Abstract
Objectives
To report outcomes following yttrium-90 microsphere brachytherapy for unresectable liver metastases from uveal melanoma and to evaluate factors predictive for overall survival (OS) and hepatic progression free survival (PFS).
Methods
Seventy-one patients were consecutively treated with microsphere brachytherapy for unresectable liver metastases from uveal melanoma between 2007 and 2012. Clinical, radiographic and positron emission tomography-derived, functional tumor parameters were evaluated by log-rank test in univariate analysis and backwards stepwise multivariate Cox proportional hazards regression. OS and hepatic PFS were estimated by Kaplan-Meier analysis.
Results
134 procedures were performed in 71 patients with median age of 63 years (range, 23–91). Fifty-eight patients (82%) received microsphere brachytherapy as a salvage therapy. Median hepatic PFS and OS following microsphere brachytherapy were 5.9 mos. (range, 1.3–19.1) and 12.3 mos. (range, 1.9–49.3), respectively. Median OS times following diagnosis of liver metastases was 23.9 mos. (range, 6.2–69.0). In univariate analysis, female sex, pre-treatment metabolic tumor volume (MTV) and total glycolic activity (TGA) were significantly correlated with hepatic PFS and OS. In multivariate analysis, female sex and TGA retained significance as independent predictors of hepatic PFS and OS. A low pre-treatment TGA (<225 g) was associated with a significantly longer median OS than was a TGA ≥225 g (17.2 vs. 9.7 mos., p=0.01)
Conclusions
90Y microsphere brachytherapy provided favorable survival times in patients with unresectable liver metastases from uveal melanoma. MTV and TGA are predictive functional tumor parameters, which may aid patient selection and risk stratification.
Keywords: Yttrium, uveal, melanoma, microsphere, brachytherapy
INTRODUCTION
Uveal melanoma is the most common primary intraocular malignant tumor among adults and represents 3% of all melanoma cases [1]. Despite treatment of the primary tumor, half of patients will develop systemic metastasis [2]. Interestingly, the liver is the first site of metastatic disease in 75–90% of patients and, ultimately, half of these patients will never develop metastatic disease beyond the liver [3, 4]. Without aggressive treatment, survival following the discovery of liver metastases ranges only 3–12 months and, unfortunately, mortality rates have not changed significantly over the past three decades [1, 4–9].
Given that the liver is the predominant organ of involvement in most patients, liver-directed therapies are paramount in the management of metastatic uveal melanoma. In fact, the reported response rates and survival times following local, liver-directed therapies are superior to those following systemic chemotherapy, which appears to have limited efficacy [4, 6, 10–12]. Thus, first line therapies for liver metastases from uveal melanoma generally include: surgical resection, transarterial chemoembolization (TACE), immunoembolization and isolated hepatic perfusion [10–16]. In many patients, surgical resection of liver metastases is not feasible due to the multiplicity of lesions or multi-segmental involvement so, catheter-directed liver therapies are frequently employed. Despite these treatment options, most patients will develop progression of their liver metastases following their first intervention.
Liver brachytherapy with yttrium-90 (90Y) microspheres, also known as selective internal radiotherapy or radioembolization, has emerged as a valuable therapeutic option for patients with liver tumors. Recently, 90Y microsphere brachytherapy has been demonstrated to be a safe and effective salvage therapy for patients with metastatic uveal melanoma who progress after standard therapies [17, 18]. Few institutions have reported their experience with microsphere brachytherapy for treatment of liver metastases from uveal melanoma. Reported local control and median survival times following treatment range from 62–77% and 7–10 months, respectively [17–21].
Compared with purely morphological imaging modalities such as computed tomography (CT) and magnetic resonance imaging (MRI), fluorodeoxyglucose positron emission tomography (FDG PET) provides the distinctive ability to characterize tumor metabolism with anatomic correlation from a co-registered CT scan (PET/CT). Recently, data have emerged to propose that PET-derived functional tumor parameters predict survival after 90Y liver brachytherapy in patients with metastases from colorectal cancer, breast cancer, cholangiocarcinoma, and melanoma from mixed cutaneous and ocular origin [21–24]. However, the predictive value of functional tumor parameters like maximum standard uptake value (SUVmax), metabolic tumor volume (MTV) and total glycolic activity (TGA) in patients with liver metastases from primary uveal melanoma is not understood. This study presents an update of our institution’s experience with microsphere brachytherapy in patients with liver metastases from uveal melanoma and has a secondary aim to evaluate whether PET-derived functional tumor parameters have predictive value for hepatic PFS and OS [17]. This report represents the largest reported series of patients treated with 90Y liver brachytherapy for metastatic uveal melanoma.
METHODS
Patients and 90Y Microsphere Brachytherapy
Following IRB approval, we reviewed the medical records of patients with biopsy-confirmed liver metastases from uveal melanoma that were consecutively treated with 90Y resin microsphere brachytherapy. 90Y resin microspheres (SIR-Spheres; SIRTEX Medical, Sydney, Australia) have been approved by the Food and Drug Administration for the treatment of unresectable, metastatic liver tumors from colorectal cancer and were used in an off-label fashion. Nine patients were treated on a clinical trial.
