457 separate transitions in post-discharge care location (median of four transitions per patient).12 Hospital readmission was reported for 68 (67%) of those not initially discharged to hospice care.12 Because caregivers of these individuals are a vital resource, examining the impact of stress on their physical health is important to identify points of greatest impact and potential targets for intervention.
In various medical and psychiatric conditions, fatigue is a highly prevalent and debilitating symptom.13 Stressful life circumstances are known as one of the multiple etiologies of fatigue.14 Studies in caregivers of persons living with chronic illness (e.g., cancer) have reported fatigue as a highly prevalent symptom associated with negative psychological responses, such as caregiver burden and depressive symptoms.14–17 However, few studies have explored fatigue in caregivers of the critically ill. In a cross-sectional study of 74 caregivers of ICU patients at high risk of dying, “feeling tired” was one of the symptoms with the highest prevalence (> 90% of caregivers).18 Notably, 80% reported the severity of their fatigue as “moderate to severe.”18 Potentially, high levels of fatigue during the acute phase of a patient’s critical illness may impair the caregiver’s ability to participate in decision making for their loved one and may be associated with negative long-term psychological outcomes.18
Because many individuals who survive critical illness experience a complex and unpredictable recovery trajectory with high morbidity and mortality, it is important to better understand caregivers’ fatigue during the post-ICU discharge period. To date, little is known about the prevalence and severity of fatigue over the course of critical illness and recovery or the impact of fatigue on caregivers’ stress responses and health outcomes. Therefore, this analysis aimed to: 1) describe the prevalence of self-reported fatigue in caregivers from patients’ ICU admission to four months post-ICU discharge; 2) explore longitudinal trends in caregiver fatigue by patient residence (home vs. institution) at each time point, and 3) compare caregivers’ psychobehavioral stress responses and patients’ physical symptom burden by presence/absence of clinically significant fatigue in caregivers at each time point.
Methods
This secondary analysis used a dataset obtained from a longitudinal study that explored biobehavioral stress responses in family caregivers of critically ill adults who required prolonged acute mechanical ventilation (≥ 4 days). The study received institutional review board approval and all participants provided written informed consent.
Site and Sample
Pairs of caregivers and patients were recruited in a medical ICU (32 beds) in a tertiary academic medical center located in western Pennsylvania. We defined caregiver as the person who provided the majority of emotional, financial, and physical support to the patient prior to ICU admission. We enrolled one caregiver per one patient. If multiple family members were available, the family was asked to identify one individual to provide study data. Caregivers were not required to have a legal relationship or to cohabitate with the patient. Caregiver eligibility criteria were: 1) non-professional, non-paid caregiver; 2) age ≥ 21 years; 3) reliable telephone access; and 4) able to read and speak English. Patient eligibility criteria were: 1) age ≥ 21 years; 2) residing at home prior to ICU admission; 3) on mechanical ventilation for ≥ 4 consecutive days in a medical ICU; and 4) not dependent on mechanical ventilation prior to this ICU admission.
Measures
Measure of Caregivers’ Fatigue
The Short-Form-36 Health Survey vitality subscale (SF-36 Vitality) was used to measure caregiver self-reported fatigue.19 The SF-36 Vitality consists of four items asking how much time over the past week caregivers: 1) felt full of pep; 2) had a lot of energy; 3) felt worn out; or 4) felt tired. Responses range from “all of the time” to “none of the time.” Scores from these items were standardized on a 0–100 metric scale, with lower scores indicating poorer functioning. A score of ≤ 45 has been identified as indicating clinically significant fatigue.20 Reliability of this four-item subscale has been well-established.19 Validity has been established with other fatigue measures in cancer patients21,22 and adults with no history of cancer.20
Measures of Caregivers’ Psychobehavioral Stress Responses
The Center for Epidemiologic Studies Short Depression Scale (CES-D 10) was used to measure depressive symptoms.23 Scores were reported using a 4-point Likert-type summative scale (score ranges 0–30), with higher scores indicating more depressive symptoms. The CES-D 10 has been validated in caregivers and healthy adults.24,25
The Brief Zarit Burden Intervie-12 items (Zarit-12) was used to measure caregiver burden.26 The Zarit-12 comprises items that describe feelings resulting from caregiving (e.g., feeling strained) using a 5-point Likert-type scale (score ranges 0–48), with higher scores indicating greater burden. Validity has been reported in caregivers of the community-dwelling elderly.27
The Caregiver Health Behavior 11-item scale (CHB) was used to measure self-reported health risk behaviors in caregivers (score ranges 0–11)28. For each item, caregivers were asked to note the presence or absence of each behavior, e.g., “not having enough rest when sick,” “missing doctor’s appointments.” Possible responses were “0 (absence of the behavior)” or “1 (presence of the behavior),” with higher scores indicating more health risk behaviors. The CHB has been used in several large population-based studies that examined caregiver health,28,29 with reported linkages between poor caregiver health behavior and high levels of care demands, mainly because of patients’ functional status29 but has not been subjected to formal psychometric testing.
