Abstract
Background
Despite national guidelines recommending bone mineral density screening with dual-energy xray absorptiometry (DXA) in women ≥65 years old, many women do not receive initial screening.
Objective
To determine the effectiveness of health system and patient-level interventions designed to increase appropriate DXA testing and osteoporosis treatment through (1) an invitation to self-refer for DXA (self-referral), (2) self-referral plus patient educational materials, and (3) usual care (UC, physician referral).
Research Design
Parallel, group-randomized, controlled trials performed at Kaiser Permanente Northwest (KPNW) and Kaiser Permanente Georgia (KPG).
Subjects
Women ≥ 65 years old without a DXA in past 5 years.
Measures
DXA completion rates 90 days after intervention mailing and osteoporosis medication receipt 180 days after initial intervention mailing.
Results
From >12,000 eligible women, those randomized to self-referral were significantly more likely to receive a DXA than UC (13.0 – 24.1% self-referral vs. 4.9 – 5.9% UC, p < 0.05). DXA rates did not significantly increase with patient educational materials. Osteoporosis was detected in a greater proportion of self-referral women compared to UC (p < 0.001). The number needed to receive an invitation to result in a DXA in KPNW and KPG regions was approximately 5 and 12, respectively. New osteoporosis prescription rates were low (0.8 – 3.4%) but significantly greater among self-referral versus UC in KPNW.
Conclusions
DXA rates significantly improved with a mailed invitation to schedule a scan without physician referral. Providing women the opportunity to self-refer may be an effective, low-cost strategy to increase access for recommended osteoporosis screening.
Keywords: osteoporosis, screening, DXA, randomized controlled trial
Introduction
The link between low bone mineral density (BMD) and increased fracture risk is well established (1). Dual-energy x-ray absorptiometry (DXA) is the current standard for identifying osteoporosis based on BMD. In the U.S., guidelines from the Surgeon General, Preventive Services Task Force, National Osteoporosis Foundation, American College of Preventive Medicine, and American Association of Clinical Endocrinologists recommend screening BMD using DXA in all women 65 years or older (2-5). However, less than one third of U.S. women ≥ 65 undergo DXA testing (6, 7). It is estimated that additional DXA screening could prevent over 35,000 fractures and save Medicare nearly $78 million over three years (7).
Women do not receive DXA testing for various reasons. Primary care providers (PCPs) and patients may be unaware of preventive screening recommendations. Screening tests recommended relatively infrequently (i.e. less than yearly) may be difficult for patients and PCPs to remember. PCPs are responsible for managing a large number of acute and chronic conditions but may be unable to address all preventive care needs in the limited time available during office visits (8).
Mammography is a screening procedure that is available to eligible patients through self-referral and without a physician order in the U.S. Similar to mammography, DXA is a screening tool associated with low radiation exposure. A pilot study of women who had PCPs in our university-based clinic found that allowing patients to self-refer for a DXA significantly improved rates of osteoporosis screening (9). However, this study (9) and other prior studies (10, 11) aimed at self-referral for DXA have not included the necessary component of health system changes allowing for direct DXA scheduling without the time consuming review process from a medical provider.
In an effort to improve primary osteoporosis screening in at-risk women, we conducted a group-randomized trial separately in two large regional health systems to test the hypothesis that the rate of DXA testing and osteoporosis treatment would improve if postmenopausal women received simple instructions to self-refer for DXA compared to the current standard of care which requires a physician referral to receive a DXA. We also anticipated that additional narrative storytelling materials would further increase DXA testing and appropriate osteoporosis medication use compared to women not receiving this intervention. Our previous research indicated that providing patients with background education, including narrative storytelling, motivates patients to seek preventive care in other chronic diseases, such as hypertension (12).
Methods
Design overview
The study protocols were approved by the Institutional Review Board at the University of Alabama at Birmingham (UAB) coordinating center and recruitment sites at Kaiser Permanente Northwest (KPNW) and Georgia (KPG). The identically designed interventions consisted of: (1) a health system redesign strategy to allow patients to self-refer and schedule a DXA (SELF), (2) SELF strategy combined with patient educational materials that included a DVD containing narrative story-telling and written materials to encourage patients to schedule a DXA and promote patient-physician communication (SELF+DVD) and, (3) a control group that required patients to obtain routine physician authorization to schedule a DXA (Usual Care, UC). All PCPs associated with study patients were offered continuing medical education (CME) materials on osteoporosis.
Settings and participants
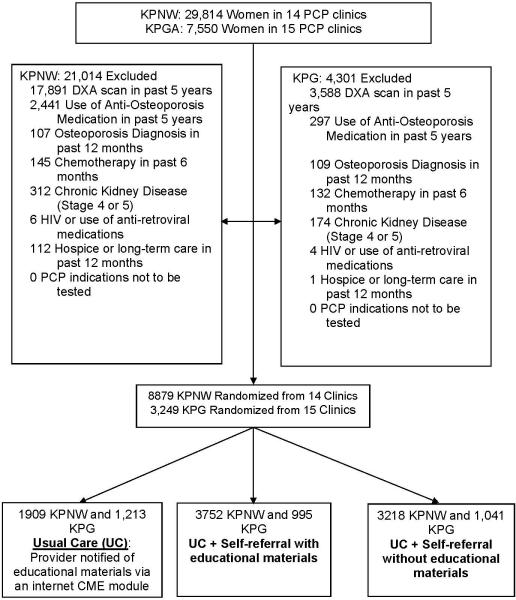
Women 65 years or older, with a KPNW or KPG PCP visit in the past 12 months and no identifiable DXA in Kaiser electronic medical records (EMR) in the past 5 years were eligible to participate. Women were excluded if there was evidence in the EMR of anti-osteoporosis medication use (except estrogen) in the previous 5 years. A full listing of the population and exclusion criteria is shown in Figure 1. Patient characteristics were obtained through EMR. Race was categorized as Caucasian, African-American or other if the race was unknown or entered as another race.
