Abstract
Background & Aims
Coffee consumption has been associated with decreased risk of liver disease and related outcomes. However, coffee drinking has not been investigated among patients with cholestatic autoimmune liver diseases, primary biliary cirrhosis (PBC), or primary sclerosing cholangitis (PSC). We investigated the relationship between coffee consumption and risk of PBC and PSC in a large North American cohort.
Methods
Lifetime coffee drinking habits were determined from responses to questionnaires from 606 patients with PBC, 480 with PSC, and 564 healthy volunteers (controls). Patients (those with PBC or PSC) were compared to controls utilizing the Wilcoxon rank sum test for continuous variables and c2 method for discrete variables. Logistic regression was used to analyze the estimate the effects of different coffee parameters (time, frequency, and type of coffee consumption) after adjusting for age, sex, smoking status, and education level.
Results
Patients with PBC and controls did not differ in coffee parameters. However, 24% of patients with PSC had never drank coffee compared to 16% of controls (P<.05), and only 67% were current drinkers compared with 77% of controls (P<.05). Patients with PSC also consumed fewer lifetime cups per month (45 vs 47 for controls, P<.05) and spent a smaller percentage of their lifetime of coffee drinking coffee (46.6% vs 66.7% for controls, P<.05). These differences remained significant in a multivariate model. Among PSC patients with concurrent ulcerative colitis, coffee protected against proctocolectomy (hazard ratio=0.34, P<.001).
Conclusions
Coffee consumption is lower among patients with PSC, but not PBC, compared to controls.
Keywords: caffeine, UC, risk factor, cholestasis, biliary inflammation
INTRODUCTION
The robust stimulant qualities of coffee have been enriching human vitality since its incarnation, however only recently have specific health benefits of this brew been reported in the scientific literature. Coffee is now considered as a medically beneficial beverage given inverse associations with metabolic syndrome1, cardiovascular disease2, and weight gain3, despite previous reports.4 A recent study by Freedman et al., revealed a strong inverse dose-dependent relationship between coffee consumption and total and cause specific mortality.5
Coffee attributes extrapolate to chronic liver disease as well; multiple studies over the last 20 years support an association between coffee consumption and decreased risk of liver disease. A sentinel study specific to alcoholic liver disease revealed an inverse relationship between coffee and risk of liver cirrhosis.6 Subsequently, coffee was linked to more favorable liver tests in high-risk patients7, as well as decreased mortality in patients with cirrhosis.8 Recently, improved liver-related outcomes and reduced hepatic fibrosis was seen in viral hepatitis C patients who consumed 2 coffee-cup equivalents per day.9 Lastly, a recent meta-analysis revealed approximately 40% risk reduction for hepatocellular carcinoma among individuals that drink coffee and 50% risk reduction for those that drink coffee heavily compared to non-drinkers.10
Despite population-based associations between coffee and liver disease risk and outcomes, an examination between coffee consumption and cholestatic liver disorders is currently lacking. PBC and PSC are both chronic autoimmune liver diseases characterized by biliary inflammation leading to biliary fibrosis and subsequent cirrhosis, liver transplant, or death.11–13 To date, the pathogenesis of PBC and PSC remains unclear, although both likely involve numerous complex interactions between predisposing genetic alleles and environmental exposures. Prior reports of fibrosis mitigation in other chronic liver disorders led us to hypothesize that coffee consumption exerts a protective role in the development of PBC and PSC. In the current study we aimed to assess patterns of coffee consumption among a cohort of PBC and PSC patients and controls drawn from our own medical center.
METHODS
Study Populations
The Mayo Cholestatic Liver Disease (MCLD) resource (Figure 1), includes PBC and PSC patients obtained from 2 cohorts recruited at our medical center as a part of the (i) Mayo Clinic PBC Genetic Epidemiology (MCPGE) Registry14,15, and (ii) PSC Resource of Genetic Risk, Environment and Synergy Studies (PROGRESS). 16 These resources were established with the aim of elucidating the genetic and environmental contributors to PBC and PSC pathogenesis. PBC and PSC patients met clinically accepted standards of each diagnosis.17 Controls were serially recruited from the Mayo Clinic Division of General Internal Medicine during annual visits for preventive medical examination. Moreover, all controls and cases were tested for anti-mitochondrial antibodies and alkaline phosphatase in serum at the time of enrollment. Control exclusion criteria included evidence of prior or current liver disease. Informed consent was obtained from all the study participants. The present study and included cohorts conform to the ethical guidelines of the 1975 Declaration of Helsinki, and have been approved by the Institutional Review Board of Mayo Clinic.
FIGURE 1.
