Abstract
Background
Optimal monitoring of vancomycin in children needs evaluation using the exposure target with area-under-the-curve of the serum concentrations vs. time over 24 hours (AUC). Our study objectives were to: (1) compare the accuracy and precision of vancomycin AUC estimations using two sampling strategies – one serum concentration sample (1S, near trough) versus two samples (2S, near peak and trough) against the rich sample (RS) method; and (2) determine the performance of these strategies in predicting future AUC against an internal validation sample (VS).
Methods
This was an retrospective cohort study using population-based pharmacokinetic modeling with Bayesian post-hoc individual estimations in NONMEM 7.2. Pediatric subjects 3 months to 21 years of age who received vancomycin ≥ 48 hours and had ≥ 3 drug samples within the first ≤ 96 hours of therapy were enrolled. Outcome measures were the accuracy, precision and internal predictive performance of AUC estimations using two monitoring strategies (i.e., 1S vs 2S) against the RS (which was derived from modeling all serum vancomycin concentrations obtained anytime during therapy), and VS (from serum concentrations obtained after 96 hours of therapy).
Results
Analysis included 138 subjects with 712 vancomycin serum concentrations. Median age was 6.1 (interquartile range [IQR] 2.2-12.2) yr, weight 22 (13-38) kg, and baseline serum creatinine 0.37 (0.30-0.50) mg/dL. Both accuracy and precision were improved with the 2S, compared to 1S, for AUC estimations (-2.0% vs -7.6 % and 10.3% vs 12.8%, respectively) against the RS. Improved accuracy and precision were also observed for 2S when evaluated against VS in predicting future AUC.
Conclusion
Compared to 1S, the 2S sampling strategy for vancomycin monitoring improved accuracy and precision in estimating and predicting future AUC. Evaluating two drug concentrations in children may be prudent to ensure adequate drug exposure.
Keywords: Accuracy, Vancomycin, Pharmacokinetics, Pediatrics, Therapeutic Drug Monitoring
BACKGROUND
Vancomycin is a bactericidal glycopeptide antibiotic with activity against Gram-positive bacteria. It is considered the drug of choice in the treatment of methicillin-resistant Staphylococcus aureus (MRSA) infections in adult and pediatric populations.1 For serious susceptible MRSA infections, studies in adults have demonstrated that a vancomycin exposure target of area-under-the-curve of the serum concentrations vs. time over 24 hours (hrs) to minimum inhibitory concentration (AUC/MIC) ratio ≥ 400 improves treatment success.2 Furthermore, vancomycin AUC/MIC ratio ≥ 400 correlates to trough concentrations between 15 and 20 mcg/mL in adults.2,3
Current Infectious Diseases Society of America (IDSA) guidelines recommend vancomycin 60 mg/kg/day empirically for treating suspected serious MRSA infections in children to optimize vancomycin exposure.1 A recent, large population-based pharmacokinetic (PK) study demonstrated that vancomycin 60 to 70 mg/kg/day, depending on age, SCr, and MIC distribution, was necessary to achieve AUC/MIC ≥ 400 in children.4 National guidelines propose to therapeutically monitor vancomycin exposure using steady-state serum trough concentrations.1,5 However, the performance of this one-sample (1S) strategy for monitoring vancomycin therapy using trough concentrations alone is unknown in children. Moreover, targeted exposure using vancomycin AUC, compared with trough concentrations, is a more achievable target in children.4,6,7 As such, traditional therapeutic drug monitoring of trough concentrations may not be optimal for estimating AUC. Measuring early post-dose concentrations, in addition to trough concentrations, may improve the ability to determine AUC, and thus adjust therapy on an individual basis.
The optimal method to monitor AUC exposure in children using 1S (i.e., trough) versus two-samples (2S consisting of concentrations near, or at trough and peak concentrations) strategies requires further exploration. This is crucial in light of recent data revealing the increased therapeutic monitoring of vancomcyin at pediatric hospitals, especially employing the 1S strategy.8 Furthermore, appropriate vancomycin therapeutic monitoring in children, particularly by pharmacy with proper healthcare provider education, may reduce hospital cost.9 Using population-based PK modeling, our study objectives were to: (1) compare the accuracy and precision of vancomycin PK parameters between two sampling strategies–1S versus 2S–against a rich sample (RS) method; and (2) determine their performance in predicting future concentrations against an internal validation sample (VS). In addition to AUC estimations (the primary endpoint), clearance (CL) and trough concentrations were also evaluated.
MATERIALS AND METHODS
Study Design
This was a two-centered, retrospective cohort study that evaluated the accuracy and precision of vancomycin monitoring strategies using population-based PK modeling. Miller Children’s Hospital (MCH) is a community-based, tertiary care, teaching hospital with 249 beds, including 34 pediatric intensive care, 69 neonatal intensive care, 94 general pediatrics, and 52 hematology/oncology beds. Rady Children’s Hospital of San Diego (RCHSD) is also a tertiary care, teaching hospital with 308 beds, including 44 pediatric intensive care, 49 neonatal intensive care, 177 general medical/surgical, and 38 hematology/oncology beds. This study was approved by the institutional review boards at both institutions with the use of a waiver of informed consent for de-identified data collection and analysis.
