Abstract
Proprotein convertases (PC), a family of serine proteases, process cancer-related substrates such as growth factors, growth factor receptors, cell adhesion molecules, metalloproteinases, etc. HIF-1α is a major transcription factor involved in tumorigenesis by sensing intratumoral hypoxia. Furin (PCSK3) is one of the numerous target genes regulated by HIF-1α transactivation and its distribution into endosomal compartments and onto the cell surface can be triggered by hypoxia via HIF-1α. siRNAs to knockdown PCs were transfected into cells alone or in combination with different drug treatments. Protein and RNA expression levels were analyzed by Western blotting or RT-PCR respectively.
PC7 (PCSK7) and furin siRNAs upregulated HIF-1α protein under normoxic condition to a level similar to that obtained by cobalt chloride treatment, eventually leading to activation of VEGF-A synthesis in two human head and neck squamous cell carcinoma cell lines. The unchanged levels of HIF-1α mRNA expression under siRNA treatment and the additive HIF-1α induction of PC siRNAs and either cobalt chloride or the 26S ribosome inhibitor, MG-132, suggested a post-transcriptional PC-mediated regulation. Furthermore, cycloheximide chase showed that PC7/furin siRNA regulation occurred at the level of HIF-1α translation. A specific IGF-1R signaling inhibitor was able to attenuate the PC siRNA induction of HIF-1α, suggesting the involvement of the IGF-1R pathway. Thus, the data show that PCs regulate HIF-1α. Furin and PC7 siRNAs induced HIF-1α protein by increasing its translation, resulting in upregulation of VEGF-A. This finding may provide insight into intricate PC functions that seem to be independent from their substrate-processing activity.
Keywords: Proprotein convertases, furin, PC7, HIF-1α regulation, cancer cells
INTRODUCTION
Proprotein convertase (PC) are a family of serine endoproteinases of the subtilisin-kexin type that can activate by cleavage several mammalian secreted proteins [1,2]. A number of PC substrates, including growth factors and their receptors, extracellular matrix proteins and proteases, play important roles in tumor growth and progression. Indeed, studies have demonstrated that some, if not all PCs are involved in tumor cell proliferation, invasion and metastasis [3-5]. This has also been demonstrated by using PC inhibitors such as α1-PDX, a broad spectrum PC inhibitor. For example, transfection of this inhibitor into a human colon carcinoma cell line reduced the tumor size of xenografts, decreased the tumor take and the formation of liver metastasis in nude mice [6,7]. The activation of IGF-1R, TGF beta and MMP-2 by furin, and the processing of PDGF-A, PDGF-B, VEGF-C by furin, PC5 and PACE4 (PCSK6) were found to be responsible for the enhanced tumor cell behavior in similar models [8-10]. The inhibition of PC processing of their substrates including IGF-1R and PDGF-A by α1-PDX overexpression in melanoma cells led to reduced cell migration and invasion [11]. PCSK9 (NARC-1) deficiency was found to result in reduced liver metastasis of melanoma cells [12]. PACE4 silencing in the DU145 prostate cancer cell line slowed proliferation rates and reduced clonogenic activity and growth capability as xenografts in nude mice [13,14]. Furin was found to play a crucial role in osteopontin-induced MKK3/6, p38-and NF-kB-dependent cervical cancer cell migration [15].
Our laboratory extensively investigated the role of PACE4 and furin in the behavior of squamous cell carcinomas. Exogenous expression of PACE4 not only enhanced in vitro and in vivo invasiveness of tumor cells of low invasive ability, but converted non-invasive keratinocytes into invasive cells as a result of increased MT2-MMP processing and MMP2 and MMP9 activation [16,17]. Furin was also implicated in regulating SCC cell growth and invasion [18-20] and its unique function was revealed by transfecting furin prosegment (ppFur) which is a more specific inhibitor of the parent PC [21]. The resultant inhibition of proliferation, invasiveness and tumorigenicity of HNSCC cells was directly linked to furin-mediated processing of cancer-related substrates including MT1-MMP, IGF-1R and TGF beta. The in vivo tumor promotion functions of both PACE4 and furin were corroborated by utilizing transgenic mice in which PC expression was targeted to the basal layer of skin and a two-stage carcinogenesis protocol [22-24]. Interestingly, a serial analysis of gene expression approach was recently attempted to uncover the mechanism of skin SCC development by comparing the global gene expression profiles of UV-induced SCC with that of normal SKH-1 mouse epidermis. More than 200 genes were identified to be differentially expressed in SCC and normal skin [25]. Of special interest was the fact that among the overexpressed genes, HIF-1α level was increased 12 fold in SCC.