Figure 1.
Screening and Randomization CONSORT Diagram
Randomization and interventions
The unit of randomization was the individual clinic
All eligible patients in 29 primary care clinics in KPNW and KPG were randomized into 1 of 3 groups: SELF, SELF + DVD, or UC (Figure 1). In order to balance the age of patients, distance to the DXA facility from the clinic, and baseline DXA rates within the Kaiser clinics, a principal component analysis was conducted. The first principal component was calculated and used to balance randomization of clinics based on these factors in each Kaiser region.
Intervention Materials
Healthcare provider materials (see below) were provided to all study groups. The health system and patient interventions were administered to the two intervention groups only. We had no contact with patients in the Usual Care group.
Healthcare Provider Materials
An Internet-based osteoporosis CME program was provided to all KPNW and KPG PCPs for several months prior to patient recruitment. The objective of the CME program was to improve recognition of osteoporosis risk and appropriate testing and treatment options.
Health System Intervention Administration at the Two Kaiser Permanente Regions
An initial letter on KP letterhead was mailed to all intervention group patients (SELF and SELF+DVD) inviting them to self-refer for a DXA (Supplement 1). Three weeks following the mailing, another similar letter was sent to intervention groups; and, additional educational materials and DVD were mailed to the SELF+DVD group. The two KP regions managed the DXA scheduling and completion process in slightly different manners due to mechanism by which DXA scans were completed.
Kaiser Permanente Northwest (KPNW)
In order to avoid overloading health system capacity to deliver DXA testing, one-seventh of the eligible population was mailed intervention materials each month during the seven month intervention period. The initial letter included a toll-free telephone number to contact KPNW study staff. After speaking with the patient, study staff generated a referral to one of four Regional Referral DXA Centers outside the KPNW system. The referral center mailed a letter to the patient that contained a second phone number for patients to call to schedule their DXA.
Kaiser Permanente Georgia (KPG)
One–ninth of the eligible population was mailed the intervention materials each month for a total of 9 months. The intervention period was longer in KPG to avoid capacity problems at the one DXA facility. The initial letter included a local number to call to schedule a DXA directly with KPG's diagnostic radiology department.
Patient Intervention Material Development and Implementation
The patient DVD and educational materials were created based on the concepts of narrative communication (13) and feedback from six focus groups (n = 12-18 patients per group) consisting of women ≥ 65 years old. Focus group participants were selected as a convenience sample from two geographical sites and were self-identified as at-risk for osteoporosis or with known osteoporosis.
From information obtained from focus groups, a DVD was created using recorded audiovisual segments of women telling their personal stories with osteoporosis, DXA screening, and osteoporosis treatments, along with summary points made by an osteoporosis educator and KP physician. Using concepts from the Chronic Care Model (14) and The Practical Robust Implementation and Sustainability Model (PRISM) (15), video segments were rated based on strength and clarity of content by the study team to produce the DVD (16). The 19 highest rated video segments were edited and compiled into a single 16 minute DVD. An educational brochure was created to mirror the DVD. We pilot tested study materials through one-on-one interviews with women at risk of osteoporosis to ensure completeness, comprehension, and readability. Additional pilot testing of the materials and randomization process was completed through a mailing of intervention materials to 100 eligible patients from KPNW and KPG each. Structured telephone interviews were conducted with a 20% random sample of those patients that received pilot materials to obtain feedback on content, clarity of the message, willingness to engage in the intervention (patient activation), and the DXA scheduling process. Based on results of pilot testing, we finalized the intervention.
Outcomes
The primary outcome of interest was DXA completion and the secondary outcome of interest was osteoporosis prescription medication receipt, as documented in the Kaiser EMRs and computerized pharmacy dispensing records, respectively. Following the initial mailing, observation of DXA completion rates and osteoporosis medication initiation occurred for 90 days and 180 days for all groups, respectively. All DXA results were sent to PCPs in the usual manner for interpretation and discussion with patients.
Statistical Analysis
Pre-trial power calculations indicated an 87% power to detect differences in DXA completion rates of 5% between UC vs. SELF vs. SELF+DVD. The observed intraclass correlation coefficient at study conclusion was smaller than the pre-trial estimate (0.05); therefore, the design had more statistical power than anticipated. We used descriptive statistics to compare demographic characteristics and outcomes within KPNW and KPG. To account for correlation of patients within clinics, due to nesting of patients within a clinic, we employed generalized estimating equation (GEE) models as developed by Liang and Zeger (17) to test for intervention effects for DXA receipt and osteoporosis medication treatment rates using a Type I error rate of 0.05.
We defined osteoporosis and osteopenia (low bone mass) based on BMD using the World Health Organization definition of a BMD T-score of ≤ -2.5 and between -1.0 and -2.5, respectively (18).