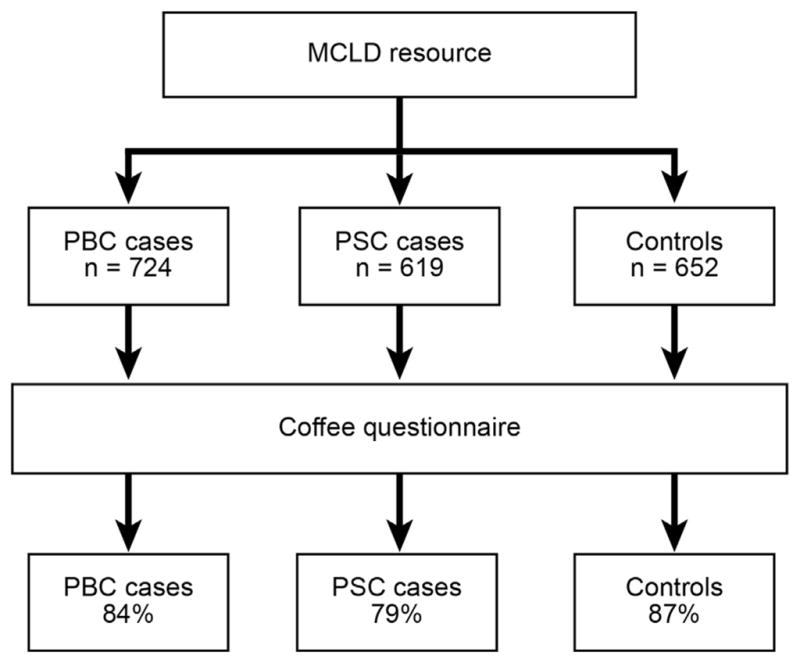
Participants and Coffee Questionnaire Response Rate within the Mayo Cholestatic Liver Disease (MCLD) Resource
Demographic, Medical History, and Lifestyle Assessment
At time of enrollment, cases and controls within the MCLD resource were supplied a study questionnaire directly or through the mail. This study instrument was developed by the Mayo Clinic Survey Center (MCSC) and collected information regarding demographics, medical history, and lifestyle exposures.15 For instance, smoking was assessed, and a positive smoking history was defined as current or past smoking of >= 100 cigarettes during the patient’s lifetime up until diagnosis of PBC or PSC18,19, or in the case of controls, up until completion of the questionnaire. A second questionnaire was mailed within two months of the initial contact in non-respondents.
Coffee Assessment
To assess coffee consumption, patients within the MCLD resource were mailed a two page, scannable questionnaire that included a prepaid return envelope. This study tool, developed with the assistance of the MCSC, was composed of ten questions highlighting current (i.e., within the past one year) and past (i.e., lifetime) coffee drinking habits. Coffee consumption was assessed using eleven frequency categories, ranging from less than one cup per month to more than 9 cups per day. The questionnaire also obtained detailed information regarding coffee start and quit ages, if available, allowing assessment of average lifetime cups of coffee per month and percentage of lifetime consuming at least one cup of coffee per month. The total reported years consuming coffee and reported cup frequencies were used to calculate “average lifetime cups of coffee”. “Percentage of lifetime consuming coffee” was calculated using age and reported duration of drinking at least one cup of coffee per month. “Lifetime coffee status” was classified by 3 separate categories: current, past, and never a coffee drinker. Parameters of coffee consumption were completed by totaling caffeinated and decaffeinated data. Participants that did not respond to the first mailing within six weeks were sent another coffee questionnaire.
Statistical Analysis
PBC or PSC patients were compared individually to a group of subjects (i.e., controls) without evidence of PBC or PSC. Continuous variables were summarized using medians and the 25th and 75th percentiles and P-values were obtained using the Wilcoxon rank sum test. P-values for discrete variables were obtained from the chi-square test. Logistic regression was used to estimate the influence of the various coffee parameters either univariately or after adjusting for age at coffee survey, sex, smoking status, and higher education. Coffee consumption was calculated using the age of initial coffee consumption and was modeled as a time-dependent covariate; only those subjects who were either non-drinkers or who included a start age were included in this analysis. Cox regression analysis was completed for pertinent clinical endpoints after adjusting for age and sex. Patients who had clinical records at Mayo Clinic were followed from diagnosis to the last clinical review of the medical charts.