The Pittsburgh Sleep Quality Index (PSQI) was used to measure caregivers’ sleep quality.30 Quality and efficiency of sleep were measured using Likert-type scales and open-ended questions (score ranges 0–21), with higher scores indicating worse sleep quality. The PSQI has been validated in other caregiver populations with chronic conditions, for example, family caregivers of cancer patients.31,32
Measure of Patients’ Agitation and Sedation
The Richmond Agitation Sedation Score (RASS)33 was used in patients prior to obtaining consent and conducting symptom measurement (score ranges −5 [unarousable] to +4 [combative]). The RASS has well-established reliability and validity in ICU patients.33,34 A RASS score of 0 (alert and calm) was used to determine patients’ ability to provide consent and participate in symptom measurement.
Measure of Patients’ Symptom Burden
The Modified Given Symptom Assessment Tool was used to measure self-reported physical symptoms in ICU survivors. Details regarding the modification of the original Given Symptom Assessment Tool and use of this modified version in a sample of ICU survivors were described in a previous publication.35 Patients were first asked about presence or absence of symptoms (e.g., pain). If present, patients were asked to rate the severity of each symptom using the scale from 0 (not present) to 10 (as severe as it could be). The total score (symptom burden index) was determined by summing severity scores for the 10 symptoms36,37 (score ranges 0–100). The original Given Symptom Assessment Tool was validated in patients with cancer38–42 but has not been subjected to psychometric testing in ICU patients during and/or after an ICU experience.
Procedure
ICU clinicians (primary nurse coordinators, social workers, case manager) identified potentially eligible caregivers and asked their permission to be approached by a research team member. If permission was granted, a research team member verified eligibility and obtained informed consent. For patients, informed consent was obtained if the RASS score was zero. If patients were unable to provide informed consent, proxy consent was obtained from caregivers. At each follow-up data collection, patient status was evaluated and retrospective consent or assent was obtained if patients were recovered sufficiently to provide consent.
At enrollment, caregiver demographic characteristics and questionnaire data were obtained during a face-to-face interview. Patient medical records were reviewed to obtain demographic and clinical characteristics. Following the patient’s discharge from the ICU, study data were obtained at two weeks or less post-ICU discharge, and two and four months post-ICU discharge (± two weeks). A research team member visited the patient’s residence (home or institution) and obtained measures of symptom burden via face-to-face interviews, except in two cases when patients relocated to another state. For caregivers, questionnaires were completed either face-to-face or by telephone interview, depending on participant preference.
Statistical Analyses
A research team member hand-entered data into IBM-SPSS v. 19.0 (SPSS, Inc., Chicago, IL), which was verified by another research team member. Descriptive statistics were reported for all variables. The Mann-Whitney U test was used to compare caregivers’ psychobehavioral responses and ICU survivors’ physical symptom burden by caregivers’ fatigue (SF-36 Vitality > 45 vs. ≤ 45), and the absolute value of r was used to report effect sizes: 0.10 (small), 0.30 (moderate), and 0.50 (large).43 To calculate the absolute value of r, the Z value was divided by the square root of the total sample size.43 The Friedman test was used to explore the changes in caregivers’ fatigue by patients’ home discharge status at four months post-ICU discharge. Statistical significance was set at α =0.05 (two-tailed).
Results
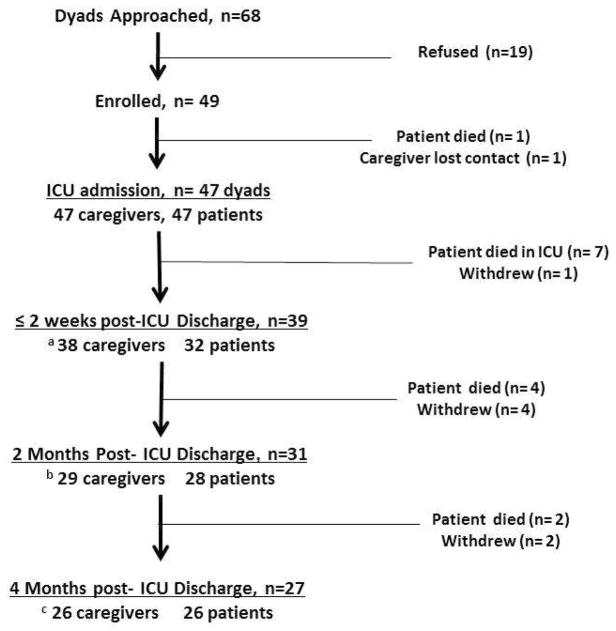
A total of 68 patient-caregiver pairs were approached between November 2008 and July 2010 during the patient’s ICU admission. Of these, 49 (72%) provided informed consent. Data regarding reasons for refusal, and the number of pairs who remained in the study and provided data at each time point are shown in Fig. 1. Patient and caregiver characteristics, including SF-36 Vitality scores, are shown in Table 1. Caregivers were mostly female and Caucasian, with a mean age of 52.3 years. Most were a spouse/significant other or adult child of the patient. Patients were mostly male and Caucasian, with mean age of 55.4 years.
Fig. 1.