All p-values to test the significance of intervention effects and all confidence intervals for proportions of individuals receiving DXA testing by intervention arm were derived directly from GEE models. Separate GEE models were developed, treating each region as an independent replication of a common protocol. We adjusted the degrees of freedom in order to account for use of GEE with a small number of clusters (19-23). When testing for differences among intervention arms, Bonferonni adjustments were employed to maintain a Type I error rate of 0.05. All analyses were conducted using SAS software 9.2 (SAS Institute Inc., Cary, NC. USA).
Results
Most patient characteristics at baseline were similar across study groups within each region (Table 1). Some differences were identified between the two KP regions. Mean distance to a DXA facility was greater at KPG than KPNW due to the difference of one DXA location at KPG vs. four at KPNW. The patient populations also differed between KPG and KPNW by race/ethnicity.
Table 1.
Baseline Characteristics of Health Plans and Women Randomized by Plan Location and Intervention Group
| Kaiser Permanente Northwest | Kaiser Permanente Georgia | |||||
|---|---|---|---|---|---|---|
| Self-Referral Only | Self-Referral With Patient Educational Materials | Usual Care | Self-Referral Only | Self-Referral with Patient Educational Materials | Usual Care | |
| Patient Characteristics | ||||||
| N | 3,218 | 3,752 | 1,909 | 1,041 | 995 | 1,213 |
| Age at randomization, years (mean± SD) | 73.8 ± 7.0 | 73.3 ± 6.9 | 73.4 ± 6.8 | 73.7 ± 6.7 | 73.5 ± 6.6 | 73.1 ± 6.3 |
| 65-74 (%) | 61.3 | 64.3 | 64.1 | 58.0 | 60.1 | 62.0 |
| 75-84 (%) | 29.2 | 27.7 | 27.8 | 33.7 | 32.3 | 31.8 |
| ≥ 85 (%) | 9.5 | 8.0 | 8.1 | 8.3 | 7.6 | 6.2 |
| Race/Ethnicity | ||||||
| White (%) | 77.1 | 84.8 | 84.4 | 40.9 | 32.7 | 41.5 |
| Black (%) | 3.2 | 0.8 | 0.5 | 36.6 | 45.5 | 36.0 |
| Other† (%) | 19.7 | 14.4 | 15.1 | 22.4 | 21.8 | 22.5 |
| Charlson co-morbidity index (51) | ||||||
| 0 (%) | 54.3 | 57.1 | 53.2 | 48.6 | 48.6 | 51.0 |
| 1 (%) | 17.1 | 16.0 | 17.7 | 17.7 | 17.7 | 20.0 |
| ≥ 2 (%) | 28.6 | 26.9 | 29.1 | 33.7 | 33.7 | 29.0 |
| Number of medications in the prior 12 months (mean ± SD) | 6.9 ± 5.5 | 6.9 ± 5.7 | 6.9 ± 5.4 | 7.4 ± 5.6 | 7.0 ± 5.4 | 6.9 ± 5.4 |
| Primary care clinic visits in prior 12 months (mean ± SD, Range) | 2.4 ± 2.4 (1-6) | 2.5 ± 2.8 (1-5) | 2.5 ± 2.4 (1-5) | 2.2 ± 2.0 (1-3) | 2.3 ± 1.9 (1-3) | 1.9 ± 1.7 (1-3) |
| Seen by a rheumatologist or an endocrinologist in the prior 12 months (%) | 1.1 | 1.0 | 1.0 | 2.3 | 1.4 | 2.2 |
| Health Plan Characteristic | ||||||
| Number of Primary Care Clinic Sites | 5 | 5 | 4 | 5 | 5 | 5 |
| Number of DXA Facilities* | 4 | 4 | 4 | 1 | 1 | 1 |
| DXA Screening Rates at Baseline (%) | 6.6 | 9.0 | 5.5 | 8.5 | 6.8 | 7.8 |
| Mean Distance from the primary care clinic to the nearest DXA facility, miles (mean ± SD) | 11.0 ± 13.2 | 9.5 ± 3.4 | 6.1 ± 4.0 | 15.4 ± 7.9 | 13.1 ± 8.1 | 14.6 ± 6.9 |
Represents the number of Dual-energy X-ray Absorptiometry (DXA) Facilities available to the entire Kaiser Permanente region not just the clinics randomized to a particular study arm.
Includes unknown race/ethnicity (range 11% - 21%).