RESULTS
Demographic, Medical History, and Lifestyle
At the time of study inception, the MCLD resource was composed of 724 PBC and 619 PSC patients, as well as 652 controls. Within 3 months of the initial coffee questionnaire distribution, the overall response rate was 84% for PBC, 79% for PSC, and 87% for controls (Figure 1). The time between enrollment and coffee survey was 5.9 years in PBC and 3.3 years in PSC patients. Responding PBC patients and controls were similar in age, yet median age in PSC patients was lower (Table 1). Both cohorts differed from the controls in terms of sex. Fewer PBC patients reported education beyond the high school level compared to controls, whereas PSC patients were similar to controls. Significantly more PBC and fewer PSC patients reported a history of smoking than controls. Inflammatory bowel disease (IBD) was not prevalent in PBC patients or controls, however 74% of PSC patients reported concurrent diagnosis of IBD. 55% of this group reported a diagnosis of Ulcerative Colitis (UC), 10% reported Crohn’s disease (CD) and 9% reported both.
TABLE 1.
Demographics and Lifestyle Assessment of PBC patients, PSC patients and Controls in the MCLD Resource
| PBC Patients (n=606) | PSC Patients (n=480) | Controls (n=564) | |
|---|---|---|---|
| Sex, % female | 542 (90%)** | 173 (36%)** | 414 (74)% |
| Age at coffee survey* | 66 (60.0, 72.0) | 55 (43.0, 65.0)** | 66 (59.0, 73.0) |
| Diagnosis of IBD, % diagnosis | 13 (3%) | 354 (74%)** | 7 (1%) |
| Higher Education, % beyond high school | 426 (70%)** | 402 (84%) | 453 (80%) |
| >100 lifetime cigarettes, n (%) | 330 (55%)** | 124 (26%)** | 230 (41%) |
Variable summarized as median (1st quartile, 3rd quartile)
Identifies P-values of < 0.05 for comparison of each patient group (PBC or PSC) versus controls
Coffee Consumption
PBC and PSC patients and controls were assessed for “lifetime coffee status”, defined as current, past, or never a coffee drinker. PBC patients and controls did not differ according to this parameter; however, 24% of PSC patients reporting reported to be never a coffee drinker compared to 16% of controls (Table 2). Similarly, fewer PSC patients than controls reported being a coffee drinker by age 18, although this finding was not statistically different. Assessment of patients and controls according to coffee drinking adjusted over lifetime included the average lifetime cups of coffee consumed per month and total percentage of lifetime drinking coffee (at least 1 cup/month). As shown in Table 2, PBC patients and controls were very similar in regards to coffee drinking adjusted over lifetime including average lifetime cups of coffee consumed per month and total percentage of lifetime drinking coffee (at least 1 cup/month). Even after adjustment for sex, a history of higher education, significant smoking exposure, body mass index (BMI), daily activity level, and duration of time since completing the MCPGE Registry survey, no differences existed between PBC patients and controls (data not shown). However, PSC patients were found to have consumed fewer cups of coffee per month (45 vs. 47, P < 0.05) and spent less total percentage of lifetime consuming coffee compared to controls (46.6% vs. 66.7%, P < 0.05). Observed differences among PSC patients and controls remained significantly different in models that included sex, a history of higher education, significant smoking exposure, BMI, daily activity level, and duration of time since completing the PROGRESS survey (data not shown).
TABLE 2.
Coffee Consumption Assessment of PBC patients, PSC patients, and Controls in the MCLD Resource
| PBC Patients (n=606) | PSC Patients (n=480) | Controls (n=564) | |
|---|---|---|---|
|
| |||
| % of life drinking coffee* | 66.7 (34.6, 74.6) | 46.6 (1.4, 66.2)** | 66.7 (34.3, 73.9) |
|
| |||
| Coffee drinker at age 18, n (%) | 225 (37%) | 151 (32%) | 203 (36%) |
|
| |||
| Lifetime cups of coffee/month (caffeinated and decaffeinated)* | 47 (45.0, 105.0) | 45 (4.0, 90.0)** | 47 (45.0,105.5) |
|
| |||
| Lifetime coffee status | ** | ||
| Never coffee drinker, n (%) | 96 (16%) | 116 (24%) | 88 (16%) |
| Past coffee drinker, n (%) | 44 (7%) | 44 (9%) | 41 (7%) |
| Current coffee drinker, n (%) | 466 (77%) | 320 (67%) | 434 (77%) |
Variable summarized as median (1st quartile, 3rd quartile)
Identifies P-values of < 0.05 for comparison of each patient group (PBC or PSC) versus controls
Logistic regression models were used to assess coffee parameters while controlling for age, sex, higher education, and smoking status in PSC patients and controls given observed differences in coffee consumption and demographics (Table 4). After adjustment, the odds of PSC was statistically lower for individuals identified as current coffee drinker vs. never a coffee drinker (OR = 0.68, CI: 0.47–0.99, P = 0.044). Also, individuals with an increase in percentage of lifetime drinking coffee (OR = 0.88 per 20 percentage increase, CI: 0.79–0.97, P = 0.013) and more average cups of coffee per month (OR = 0.89 per 20 cup increase, CI: 0.83–0.94, P < 0.001) had statistically lower odds of PSC.