Participants
Study participants included pediatric subjects who were hospitalized from September 1993 to June 2011 at MCH or RCHSD. Participants were included if they were between the ages of 3 months to 21 years; received intravenous vancomycin therapy for a minimum of 48 hrs; had a minimum of 3 vancomycin serum concentrations including an initial concentration obtained at or near trough concentrations (within 2 hr before next dose), an initial serum concentration obtained near peak concentrations (1-3 hr after end of dose infusion) and subsequent trough concentrations. Trough was defined as the minimum concentration right before the next dose; peak as the maximum concentration at the end of infusion. The initial concentrations must have been obtained within the first 96 hrs of therapy. Participants were excluded if they had < 3 vancomycin serum concentrations, received hemodialysis, or had a history of cystic fibrosis, or on certain concurrent medications (i.e., tacrolimus, cyclosporine, sirolimus, and amphotericin B).
As part of routine patient care, clinical pharmacists monitored drug concentrations in all subjects receiving vancomycin. Subjects were monitored daily while on vancomycin; blood samples to evaluate vancomycin trough concentrations were usually obtained after the third vancomycin dose to determine steady-state concentrations. The entire dosing history and measured serum concentrations, in the context of the timing of the blood sample after vancomycin infusion, were used in the PK modeling. Finally, renal function was also monitored closely using baseline and subsequent serum creatinine (SCr) values during therapy.
Population-based Pharmacokinetic Model Development and Bayesian Estimation
The methodologies for the serum vancomycin and SCr assays have been described previously.4 Nonlinear Mixed Effects Modeling (NONMEM) version 7.2 (Icon, Dublin, Ireland) was used for population-based PK modeling. One-compartment models were fitted using the first-order conditional estimation subroutine and the interaction option. Covariates, including age, weight, and SCr, were incorporated in the modeling. Details of the PK modeling were reported previously.4 The maximum a posteriori Bayesian analysis of each subject’s data using the final population model and the POSTHOC option were used to generate each individual subject’s parameter estimate for clearance (CL). The AUC (mg-hr/L) was calculated by 24-hr dose (mg/kg/day) ÷ CL (L/hr); and steady-state trough concentrations determined by the intermittent short infusion model with a 1-hr infusion time (Dose = [(Trough concentration)(CL)(tin)(1–e−kτ)] / [(1–e−ktin)(e−ktmin)] where tin = infusion time, τ = dosing interval, k = elimination rate constant, tmin = time to trough).
Four different NONMEM models with Bayesian estimations were performed based on the following sampling strategies, which comprised of all subjects who met study criteria and were defined based on the number and timing of vancomycin serum concentrations:
Rich sample (RS): All serum concentrations (including peak, trough and random samples) obtained anytime during therapy for each subject. RS was used to generate expected PK values, which were compared to those generated from 1S vs. 2S.
One-sample (1S): First initial serum concentration near, or at trough obtained within the first 96 hrs of therapy. All other samples were removed.
Two-samples (2S): First two initial serum concentrations near, or at peak and trough concentrations, both obtained within the first 96 hrs of therapy. All other samples were removed.
Validation sample (VS): Any serum concentrations obtained after the first two initial serum concentrations near, or at peak and trough concentrations (i.e., excluded samples in 1S and 2S). The VS was used to generate internal predictive PK values, which were compared to those generated from 1S vs. 2S. This internal validation set represented samples that were obtained from the each subject later during the course of vancomycin therapy (i.e., excluding samples for 1S and 2S).
After completing the PK estimations, the 1S and 2S sampling strategies were compared against the RS and VS. The accuracy and precision of PK estimations for the 1S or 2S versus RS or VS groups were determined. Accuracy represents systematic error, which is the tendency to over- or under-predict a parameter. Precision is the random error that characterizes the degree of variation in the estimation. The accuracy and precision for these comparisons were calculated using the following equations:
- Accuracy, defined as the median % predicted error:
- Precision, defined as median % predicted absolute error:
The values for the X-estimated were AUC, CL, or trough derived from 1S or 2S sampling strategies; and the values for X-actual were AUC, CL, or trough derived from RS or VS. Median values of accuracy and precision are presented to describe the performance of the models.
Statistical Analysis
All data was entered into an Excel database and reformatted for analysis in SPSS GradPack version 19 (IBM, Chicago, Illinois). Statistical significance was set a priori at p < 0.05. A paired t-test was used to detect for significant differences in accuracy and precision between 1S or 2S and RS or VS. Coefficient of determination (R2) was determined for the different sampling strategies against RS or VS for AUC, CL, and trough concentration.
RESULTS
During the study period, vancomycin therapy was initiated in 1604 pediatric subjects. A remaining total of 138 subjects with 712 vancomycin serum concentrations were eligible for this study (Figure 1). The median age was 6.1 years, median weight was 22.2 kg, and median SCr was 0.37 mg/dL (Table 1).
Figure 1.