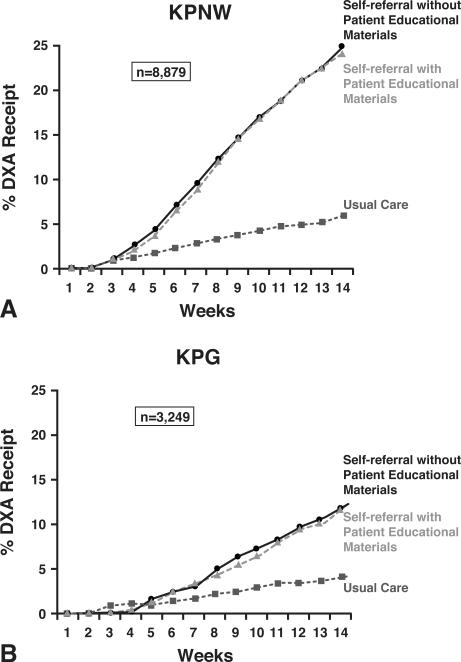
DXA completion rates for SELF, SELF+DVD, and UC are shown for each region in Figure 2. DXA completion rates over the 90 days following randomization were as great as 24% and were approximately five-fold higher in the self-referral groups compared to UC at KPNW; Odds Ratios (ORs): 4.9 (95% Confidence Interval {CI}; 3.3-7.1) for SELF vs. UC and 4.8 (95% CI 3.3-6.9) for SELF+DVD vs. UC (data not shown). At KPG, DXA completion rates were somewhat lower than KPNW (approximately 13% completion in self-referral groups). Within KPG, DXA completion rates were approximately two- to three-fold higher in the two self-referral groups compared to UC; ORs: 2.7 (95% CI 1.5-4.8) for SELF vs. UC and 2.2 (95% CI 1.1-4.4) for SELF+DVD vs. UC. These findings of greater DXA completion in the intervention groups vs. the UC group persisted beyond the 90-days following the intervention, as reflected in 12-month DXA completion rates for KPNW: 37% SELF, 35% SELF+DVD, and 18% for UC; OR comparing self-referral to usual care: 2.6 (95% CI 2.3 – 3.0).
Figure 2.
Cumulative incidence of DXA receipt in Kaiser Permanente Northwest (KPNW) (2a) and Kaiser Permanente Georgia (KPG) (2b) after two intervention strategies compared to usual care.
We also determined the number of women needed to receive an invitation to self-refer with or without additional educational materials to lead to one completed DXA and one DXA showing osteoporosis. The numbers needed to contact with our mailed self-referral invitation ranged from just over 5 in the KPNW region for DXA completion to just over 100 to obtain a DXA showing osteoporosis in the KPG region (Table 2).
Table 2.
Rates of Dual-energy X-ray Absorptiometry (DXA) Completion and Osteoporosis Detection and Number Needed to Self-Refer for One Additional DXA and One Additional Osteoporosis Diagnosis by Each Health Plan Location and by Intervention Group
| Outcome: Any DXA | Outcome: DXA Showing Osteoporosis | |||
|---|---|---|---|---|
| Health Plan Location, Intervention Group, and Type of Contact | DXA Completion Rate, % | Number of Women Needed to Contact to Identify One DXA (95% CI) | Osteoporosis Diagnosis Rate, % | Number of Women Needed to Contact to Identify One Woman with Osteoporosis (95% CI) |
| Kaiser Permanente Northwest | n = 8,879 | |||
| Letter inviting self-referral | 24.1* | 5.4 (4.9-6.0) | 1.6* | 43.3 (33.1-62.3) |
| Letter Inviting Self-Referral with Patient Educational Materials | 23.4* | 5.5 (5.0-6.1) | 1.4* | 43.9 (34.0-62.1) |
| Usual Care (none) | Reference | - | - | - |
| Kaiser Permanente Georgia | n = 3,249 | |||
| Letter Inviting Self-Referral Only | 13.4† | 11.8 (9.1-16.4) | 1.0* | 81.9 (48.3-269.8) |
| Letter inviting Self-Referral with Patient Educational Materials | 13.0‡ | 12.3 (9.9-17.6) | 0.9* | 100.5 (55.2-558.7) |
| Usual Care | Reference | - | - | - |
p < 0.001 versus usual care
p = 0.003 versus usual care
p = 0.04 versus usual care
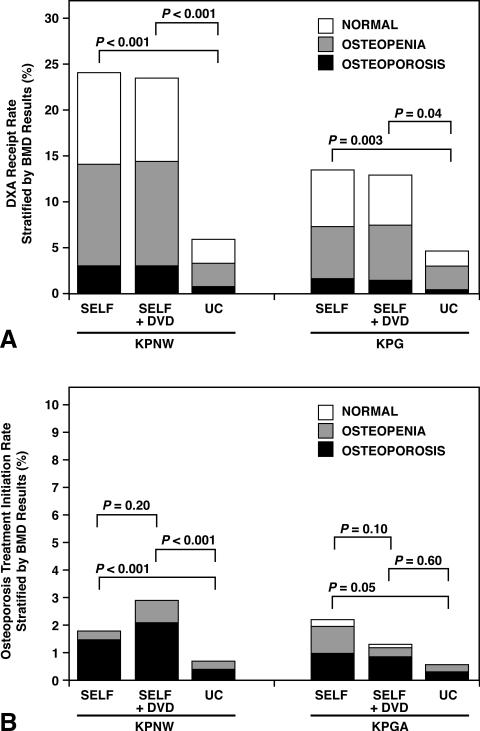
Detection of osteoporosis for SELF, SELF+DVD, and UC were 3.0%, 3.0%, and 0.7% at KPNW, respectively, and 1.6%, 1.4%, and 0.4% at KPG, respectively (Figure 3a). Although all osteoporosis detection rates were low, detection of osteoporosis among women in either the SELF or SELF+DVD groups was four- to five-fold higher than for women in the UC group in both health plans: ORs for osteoporosis diagnosis at KPNW were 4.2 (95% CI 2.2-8.2) for SELF vs. UC and 3.8 (95% CI 2.0-7.3) for SELF+DVD vs. UC; and, at KPG were 5.2 (95% CI 3.3-8.1) for SELF vs. UC and 5.0 (95% CI 3.1-8.1) for SELF+DVD vs. UC (data not shown). Combining the three study groups and the two health plans, 12.5% (261 of 2,091) of women had evidence of osteoporosis based on DXA results. An additional 47.2% (987 of 2,091) of women had low BMD (osteopenia) and the remaining had BMD in the normal range.