Participant enrollment and follow-up. Participant enrollment occurred between November 2008 and July 2010 (over 21 months). In 19 pairs who refused, reasons for refusal include: “too busy” (n=10, 53%), “feel stressed” (n=4, 21%), “other family members disagree” (n=2, 10.5%), “not interested” (n=2, 10.5%), and “do not feel comfortable” (n=1, 5%). a A total of 38 caregivers and 32 patients participated in data collection because: a caregiver skipped measurement (n=1); patients were unable to answer (n=5, RASS score −3 to −1); a patient refused to answer (n=1). b A total of 29 caregivers and 28 patients participated in data collection because: missing data occurred because: caregivers skipped measurement (n=2); a patient was unable to answer (n=1, RASS score −3 to −1); patients refused to answer (n=2). c A total of 26 caregivers and 26 patients participated in data collection because: missing data occurred because: a caregiver was unavailable (n=1); a patient refused to answer (n=1). ICU = intensive care unit; RASS = Richmond Agitation Sedation Score.
Table 1.
Demographic and Clinical Characteristics of Caregiver-Patient Dyads from ICU Admission to Four Months Post-ICU Discharge
| ICU Admission (Enrollment) (n=47) | ≤ 2 Weeks Post-ICU Discharge (n=39) | 2 Months Post-ICU Discharge (n=31) | 4 Months Post-ICU discharge (n=27) | |
|---|---|---|---|---|
| Caregiver Characteristics | ||||
| Age (yrs), mean (SD) | 51.9 (12.1) | 51.1 (12.3) | 49.5 (12.4) | 50.6 (11.1) |
| Gender (male) | 12 (25.5) | 9 (23.1) | 7 (22.6) | 6 (22.2) |
| Ethnicity, Caucasian, n (%) | 44 (93.6) | 36 (92.3) | 30 (96.8) | 27 (100.0) |
| Relationship to patient, n (%) | ||||
| Spouse or significant other | 27 (57.4) | 22 (56.4) | 17 (54.8) | 15 (55.6) |
| Adult child | 12 (25.5) | 10 (25.6) | 8 (25.8) | 6 (22.2) |
| Parent or sibling | 8 (17.0) | 7 (18.0) | 6 (19.4) | 6 (22.2) |
| SF-36 Vitality, mean (SD) | 45.9 (23.3) | 42.2 (24.7) b | 48.6 (24.0) c | 48.5 (24.6) d |
| SF-36 Vitality ≤ 45, n (%) | 20 (43) | 20 (53) b | 13 (45) c | 13 (50) d |
| Patient Characteristics | ||||
| Age (yrs), mean (SD) | 55.5 (16.7) | 54.9 (16.9) | 53.0 (16.9) | 52.2 (15.6) |
| Gender (male), n (%) | 31 (66.0) | 26 (66.7) | 21 (67.7) | 19 (70.4) |
| Ethnicity, Caucasian, n (%) | 44 (93.6) | 36 (92.3) | 30 (96.8) | 27 (100.0) |
| Primary diagnosis, n (%) | ||||
| Respiratory | 26 (55.3) | 23 (59.0) | 19 (61.3) | 17 (63.0) |
| Sepsis, multisystem failure | 9 (19.2) | 6 (15.4) | 3 (9.7) | 1 (3.7) |
| Gastrointestinal, hepatic | 8 (17.0) | 6 (15.4) | 5 (16.1) | 5 (18.5) |
| Others | 4 (8.5) | 4 (10.2) | 4 (12.9) | 4 (14.8) |
| Charlson Comorbidity Score, mean (SD)a | 4.1 (3.3) | 3.8 (3.4) | 3.5 (3.5) | 3.1 (3.0) |
| APACHE II score, mean (SD) | 21.6 (8.0) | 21.6 (7.8) | 21.7 (8.4) | 20.4 (7.5) |
| ICU length of stay (days), mean (SD) | 22.9 (13.7) | 24.3 (13.5) | 23.7 (12.5) | 22.0 (10.2) |
| Days on mechanical ventilation, mean (SD) | 20.1 (13.1) | 21.4 (13.4) | 20.8 (12.2) | 18.9 (9.7) |
SD = standard deviation; SF-36 Vitality = Short Form-36 Health Survey Vitality subscale; APACHE = acute physiology and chronic health evaluation; ICU = intensive care unit.
Charlson Comorbidity Score identifies the presence of nine disease/conditions: cardiac, pulmonary, neurologic, renal, gastrointestinal, hematologic/oncologic, rheumatologic, diabetes and peripheral vascular disease. Total score ranges 0–37 and a higher score indicates a greater number and seriousness of comorbid conditions.
n=38 because of missing data; caregiver skipped measurement (n=1).
n=29 because of missing data; caregiver skipped measurement (n=2).
n=26 because of missing data; caregiver was unavailable (n=1).
Patient death was the main reason for attrition. There were no significant differences between pairs who remained in the study or were lost to attrition, with two exceptions. Patients lost to attrition (n=20, 43%) had a higher mean Charlson Comorbidity Index score (mean ± SD = 5.4 ± 3.4) than those who remained in the study (n=27, mean ± SD = 3.1 ± 3.0; independent sample t-test, P=0.02). Also, all participants who were African American either died (n=2) or withdrew (n=1) by four months.