TABLE 4.
Logistic Regression Analysis with and without Adjustment for Patient Characteristics: Age, Sex, Higher Education, and Smoking Exposure.
| Odds Ratios (95% Confidence Interval) unadjusted | Odds Ratios (95% Confidence Interval) after adjustment * | |||
|---|---|---|---|---|
|
| ||||
| Risk Factor | PSC Patients vs. Controls | PBC Patients vs. Controls | PSC Patients vs. Controls | PBC Patients vs. Controls |
|
| ||||
| % of life drinking coffee, per increase of 20% | 0.73 (0.67, 0.80) | 1.00 (0.92, 1.08) | 0.88 (0.79, 0.97) | 0.98 (0.90, 1.06) |
|
| ||||
| Coffee drinker at age 18 | 0.82 (0.63, 1.06) | 1.05 (0.83, 1.34) | 0.88 (0.64, 1.20) | 0.98 (0.76, 1.26) |
|
| ||||
| Lifetime cups of coffee/month (caffeinated and decaffeinated), per increase of 20 cups | 0.83 (0.79, 0.88) | 1.00 (0.97, 1.05) | 0.89 (0.83, 0.94) | 0.99 (0.95, 1.03) |
|
| ||||
| Current coffee status | ||||
| - Past coffee drinker versus Never | 0.81 (0.49, 1.35) | 0.98 (0.59, 1.65) | 1.05 (0.58, 1.92) | 1.03 (0.60, 1.77) |
| - Current coffee drinker versus Never | 0.56 (0.41, 0.77) | 0.98 (0.72, 1.35) | 0.68 (0.47, 0.99) | 0.94 (0.67, 1.31) |
Each of the shown risk factors is from a separate logistic regression model.
The adjusted models show the Odds Ratios for each risk factor after adjusting for survey age, sex higher education and significant smoking exposure.
PSC, Inflammatory Bowel Disease, and Lifetime Coffee Status
We performed additional analyses to clarify the contribution of concurrent IBD on the observed effect of decreased coffee consumption in PSC. At the time of enrollment, 74% of PSC patients had IBD with self-reported diagnosis of either UC (PSC-UC) or CD (PSC-CD). Among PSC-UC patients, there were a total of 95 (60%) proctocolectomies (prior to coffee survey). Further analysis of these groups according to age at onset and proctocolectomy per lifetime coffee drinking status was completed (Table 3). There were no differences between median age of disease onset (PSC, PSC-UC, or PSC-CD) or proctocolectomy according to lifetime coffee drinking groups. However, 63% of PSC-UC and 55% of PSC-CD patients reported current coffee drinking at the time of survey completion. Only 27% of PSC-UC patients and 31% of PSC-CD patients reported never drinking coffee in their lifetime. Cessation of coffee in the past for any reason included few patients within each group (10% PSC-UC and 14% PSC-CD). Interestingly, 55% of PSC-UC patients with proctocolectomy were current drinkers of coffee, whereas 44% never consumed coffee.
TABLE 3.
PSC and Concurrent Diagnosis of IBD According to Lifetime Coffee Status: Never, Past, and Current Coffee Drinking
| Never a Coffee Drinker | Past Coffee Drinker | Current Coffee Drinker | ||||
|---|---|---|---|---|---|---|
| n | Age* | n | Age* | n | Age* | |
|
| ||||||
| PSC (n =474) | 114 (24%) | 40 (31, 50) | 43 (9%) | 46 (35, 55) | 317 (67%) | 43 (33, 53) |
|
| ||||||
| Concurrent IBD | ||||||
| PSC-UC (n = 303) | 81 (27%) | 39 (29, 49) | 30 (10%) | 45 (35, 55) | 192 (63%) | 41 (30, 51) |
| PSC-CD (n = 91) | 28 (31%) | 44 (36, 58) | 13 (14%) | 53 (33, 55) | 50 (55%) | 46 (32, 55) |
| No Concurrent IBD (n=125) | 19 (15%) | 40 (33, 57) | 9 (7%) | 47 (37, 55) | 97 (78%) | 46 (37, 54) |
|
| ||||||
| Proctocolectomy for PSC-UC (n = 146) | 43 (29%) | 38 (28, 51) | 9 (6%) | 55 (46, 61) | 94 (64%) | 40 (30, 53) |
Age at PSC diagnosis summarized as median in years (1st quartile, 3rd quartile). The ages were not statistically different among the coffee status groups (P > 0.10) for any of the subsets of the PSC cohort.