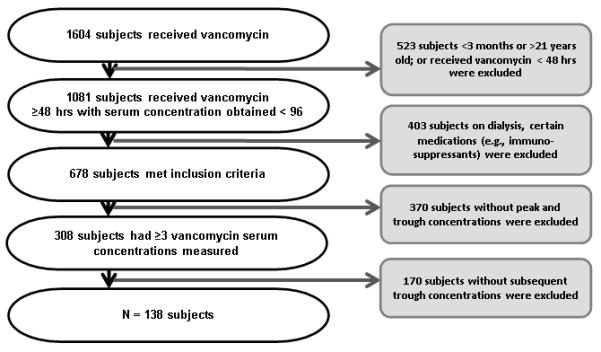
Inclusion Criteria Algorithm
Table 1.
Baseline Characteristics1
| Demographics | N = 138 |
|---|---|
|
| |
| Age, years (IQR) | 6.1 (2.2 - 12.2) |
| 3 months - < 2 years, no. (%) | 30 (22) |
| 2 years - < 12 years, no. (%) | 71 (51) |
| ≥ 12 years, no. (%) | 37 (27) |
|
| |
| Male gender, no. (%) | 72 (52) |
|
| |
| Race/Ethnicity, no. (%) | |
| Hispanic | 58 (42) |
| Caucasian | 39 (28) |
| African American | 8 (6) |
| Asian | 3 (2) |
| Other/Unknown | 30 (22) |
|
| |
| Weight, kg (IQR) | 22.2 (13.2 - 37.9) |
| Overweight or obese, no. (%)2 | 23 (17) |
|
| |
| Serum creatinine, mg/dL (IQR) | 0.37 (0.30 - 0.50) |
|
| |
| Concurrent use of nephrotoxic medications, no. (%) | 67 (49) |
|
| |
| Stay in intensive care unit, no. (%) | 67 (49) |
|
| |
| Concurrent use of chemotherapeutic agents, no. (%) | 12 (9) |
|
| |
| Initial vancomycin dose, mg/kg/day (IQR) | 44 (39 - 47) |
| Every 6 hr, no. (%) | 17 (12) |
| Every 8 hr, no. (%) | 100 (72) |
| Every 12 hr, no. (%) | 14 (10) |
Values are medians (interquartile range, IQR), unless otherwise stated.
Using the definitions and growth charts established by the Centers for Disease Control and Prevention, overweight was classified as 85-94% and obese as ≥ 95% of body mass index (for children ≥ 2 years old), or actual weight (for infants and children younger than 2 years).
Age, weight, and SCr were independent covariates for CL, and were incorporated in the population-based PK modeling using the four different sampling groups (i.e., 1S, 2S, RS and VS) to derive AUC (primary endpoint), and CL and trough (secondary endpoints) estimations. The number of samples included in the analysis for 1S, 2S, RS and VS were 138, 276, 712, and 436 respectively. The number of drug samples measured from the end of infusion to 1 hr was 27 (3%); 1.1- 2 hrs, 209 (29%); 2.1-3 hrs, 64 (9%); 3.1-4 hrs, 29 (4%); 4.1-5 hrs, 38 (5%); and >5 hrs, 345 (49%). The infusion times were 1 to 1.5 hrs.
The final pharmacokinetic model using the RS method incorporated covariates age, SCr and allometric weight for CL, and weight for Vd (Table 2). Minimization and the covariance step were successful for this model. The median estimates for AUC, CL, and trough concentration were derived using different sampling groups (Table 3). The median estimates for AUC and CL were most similar among the 2S, RS, and VS groups, indicating improved accuracy using the 2S, rather than 1S, scheme to estimate PK parameters. However, improved accuracy was not evident for trough using the 2S strategy.
Table 2.
Vancomycin Final Pharmacokinetic Model using Rich Sample Method
| Parameter Estimates | Intersubject Variability |
Residual Error |
|---|---|---|
| CL (L/hr) = 0.258 * Wt0.75 * (0.4/SCr)0.431 * [ln(Age)/7.7] 0.808 | 41% | 32% |
| Vd (L) = 0.644 * Wt | 12% |
Abbreviations and units: Wt = Weight in kg, Age in days, and SCr = Serum creatinine in mg/dL
Table 3.
Pharmacokinetic and Exposure Parameter Estimates using Two Vancomycin Monitoring Strategies: 1S vs. 2S
| Sample Type | CL (L/kg/hr) | AUC (mg-hr/L) | Trough Concentrations (mcg/mL) |
|---|---|---|---|
| Monitoring Strategy | |||
| One Sample (1S)1 | 0.14 (0.10 – 0.18) | 329 (265 – 406) | 4.2 (2.6 – 6.9) |
| Two Samples (2S)2 | 0.13 (0.10 – 0.16) | 356 (287 – 451) | 3.3 (2.2 – 6.1) |
| Expected and Predictive Values | |||
| Rich Sample (RS) | 0.13 (0.09 – 0.17) | 365 (292 – 478) | 5.0 (2.8 – 9.7) |
| Validation Sample (VS) | 0.13 (0.09 – 0.16) | 369 (294 – 491) | 5.2 (2.9 – 9.9) |
Numbers are expressed as median (interquartile).
One vancomycin serum concentration obtained near trough concentration.