HIF-1α heterodimerizes with HIF-1β to form the HIF-1 transcriptional factor that plays complex roles in multiple aspects of cellular behavior [26,27]. Whereas HIF-1β is stably expressed, HIF-1α expression varies with oxygen levels. One of the major mechanisms of oxygen-dependent regulation of HIF activity is through hydroxylation of two conserved proline residues within HIF-1α by specific prolyl hydroxylases. HIF-1α hydroxylation facilitates the binding of the von Hippel Lindau protein which directs the HIF-1α poly-ubiquitylation and proteasomal degradation. The regulation of HIF-1α also occurs at the level of HIF-1α protein synthesis, HIF-1 DNA binding and transactivation [27,28]. HIF-1α has been observed to be elevated in a broad range of human cancer cell types [28,29] and promotes tumor progression through target genes including those active in regulating angiogenesis, growth factor signaling, metabolic reprogramming, epithelial-mesenchymal transition, invasion and metastasis [30].
Of particular importance was the demonstration that furin mRNA was rapidly increased in oxygen-deprived cells and HIF-1α was responsible for driving furin transcription by binding to a consensus hypoxia response element of the furin promoter [31,32]. This association between HIF-1α and PCs and the fact that HIF-1α was overexpressed in experimental mouse SCCs [25] prompted us to further evaluate the status of HIF-1α in murine SCC cells. In the present study, we uncovered a novel and opposite mechanism by which furin could downregulate HIF-1 as demonstrated by silencing of two PCs (furin and PC7). These experiments, using siRNAs, resulted in enhanced HIF-1α protein, probably through a translational activation mechanism, thus adding to the complexity of the interplay between HIF-1α and PCs in the tumor environment.
MATERIALS AND METHODS
Materials
Cobalt chloride (CoCl2, #C8661) and cycloheximide (CHX, #C4859) were purchased from SIGMA-ALDRICH (St. Louis, MO). MG-132 (#474790) and picropodophyllin (PPP, #407247) was purchased from CALBIOCHEM (EMD Chemicals, San Diego, CA). The convertase inhibitor, CMK (Val-Lys-Arg-chloromethylketone, ALX-260-022) was obtained from Enzo Life Sciences (Plymouth Meeting, PA).
Cell culture, siRNA transfection and Western blot analysis
A253 and Detroit 562 (D562) are human head and neck squamous cell carcinoma cell lines, cultured in S-MEM with 10% FBS and 2 mM L-glutamine [20]. For all SiRNA experiments, cells were transfected by Lipofectamine® RNAiMAX Reagent (Invitrogen, Carlsbad, CA) using the manufacturer’s protocol. The following SiRNAs were purchased from Thermo Scientific (Lafayette, CO): ON-TARGETplus Non-targeting siRNA #1 (#D-001810-01-0005) for control siRNA, ON-TARGETplus PCSK7 siRNA (#J-005988-07-0005) for PC7, ON-TARGETplus FURIN siRNA-SMARTpool (#L-005882-00-0005) for Furin, and ON-TARGETplus PCSK6 siRNA (#J-005983-05-0002) for PACE4.
Cell lysates were harvested two days after siRNA transfection and subjected to Western blot analysis as described [33]. For CoCl2 and PPP treatments, the transfected or non-transfected cells were incubated with drugs overnight. The treatment time with MG-132 was 30 min. For CHX treatment, the cells were incubated for different times as labeled in the corresponding figures. Regarding VEGF-A and VEGF-C analysis, the cells were first transfected with SiRNA for 2 days and serum-free media were collected after 1-day incubation. The conditioned media were concentrated with Microcon centrifugal filters (#42407) from Millipore (Billerica, MA) and used for Western blot analysis. The following antibodies were used: HIF-1α (#610958) from BD Biosciences (San Jose, CA), VEGF-A (#sc-152), VEGF-C (#sc-1881) and IGF-1Rβ (#sc-713) from Santa Cruz Biotechnology (Santa Cruz, CA), and GAPDH (#ab8245) from Abcam (Cambridge, MA). The quantification of Western blot results was performed by using ImageJ developed by the National Institutes of Health.
RNA extraction and RT-PCR
The total RNA was extracted by using RNAqueous kit (AM1912) provided by Life Technologies (Carlsbad, CA). The RT-PCR analysis of mRNA of different PC was performed by using SuperScript™ One-Step RT-PCR with Platinum® Taq kit (#10928) from Life Technologies (Carlsbad, CA). The following primers were used for different PCs: PC7 forward: 5′GCAATGGCACCTGAATAACC; PC7 reverse: 5′GTGGTTGCCATTCTCCACAT; PACE4 forward: 5′GCAAACAATTCCTACTGCATC; PACE4 reverse: 5′CAGGGCTTGTAGCCATTCTC; Furin forward: 5′TGAGCCATTCGTATGGCTACG; Furin reverse: 5′GGACACAGCTTTTCTGGTGCA; HIF-1α forward: 5′TCAAAGTCGGACAGCCTCA; HIF-1α reverse: 5′CCCTGCAGTAGGTTTCTGCT; GAPDH forward: 5′ATCCCATCACCATCTTCCAG-3′; GAPDH reverse: ATGACCTTGCCCACAGCCT.