A multidisciplinary team selected patients eligible for this treatment including: radiation oncology, interventional radiology, and medical oncology. This treatment was frequently offered as a salvage therapy for progressive disease following immunoembolization or TACE [10, 14]. Eligibility criteria have previously been described and included patients with liver-dominant, unresectable disease, and typically with less than 50% liver tumor burden [17, 25]. Standard exclusion criteria for this procedure have previously been described and were used during patient selection [17, 25]. Patients eligible for this therapy had adequate liver (bilirubin<1.8 mg/dL, albumin >3.0 g/dL, no ascites) and renal function (creatinine≤ 2.0 mg/dL). Patients who had received previous radiation therapy to the liver were excluded; however, re-treatment with microspheres was permitted in select cases.
Preparatory three-phase, contrast-enhanced CT, MRI and PET/CT were performed to assess tumor volume. Pretreatment arteriogram, embolization of non-target, extra-hepatic vessels, and prescription of 90Y resin microsphere activity using the body surface area (BSA) method have previously been described [17]. A dose reduction of 25% was applied to patients who had received prior catheter-directed liver therapies. 99mTc-macroaggregated albumin single-photon emission CT (SPECT) was also performed to determine lung shunt fraction, tumor-to-normal liver uptake ratio, and subsequently calculate nominal dose to the tumor, lung, and liver. Depending on the anatomic distribution of tumor, patients received either whole liver or lobar delivery of microspheres. Additionally, whole liver therapy performed in a staged fashion with sequential embolization of individual lobes was permitted and has been described in the literature [25].
Assessment of Treatment Response
Clinical follow up included comprehensive history and physical examinations, toxicity evaluation, and laboratory studies one month after treatment and then every three months that followed. The degree of toxicity was determined with the Common Terminology Criteria for Adverse Events (CTCAE). PET/CT, CT and MRI were also performed at these time intervals and were assessed by nuclear medicine and radiology specialists. Treatment response with anatomic imaging was assessed using Response Evaluation Criteria in Solid Tumors (RECIST), assessing up to five tumor foci [26]. Treatment response was also assessed in up to five lesions on PET/CT, where available, because standard criteria may be insensitive when assessing response to interventional treatments, including 90Y radioembolization [26–29]. Response assessment criteria of PET/CT continue to evolve in the literature with no current standard. In this study, response assessment criteria for PET/CT were similar to those used in prior studies: patients had a partial response if >25% decrease in SUVmax at three months following treatment, complete response if there was normalization of SUVmax and progressive disease if >25% increase in SUVmax [23, 24].
Measurement of Functional Tumor Parameters
Following administration of 13–17 mCi of 18F-FDG, whole body PET/CT was obtained 75 minutes after injection using a Biograph 6 (Siemens Medical Solutions, Knoxville, TN). A low dose, co-registered CT was obtained on the same area and emission data were reconstructed with attenuation correction based on the co-registered CT. Commercially-available software (MIM Maestro, MIM Software Inc., Cleveland, OH) was used to delineate liver tumors on PET/CT. To reduce observer bias, tumors were automatically contoured using a previously described threshold technique [24]. The SUV threshold was determined individually for each patient and was defined to be the SUVmax of normal, uninvolved liver parenchyma plus two standard deviations. The median SUV threshold was 3.3 (range, 2.0–4.6). Tumor volumes measuring less than 2 cc were excluded from analysis. The results of the auto-contouring were verified on the co-registered CT to confirm exclusion of physiologic FDG uptake in the nearby kidneys and gastrointestinal tract. MTV, TGA and SUVmax were assessed from the summation of the intra-hepatic tumor volumes. Liver tumors were not counted or considered individually due to the large number of patients with innumerable liver metastases or multiple, confluent tumors. MTV defines the volume of tumor on the basis of metabolic activity that is higher than the surrounding normal tissue uptake. SUVmax is the portion of the tumor that demonstrates maximum metabolic activity. TGA is the product of the MTV and the mean standard uptake value in the tumor(s).
Statistical methods
Survival analyses were conducted by using time from treatment until progression or death. Patients alive or without progression are censored at the date of last clinical follow up. Survival times were analyzed by Kaplan-Meier method. Log-rank test was used for univariate analysis and multivariate analysis was performed on significant univariate predictors using the Cox proportional hazards regression. Measures of MTV were excluded from multivariate testing as they are highly correlated with TGA. Statistical tests were performed at the 5% level of significance.
RESULTS
Patient and Treatment Characteristics
Seventy one patients were consecutively treated with 90Y microsphere brachytherapy from May 2007 through December 2012. Patients were followed for a median of 9.9 months (95% CI, 7.8 to 12.0) after treatment. Patient and treatment characteristics are presented in Table 1. Gender was well balanced and patients had excellent performance status. Extra-hepatic metastases and monosomy 3 genetic aberrations were common.
Table 1.