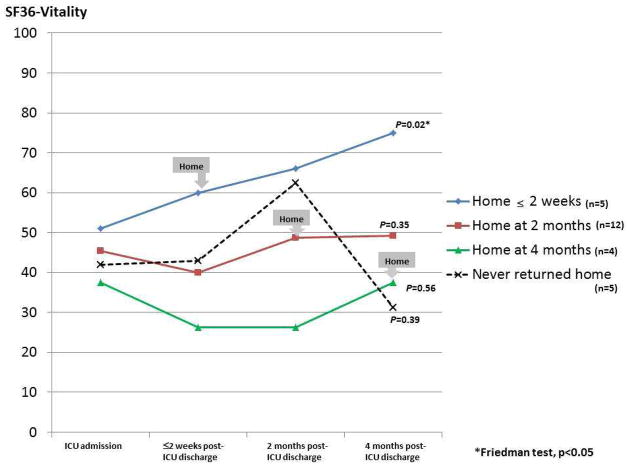
To explore changes in caregiver fatigue depending on patients’ residence, we explored longitudinal trends in SF-36 Vitality scores in the 26 pairs who provided data at all collection points (Fig. 2). Of these patients, 21 returned home by four months post-ICU discharge and five never returned home. At four months post-ICU discharge, mean SF-36 Vitality scores were lower (less vitality) in caregivers of patients who never returned home. In contrast, scores for caregivers of patients who returned home within two weeks of ICU discharge improved significantly (Friedman’s test, P <0.05). With these exceptions, there were no significant differences dependent on time of return to home.
Fig. 2.
Description of the trends in SF-36 Vitality scores by timing of patients’ home discharge in a subsample of caregivers (n=26) who participated at all data follow-up points.
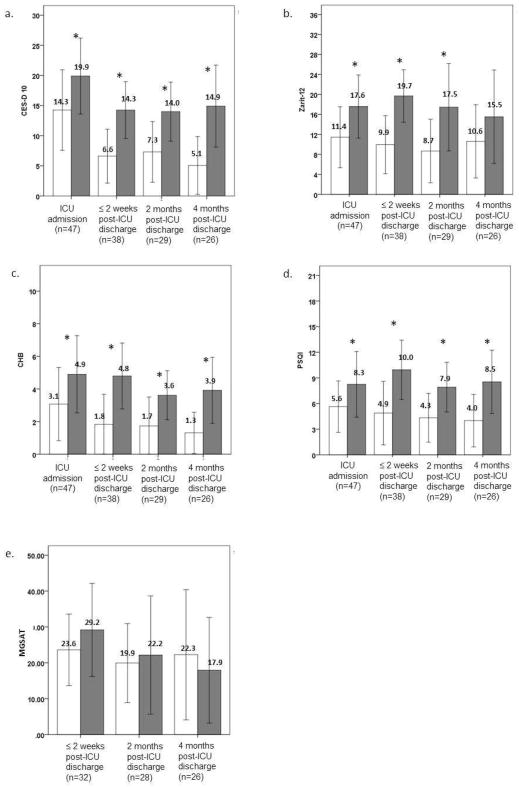
Scores of measures that examined caregivers’ psychobehavioral stress responses and patients’ physical symptoms by presence/absence of clinically significant fatigue (SF-36 Vitality ≤ 45) are shown in Fig. 3. During ICU admission, 20 caregivers (43%) reported SF-36 Vitality scores indicating clinically significant fatigue. This percentage remained essentially the same two weeks or less post-ICU discharge (53%), two months post-ICU discharge (45%), and four months post-ICU discharge (50%). Caregivers who reported clinically significant fatigue also reported more depressive symptoms, health risk behaviors, and poorer sleep quality at ICU admission, which persisted over four months post-ICU discharge (Mann-Whitney U test, all P< 0.05). Greater caregiver burden also tended to be associated with clinically significant fatigue from patients’ ICU admission to two months post-ICU discharge (Mann-Whitney U test, all P< 0.05).
Fig. 3.
Comparison of the scores of measure indicating caregivers’ psychobehavioral responses (a–d) and patients’ symptom burden (e) by caregivers grouped based upon SF-36 Vitality scores. Black blocks indicate scores reported in caregivers with SF-36 Vitality scores ≤ 45 (scores indicating clinically significant fatigue). White blocks indicate scores reported in caregivers with SF-36 Vitality scores of > 45. CES-D 10 = Center for Epidemiologic Studies-Depression 10 items; Zarit-12 = Brief Zarit Burden Score; CHB = caregiver health behavior; PSQI = Pittsburgh sleep quality index; MGSAT = Modified Given Symptom Assessment Tool.
Few patients were unable to self-report symptoms. Based on those patients who were able to provide symptom data at the each time point, caregiver fatigue appeared to be associated with greater patient symptom burden (Fig. 3e).
Discussion
To our knowledge, this analysis is among the first that longitudinally explored fatigue in family caregivers of adults who received prolonged mechanical ventilation in an ICU. Three major findings can be highlighted. First, caregiver fatigue was associated with prolonged requirement for institutional care and displayed a worsening trend if patients were unable to return home during the observation period. If patients returned home at any point, fatigue tended to decrease. Second, approximately half of caregivers reported SF-36 Vitality scores lower than the cut-off score indicating clinically significant fatigue (≤ 45) at each data collection point. Notably, reported fatigue exceeded that reported in the general population and caregivers of persons living with other chronic illness.44–50 Third, caregivers with clinically significant fatigue consistently reported worse depressive symptoms, burden, health risk behaviors, and sleep quality.