Cox Regression
Cox regression analysis was completed for outcomes among PSC patients including liver transplant, cholangiocarcinoma (CCA), and proctocolectomy adjusting for diagnosis age and sex. At the time of PSC diagnosis, 47 (10%) patients had proctocolectomy, this was not associated with coffee exposure at the time of PSC diagnosis (P = 0.33). There were 45 PSC patients who underwent a proctocolectomy following diagnosis, coffee consumption was associated with a decreased risk of surgery (HR = 0.34, CI = 0.19–0.62, P < 0.001). There was no significant predictive relationship of coffee exposure with liver transplant (74 patients, HR = 0.91, CI = 0.55–1.53) or with CCA (33 patients, HR = 0.64, CI = 0.31–1.34). Coffee consumption at diagnosis was present in approximately 65% of patients, therefore there was 80% power to detect hazards ratios of 0.41 (endpoint of proctocolectomy), 0.51 (liver transplant), and 0.36 (CCA).
DISCUSSION
This large, observational study of coffee consumption in both PBC and PSC patients from North America demonstrated less coffee consumption among individuals with PSC, but not PBC. Specifically, PSC patients were more likely to have never consumed coffee in their lifetime as compared to controls. Assessment of other coffee drinking behavior revealed fewer lifetime cups of coffee per month and less percentage of life spent consuming at least 1 cup of coffee per month in PSC patients compared to controls, but not in PBC patients. The observation of reduced coffee consumption in PSC remained statistically significant after adjusting for age, sex, higher education and significant history of smoking.
Our study represents the first quantitative assessment of coffee drinking among a large cohort of both PBC and PSC patients. PBC and PSC are rare, however they both may result in significant liver-related morbidities11–13 and no detailed assessment of coffee use has been completed. PBC and PSC pathogenesis is unclear, yet both are likely the result of complex interactions between genes and environment. Identification of modifiable exposures affecting disease risk is enticing for obvious clinical implications, but also disparity between PBC and PSC among risk exposures may provide insight into potential cellular mechanisms in disease pathogenesis.
The observed finding of reduced coffee consumption among PSC patients within our study may represent effective attenuation of genetic or environmental risks, immune system activation, or progression of fibrosis. Recently, a study of nongenetic risk factors among a modestly sized Norwegian PSC cohort revealed similar findings.20 Despite only assessing patients with PSC, the negative association between disease and coffee consumption in this cohort was driven specifically by findings in male patients. Nonetheless, in our study it remains remarkable that the observed association between PSC and coffee was not apparent in those with PBC. A potential explanation may be elucidated by the seemingly important intricacies of the gut-liver axis present in PSC. This hypothesized mechanism underscoring PSC pathogenesis has renewed interest given advancement in the characterization of bowel microbiota via genomic technology21 A possible genetic constituent underscoring the importance of intestinal microbiota in PSC was recently observed in a large PSC genome-wide association study.22 Polymorphisms strongly associated with PSC located within the gene FUT2, a protein important in protein glycosylation, were particularly interesting given a prior locus association with CD and other infectious diseases such as streptococcus23 and candida.24 Coffee has also been recently shown to have significant influence at the level of bowel flora, as reported by Nakayama.25 Coffee fed mice displayed altered galacto-oligosaccharide consumption by gut bacteria as well altered populations of E. coli, Clostridium, and Bifidiobacterium species. Furthermore, the importance of the gut-liver axis in PSC pathogenesis is also supported by the evidence of decreased hazard of proctocolectomy in PSC-UC patients that were identified as previous or current coffee drinkers.
PSC has strong HLA associations and shared genetic risk loci with other autoimmune disease, suggesting an underlying inflammatory dysregulation as a component of disease pathogenesis.22 Polyphenols, present at high concentrations in coffee, are bioactive substances associated with potent anti-oxidant activity in experimental models.9 These compounds exert an effect through signaling cascades that can modulate gene expression and promote anti-inflammatory mediators.26 Thus, coffee consumption may exert balance to unregulated immune activation in PSC via alteration of gene expression and down regulation of inflammation pathways. Possible cellular mediators were recently highlighted by Vitaglione.27 A reduction in hepatic concentrations of pro-inflammatory molecules TNF-α and interferon-γ with concurrently increased anti-inflammatory mediators interleukin-4 and interleukin-10 were seen in mice fed the equivalent of 2 cups of filtered coffee daily.