Figure 3.
Rates of DXA Testing (3a) and Osteoporosis Prescription Treatment (3b) stratified by Bone Mineral Density and Treatment Groups for Each Study Region.
SELF: self-referral without patient educational materials; SELF+DVD: self-referral with patient educational materials; UC: Usual Care. KPNW: Kaiser Permanente Northwest. KPG: Kaiser Permanente Georgia
Treatment rates with prescription osteoporosis medications (other than hormone therapy) for the entire population for SELF, SELF+DVD, and UC were 2.8%, 3.4%, and 1.4% in KPNW, respectively, and 3.4%, 1.8%, and 0.8% in KPG, respectively (Figure 3b). Although all treatment rates were low, KPNW women in either intervention group were significantly more likely than UC women to initiate prescription osteoporosis treatment. However, rates for osteoporosis treatment initiation did not differ significantly between the intervention groups (SELF compared to SELF+DVD).
Comment
Allowing patients to schedule their own DXA without a physician order (i.e. “self-refer”) in two large regional health plans located in the Northwest and the Southeast U.S. significantly increased DXA completion rates over 3 months by a relative 18% compared to usual care. The addition of an educational DVD, which contained patient story-telling aimed to promote patient-provider communication, did not significantly augment screening rates over self-referral alone. The simple intervention of a letter and authorization to self-refer for DXA resulted in >50% screened patients being recognized as having an increased risk of fracture based on a T-score in the osteopenia or osteoporosis range. Osteoporosis medication use increased significantly, albeit slightly, among women in the intervention groups when compared to usual care in the larger KPNW health plan.
Similar to DXA, screening mammograms are recommended and covered by Medicare and most private medical insurers for all women age 65 and older. The risks associated with obtaining a DXA are low. Radiation exposure during a DXA is approximately one-fifth of the exposure amount of a mammogram (24). In 1992, women were provided the option of self-scheduling mammograms without a physician order (25). Although it is unclear how much this policy alone has influenced mammography utilization, mammography rates increased by over 160% between 1996 - 2005 (26). A program allowing patients to obtain an influenza vaccine without a physician order was similarly associated with a 30% increase in vaccination rates of high-risk patients when compared to usual care (27). These mammography and vaccination health policy changes suggest that overreliance on heavily burdened physicians to perform preventive services is one of the major barriers to preventive health care. Limited physician time is a major impediment to osteoporosis screening (28). Direct involvement of the patient may diminish the burden on providers and may achieve improved rates of recommended preventive measures.(29). However, not all self-referral screening services are likely to yield outcomes where benefits greatly exceed risks.
Other primary osteoporosis screening programs have led to improved DXA rates. In a pilot study completed at our university-based clinic, a simple letter intervention led to an approximate 13% increase in DXA utilization when compared to usual care but required an extra step from physicians to approve DXA screening (9). In a large, randomized controlled study from a Midwestern U.S. multi-specialty clinic (11), DXA completion increased by nearly 11% after mailing a letter that offered self-scheduling and by 18% with the mailing plus provider prompting, when compared to usual care. Two smaller, non-randomized studies that also mailed letters to women and allowed patients to self-schedule a DXA were associated with a 13-26% increase in DXA completion (10, 30). Both studies included follow-up calls to non-responders, and 60-70% of patients scheduled a DXA only after the follow-up phone call.
Our large randomized controlled trials were somewhat similar to these studies in that we used a letter to activate patients to schedule a DXA. However, our study did not include any follow-up calls to non-responders or provider prompting. Our simple, generalizable intervention achieved an increase in DXA receipt similar in magnitude to these earlier investigations and also showed an increase in treatment rates in one region. The number of patients that needed to be contacted to yield a DXA and to identify osteoporosis was not excessive compared to the very modest mailing costs of such a system intervention. Collectively, our results, and the results of prior studies, suggest that more patients obtain preventive care when given direct access to these services. We recognize that self-referral does not fully resolve the issue of underutilization of DXA screening for all women at risk of osteoporosis as not all will take advantage of the self-referral option. However, we believe the benefits of the opportunity to self-refer outweigh any risks of this self-referral process and DXA procedure.
We observed a somewhat greater response to completing DXA scans in women in the KPNW region when compared to the KPG region. We do not fully understand the reason for this but it may be related to the wider availability of DXA facilities in the KPNW region, differing scheduling techniques between the regions, and differences in patient characteristics. Increased distance and a lack of familiarity with imaging centers can be barriers to care (31). Furthermore, in many studies, particularly in the southern U.S., Caucasian women are more likely to receive DXA tests than women of other racial/ethnic groups (32-34), and this may have played a role in the relatively larger proportion of women undergoing DXA imaging at KPNW.