In the U.S., it is a common practice for ICU survivors such as those recruited for this study to be transferred to a long-term care facility for extended rehabilitation following the acute phase of their illness.12,51 Although it might be expected that caregiver fatigue would be less during the time the patient was institutionalized, our findings and others have reported the opposite. Prior studies have reported that caregivers’ psychological responses varied depending on patients’ discharge status and tended to be more positive following home discharge.52 Although we did not explore causes of this response, possible reasons might include the time required for travel to the institution, concerns related to a slower than expected recovery, and/or fear that home discharge might never occur. Further research is warranted to identify strategies to support caregivers of ICU survivors who require extended institutional care. It would be important to examine how unresolved fatigue in caregivers affects the quality of support they provide to patients, long-term recovery in ICU survivors, and caregivers’ own physical health.
We found approximately half of family caregivers reported vitality scores below 45, indicating clinically significant fatigue at all time points in our study. This is consistent with Lemiale et al. who reported similar findings in caregivers at three months post-ICU discharge.53 Cameron et al. also reported fatigue in caregivers of survivors of acute respiratory distress syndrome from six to 53 months (average 23 months) post-ICU discharge.54 Similar to the caregivers of persons living with other chronic conditions, such as dementia,44–46 post-stroke,47 psychiatric diagnoses (e.g., schizophrenia),48 and cancer,49,50 fatigue may be an important yet under-recognized problem experienced by caregivers of ICU survivors. More research is needed to better understand and manage fatigue in this caregiver population.
In our study, as in others that examined post-ICU outcomes in patients who required prolonged mechanical ventilation, mortality was high. Prior studies have reported patient mortality that ranged from 28% to 36% at two to four months post ICU discharge.35,53,55 Because of complicated and fluctuating recovery trajectories and high mortality in this patient population, family caregivers experience substantial stress after their loved one is discharged from the ICU.4 We previously reported that caregiver depressive symptoms and health risk behaviors were highly prevalent and correlated with each other while their loved ones were in the ICU.56 During the initial two months following ICU discharge, close to half of caregivers continued to report high levels of depressive symptoms, greater burden, and more health risk behaviors.7 If patient status continues to deteriorate, discussions may be initiated regarding treatment limitation, a factor that may cause increased caregiver stress. Findings from our study suggest that these caregivers are highly vulnerable to develop negative health consequences. Additional work is necessary to identify modifiable risk factors and critical time points to initiate supportive interventions. Options such as earlier integration of palliative or hospice care, which may assist in better management of patient symptoms,57,58 can provide needed support to caregivers. The availability of technology that can support patients and caregivers in their home through interventions, such as telerehabilitation, might be an option. With this technology, support can be provided on a sustained basis after hospital discharge in a more accessible and less expensive manner compared with traditional approaches involving in-person visits in home settings or outpatient clinics.59 It would be valuable in future studies to determine the impact of changes in patients’ status on caregiver long-term psychobehavioral responses and their health outcomes.
Our results need cautious interpretation because our sample was limited to caregivers of ICU survivors who were available at four months post-ICU discharge. To evaluate caregiver fatigue, we used SF-36 Vitality scores. This measure and other currently available fatigue measures were developed and tested in those living with chronic health conditions and may not be sufficiently sensitive to measure fatigue of ICU caregivers.60 For future studies, it will be important to determine which specific aspects of fatigue are worthy of further investigation in ICU caregivers. For example, knowing triggers of fatigue and impact of fatigue on caregivers’ daily life and quality of interaction with patients at different time points post-ICU discharge may be valuable in designing interventions that ultimately reduce the negative impact of fatigue on physical and mental health.
We were unable to obtain measures of fatigue from caregivers prior to the time of ICU admission or immediately after ICU admission. Despite efforts to enroll participants as early as possible, caregivers were enrolled after an average of 13 days following initiation of mechanical ventilation for their loved one. Therefore, our findings are limited in explaining whether low vitality scores in our sample derived from involvement prior to ICU admission or over the course of patients’ ICU admission and survival, or a combination of these factors. Notably, 10 of 28 caregivers in this sample (36%) reported the patient had one or more impairments in activities of daily living and/or instrumental activities of daily living that required caregiver assistance prior to ICU admission. This suggests the need to consider both the impact of ICU admission and pre-existing care demands in measuring caregiver response.
Conclusion
Our findings introduce another important area for study to explain mechanisms and design future interventions to support family caregivers of ICU survivors. Findings from this secondary analysis highlight fatigue as common in caregivers of ICU survivors and potentially linked with caregivers’ reports of psychobehavioral stress responses. Our data also suggest worsening trends in caregivers’ fatigue when there is greater symptom distress and long-term institutionalization of a loved one after ICU discharge. For future studies, we suggest including fatigue as one of the health indicators to elucidate its role in explaining stress impact on caregivers’ physical and mental health and guidance in identifying the main foci of interventions to support both ICU survivors and their family caregivers.
Acknowledgments
Funding was provided by the National Institutes of Health, National Institute of Nursing Research, U.S. Public Health Service (F32 NR 011271 and T32 NR 008857) and Rehabilitation Nursing Foundation, Fellow Research Award (FEL-0905). Dr. Tate is funded through the National Institute of Mental Health institutional post-doctoral fellowship (T32 MH19986).