Two vancomycin serum concentrations obtained near peak and trough concentrations.
For AUC and CL, the accuracy and precision of the 2S scheme was significantly higher than 1S when both were evaluated against RS (Table 4). A similar statistically significant finding of improved accuracy with 2S was observed with VS. While the accuracy of trough was improved with the 1S strategy, as compared to 2S, the percent error exceeded 10% for both RS and VS. The precision of trough concentration was not statistically different between 1S and 2S (Table 4).
Table 4.
Accuracy and Precision of Two Vancomycin Monitoring Strategies: 1S vs 2S1
| Parameter | Rich Sample | Validation Sample | ||||
|---|---|---|---|---|---|---|
| 1S | 2S | p-Value | 1S | 2S | p-Value | |
| Accuracy (%) | ||||||
| CL | 9.5 | 2.7 | <0.001 | 15.8 | 6.3 | <0.001 |
| AUC | −7.6 | −2.0 | 0.020 | −11.6 | −6.1 | 0.032 |
| Trough Concentratio n |
−11.2 | −24.2 | 0.009 | −19.0 | −29.6 | 0.009 |
| Precision (%) | ||||||
| CL | 14.8 | 10.1 | <0.001 | 21.6 | 15.7 | 0.002 |
| AUC | 12.8 | 10.3 | 0.013 | 18.8 | 15.8 | 0.325 |
| Trough Concentratio n |
26.0 | 28.2 | 0.429 | 32.4 | 37.0 | 0.353 |
Median values are presented. One vancomycin serum concentration strategy (1S) obtained near trough concentration. Two vancomycin serum concentrations strategy (2S) obtained near peak and trough concentrations.
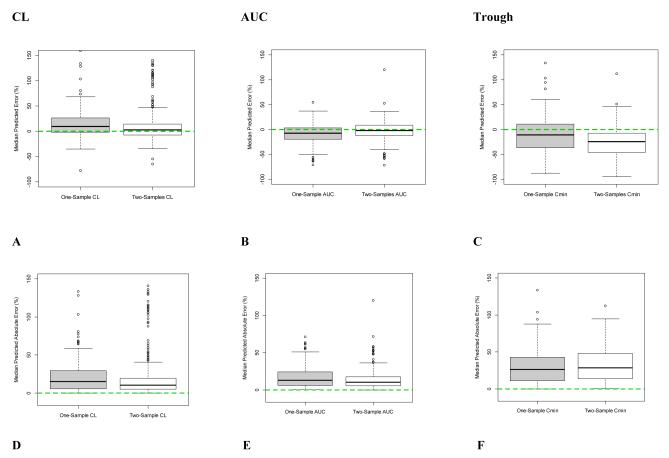
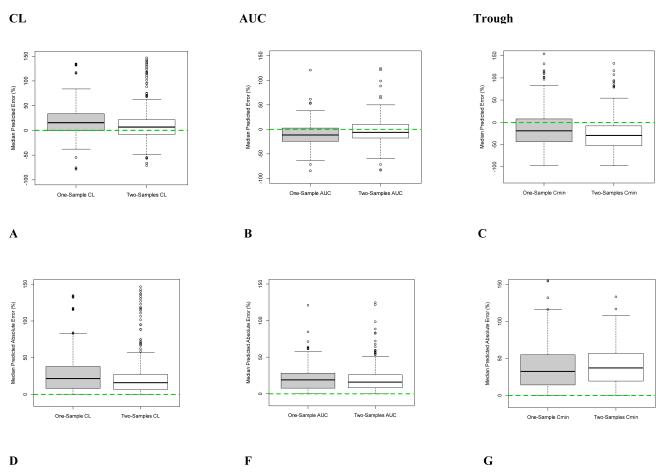
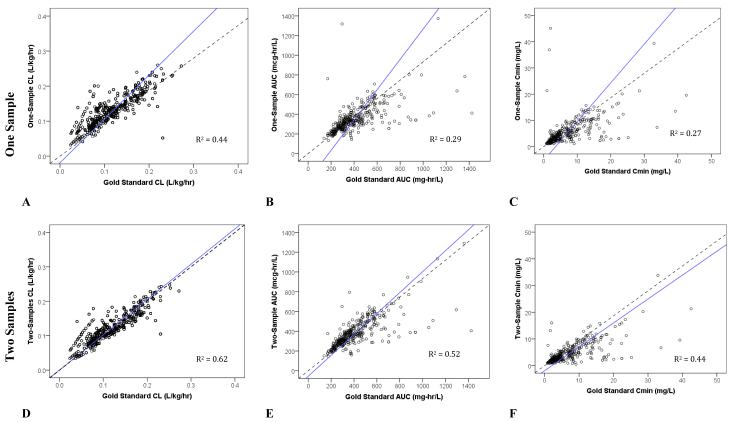
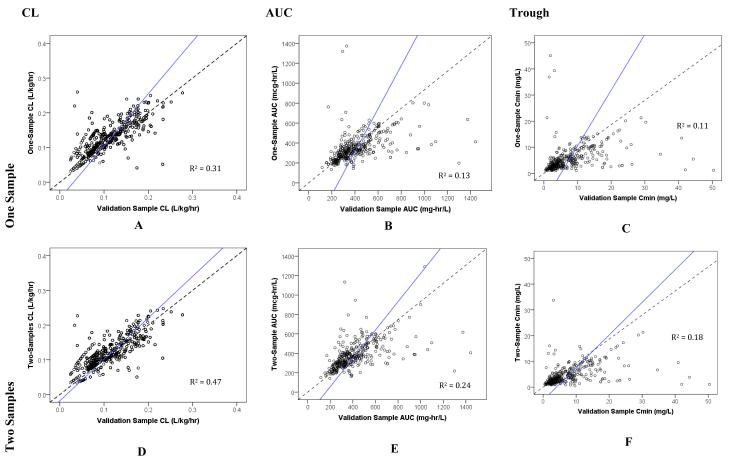
When evaluated against the RS and in predicting future concentrations with VS, the 2S (white boxplot) sampling scheme, compared to the 1S (grey boxplot), had improved accuracy and precision for both CL and AUC since the median values were closer to 0% error (dotted line; Figures 2A, 2B, 2D, 2E, 3A, 3B, 3D, and 3E). However, the same was not observed with troughs (Figures 2C, 2F, 3C, and 3F). For all PK parameters including AUC, CL, and trough, scatter plots demonstrated better fit for 2S, as compared to 1S, for both RS and VS (Figures 4A-F and 5A-F). Similarly, correlation analyses with R2 values demonstrated improved fit with 2S versus 1S against both RS and VS for AUC, CL, and trough (Figures 4A-F and 5A-F).
Figure 2. Accuracy and Precision of One-Sample (1S) versus Two-Sample (2S) Vancomycin Monitoring Strategies Compared to the Rich Sample Method.
Median predicted error represents accuracy; and median predicted absolute error, precision.
Dashed line at zero represents no difference between compared samples, hence serves as the threshhold for best accuracy or precision.
Figure 3. Accuracy and Precision of One-Sample (1S) versus Two-Sample (2S) Vancomycin Monitoring Strategies in Internally Predicting Future Concentrations using Validation Samples.
Median predicted error represents accuracy; and median predicted absolute error, precision.
Dashed line at zero represents no difference between compared samples, hence serves as the threshhold for best accuracy or precision.
Figure 4. Scatter Plots of One- versus Two-Sample Vancomycin Monitoring Strategies against the Rich Sample (or Gold Standard) Method.
Dotted line represents line of unity; and solid line, line of regression.
Figure 5. Scatter Plots of One- versus Two-Sample Vancomycin Monitoring Strategies against the Validation Sample.