Suboptimal patient understanding of osteoporosis risks, diagnosis, and effective treatment options contribute to overall low DXA screening rates (28). In our study, we incorporated peer storytelling via an educational DVD in one group to promote patient activation. The use of peer modeling has been effective in other areas of medicine, including smoking cessation and treatment of cancer patients (35, 36). Narrative communications (13, 37), delivered in a manner similar to our intervention, have led to significantly better blood pressure management (12). The latter was culturally tailored using patient stories drawn from the local population (12). Our study featured stories from culturally diverse patients, but cultural tailoring may have been muted since the intervention was distributed in two culturally dissimilar geographic regions. Similar to our experience, no increase in DXA completion rates was seen in another randomized study of mailed patient educational materials without a component of self-referral (38). It is possible that our patient educational approach using DVD narratives may be of lesser value with certain patients, in some disease states, or in particular clinical settings. Further, we were unable to verify how many women watched the DVD or read our educational materials.
Our study included large primary care populations treated at two geographically distinct health plans. Despite its consistent findings across the health systems, there are several potential limitations. Due to protocol within the health systems, DXA orders were only active for 90-days, limiting use of the self-referral process to three-months. However, we found persistent effects for up to one year in at least one of the plans. In addition, the letter we sent inviting women to self-refer for a DXA provided minimal educational information about osteoporosis and DXA screening. Although limited in its educational value, and given findings that education interventions alone seldom change patient behavior (38), the information in the letter was not provided to the usual care group, and this omission could have impacted the differences seen in DXA completion rates between the groups. For operational reasons, we also delayed distribution of educational materials to the SELF+DVD group. These were sent after the initial invitation for self-referral and this may have negatively influenced their effectiveness on promoting DXA completion. When evaluating osteoporosis treatment initiation rates, we limited assessment of “appropriate osteoporosis treatment” to those diagnosed with osteoporosis by DXA criteria. There are some women with DXA T-scores better than -2.5 that may benefit from osteoporosis treatment based on clinical information, but these decisions require more criteria than those easily captured through electronic health records. Lastly, we randomized intervention assignment by health plan clinic rather than by patient or by physician to prevent a potential contaminating influence of a change in behavior by physicians due to the intervention (39). It is possible that this approach could have increased the patient variability between clinics and decreased statistical power somewhat.
With osteoporosis prevalence increasing with aging populations, there is a growing need to better identify osteoporosis prior to fragility fractures. Yet, most women do not undergo DXA testing (6), despite its low risk and the national recommendations (2-4, 40-42). Newer scrutiny about too frequent repeat osteoporosis testing (43) and new concerns about osteoporosis treatments (44, 45), coupled with decreasing DXA reimbursement, may foster lower interest in initial DXA testing and lead to an even larger number of women not receiving an initial DXA. We found that creating an opportunity for patients to self-refer for DXAs led to significantly greater DXA completion and osteoporosis treatment. Allowing women to self-refer and schedule their own DXAs is similar to current health policy for screening mammography and some vaccinations. It should be considered as a low cost approach for wider national implementation and dissemination of this recommended preventive service.
Supplementary Material
Acknowledgements
This project was supported by the NIH/NIAMS (P60AR048095-09)
Dr. Warriner is supported by AHRQ (K12HS019465-01)
Dr. Curtis is supported by the NIH (AR053351)
Dr. Saag is supported by the NIH (K24AR052361-07, P60AR048095-09, UL1 TR000165).
Dr. Saag had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Funding: NIH/NIAMS
Footnotes
Clinicaltrials.gov reference number: NCT00788632
Disclosures
AHW: research: Amylin (subsidiary of Bristol-Myers Squibb)
RCO: No disclosures
ACF: No disclosures
DWR: No disclosures
JJA: External Medical Education Review Panel: Pfizer
JRC: research and/or consulting: Merck, Roche, Eli Lilly, Amgen
DTR: research: GSK
MMR: No disclosures
BER: No disclosures
AGR: No disclosures
MMS: research: Amgen, Pfizer
KGS: research and/or consulting: Amgen, Eli Lilly, Merck
References
- 1.Marshall D, Johnell O, Wedel H. Meta-analysis of how well measures of bone mineral density predict occurrence of osteoporotic fractures. Bmj. 1996;312(7041):1254–9. doi: 10.1136/bmj.312.7041.1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Surgeon General's Report on bone health and osteoporosis. Office of the Surgeon General; Rockville (MD): 2004. 2010/10/15. [PubMed] [Google Scholar]
- 3.2010 Pages http://www.nof.org/sites/default/files/pdfs/NOF_ClinicianGuide2009_v7.pdf.