Footnotes
Disclosures
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Society of Critical Care Medicine. [Accessed October 10, 2012];Critical care statistics in the United States. 2012 Available from http://www.sccm.org/Public_Health_and_Policy/Pages/Statistics-Brochure.aspx.
- 2.Needham DM, Davidson J, Cohen H, et al. Improving long-term outcomes after discharge from intensive care unit: report from a stakeholders’ conference. Crit Care Med. 2012;40:502–509. doi: 10.1097/CCM.0b013e318232da75. [DOI] [PubMed] [Google Scholar]
- 3.Desai SV, Law TJ, Needham DM. Long-term complications of critical care. Crit Care Med. 2011;39:371–379. doi: 10.1097/CCM.0b013e3181fd66e5. [DOI] [PubMed] [Google Scholar]
- 4.Davidson JE, Jones C, Bienvenu OJ. Family response to critical illness: postintensive care syndrome-family. Crit Care Med. 2012;40:618–624. doi: 10.1097/CCM.0b013e318236ebf9. [DOI] [PubMed] [Google Scholar]
- 5.Azoulay E, Pochard F, Kentish-Barnes N, et al. Risk of post-traumatic stress symptoms in family members of intensive care unit patients. Am J Respir Crit Care Med. 2005;171:987–994. doi: 10.1164/rccm.200409-1295OC. [DOI] [PubMed] [Google Scholar]
- 6.Anderson WG, Arnold RM, Angus DC, Bryce CL. Posttraumatic stress and complicated grief in family members of patients in the intensive care unit. J Gen Intern Med. 2008;23:1871–1876. doi: 10.1007/s11606-008-0770-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choi J, Sherwood PR, Schulz R, et al. Patterns of depressive symptoms in caregivers of mechanically ventilated critically ill adults from intensive care unit admission to 2 months postintensive care unit discharge: a pilot study. Crit Care Med. 2012;40:1546–1553. doi: 10.1097/CCM.0b013e3182451c58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vitaliano PP, Zhang J, Scanlan JM. Is caregiving hazardous to one’s physical health? A meta-analysis Psychol Bull. 2003;129:946–972. doi: 10.1037/0033-2909.129.6.946. [DOI] [PubMed] [Google Scholar]
- 9.Schulz R, Beach SR. Caregiving as a risk factor for mortality: the Caregiver Health Effects Study. JAMA. 1999;282:2215–2219. doi: 10.1001/jama.282.23.2215. [DOI] [PubMed] [Google Scholar]
- 10.Pinquart M, Sorensen S. Differences between caregivers and noncaregivers in psychological health and physical health: a meta-analysis. Psychol Aging. 2003;18:250–267. doi: 10.1037/0882-7974.18.2.250. [DOI] [PubMed] [Google Scholar]
- 11.Pinquart M, Sorensen S. Correlates of physical health of informal caregivers: a meta-analysis. J Gerontol B Psychol Sci Soc Sci. 2007;62:P126–137. doi: 10.1093/geronb/62.2.p126. [DOI] [PubMed] [Google Scholar]
- 12.Unroe M, Kahn JM, Carson SS, et al. One-year trajectories of care and resource utilization for recipients of prolonged mechanical ventilation: a cohort study. Ann Intern Med. 2010;153:167–175. doi: 10.1059/0003-4819-153-3-201008030-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Whitehead L. The measurement of fatigue in chronic illness: a systematic review of unidimensional and multidimensional fatigue measures. J Pain Symptom Manage. 2009;37:107–128. doi: 10.1016/j.jpainsymman.2007.08.019. [DOI] [PubMed] [Google Scholar]
- 14.Teel CS, Press AN. Fatigue among elders in caregiving and noncaregiving roles. West J Nurs Res. 1999;21:498–514. doi: 10.1177/01939459922044009. discussion 514–520. [DOI] [PubMed] [Google Scholar]
- 15.Clark PC. Effects of individual and family hardiness on caregiver depression and fatigue. Res Nurs Health. 2002;25:37–48. doi: 10.1002/nur.10014. [DOI] [PubMed] [Google Scholar]
- 16.Gaston-Johansson F, Lachica EM, Fall-Dickson JM, Kennedy MJ. Psychological distress, fatigue, burden of care, and quality of life in primary caregivers of patients with breast cancer undergoing autologous bone marrow transplantation. Oncol Nurs Forum. 2004;31:1161–1169. doi: 10.1188/04.ONF.1161-1169. [DOI] [PubMed] [Google Scholar]
- 17.Passik SD, Kirsh KL. A pilot examination of the impact of cancer patients’ fatigue on their spousal caregivers. Palliat Support Care. 2005;3:273–279. doi: 10.1017/s1478951505050431. [DOI] [PubMed] [Google Scholar]
- 18.McAdam JL, Dracup KA, White DB, Fontaine DK, Puntillo KA. Symptom experiences of family members of intensive care unit patients at high risk for dying. Crit Care Med. 2010;38:1078–1085. doi: 10.1097/CCM.0b013e3181cf6d94. [DOI] [PubMed] [Google Scholar]
- 19.Ware J, Jr, Kosinski M, Gandek B. SF-36 ® Health Survey: Manual and interpretation guide. Lincoln, RI: Quality Metric Inc; 1993. [Google Scholar]