Patient demographics and treatment
| Parameter | Data* |
|---|---|
| Sex | |
| Male | 34 (48) |
| Female | 37 (52) |
| Age at treatment (years) | |
| Median | 63 |
| Range | 23–91 |
| Karnofsky Performance Status | |
| Median | 90 |
| Range | 70–100 |
| Extrahepatic Metastases | |
| Present | 39 (55) |
| Absent | 29 (41) |
| Unknown | 3 (4) |
| Monosomy 3 genetic aberration in tumor | |
| Present | 14 (20) |
| Absent | 3 ( 4 ) |
| Unknown | 54 (76) |
| Prior treatment for metastatic disease | |
| None | 13 (18) |
| Total | 58 (82) |
| Immunoembolization | 50 (70) |
| Chemoembolization | 31 (44) |
| Systemic therapy† | 10 (14) |
| Partial hepatectomy | 2 (3) |
| Time from diagnosis of ocular tumor to diagnosis of liver metastases (months) | |
| Median ± standard deviation | 25.1 ±36.3 |
| 95% CI†† | 16.6–33.6 |
| Range | 3.1–190.5 |
| Time from diagnosis of liver metastases to 90Y microsphere brachytherapy (months) † | |
| Median ± standard deviation | 9.8 ±10.3 |
| 95% CI | 7.4–12.2 |
| Range | 1.1–53.5 |
| 90Y Embolization procedures | |
| Total | 134 |
| Mean procedures per patient | 1.9 |
| Number of treatments per patient | |
| 1 | 18 (25) |
| 2 | 46 (65) |
| 3 | 4 (6) |
| 4 | 3 ( 4) |
| Treatment site (n=134 procedures) | |
| Right lobe | 64 (48) |
| Left lobe | 51 (38) |
| Whole liver, single fraction | 5 (4) |
| Whole liver, fractionated | 14 (10) |
| Delivered activity (GBq) † | |
| Right Lobar Administration | |
| Median ± standard deviation | 0.88±0.22 |
| Range | 0.33–1.33 |
| Left Lobar Administration | |
| Median ± standard deviation | 0.33±0.13 |
| Range | 0.07–0.68 |
| Whole liver embolization, single fraction | |
| Median ± standard deviation | 0.84±0.40 |
| Range | 0.63–1.59 |
| Whole liver embolization, fractionated | |
| Median ± standard deviation | 0.59±0.22 |
| Range | 0.19–1.07 |
| Dose to tumor (Gy) | |
| Mean ± standard deviation | 36.3±35.4 |
| Range | 8.5–178 |
| Dose to liver (Gy) | |
| Mean ± standard deviation | 15.8±7.9 |
| Range | 3.7–33.7 |
| Dose to lung (Gy) | |
| Mean ± standard deviation | 1.6±1.8 |
| Range | 0.3–4.7 |
Unless otherwise indicated, data are numbers of patients with percentages in parentheses.
Systemic therapies received: Sunitinib (n=6), Ipilimumab (n=1), Tremelimumab (n=1), other (n=2)
Abbreviations: CI=confidence interval, 90Y=yttrium-90, GBq=gigabecquerel, Gy=Gray
The median time from the diagnosis of liver metastases to 90Y microsphere brachytherapy was 9.8 months (95% CI, 7.4 to 12.2) and 82% of patients had received prior liver-directed therapies (Table 1). Five patients were treated to the whole liver in a single fraction and 14 received unilobar delivery only. The remainder of the patients received fractionated treatment to the whole liver, most commonly through selective, lobar embolization of the left and right hepatic artery in staged, two fraction treatment courses (Table 1). Seven patients underwent three or more radioembolization procedures during the period of follow up. Such retreatments were performed in the event of disease recurrence or progression (n=4), due to anatomic considerations (n=2), and due to arterial dissection resulting in premature termination of a planned delivery with repeat treatment at a later date (n=1). There was a wide range of delivered activities for lobar and whole-liver treatments, with a median of 36.3 Gy delivered to tumor (Table 1).
Clinical Response and Patient Survival
There were no treatment-related deaths. Prior to treatment, 17 patients had pre-existing liver dysfunction (grade 1 or 2 elevation of liver function tests (LFT)) due to tumor burden or prior liver-directed therapies. LFT elevation was observed in 34 patients (48%) after radioembolization, including five patients (7%) with grade two or three toxicity. Non-hepatic toxicities were frequent but self-limiting: grade ≤2 fatigue in 31 (44%), grade ≤2 nausea in 11 (15%), and grade ≤2 abdominal discomfort in 18 (25%). The temporal responses of liver toxicities in patients with uveal melanoma following 90Y microsphere brachytherapy administered at our institution have previously been reported [18].
Treatment response of target lesions at three months following treatment was evaluated with CT or MRI in 61 patients (86%) and was as follows: partial response (PR) in five (8%), stable in 32 (52%), and progression in 24 (39%). Treatment response on PET/CT at three months following treatment was evaluable in 29 patients and was more favorable: PR in 10 (34%), stable in 14 (48%), and progression in five (17%). At the time of analysis, 52 patients had progression in the liver with a median hepatic PFS of 5.9 months (range, 1.3 to 19.1).
Survival data were available for all 71 patients and 56 had died at time of analysis. Median OS following microsphere brachytherapy was 12.3 months (range, 1.9–49.3). Median OS after the diagnosis of the primary ocular tumor and liver metastases was 55.6 months (range, 22–226) and 23.9 months (range, 6.2–69.0), respectively.
Outcomes were compared among patients receiving brachytherapy as a salvage therapy (n=58) or as a first-line therapy following diagnosis of liver metastases (n=13). OS following liver brachytherapy was significantly longer in the non-salvage group compared to the salvage group (median OS not reached vs. 10.8 mos., p=0.01). While this could reflect different sensitivities to therapy among the subgroups, it may also be related to the time interval between diagnosis of liver metastases and brachytherapy, which was longer in the salvage group (11.3 vs. 2.1 mos.). Regardless, there was no detectable difference in radiographic response to therapy or hepatic PFS (p=0.37) among the subgroups. Additionally, there was no discernable difference in OS following the diagnosis of liver metastases among the two subgroups (p=0.57).