- 4.Lim LS, Hoeksema LJ, Sherin K. Screening for osteoporosis in the adult U.S. population: ACPM position statement on preventive practice. Am J Prev Med. 2009;36(4):366–75. doi: 10.1016/j.amepre.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 5.Watts NB, Bilezikian JP, Camacho PM, Greenspan SL, Harris ST, Hodgson SF, et al. American Association of Clinical Endocrinologists Medical Guidelines for Clinical Practice for the diagnosis and treatment of postmenopausal osteoporosis. Endocr Pract. 2010;16(Suppl 3):1–37. doi: 10.4158/ep.16.s3.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Curtis JR, Carbone L, Cheng H, Hayes B, Laster A, Matthews R, et al. Longitudinal trends in use of bone mass measurement among older americans, 1999-2005. J Bone Miner Res. 2008;23(7):1061–7. doi: 10.1359/JBMR.080232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.King AB, Saag KG, Burge RT, Pisu M, Goel N. Fracture Reduction Affects Medicare Economics (FRAME): impact of increased osteoporosis diagnosis and treatment. Osteoporos Int. 2005;16(12):1545–57. doi: 10.1007/s00198-005-1869-5. [DOI] [PubMed] [Google Scholar]
- 8.Majumdar SR, Soumerai SB. Why most interventions to improve physician prescribing do not seem to work. CMAJ. 2003;169(1):30–1. [PMC free article] [PubMed] [Google Scholar]
- 9.Warriner AH. A Randomized Trial of a Mailed Intervention and Self-Scheduling to Improve Osteoporosis Screening in Postmenopausal Women. J Bone Miner Res. 2012 doi: 10.1002/jbmr.1720. Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Denberg TD, Myers BA, Lin CT, Libby AM, Min SJ, McDermott MT, et al. An outreach intervention increases bone densitometry testing in older women. J Am Geriatr Soc. 2009;57(2):341–7. doi: 10.1111/j.1532-5415.2008.02111.x. [DOI] [PubMed] [Google Scholar]
- 11.Lafata JE, Kolk D, Peterson EL, McCarthy BD, Weiss TW, Chen YT, et al. Improving osteoporosis screening: results from a randomized cluster trial. J Gen Intern Med. 2007;22(3):346–51. doi: 10.1007/s11606-006-0060-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Houston TK, Allison JJ, Sussman M, Horn W, Holt CL, Trobaugh J, et al. Culturally appropriate storytelling to improve blood pressure: a randomized trial. Ann Intern Med. 2011;154(2):77–84. doi: 10.7326/0003-4819-154-2-201101180-00004. [DOI] [PubMed] [Google Scholar]
- 13.Hinyard LJ, Kreuter MW. Using narrative communication as a tool for health behavior change: a conceptual, theoretical, and empirical overview. Health Educ Behav. 2007;34(5):777–92. doi: 10.1177/1090198106291963. [DOI] [PubMed] [Google Scholar]
- 14.Bodenheimer T, Wagner EH, Grumbach K. Improving primary care for patients with chronic illness. Jama. 2002;288(14):1775–9. doi: 10.1001/jama.288.14.1775. [DOI] [PubMed] [Google Scholar]
- 15.Feldstein AC, Glasgow RE. A practical, robust implementation and sustainability model (PRISM) for integrating research findings into practice. Jt Comm J Qual Patient Saf. 2008;34(4):228–43. doi: 10.1016/s1553-7250(08)34030-6. [DOI] [PubMed] [Google Scholar]
- 16.Kreuter MW, Buskirk TD, Holmes K, Clark EM, Robinson L, Si X, et al. What makes cancer survivor stories work? An empirical study among African American women. J Cancer Surviv. 2008;2(1):33–44. doi: 10.1007/s11764-007-0041-y. [DOI] [PubMed] [Google Scholar]
- 17.Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73(1):13–22. [Google Scholar]
- 18.Kanis JA, Melton LJ, 3rd, Christiansen C, Johnston CC, Khaltaev N. The diagnosis of osteoporosis. J Bone Miner Res. 1994;9(8):1137–41. doi: 10.1002/jbmr.5650090802. [DOI] [PubMed] [Google Scholar]
- 19.Fay MP, Graubard BI. Small-sample adjustments for Wald-type tests using sandwich estimators. Biometrics. 2001;57(4):1198–206. doi: 10.1111/j.0006-341x.2001.01198.x. [DOI] [PubMed] [Google Scholar]
- 20.Mancl LA, DeRouen TA. A covariance estimator for GEE with improved small-sample properties. Biometrics. 2001;57(1):126–34. doi: 10.1111/j.0006-341x.2001.00126.x. [DOI] [PubMed] [Google Scholar]
- 21.Pan W, Wall MM. Small-sample adjustments in using the sandwich variance estimator in generalized estimating equations. Stat Med. 2002;21(10):1429–41. doi: 10.1002/sim.1142. [DOI] [PubMed] [Google Scholar]
- 22.Morel JG. Logistic Regression Under Complex Survey Designs. Survey Methodology. 1989;15:203–23. [Google Scholar]
- 23.Morel JG, Bokossa MC, Neerchal NK. Small Sample Correction for the Variance of GEE Estimators. Biometrical Journal. 2003;4:395–409. [Google Scholar]
- 24.Damilakis J, Adams JE, Guglielmi G, Link TM. Radiation exposure in X-ray-based imaging techniques used in osteoporosis. Eur Radiol. 2010;20(11):2707–14. doi: 10.1007/s00330-010-1845-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.2011 Pages http://www.fda.gov/Radiation-EmittingProducts/MammographyQualityStandardsActandProgram/ConsumerInformation/ucm11 3968.htm on May 9 2012.