- 20.Donovan KA, Jacobsen PB, Small BJ, Munster PN, Andrykowski MA. Identifying clinically meaningful fatigue with the Fatigue Symptom Inventory. J Pain Symptom Manage. 2008;36:480–487. doi: 10.1016/j.jpainsymman.2007.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hann DM, Jacobsen PB, Azzarello LM, et al. Measurement of fatigue in cancer patients: development and validation of the Fatigue Symptom Inventory. Qual Life Res. 1998;7:301–310. doi: 10.1023/a:1024929829627. [DOI] [PubMed] [Google Scholar]
- 22.Stein KD, Martin SC, Hann DM, Jacobsen PB. A multidimensional measure of fatigue for use with cancer patients. Cancer Pract. 1998;6:143–152. doi: 10.1046/j.1523-5394.1998.006003143.x. [DOI] [PubMed] [Google Scholar]
- 23.Radloff L. The CES-D scale: a self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- 24.Logsdon RG, Teri L. Depression in Alzheimer’s disease patients: caregivers as surrogate reporters. J Am Geriatr Soc. 1995;43:150–155. doi: 10.1111/j.1532-5415.1995.tb06380.x. [DOI] [PubMed] [Google Scholar]
- 25.Andresen EM, Malmgren JA, Carter WB, Patrick DL. Screening for depression in well older adults: evaluation of a short form of the CES-D (Center for Epidemiologic Studies Depression Scale) Am J Prev Med. 1994;10:77–84. [PubMed] [Google Scholar]
- 26.Bedard M, Molloy DW, Squire L, et al. The Zarit Burden Interview: a new short version and screening version. Gerontologist. 2001;41:652–657. doi: 10.1093/geront/41.5.652. [DOI] [PubMed] [Google Scholar]
- 27.O’Rourke N, Tuokko HA. Psychometric properties of an abridged version of The Zarit Burden Interview within a representative Canadian caregiver sample. Gerontologist. 2003;43:121–127. doi: 10.1093/geront/43.1.121. [DOI] [PubMed] [Google Scholar]
- 28.Schulz R, Newsom J, Mittelmark M, et al. Health effects of caregiving: the caregiver health effects study: an ancillary study of the Cardiovascular Health Study. Ann Behav Med. 1997;19:110–116. doi: 10.1007/BF02883327. [DOI] [PubMed] [Google Scholar]
- 29.Burton LC, Newsom JT, Schulz R, Hirsch CH, German PS. Preventive health behaviors among spousal caregivers. Prev Med. 1997;26:162–169. doi: 10.1006/pmed.1996.0129. [DOI] [PubMed] [Google Scholar]
- 30.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 31.Carter PA. Caregivers’ descriptions of sleep changes and depressive symptoms. Oncol Nurs Forum. 2002;29:1277–1283. doi: 10.1188/02.ONF.1277-1283. [DOI] [PubMed] [Google Scholar]
- 32.Carter PA, Chang BL. Sleep and depression in cancer caregivers. Cancer Nurs. 2000;23:410–415. doi: 10.1097/00002820-200012000-00002. [DOI] [PubMed] [Google Scholar]
- 33.Sessler CN, Gosnell MS, Grap MJ, et al. The Richmond Agitation-Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002;166:1338–1344. doi: 10.1164/rccm.2107138. [DOI] [PubMed] [Google Scholar]
- 34.Ely EW, Truman B, Shintani A, et al. Monitoring sedation status over time in ICU patients: reliability and validity of the Richmond Agitation-Sedation Scale (RASS) JAMA. 2003;289:2983–2991. doi: 10.1001/jama.289.22.2983. [DOI] [PubMed] [Google Scholar]
- 35.Choi J, Hoffman LA, Schulz R, et al. Self-reported physical symptoms in intensive care unit (ICU) survivors: pilot exploration over four months post-ICU discharge. J Pain Symptom Manage. 2013 Jul 12; doi: 10.1016/j.jpainsymman.2013.03.019. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Given B, Given CW, McCorkle R, et al. Pain and fatigue management: results of a nursing randomized clinical trial. Oncol Nurs Forum. 2002;29:949–956. doi: 10.1188/02.ONF.949-956. [DOI] [PubMed] [Google Scholar]
- 37.Kurtz ME, Kurtz JC, Given CW, Given B. A randomized, controlled trial of a patient/caregiver symptom control intervention: effects on depressive symptomatology of caregivers of cancer patients. J Pain Symptom Manage. 2005;30:112–122. doi: 10.1016/j.jpainsymman.2005.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Given B, Given CW, Sikorskii A, et al. Establishing mild, moderate, and severe scores for cancer-related symptoms: how consistent and clinically meaningful are interference-based severity cut-points? J Pain Symptom Manage. 2008;35:126–135. doi: 10.1016/j.jpainsymman.2007.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Given B, Given CW, Sikorskii A, et al. Analyzing symptom management trials: the value of both intention-to-treat and per-protocol approaches. Oncol Nurs Forum. 2009;36:E293–302. doi: 10.1188/09.ONF.E293-E302. [DOI] [PubMed] [Google Scholar]