Dotted line represents line of unity; and solid line, line of regression.
DISCUSSION
Vancomycin remains as the primary treatment for serious MRSA infections, yet current data are insufficient to support the optimal method for therapeutic drug monitoring in children.1,2 With the use of high-dose vancomycin to augment treatment success for invasive MRSA infections coupled to the emerging evidence correlating vancomycin use to the development of nephrotoxicity in children, it is prudent to ensure accuracy and precision of therapeutic monitoring of vancomycin.1,4,10-12 To our knowledge, this is the largest population-based pharmacokinetic study that attempts to ascertain the optimal method to monitor vancomycin therapy in children. Specifically, this study evaluated the accuracy and precision of the therapeutic monitoring of vancomycin using 1S (i.e., single concentration near trough) versus 2S (i.e., two concentrations near peak and trough) to derive Bayesian estimations in children.
Our study demonstrated that estimating PK parameters, specifically AUC and CL, derived from 2S strategy was more accurate and precise than 1S (i.e., compared against RS) in children. This corroborates with recent evidence in adults to suggest that trough concentrations alone are poor surrogates for vancomycin exposure by AUC, underestimating it by ~25%.13 In this study, the 2S sampling strategy also had improved internal predictive performance for AUC and CL (i.e., compared against VS). In contrast, the 1S strategy had improved accuracy, but not precision, for trough. Based on one study, the 1S scheme may be advantageous since minimizing the number of vancomycin concentrations may confer convenience and considerable cost savings.14 Despite the improved accuracy for trough and curtailing the need for drug sampling using the 1S strategy, there was a stronger correlation between 2S and RS (R2 = 0.44) than 1S and RS (R2 = 0.27) for trough. This implies that, while 2S probably under-estimates trough consistently at all concentrations (Figure 4F), estimation of trough remains poor using 1S especially in subjects at extreme trough concentrations (i.e., very high and very low values) who are most in need of dose modification (Figure 4C). As such, the use of 1S strategy for dose adjustment is sub-optimal.
Despite AUC/MIC being the PD measure of vancomycin exposure evaluated in studies to optimize treatment success, trough concentration has been the common target used in clinical practice for adults and children.3,15,16 In adults, troughs ranging from 15 to 20 mcg/mL are generally required to attain AUC/MIC ≥ 400, assuming a MIC of ≤ 1 mcg/mL.2,3 This trough range is recommended for vancomycin dosing to treat invasive MRSA infections in current guidelines by IDSA.1 Furthermore, this strong correlation between trough and AUC/MIC values in adults decreases the reliance on monitoring peak concentrations in adults.17 However, emerging evidence in children suggests that AUC ~ 400 (assuming MIC of ~ 1 mcg/mL) corresponds to troughs ~ 8 to 9 mcg/mL for regimens 60 to 70 mg/kg/day.6,18 This complicates monitoring vancomycin using only trough concentrations, despite the enhancement of accuracy with 1S, compared to 2S, for troughs observed in our study. Furthermore, a minimum of 10% error (up to ~20%) for troughs using the 1S scheme (compared against both RS and VS) should be underscored.