- 26.Rao VM, Levin DC, Parker L, Frangos AJ. Recent trends in mammography utilization in the Medicare population: is there a cause for concern? J Am Coll Radiol. 2008;5(5):652–6. doi: 10.1016/j.jacr.2008.01.023. [DOI] [PubMed] [Google Scholar]
- 27.Nichol KL, Korn JE, Margolis KL, Poland GA, Petzel RA, Lofgren RP. Achieving the national health objective for influenza immunization: success of an institution-wide vaccination program. Am J Med. 1990;89(2):156–60. doi: 10.1016/0002-9343(90)90293-m. [DOI] [PubMed] [Google Scholar]
- 28.Feldstein AC, Schneider J, Smith DH, Vollmer WM, Rix M, Glauber H, et al. Harnessing stakeholder perspectives to improve the care of osteoporosis after a fracture. Osteoporos Int. 2008;19(11):1527–40. doi: 10.1007/s00198-008-0605-3. [DOI] [PubMed] [Google Scholar]
- 29.Bergeson SC, Dean JD. A systems approach to patient-centered care. Jama. 2006;296(23):2848–51. doi: 10.1001/jama.296.23.2848. [DOI] [PubMed] [Google Scholar]
- 30.Ayoub WT, Newman ED, Blosky MA, Stewart WF, Wood GC. Improving detection and treatment of osteoporosis: redesigning care using the electronic medical record and shared medical appointments. Osteoporos Int. 2009;20(1):37–42. doi: 10.1007/s00198-008-0635-x. [DOI] [PubMed] [Google Scholar]
- 31.Curtis JR, Laster A, Becker DJ, Carbone L, Gary LC, Kilgore ML, et al. The geographic availability and associated utilization of dual-energy X-ray absorptiometry (DXA) testing among older persons in the United States. Osteoporos Int. 2009;20(9):1553–61. doi: 10.1007/s00198-008-0821-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Neuner JM, Zhang X, Sparapani R, Laud PW, Nattinger AB. Racial and socioeconomic disparities in bone density testing before and after hip fracture. J Gen Intern Med. 2007;22(9):1239–45. doi: 10.1007/s11606-007-0217-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mikuls TR, Saag KG, George V, Mudano AS, Banerjee S. Racial disparities in the receipt of osteoporosis related healthcare among community-dwelling older women with arthritis and previous fracture. J Rheumatol. 2005;32(5):870–5. [PubMed] [Google Scholar]
- 34.Mudano AS, Casebeer L, Patino F, Allison JJ, Weissman NW, Kiefe CI, et al. Racial disparities in osteoporosis prevention in a managed care population. South Med J. 2003;96(5):445–51. doi: 10.1097/01.SMJ.0000053918.93363.B0. [DOI] [PubMed] [Google Scholar]
- 35.Stanton AL, Ganz PA, Kwan L, Meyerowitz BE, Bower JE, Krupnick JL, et al. Outcomes from the Moving Beyond Cancer psychoeducational, randomized, controlled trial with breast cancer patients. J Clin Oncol. 2005;23(25):6009–18. doi: 10.1200/JCO.2005.09.101. [DOI] [PubMed] [Google Scholar]
- 36.Rabius V, Pike KJ, Wiatrek D, McAlister AL. Comparing internet assistance for smoking cessation: 13-month follow-up of a six-arm randomized controlled trial. J Med Internet Res. 2008;10(5):e45. doi: 10.2196/jmir.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Houston TK, Cherrington A, Coley HL, Robinson KM, Trobaugh JA, Williams JH, et al. The art and science of patient storytelling-harnessing narrative communication for behavioral interventions: the ACCE project. J Health Commun. 2011;16(7):686–97. doi: 10.1080/10810730.2011.551997. [DOI] [PubMed] [Google Scholar]
- 38.Solomon DH, Finkelstein JS, Polinski JM, Arnold M, Licari A, Cabral D, et al. A randomized controlled trial of mailed osteoporosis education to older adults. Osteoporos Int. 2006;17(5):760–7. doi: 10.1007/s00198-005-0049-y. [DOI] [PubMed] [Google Scholar]
- 39.Fayers PM, Jordhoy MS, Kaasa S. Cluster-randomized trials. Palliat Med. 2002;16(1):69–70. doi: 10.1191/0269216302pm503xx. [DOI] [PubMed] [Google Scholar]
- 40.Hodgson SF, Watts NB, Bilezikian JP. American Association of Clinical Endocrinologists Medical Guidelines for clinical practice for the prevention and treatment of postmenopausal osteoporosis: 2001 edition with selected updates for 2003. Endocr Pract. 2003;9(6):544–64. doi: 10.4158/EP.9.6.544. [DOI] [PubMed] [Google Scholar]
- 41.Force USPST Screening for osteoporosis in postmenopausal women: recommendations and rationale. Ann Intern Med. 2002;137(6):526–8. doi: 10.7326/0003-4819-137-6-200209170-00014. [DOI] [PubMed] [Google Scholar]
- 42.Screening for osteoporosis: U.S. preventive services task force recommendation statement. Ann Intern Med. 2011;154(5):356–64. doi: 10.7326/0003-4819-154-5-201103010-00307. [DOI] [PubMed] [Google Scholar]
- 43.Gourlay ML, Fine JP, Preisser JS, May RC, Li C, Lui LY, et al. Bone-density testing interval and transition to osteoporosis in older women. N Engl J Med. 2012;366(3):225–33. doi: 10.1056/NEJMoa1107142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Whitaker M, Guo J, Kehoe T, Benson G. Bisphosphonates for osteoporosis--where do we go from here? N Engl J Med. 2012;366(22):2048–51. doi: 10.1056/NEJMp1202619. [DOI] [PubMed] [Google Scholar]
- 45.Black DM, Bauer DC, Schwartz AV, Cummings SR, Rosen CJ. Continuing bisphosphonate treatment for osteoporosis--for whom and for how long? N Engl J Med. 2012;366(22):2051–3. doi: 10.1056/NEJMp1202623. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.