Metabolic Response and the Predictive Value of PET/CT
Fifty patients (21 men and 29 women) had pre-treatment PET/CT and 29 patients (7 men and 22 women) had both pre- and post-treatment PET/CT scans available for review. OS in these 50 patients was highly similar to the study cohort (log-rank p=0.87) indicating that this is a representative population of patients treated.
Pretreatment PET/CT scans performed at a median of 16 days prior to microsphere brachytherapy demonstrated a median SUVmax of 6.9 (range, 0–40.3), median MTV 57.2 cc (range, 0–1570.2) and median TGA of 221.8 cc (range, 0–5733.0). Pre-treatment metabolic tumor volumes were similar to contoured tumor volume on anatomic imaging (paired t-test, p=0.34). Post-treatment PET/CT performed at a median of 89 days after therapy demonstrated a median SUVmax of 5.9 (range, 0–12.2), median MTV 30.1 cc (range, 0–1198.3) and median TGA of 157.7 cc (range, 0–6527.1). The changes in functional tumor parameters in patients who responded to microsphere brachytherapy were (mean ± standard deviation) SUVmax decrease of 1.5±1.8, MTV decrease of 203.7±437.0 cc and TGA decrease of 527.1±1098.3 cc (Figure 1).
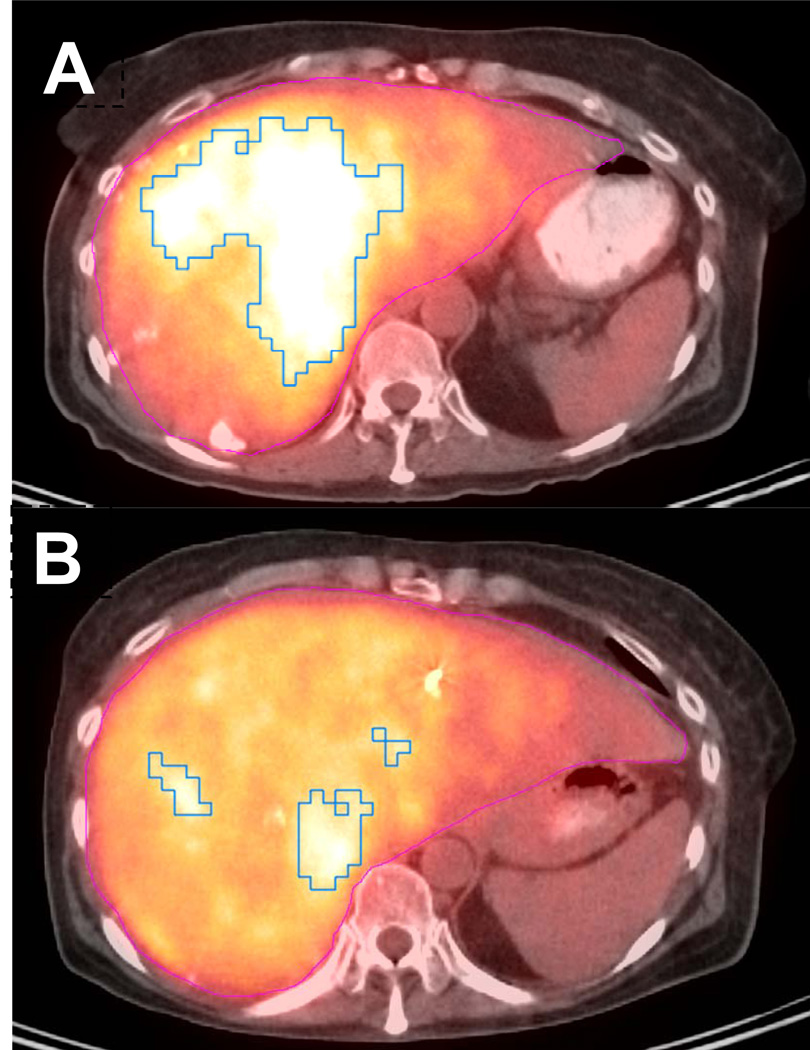
Figure 1.
Representative axial FDG PET/CT image from a patient with a partial response following 1.59 GBq of 90Y microspheres embolized via the left hepatic artery. (A) Pre-treatment functional tumor parameters improved from SUVmax 6.0, MTV 325 cc, and TGA 1667 cc to (B) SUVmax 5.2, MTV 117 cc, and TGA 551 cc on FDG PET/CT completed 88 days after treatment.