In this study, we did not establish a priori thresholds for accuracy and precision as we strived to determine the optimal monitoring strategy between 1S and 2S, which are both commonly used in clinical practice, to estimate and predict AUC, CL, and trough. The boundaries for accuracy and precision utilized in previously published reports have been < 5 to 20%.19-22 Using the upper limit of < 20%, both 1S and 2S schemes achieved good accuracy and precision for AUC and CL estimations (via RS) and future predictions (via VS). However, the 2S sampling had consistently improved accuracy (< 5%) and precision (~10-15%) for AUC and CL. In contrast, the 2S strategy produced relatively poor (i.e., > 20%) accuracy and precision for trough since it under-estimated trough concentrations when compared against RS and VS. Imprecision was also observed for troughs with 1S strategy. Collectively, these observations endorse the 2S sampling strategy to monitor vancomycin AUC since the estimation and internal prediction of trough is unreliable.
In our study cohort, the median initial vancomycin dose was approximately 40 mg/kg/day, which is below the recommended 60 mg/kg/day.1,4,23 Consequently, this regimen produced low AUC ~350 mghr/L and trough ~ 4 mcg/mL. In addition to increasing the total daily dose, augmenting the frequency of administration may achieve higher troughs and this may result in different trough accuracy determination.
Several limitations exist in our study. A small sample size of hospitalized infants and children > 3 months old (excluding neonates) was used to develop the models. Findings cannot be extrapolated to neonates as this population exhibits different PK profile of vancomycin.24,25 In addition, the opportunistic design of our study resulted in different schedules for blood sampling that was based on the standard of practice at the two study sites. The PK models were inevitably derived from limited sampling strategies. This may have compromised our internal prediction of AUC values since accurate determination of AUC classically requires intensive sampling. Furthermore, our sparse data, especially for peak concentrations (i.e., ~ 3% measured within first hour from the end of infusion), encumbered the estimation of the volume of distribution of vancomycin; thus, accuracy and precision of volume of distribution estimations were not determined.
While AUC precision and accuracy was improved with 2S, there appears to be some loss in trough accuracy. This study limitation was not totally unexpected since sampling for 1S is entirely at trough concentrations. The use of a one-compartment model may have contributed to this observation as well. Despite this, recent studies have demonstrated that targeted exposure using vancomycin AUC, compared with trough, is a more achievable target in children.4,6,7 Per se, for clinical application in treating invasive MRSA infections that require targeted exposure using AUC/MIC ≥ 400, drug sampling using 2S strategy, as opposed to 1S, may provide more accurate and precise estimation of AUC. Furthermore, the integration of peak, along with trough concentrations, improves AUC estimation especially in the setting when the Bayesian approach is unavailable.13
Future exploration of vancomycin PK studies in children should incorporate intensive sampling (e.g., three or more samples) to validate that the 2S strategy is a reasonable scheme to optimize accuracy and precision of AUC and CL estimations, and, as equally important, to balance it with practice convenience, which is essential for pediatric care. In addition, well-designed studies to investigate the association between drug exposure and clinical outcomes are necessary to confirm the preferential PD target (i.e., AUC/MIC or trough) for treatment success. This information is imperative in light of recent evidence in children suggesting that AUC/MIC ~ 400 corresponds to troughs ~ 8 to 9 mcg/mL for certain vancomycin dosing regimens.4,6
With minimal evidence to support targeting troughs for treatment success specifically in children, it is prudent to monitor vancomycin exposure by the more achievable target AUC (along with MIC, if available) to prevent drug over-exposure and potentially adverse effects.4,10 In addition, the AUC/MIC is the PD target linked to efficacy in adult studies, with MIC ≥ 1.5 mg/L predictive of treatment failure.26,27 Furthermore, targeting troughs above 15 mg/L should prompt consideration for evaluating post-dose (i.e., near peak) concentrations to potentially avert drug toxicity.28 Consequently, incorporating the computation of AUC in the therapeutic monitoring of vancomycin can be optimized using the 2S approach for children with suspected or confirmed invasive MRSA infections. In our study, most drug concentrations were measured close to peak, or trough concentrations (i.e., within the first 2 to 3 hrs or > 5 hrs, respectively, from the end of dose administration). The 2S scheme proposed in our study is similar to the strategy previously recommended for once-daily aminoglycoside monitoring using an AUC target.29
CONCLUSION
Population-based PK studies to evaluate optimal vancomycin monitoring strategies to target AUC in the children are limited. Our study demonstrated that the 2S sampling strategy, accompanied by the Bayesian approach, for vancomycin monitoring was accurate and precise in estimating and internally predicting AUC and CL. As such, therapeutic monitoring targeting AUC/MIC using two vancomycin concentrations (i.e., measured within the first 2-3 hrs and ~ > 5 hrs from the end of infusion, depending on dosing interval) is a vigilant approach for children with suspected or confirmed invasive MRSA infections.