Coffee consumption and smoking habits have been shown to be highly correlative28, and prior studies have identified a significant smoking history associated with increased risk of PBC15 yet a decreased risk in PSC.29 Our data support these prior findings (Table 1), and we show that the effects of coffee remained independent after adjusting for smoking. No evidence of an association between coffee consumption and PBC is particularly interesting given the lack of concurrent IBD association. However, the clinical nature of IBD and stimulant effects of coffee could integrate as a potential confounder in the observed study effect. Recently, Cohen et al.30, published findings of significant dietary perceptions of patients with IBD. Soda, red meat, leafy and non-leafy vegetables, dairy products, fruit, fried foods, and alcohol were all more frequently reported as worsening symptoms of UC compared to coffee. In this study, less than 5% of patients with UC and proctocolectomy reported any change of bowel symptoms with coffee.
Nonetheless, we assessed for modified patient behavior in patients with past coffee consumption without being a current coffee drinker. We saw that PBC (7%), PSC (9%) patients and controls (7%) had similar numbers of past coffee drinkers. In a sub-analysis (Table 3), past coffee drinkers were similar within the PSC-UC (9%), PSC-CD (13%), and PSC-UC with proctocolectomy (6%) groups as well. In order to assess for confounding from altered gastrointestinal transit, a multivariable model was completed that included age, sex, higher education and significant smoking history among patients without a history of proctocolectomy. This adjusted model revealed that the odds of PSC were statistically lower for patients that drank more average cups of coffee per month (OR = 0.89, CI: 0.83–0.94, P < 0.001). However, a longitudinal study is warranted to further address this issue.
Despite including a large group of PBC and PSC patients as well as controls, the MCLD resource still has potential limitations. For example, patient self-reporting of coffee consumption history has conceivable memory bias and the survey term “coffee” represents a variable product. We were unable to assess for brands, preparations, and additives. Measurement variability also exists in “what defines a cup of coffee” as well as other product consumption containing important bioactive substances. Given the rarity of PSC, overcoming these limitations by utilizing a prospective design would be challenging.
In conclusion, study of coffee in the MCLD resource revealed decreased coffee consumption among patients with PSC, but not PBC. Our results demonstrate that PSC patients were more likely to have never consumed coffee in their lifetime, spent less percentage of their life consuming coffee and drank fewer average cups of coffee per month compared to controls. Despite classification of PBC and PSC as autoimmune cholestatic liver disorders, coffee consumption represents another key disparity between these diseases. Nonetheless, the benefit of coffee in PSC is congruent with other chronic liver disorders, therefore, prospective study of liver-related outcomes and survival in PSC should be completed.
Acknowledgments
Grant Support: This research was supported by grants to Dr. Lazaridis from the NIH (RO1 DK80670 and DK84960) and A. J. and Sigismunda Palumbo Charitable Trust. Support was also provided to Dr. Lammert from the American Liver Foundation.
Abbreviations
- PBC
Primary Biliary Cirrhosis
- PSC
Primary Sclerosing Cholangitis
- MCLD
Mayo Cholestatic Liver Disease
- MCPGE Registry
Mayo Clinic PBC Genetic Epidemiology Registry
- PROGRESS
PSC Resource of Genetic Risk, Environment and Synergy Studies
- UC
Ulcerative Colitis
- MCSC
Mayo Clinic Survey Center
- IBD
Inflammatory Bowel Disease
- CD
Crohn’s disease
- PSC-UC
Concurrent PSC and UC
- PSC-CD
Concurrent PSC and CD
- HR
Hazard Ratio
- CCA
Cholangiocarcinoma
Footnotes
Author Contributions: Craig Lammert: assembly/interpretation of data, drafting and revision of manuscript; Brian D. Juran: critical revision of manuscript, data interpretation; Erik Schlicht: collection and assembly of data; Xiao Xie: statistical analysis, assembly of data; Elizabeth J. Atkinson: statistical analysis, critical revision of statistics and results; Mariza de Andrade: critical revision of statistics and results; Konstantinos N. Lazaridis: Conception of study and design, collection and assembly of data, drafting and approval of final manuscript version.