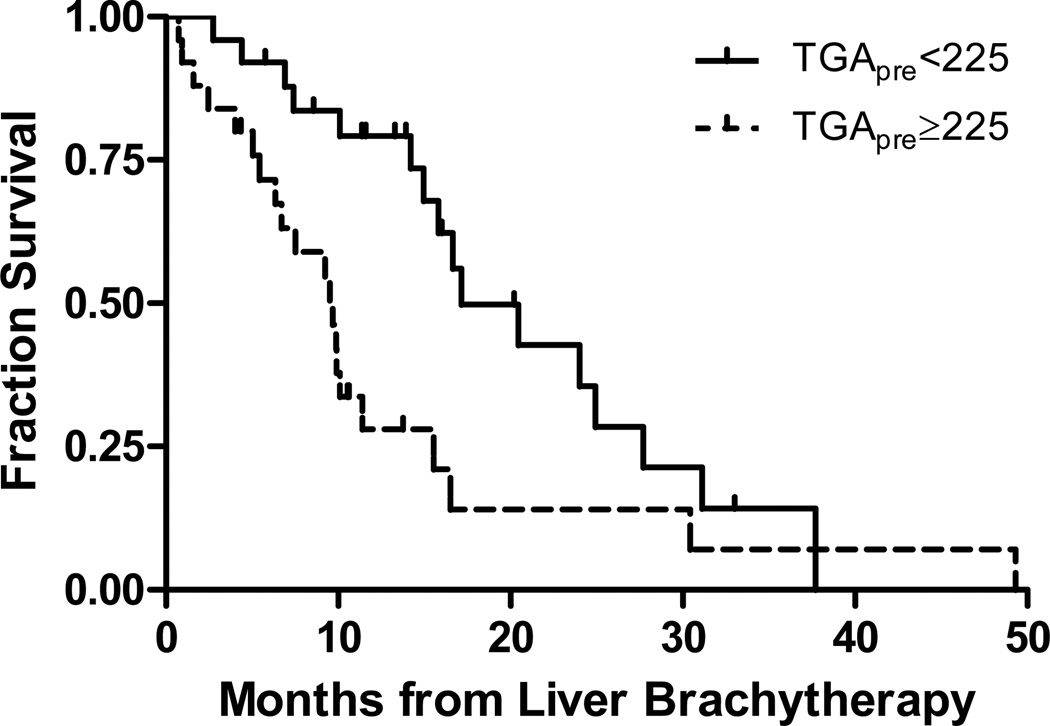
Univariate analysis of pre-treatment clinical features demonstrated that female sex, MTV and TGA are significantly correlated with both hepatic PFS and OS following microsphere brachytherapy (Table 2). Female sex and TGA retained significance in a multivariate Cox proportional hazards model after adjusting for age, KPS, the presence of extra-hepatic disease and SUVmax. MTV was excluded from multivariate analysis as the data are highly correlated with TGA. Patients were stratified by TGA less than or greater than or equal to 225 g, which approximates the median value. Patients with low TGA had a median OS of 17.2 months versus 9.7 months observed in those patients with high TGA (HR for death 0.40, 95% CI 0.20 to 0.81, p=0.01) (Figure 2).
Table 2.
Analysis of factors and values as predictors of overall survival (top) and hepatic progression free survival (bottom) in 50 patients with analyzed pre-treatment FDG PET/CT. Metabolic tumor volume (MTVpre) was omitted from multivariate analysis as it is highly correlated with total glycolic activity (TGApre).
| Overall survival | |||||||
|---|---|---|---|---|---|---|---|
| Univariate analysis | Multivariate analysis | ||||||
| Parameter | Hazard ratio (HR) |
p | HR per 100 units |
Parameter | Hazard ratio (HR) |
p | HR per 100 units |
| Age | 1.0011 | 0.9206 | - | Age | - | - | - |
| Female | 0.374 | 0.0123 | - | Female | 0.3373 | 0.0007 | - |
| KPS* | 0.9642 | 0.0756 | - | KPS | - | - | - |
| Extrahepatic Disease | 1.333 | 0.4083 | - | Extrahepatic Disease | - | - | - |
| SUVmax, pre | 1.0483 | 0.3098 | - | SUVmax, pre | - | - | - |
| MTVpre | 1.0017 | 0.0003 | 1.1888 | MTVpre | Omit | - | - |
| TGApre | 1.0006 | <0.0001 | 1.0668 | TGApre | 1.0007 | <0.0001 | 1.0714 |
| Hepatic progression free survival | |||||||
| Univariate analysis | Multivariate analysis | ||||||
| Parameter | HR | p |
HR per 100 units |
Parameter | HR | p |
HR per 100 units |
| Age | 0.9965 | 0.7517 | - | Age | - | - | - |
| Female | 0.4644 | 0.0267 | - | Female | 0.4492 | 0.0217 | - |
| KPS | 0.9849 | 0.4875 | - | KPS | - | - | - |
| Extrahepatic Disease | 1.917 | 0.0564 | - | Extrahepatic Disease | - | - | - |
| SUVmax, pre | 1.0352 | 0.3412 | - | SUVmax, pre | - | - | - |
| MTVpre | 1.0011 | 0.0367 | 1.1142 | MTVpre | Omit | - | - |
| TGApre | 1.0004 | 0.007 | 1.0443 | TGApre | 1.0005 | 0.0055 | 1.0469 |
Abbreviations: KPS=Karnofsky performance status, SUVmax=standard uptake value, MTV=metabolic tumor volume, TGA=total glycolic activity
Figure 2.
Kaplan-Meier survival curves as a function of pre-treatment total glycolic activity (TGApre) (log rank p=0.01).
DISCUSSION
Uveal melanoma is remarkable for its predilection to metastasize to the liver. Despite routine clinical surveillance for early detection of metastatic disease, recent series continue to report unfavorable survival times, thus highlighting the need for liver-targeted therapies [4, 30, 31]. This study describes clinical outcomes for a large series of patients receiving a novel, liver-directed therapy and substantiates preliminary reports proposing that 90Y microsphere brachytherapy is an effective treatment for liver metastases from uveal melanoma [19–21]. The rates of response and PFS in our patient population are comparable to previously published studies [17, 19–21].