Acknowledgements
The authors would like to thank and acknowledge the following groups or individuals who contributed to this research:
Jane Hodding, PharmD for engaging the Pharmacy Department at Miller Children’s Hospital to support this study;
Pharmacy staff at Miller Children’s Hospital and Rady Children’s Hospital for providing the PK information; and
Andrew Vo and Rebecca Kandillian for data collection, entry, and/or management.
Financial Disclosure The project described was supported by Grant Number K23AI089978 to J.L. from the National Institute of Allergy and Infectious Diseases; and U54HD071600 to E.V.C. and J.S.B from the Eunice Kennedy Shriver National Institute of Child Health and Human Development. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases, the Eunice Kennedy Shriver National Institute of Child Health and Human Development, or the National Institutes of Health.
Footnotes
Conflict of Interest J.L. has previously served on the speaker’s bureau for Pfizer and received investigator-initiated grants from Pfizer, Astellas and Cubist. E.V.C. has served as a consultant to Trius, Cerexa and Abbott Pharmaceuticals. All other authors disclose no conflict of interest.
This work was presented at the 51st annual meeting of the Infectious Diseases Society of America (ID Week) on October 20, 2012 in San Diego, CA.
REFERENCES
- 1.Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the infectious diseases society of america for the treatment of methicillin-resistant staphylococcus aureus infections in adults and children: Executive summary. Clin Infect Dis. 2011;52:285–292. doi: 10.1093/cid/cir034. [DOI] [PubMed] [Google Scholar]
- 2.Rybak M, Lomaestro B, Rotschafer JC, et al. Therapeutic monitoring of vancomycin in adult patients: A consensus review of the american society of health-system pharmacists, the infectious diseases society of america, and the society of infectious diseases pharmacists. Am J Health Syst Pharm. 2009;66:82–98. doi: 10.2146/ajhp080434. [DOI] [PubMed] [Google Scholar]
- 3.Kullar R, Davis SL, Taylor TN, et al. Effects of targeting higher vancomycin trough levels on clinical outcomes and costs in a matched patient cohort. Pharmacotherapy. 2012;32:195–201. doi: 10.1002/j.1875-9114.2011.01017.x. [DOI] [PubMed] [Google Scholar]
- 4.Le J, Bradley JS, Murray W, et al. Improved vancomycin dosing in children using area under the curve exposure. Pediatr Infect Dis J. 2013;32:e155–163. doi: 10.1097/INF.0b013e318286378e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rybak MJ, Lomaestro BM, Rotschafer JC, et al. Therapeutic monitoring of vancomycin in adults summary of consensus recommendations from the american society of health-system pharmacists, the infectious diseases society of america, and the society of infectious diseases pharmacists. Pharmacotherapy. 2009;29:1275–1279. doi: 10.1592/phco.29.11.1275. [DOI] [PubMed] [Google Scholar]
- 6.Frymoyer A, Guglielmo BJ, Hersh AL. Desired vancomycin trough serum concentration for treating invasive methicillin-resistant staphylococcal infections. Pediatr Infect Dis J. 2013 doi: 10.1097/INF.0b013e318299f75c. [DOI] [PubMed] [Google Scholar]
- 7.Gordon CL, Thompson C, Carapetis JR, et al. Trough concentrations of vancomycin: Adult therapeutic targets are not appropriate for children. Pediatr Infect Dis J. 2012;31:1269–1271. doi: 10.1097/INF.0b013e31826a3eaf. [DOI] [PubMed] [Google Scholar]
- 8.Moffett BS, Edwards MS. Analysis of vancomycin therapeutic drug monitoring trends at pediatric hospitals. Pediatr Infect Dis J. 2013;32:32–35. doi: 10.1097/INF.0b013e31826fd98d. [DOI] [PubMed] [Google Scholar]
- 9.Suryadevara M, Steidl KE, Probst LA, et al. Inappropriate vancomycin therapeutic drug monitoring in hospitalized pediatric patients increases pediatric trauma and hospital costs. J Pediatr Pharmacol Ther. 2012;17:159–165. doi: 10.5863/1551-6776-17.2.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McKamy S, Hernandez E, Jahng M, et al. Incidence and risk factors influencing the development of vancomycin nephrotoxicity in children. J Pediatr. 2011;158:422–426. doi: 10.1016/j.jpeds.2010.08.019. [DOI] [PubMed] [Google Scholar]
- 11.Cies JJ, Shankar V. Nephrotoxicity in patients with vancomycin trough concentrations of 15-20 mug/ml in a pediatric intensive care unit. Pharmacotherapy. 2013;33:392–400. doi: 10.1002/phar.1227. [DOI] [PubMed] [Google Scholar]