Conflict of Interest: All included authors have no conflicts to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Matsuura H, Mure K, Nishio N, Kitano N, Nagai N, Takeshita T. Relationship between coffee consumption and prevalence of metabolic syndrome among Japanese civil servants. J Epidemiol. 2012;22(2):160–6. doi: 10.2188/jea.JE20110068. Epub 2012/02/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andersen LF, Jacobs DR, Jr, Carlsen MH, Blomhoff R. Consumption of coffee is associated with reduced risk of death attributed to inflammatory and cardiovascular diseases in the Iowa Women’s Health Study. Am J Clin Nutr. 2006;83(5):1039–46. doi: 10.1093/ajcn/83.5.1039. Epub 2006/05/11. [DOI] [PubMed] [Google Scholar]
- 3.Pan A, Malik VS, Hao T, Willett WC, Mozaffarian D, Hu FB. Changes in water and beverage intake and long-term weight changes: results from three prospective cohort studies. Int J Obes (Lond) 2013 doi: 10.1038/ijo.2012.225. Epub 2013/01/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Noordzij M, Uiterwaal CS, Arends LR, Kok FJ, Grobbee DE, Geleijnse JM. Blood pressure response to chronic intake of coffee and caffeine: a meta-analysis of randomized controlled trials. J Hypertens. 2005;23(5):921–8. doi: 10.1097/01.hjh.0000166828.94699.1d. Epub 2005/04/19. [DOI] [PubMed] [Google Scholar]
- 5.Freedman ND, Park Y, Abnet CC, Hollenbeck AR, Sinha R. Association of coffee drinking with total and cause-specific mortality. N Engl J Med. 2012;366(20):1891–904. doi: 10.1056/NEJMoa1112010. Epub 2012/05/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klatsky AL, Armstrong MA. Alcohol, smoking, coffee, and cirrhosis. Am J Epidemiol. 1992;136(10):1248–57. doi: 10.1093/oxfordjournals.aje.a116433. Epub 1992/11/15. [DOI] [PubMed] [Google Scholar]
- 7.Ruhl CE, Everhart JE. Coffee and caffeine consumption reduce the risk of elevated serum alanine aminotransferase activity in the United States. Gastroenterology. 2005;128(1):24–32. doi: 10.1053/j.gastro.2004.09.075. Epub 2005/01/06. [DOI] [PubMed] [Google Scholar]
- 8.Tverdal A, Skurtveit S. Coffee intake and mortality from liver cirrhosis. Ann Epidemiol. 2003;13(6):419–23. doi: 10.1016/s1047-2797(02)00462-3. Epub 2003/07/24. [DOI] [PubMed] [Google Scholar]
- 9.Modi AA, Feld JJ, Park Y, Kleiner DE, Everhart JE, Liang TJ, et al. Increased caffeine consumption is associated with reduced hepatic fibrosis. Hepatology. 2010;51(1):201–9. doi: 10.1002/hep.23279. Epub 2009/12/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bravi F, Bosetti C, Tavani A, Gallus S, La Vecchia C. Coffee reduces risk for hepatocellular carcinoma: an updated meta-analysis. Clin Gastroenterol Hepatol. 2013;11(11):1413–21. e1. doi: 10.1016/j.cgh.2013.04.039. Epub 2013/05/11. [DOI] [PubMed] [Google Scholar]
- 11.Kaplan MM, Gershwin ME. Primary biliary cirrhosis. N Engl J Med. 2005;353(12):1261–73. doi: 10.1056/NEJMra043898. Epub 2005/09/24. [DOI] [PubMed] [Google Scholar]
- 12.Broome U, Olsson R, Loof L, Bodemar G, Hultcrantz R, Danielsson A, et al. Natural history and prognostic factors in 305 Swedish patients with primary sclerosing cholangitis. Gut. 1996;38(4):610–5. doi: 10.1136/gut.38.4.610. Epub 1996/04/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Farrant JM, Hayllar KM, Wilkinson ML, Karani J, Portmann BC, Westaby D, et al. Natural history and prognostic variables in primary sclerosing cholangitis. Gastroenterology. 1991;100(6):1710–7. doi: 10.1016/0016-5085(91)90673-9. Epub 1991/06/01. [DOI] [PubMed] [Google Scholar]