In previous observational studies, the median OS of patients with metastatic uveal melanoma ranges from two months in those patients receiving no treatment to 12.5 months in unselected patients receiving therapies like surgical resection, palliative external beam radiotherapy, TACE, isolated hepatic perfusion, systemic chemotherapy or immunotherapy [4, 9–16, 30]. Furthermore, modern prognostic models for metastatic uveal melanoma estimate median survival to be only 14.9 mos. in a subset of patients with the most favorable prognostic features [31]. Our observed survival of 23.9 months following the diagnosis of liver metastases compares favorably with these series. This figure also compares favorably to our institution’s historic control from a phase I study of liver immunoembolization for uveal melanoma in which median OS was 14.4 months in 34 patients, most of whom had not received prior therapy for liver metastases [14]. Furthermore, the observed median OS of 12.3 months following microsphere brachytherapy is especially encouraging when compared with a second institutional historic control from a phase II trial of liver chemoembolization in which median OS was 5.2 months for all patients and 9.8 months in those patients with limited tumor burden [10, 32]. While direct comparison between treatment modalities may not be warranted outside the realm of a randomized trial, the survival times reported in the current series are promising.
While local control was achieved in 60% of patients at three months following 90Y microsphere brachytherapy, rates of measurable, objective response were lower than described in other series [20]. This may be due to inclusion of previously treated patients with considerable tumor burden, or the relative insensitivity of standard RECIST criteria in assessing response to 90Y microsphere brachytherapy on CT and MRI. Additionally, relatively low absorbed doses to the hepatic tumors were delivered in the present series, as a dose reduction was applied in patients who were heavily pretreated with liver-directed therapies. In theory, absorbed dose to the tumor represents an important predictor for treatment response and survival, and there may be a role for dose escalation in future studies.
To date, only a limited number of prognostic factors have been identified to predict poor clinical outcomes in patients with metastatic uveal melanoma [2, 4, 7, 9, 10, 17, 31]. In the current study, we hypothesized that functional tumor parameters are predictive for clinical outcomes following microsphere brachytherapy in patients with liver-dominant disease from metastatic uveal melanoma. Pre-treatment TGA has been found to be an independent, negative predictive factor for hepatic PFS and OS, indicating that larger volume and more metabolically-active tumors portend for poor outcomes. On univariate analysis, pre-treatment MTV was found to be a negative predictive factor for OS and PFS, which is consistent with findings in a separate small series of seven patients with liver metastases from uveal melanoma [21]. In clinical practice, contoured tumor volume may approximate MTV. Interestingly, SUVmax did not demonstrate any predictive value despite its common use in clinical practice. This finding is in agreement with a previously published series and suggests that MTV and TGA are more informative, functional tumor parameters than SUVmax, which represents a point measurement [20].
Microsphere liver brachytherapy has significant associated risks of gastrointestinal, liver and pulmonary toxicity, so patient prognosis should be considered when selecting patients for this complex treatment. Traditionally, physicians have relied on cross-sectional imaging during patient selection for this therapy. Alternative methods to assess liver disease burden and prognosis, such as with PET/CT, could lead to more appropriate utilization microsphere brachytherapy.
Multivariate analysis demonstrated that female sex is an independent predictor of OS and hepatic PFS following microsphere brachytherapy. Similar gender-based findings have been reported in other studies of patients with metastatic uveal melanoma but, until recently, it was not clear whether sex truly carries such strong prognostic weight [4, 11]. Zloto et al. recently reviewed a large database of uveal melanoma patients from a national referral center and found that men not only suffered a higher rate of metastases but had an almost two-fold excess of melanoma-related mortality in the first 10 years after diagnosis. There was no apparent difference in OS among the sexes due to competing risks in female patients. While the biologic reasons for these gender differences are not yet understood, these data have implications for clinical trial planning and testing of new therapies in patients with metastatic uveal melanoma [33].
Our study is limited by the inherent bias in retrospective and non-randomized analysis. The widespread application of surveillance-imaging for detection of liver metastases in this patient population could also contribute to lead-time bias. Our study is also somewhat limited by the use of a threshold method when defining tumor volume on PET/CT. It is possible that the threshold definition will influence measures of SUVmax, MTV and TGA, so we used a previously adopted threshold to determine tumor volume in order to reduce bias [24]. Additionally, only 29 patients had uniformly available pre- and post-treatment PET/CT scans, so the data are underpowered to determine whether the degree of metabolic response to treatment on PET/CT predicts for OS or PFS. Finally, we were unable to detect an overt difference in response to therapy among the treatment-naïve and salvage subgroups. The present series may have been underpowered to complete this task and this question should remain an active area of investigation. In the salvage setting, tumors could be inherently radio-resistant or there may be impaired delivery of microspheres to target tumors.
The efficacy of microsphere brachytherapy in patients with metastatic uveal melanoma warrants further study. Currently, our institution is conducting a phase II, open-label study (ClinicalTrials.gov identifier: NCT01473004) to investigate clinical response and local control following local, high-dose radiotherapy with 90Y microspheres in patients with liver metastases from uveal melanoma. Pre- and post-treatment PET/CT will be routinely performed in this study. Additional study is also necessary on the subject of non-random karyotypic abnormalities like monosomy 3, duplications in 8q and other aneuploidy which are commonly identified in uveal melanoma [34]. Such chromosomal alterations may portend worse survival or correlate with response to local radiotherapy, but numbers were too few in the present series to conclude.
In conclusion, this series demonstrates efficacy local radiotherapy delivered with 90Y liver microspheres in patients with metastatic uveal melanoma. This treatment is a promising therapeutic option in this patient population and further investigation is warranted. MTV and TLG provide both metabolic and volumetric information that reflect whole-liver tumor burden. These metrics are predictive of clinical outcomes and may be useful quantitative criteria for patient selection when microsphere brachytherapy is contemplated.