- 12.Totapally BR, Machado J, Lee H, et al. Acute kidney injury during vancomycin therapy in critically ill children. Pharmacotherapy. 2013;33:598–602. doi: 10.1002/phar.1259. [DOI] [PubMed] [Google Scholar]
- 13.Neely MN, Youn G, Jones B, et al. Are vancomycin troughs adequate for optimal dosing? Antimicrob Agents Chemother. 2013 [Google Scholar]
- 14.Rybak MJ, Boike SC. Monitoring vancomycin therapy. Drug Intell Clin Pharm. 1986;20:757–761. doi: 10.1177/106002808602001003. [DOI] [PubMed] [Google Scholar]
- 15.Hidayat LK, Hsu DI, Quist R, et al. High-dose vancomycin therapy for methicillin-resistant staphylococcus aureus infections: Efficacy and toxicity. Arch Intern Med. 2006;166:2138–2144. doi: 10.1001/archinte.166.19.2138. [DOI] [PubMed] [Google Scholar]
- 16.Moise-Broder PA, Forrest A, Birmingham MC, et al. Pharmacodynamics of vancomycin and other antimicrobials in patients with staphylococcus aureus lower respiratory tract infections. Clin Pharmacokinet. 2004;43:925–942. doi: 10.2165/00003088-200443130-00005. [DOI] [PubMed] [Google Scholar]
- 17.Suzuki Y, Kawasaki K, Sato Y, et al. Is peak concentration needed in therapeutic drug monitoring of vancomycin? A pharmacokinetic-pharmacodynamic analysis in patients with methicillin-resistant staphylococcus aureus pneumonia. Chemotherapy. 2012;58:308–312. doi: 10.1159/000343162. [DOI] [PubMed] [Google Scholar]
- 18.Le J, Bradley JS, Murray W, et al. Vancomycin dosing in children by area-under-the curve (auc). Platform presentation at: 51st annual interscience conference on antimicrobial agents and chemotherapy; chicago, il. 2011 sept 17-20. [Google Scholar]
- 19.Al-Khatib M, Shapiro RJ, Partovi N, et al. Limited sampling strategies for predicting area under the concentration-time curve of mycophenolic acid in islet transplant recipients. Ann Pharmacother. 2010;44:19–27. doi: 10.1345/aph.1M511. [DOI] [PubMed] [Google Scholar]
- 20.Kim JS, Nafziger AN, Tsunoda SM, et al. Limited sampling strategy to predict auc of the cyp3a phenotyping probe midazolam in adults: Application to various assay techniques. J Clin Pharmacol. 2002;42:376–382. [PubMed] [Google Scholar]
- 21.Musuamba FT, Rousseau A, Bosmans JL, et al. Limited sampling models and bayesian estimation for mycophenolic acid area under the curve prediction in stable renal transplant patients co-medicated with ciclosporin or sirolimus. Clin Pharmacokinet. 2009;48:745–758. doi: 10.2165/11318060-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 22.Ting LS, Villeneuve E, Ensom MH. Beyond cyclosporine: A systematic review of limited sampling strategies for other immunosuppressants. Ther Drug Monit. 2006;28:419–430. doi: 10.1097/01.ftd.0000211810.19935.44. [DOI] [PubMed] [Google Scholar]
- 23.Frymoyer A, Hersh AL, Benet LZ, et al. Current recommended dosing of vancomycin for children with invasive methicillin-resistant staphylococcus aureus infections is inadequate. Pediatr Infect Dis J. 2009;28:398–402. doi: 10.1097/INF.0b013e3181906e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Allegaert K, van den Anker JN. Creatinine-based vancomycin dosing regimens in neonates: There is more to consider than the variation in drug assay. Pharmacotherapy. 2012;32:e174. doi: 10.1002/j.1875-9114.2012.01180.x. discussion e175. [DOI] [PubMed] [Google Scholar]
- 25.Mehrotra N, Tang L, Phelps SJ, et al. Evaluation of vancomycin dosing regimens in preterm and term neonates using monte carlo simulations. Pharmacotherapy. 2012;32:408–419. doi: 10.1002/j.1875-9114.2012.01029.x. [DOI] [PubMed] [Google Scholar]
- 26.Patel N, Pai MP, Rodvold KA, et al. Vancomycin: We can’t get there from here. Clin Infect Dis. 2011;52:969–974. doi: 10.1093/cid/cir078. [DOI] [PubMed] [Google Scholar]
- 27.van Hal SJ, Lodise TP, Paterson DL. The clinical significance of vancomycin minimum inhibitory concentration in staphylococcus aureus infections: A systematic review and meta-analysis. Clin Infect Dis. 2012;54:755–771. doi: 10.1093/cid/cir935. [DOI] [PubMed] [Google Scholar]
- 28.Saunders NJ. Why monitor peak vancomycin concentrations? Lancet. 1994;344:1748–1750. doi: 10.1016/s0140-6736(94)92890-8. [DOI] [PubMed] [Google Scholar]
- 29.Barclay ML, Duffull SB, Begg EJ, et al. Experience of once-daily aminoglycoside dosing using a target area under the concentration-time curve. Aust N Z J Med. 1995;25:230–235. doi: 10.1111/j.1445-5994.1995.tb01529.x. [DOI] [PubMed] [Google Scholar]