- 14.Juran BD, Hirschfield GM, Invernizzi P, Atkinson EJ, Li Y, Xie G, et al. Immunochip analyses identify a novel risk locus for primary biliary cirrhosis at 13q14, multiple independent associations at four established risk loci and epistasis between 1p31 and 7q32 risk variants. Hum Mol Genet. 2012;21(23):5209–21. doi: 10.1093/hmg/dds359. Epub 2012/09/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lammert C, Nguyen DL, Juran BD, Schlicht E, Larson JJ, Atkinson EJ, et al. Questionnaire based assessment of risk factors for primary biliary cirrhosis. Dig Liver Dis. 2013;45(7):589–94. doi: 10.1016/j.dld.2013.01.028. Epub 2013/03/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Juran BD, Atkinson EJ, Schlicht EM, Larson JJ, Ellinghaus D, Franke A, et al. Genetic polymorphisms of matrix metalloproteinase 3 in primary sclerosing cholangitis. Liver Int. 2011;31(6):785–91. doi: 10.1111/j.1478-3231.2010.02420.x. Epub 2010/12/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lindor KD, Gershwin ME, Poupon R, Kaplan M, Bergasa NV, Heathcote EJ. Primary biliary cirrhosis. Hepatology. 2009;50(1):291–308. doi: 10.1002/hep.22906. Epub 2009/06/26. [DOI] [PubMed] [Google Scholar]
- 18.Zein CO, Beatty K, Post AB, Logan L, Debanne S, McCullough AJ. Smoking and increased severity of hepatic fibrosis in primary biliary cirrhosis: A cross validated retrospective assessment. Hepatology. 2006;44(6):1564–71. doi: 10.1002/hep.21423. Epub 2006/11/30. [DOI] [PubMed] [Google Scholar]
- 19.Cigarette smoking among adults--United States, 2004. MMWR Morb Mortal Wkly Rep. 2005;54(44):1121–4. Epub 2005/11/11. [PubMed] [Google Scholar]
- 20.Andersen IM, Tengesdal G, Lie BA, Boberg KM, Karlsen TH, Hov JR. Effects of Coffee Consumption, Smoking, and Hormones on Risk for Primary Sclerosing Cholangitis. Clin Gastroenterol Hepatol. 2013 doi: 10.1016/j.cgh.2013.09.024. Epub 2013/10/01. [DOI] [PubMed] [Google Scholar]
- 21.Weinstock GM. Genomic approaches to studying the human microbiota. Nature. 2012;489(7415):250–6. doi: 10.1038/nature11553. Epub 2012/09/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu JZ, Hov JR, Folseraas T, Ellinghaus E, Rushbrook SM, Doncheva NT, et al. Dense genotyping of immune-related disease regions identifies nine new risk loci for primary sclerosing cholangitis. Nat Genet. 2013;45(6):670–5. doi: 10.1038/ng.2616. Epub 2013/04/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blackwell CC, Jonsdottir K, Hanson M, Todd WT, Chaudhuri AK, Mathew B, et al. Non-secretion of ABO antigens predisposing to infection by Neisseria meningitidis and Streptococcus pneumoniae. Lancet. 1986;2(8501):284–5. doi: 10.1016/s0140-6736(86)92103-3. Epub 1986/08/02. [DOI] [PubMed] [Google Scholar]
- 24.Thom SM, Blackwell CC, MacCallum CJ, Weir DM, Brettle RP, Kinane DF, et al. Non-secretion of blood group antigens and susceptibility to infection by Candida species. FEMS Microbiol Immunol. 1989;1(6–7):401–5. doi: 10.1111/j.1574-6968.1989.tb02428.x. Epub 1989/06/01. [DOI] [PubMed] [Google Scholar]
- 25.Nakayama T, Oishi K. Influence of coffee (Coffea arabica) and galacto-oligosaccharide consumption on intestinal microbiota and the host responses. FEMS Microbiol Lett. 2013;343(2):161–8. doi: 10.1111/1574-6968.12142. Epub 2013/04/05. [DOI] [PubMed] [Google Scholar]
- 26.Tangney CC, Rasmussen HE. Polyphenols, inflammation, and cardiovascular disease. Curr Atheroscler Rep. 2013;15(5):324. doi: 10.1007/s11883-013-0324-x. Epub 2013/03/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vitaglione P, Morisco F, Mazzone G, Amoruso DC, Ribecco MT, Romano A, et al. Coffee reduces liver damage in a rat model of steatohepatitis: the underlying mechanisms and the role of polyphenols and melanoidins. Hepatology. 2010;52(5):1652–61. doi: 10.1002/hep.23902. Epub 2010/11/03. [DOI] [PubMed] [Google Scholar]
- 28.Swanson JA, Lee JW, Hopp JW. Caffeine and nicotine: a review of their joint use and possible interactive effects in tobacco withdrawal. Addict Behav. 1994;19(3):229–56. doi: 10.1016/0306-4603(94)90027-2. Epub 1994/05/01. [DOI] [PubMed] [Google Scholar]
- 29.Loftus EV, Jr, Sandborn WJ, Tremaine WJ, Mahoney DW, Zinsmeister AR, Offord KP, et al. Primary sclerosing cholangitis is associated with nonsmoking: a case-control study. Gastroenterology. 1996;110(5):1496–502. doi: 10.1053/gast.1996.v110.pm8613055. Epub 1996/05/01. [DOI] [PubMed] [Google Scholar]
- 30.Cohen AB, Lee D, Long MD, Kappelman MD, Martin CF, Sandler RS, et al. Dietary patterns and self-reported associations of diet with symptoms of inflammatory bowel disease. Dig Dis Sci. 2013;58(5):1322–8. doi: 10.1007/s10620-012-2373-3. Epub 2012/08/28. [DOI] [PMC free article] [PubMed] [Google Scholar]