Acknowledgments
Funding: National Cancer Institute Cancer Center Support grant (P30 CA56036-11).
Footnotes
Conflict of interest: none
REFERENCES
- 1.Singh AD, Turell ME, Topham AK, et al. Uveal melanoma: trends in incidence, treatment and survival. Ophthalmology. 2011;118:1881–1885. doi: 10.1016/j.ophtha.2011.01.040. [DOI] [PubMed] [Google Scholar]
- 2.Kath R, Hayungs J, Bornfeld N, et al. Prognosis and treatment of disseminated uveal melanoma. Cancer. 1993;72:2219–2223. doi: 10.1002/1097-0142(19931001)72:7<2219::aid-cncr2820720725>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 3.The Collaborative Ocular Melanoma Study Group. Assessment of metastatic disease status at death in 435 patients with large choroidal melanoma in the Collaborative Ocular Melanoma Study (COMS) COMS report no. 15. Arch Ophthalmol. 2001;119:670–676. doi: 10.1001/archopht.119.5.670. [DOI] [PubMed] [Google Scholar]
- 4.Rietschel P, Panageas KS, Hanlon C, et al. Variates of survival in metastatic uveal melanoma. J Clin Oncol. 2005;23:8076–8080. doi: 10.1200/JCO.2005.02.6534. [DOI] [PubMed] [Google Scholar]
- 5.Augsburger JJ, Correa ZM, Shaikh AH. Effectiveness of treatments for metastatic uveal melanoma. Am J Ophthalmol. 2009;148:119–127. doi: 10.1016/j.ajo.2009.01.023. [DOI] [PubMed] [Google Scholar]
- 6.Bedikian AY, Legha SS, Mavligit G, et al. Treatment of uveal melanoma metastatic to the liver: A review of the M.D. Anderson Cancer Center experience and prognostic factors. Cancer. 1995;76:1665–1670. doi: 10.1002/1097-0142(19951101)76:9<1665::aid-cncr2820760925>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 7.Flaherty LE, Unger JM, Liu PY, et al. Metastatic melanoma from intraocular primary tumors: The Southwest Oncology Group experience in phase II advanced melanoma clinical trials. Am J Clin Oncol. 1998;21:568–572. doi: 10.1097/00000421-199812000-00008. [DOI] [PubMed] [Google Scholar]
- 8.Gragoudas ES, Egan KM, Seddon JM, et al. Survival of patients with metastases from uveal melanoma. Ophthalmology. 1991;98:383–389. doi: 10.1016/s0161-6420(91)32285-1. [DOI] [PubMed] [Google Scholar]
- 9.Rajpal S, Moore R, Karakousis CP. Survival in metastatic ocular melanoma. Cancer. 1983;52:334–336. doi: 10.1002/1097-0142(19830715)52:2<334::aid-cncr2820520225>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 10.Yamamoto A, Chervoneva I, Sullivan KL, et al. High-dose immunoembolization: Survival benefit in patients with hepatic metastases from uveal melanoma. Radiology. 2009;252:290–298. doi: 10.1148/radiol.2521081252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hsueh EC, Essner R, Foshag LJ, et al. Prolonged survival after complete resection of metastases from intraocular melanoma. Cancer. 2004;100:122–129. doi: 10.1002/cncr.11872. [DOI] [PubMed] [Google Scholar]
- 12.Aoyama T, Mastrangelo MJ, Berd D, et al. Protracted survival after resection of metastatic uveal melanoma. Cancer. 2000;89:1561–1568. doi: 10.1002/1097-0142(20001001)89:7<1561::aid-cncr21>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 13.Alexander HR, Libutti SK, Pingpank JF, et al. Hyperthermic isolated hepatic perfusion using melphalan for patients with ocular melanoma metastatic to the liver. Clin Cancer Res. 2003;9:6343–6349. [PubMed] [Google Scholar]
- 14.Sato T, Eschelman DJ, Gonsalves CF, et al. Immunoembolization of malignant liver tumors, including uveal melanoma using granulocyte-macrophage colony-stimulating factor. J Clin Oncol. 2008;26:5436–5442. doi: 10.1200/JCO.2008.16.0705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Venturini M, Pilla L, Agostini G, et al. Transarterial chemoembolization with drug-eluting beads preloaded with irinotecan as a first-line approach in uveal melanoma liver metastases: Tumor response and predictive value of diffusion-weighted MR imaging in five patients. J Vasc Interv Radiol. 2012;23:937–941. doi: 10.1016/j.jvir.2012.04.027. [DOI] [PubMed] [Google Scholar]
- 16.Sharma KV, Gould JE, Harbour JW, et al. Hepatic arterial chemoembolization for management of metastatic melanoma. AJR Am J Roentgenol. 2008;190:99–104. doi: 10.2214/AJR.07.2675. [DOI] [PubMed] [Google Scholar]
- 17.Gonsalves CF, Eschelman DJ, Sullivan KL, et al. Radioembolization as salvage therapy for hepatic metastasis of uveal melanoma: A single-institution experience. Am J Roentgenol. 2011;196:468–473. doi: 10.2214/AJR.10.4881. [DOI] [PubMed] [Google Scholar]