- 40.Jeon S, Given CW, Sikorskii A, Given B. The utility of screening in the design of trials for symptom management in cancer. J Pain Symptom Manage. 2009;38:606–614. doi: 10.1016/j.jpainsymman.2009.02.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sikorskii A, Given CW, Given B, et al. Symptom management for cancer patients: a trial comparing two multimodal interventions. J Pain Symptom Manage. 2007;34:253–264. doi: 10.1016/j.jpainsymman.2006.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sikorskii A, Given CW, You M, Jeon S, Given BA. Response analysis for multiple symptoms revealed differences between arms of a symptom management trial. J Clin Epidemiol. 2009;62:716–724. doi: 10.1016/j.jclinepi.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Field A. Discovering statistics using SPSS. 2. Thousand Oaks, CA: Sage Publications Ltd; 2005. [Google Scholar]
- 44.Arango-Lasprilla JC, Lehan T, Drew A, et al. Health-related quality of life in caregivers of individuals with dementia from Colombia. Am J Alzheimers Dis Other Demen. 2010;25:556–561. doi: 10.1177/1533317510382287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Argimon JM, Limon E, Vila J, Cabezas C. Health-related quality of life in carers of patients with dementia. Fam Pract. 2004;21:454–457. doi: 10.1093/fampra/cmh418. [DOI] [PubMed] [Google Scholar]
- 46.Gusi N, Prieto J, Madruga M, Garcia JM, Gonzalez-Guerrero JL. Health-related quality of life and fitness of the caregiver of patient with dementia. Med Sci Sports Exerc. 2009;41:1182–1187. doi: 10.1249/MSS.0b013e3181951314. [DOI] [PubMed] [Google Scholar]
- 47.McPherson CJ, Wilson KG, Chyurlia L, Leclerc C. The caregiving relationship and quality of life among partners of stroke survivors: a cross-sectional study. Health Qual Life Outcomes. 2011;9:29. doi: 10.1186/1477-7525-9-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zendjidjian X, Richieri R, Adida M, et al. Quality of life among caregivers of individuals with affective disorders. J Affect Disord. 2012;136:660–665. doi: 10.1016/j.jad.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 49.Petruzzi A, Finocchiaro CY, Lamperti E, Salmaggi A. Living with a brain tumor: reaction profiles in patients and their caregivers. Support Care Cancer. 2013;21:1105–1111. doi: 10.1007/s00520-012-1632-3. [DOI] [PubMed] [Google Scholar]
- 50.Papadopoulos A, Vrettos I, Kamposioras K, et al. Impact of cancer patients’ disease awareness on their family members’ health-related quality of life: a cross-sectional survey. Psychooncology. 2011;20:294–301. doi: 10.1002/pon.1731. [DOI] [PubMed] [Google Scholar]
- 51.Scheinhorn DJ, Hassenpflug MS, Votto JJ, et al. Post-ICU mechanical ventilation at 23 long-term care hospitals: a multicenter outcomes study. Chest. 2007;131:85–93. doi: 10.1378/chest.06-1081. [DOI] [PubMed] [Google Scholar]
- 52.Van Pelt DC, Schulz R, Chelluri L, Pinsky MR. Patient-specific, time-varying predictors of post-ICU informal caregiver burden: the caregiver outcomes after ICU discharge project. Chest. 2010;137:88–94. doi: 10.1378/chest.09-0795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lemiale V, Kentish-Barnes N, Chaize M, et al. Health-related quality of life in family members of intensive care unit patients. J Palliat Med. 2010;13:1131–1137. doi: 10.1089/jpm.2010.0109. [DOI] [PubMed] [Google Scholar]
- 54.Cameron JI, Herridge MS, Tansey CM, McAndrews MP, Cheung AM. Well-being in informal caregivers of survivors of acute respiratory distress syndrome. Crit Care Med. 2006;34:81–86. doi: 10.1097/01.ccm.0000190428.71765.31. [DOI] [PubMed] [Google Scholar]
- 55.Chelluri L, Im KA, Belle SH, et al. Long-term mortality and quality of life after prolonged mechanical ventilation. Crit Care Med. 2004;32:61–69. doi: 10.1097/01.CCM.0000098029.65347.F9. [DOI] [PubMed] [Google Scholar]
- 56.Choi J, Hoffman LA, Schulz R, et al. Health risk behaviors in family caregivers during patients’ stay in intensive care units: a pilot analysis. Am J Crit Care. 2013;22:41–45. doi: 10.4037/ajcc2013830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bakitas M, Lyons KD, Hegel MT, et al. Effects of a palliative care intervention on clinical outcomes in patients with advanced cancer: the Project ENABLE II randomized controlled trial. JAMA. 2009;302:741–749. doi: 10.1001/jama.2009.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med. 2010;363:733–742. doi: 10.1056/NEJMoa1000678. [DOI] [PubMed] [Google Scholar]
- 59.Schein RM, Schmeler MR, Holm MB, Saptono A, Brienza DM. Telerehabilitation wheeled mobility and seating assessments compared with in person. Arch Phys Med Rehabil. 2010;91:874–878. doi: 10.1016/j.apmr.2010.01.017. [DOI] [PubMed] [Google Scholar]
- 60.Dittner AJ, Wessely SC, Brown RG. The assessment of fatigue: a practical guide for clinicians and researchers. J Psychosom Res. 2004;56:157–170. doi: 10.1016/S0022-3999(03)00371-4. [DOI] [PubMed] [Google Scholar]