- 18.Piana PM, Gonsalves CF, Sato T, et al. Toxicities after radioembolization with Yttrium-90 SIR-Spheres: Incidence and contributing risk factors at a single center. J Vasc Interv Radiol. 2011;22:1373–1379. doi: 10.1016/j.jvir.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 19.Kennedy AS, Nutting C, Jakobs T, et al. A first report of radioembolization for hepatic metastases from ocular melanoma. Cancer Investigation. 2009;27:682–690. doi: 10.1080/07357900802620893. [DOI] [PubMed] [Google Scholar]
- 20.Klingenstein A, Haug AR, Zech CJ, et al. Radioembolization as locoregional therapy of hepatic metastases in uveal melanoma patients. Cardiovasc Intervent Radiol. 2013;36:158–165. doi: 10.1007/s00270-012-0373-5. [DOI] [PubMed] [Google Scholar]
- 21.Piduru SM, Schuster DM, Barron BJ, et al. Prognostic value of 18F-fluorodeoxyglucose positron emission tomography-computed tomography in predicting survival in patients with unresectable metastatic melanoma to the liver undergoing yttrium-90 radioembolization. J Vasc Interv Radiol. 2012;23:943–948. doi: 10.1016/j.jvir.2012.04.010. [DOI] [PubMed] [Google Scholar]
- 22.Gulec SA, Suthar RR, Barot TC, et al. The prognostic value of functional tumor volume and total lesion glycolysis in patients with colorectal cancer liver metastases undergoing 90Y selective internal radiation therapy plus chemotherapy. Eur J Nucl Med Mol Imaging. 2011;38:1289–1295. doi: 10.1007/s00259-011-1758-4. [DOI] [PubMed] [Google Scholar]
- 23.Haug AR, Donfack BPT, Trumm C, et al. 18FDG PET/CT predicts survival after radioembolization of hepatic metastases from breast cancer. J Nucl Med. 2012;53:371–377. doi: 10.2967/jnumed.111.096230. [DOI] [PubMed] [Google Scholar]
- 24.Haug AR, Heinemann V, Bruns CJ, et al. 18-FDG PET independently predicts survival in patients with cholangiocellular carcinoma treated with Y-90 microspheres. Eur J Nucl Med Mol Imaging. 2011;38:1037–1045. doi: 10.1007/s00259-011-1736-x. [DOI] [PubMed] [Google Scholar]
- 25.Kennedy A, Nag S, Salem R, et al. Recommendations for radioembolization of hepatic malignancies using yttrium-90 microsphere brachytherapy: a consensus panel report from the radioembolization brachytherapy oncology consortium. Int J Radiat Oncol Biol Phys. 2007;68:13–23. doi: 10.1016/j.ijrobp.2006.11.060. [DOI] [PubMed] [Google Scholar]
- 26.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 27.Szyszko T, Al-Nahhas A, Canelo R, et al. Assessment of response to treatment of unresectable liver tumors with 90Y microspheres: Value of FDG PET versus computer tomography. Nucl Med Commun. 2007;28:15–20. doi: 10.1097/MNM.0b013e328011453b. [DOI] [PubMed] [Google Scholar]
- 28.Zerizer I, Al-Nahhas A, Towey, et al. The role of early (18)F-FDG PET/CT in prediction of progression-free survival after (90)Y radioembolization: Comparison with RECIST and tumor density criteria. Eur J Nucl Med Mol Imaging. 2012;39:1391–1399. doi: 10.1007/s00259-012-2149-1. [DOI] [PubMed] [Google Scholar]
- 29.Lewandowski RJ, Thurston KG, Goin JE, et al. 90Y microsphere (TheraSphere) treatment for unresectable colorectal cancer metastases of the liver: response to treatment at targeted doses of 135–150 Gy as measured by [18F] fluorodeoxyglucose positron emission tomography and computer tomographic imaging. J Vasc Interv Radiol. 2005;16:1641–1651. doi: 10.1097/01.RVI.0000179815.44868.66. [DOI] [PubMed] [Google Scholar]
- 30.Diener-West M, Reynolds SM, Agugliaro DJ, et al. Development of metastatic disease after enrollment in the COMS trials for treatment of choroidal melanoma: Collaborative Ocular Melanoma Study Group report No. 26. Arch Ophthalmol. 2005;23:1639–1643. doi: 10.1001/archopht.123.12.1639. [DOI] [PubMed] [Google Scholar]
- 31.Eskelin S, Pyrhonen S, Hahka-Kemppinen M, et al. A prognostic model and staging for metastatic uveal melanoma. Cancer. 2003;97:465–475. doi: 10.1002/cncr.11113. [DOI] [PubMed] [Google Scholar]
- 32.Patel K, Sullivan K, Berd D, et al. Chemoembolization of the hepatic artery with BCNU for metastatic uveal melanoma: Results of a phase II study. Melanoma Res. 2005;15:297–304. doi: 10.1097/00008390-200508000-00011. [DOI] [PubMed] [Google Scholar]
- 33.Zloto O, Pe’er J, Frenkel S. Gender differences in clinical presentation and prognosis of uveal melanoma. Invest Ophthalmol Vis Sci. 2013;54:652–656. doi: 10.1167/iovs.12-10365. [DOI] [PubMed] [Google Scholar]
- 34.Prescher G, Bornfeld N, Hirche H, et al. Prognostic implications of monosomy 3 in uveal melanoma. Lancet. 1996;347:1222–1225. doi: 10.1016/s0140-6736(96)90736-9. [DOI] [PubMed] [Google Scholar]