RESULTS
Downregulation of PC7 or Furin Induces HIF-1α
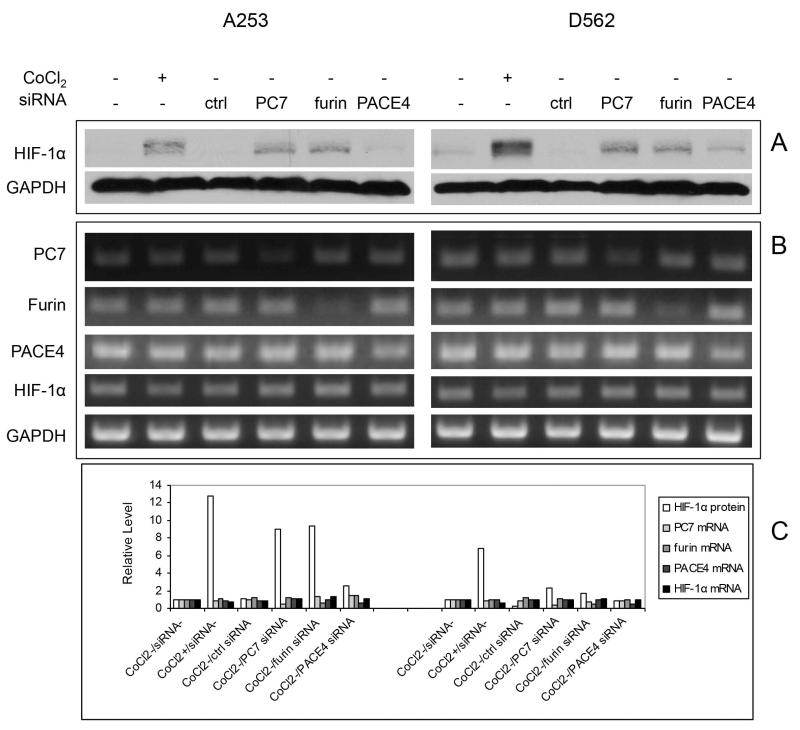
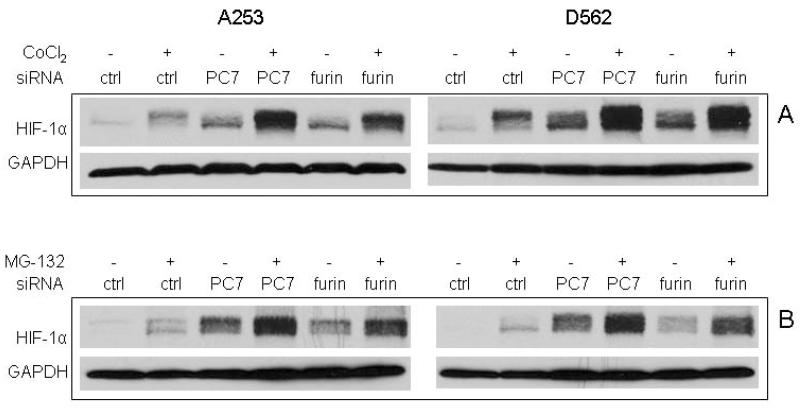
PCs regulate tumor cell biology through processing a number of substrates, some of which including IGF-1 and TGFbeta can alter HIF-1α expression under normoxic conditions [34-36]. PCs and HIF-1α appear to be upregulated during SCC development [20,25]. We first examined if alteration of PC expression could affect HIF-1α by using the human A253 and D562 cell lines, two aggressive HNSCC cell lines characterized by high furin expression levels [20]. SiRNAs for PC7, furin and PACE4 were transfected into these cells, and after two days, Western blot analysis displayed a strong upregulation of HIF-1α protein induced by either PC7 or furin SiRNAs. A milder induction by PACE4 was seen in both cell lines as compared to control SiRNA (Fig. 1A). This level of induction is comparable to the one induced by CoCl2, a drug that can mimic hypoxia in cells. The specific silencing of PC7, furin and PACE4 mRNA by their respective SiRNA was confirmed by RT-PCR analysis (Figure 1B and 1C) that also showed no changes of the expression of other PCs by individual SiRNA used in this experiment. Although HIF-1α protein was significantly elevated by treatment with CoCl2, or by PC7 or furin knockdown, its mRNA levels remained unchanged during these experiments. This is consistent with the fact that CoCl2 interferes with HIF-1α degradation and indicates that the HIF-1α regulation by either PC7 or furin may happen at a non-transcriptional level. PC7 or furin SiRNA mediated induction of HIF-1α protein in synergy with CoCl2 in both cell lines (Fig. 2A). When the control-SiRNA-transfected cells were exposed to MG-132, an inhibitor of 26s proteasome, critical for HIF-1α degradation, HIF-1α protein accumulated as expected (Fig. 2B). This also points to PC7 or furin SiRNA being able to synergize with MG-132 to efficiently increase HIF-1α protein level.
Figure 1.
The effect of PC knockdown on the HIF-1α expression in A253 and D562 cells as compared to that of CoCl2. A. The cells were treated 200 μM overnight or transfected with different PC SiRNAs for 2 days and the cell lysates were collected for Western blot analysis. CoCl2 treatment, as well as SiRNA transfections of PC7 and furin upregulated HIF-1α expression. GAPDH was used as loading control. B. RT-PCR analysis of the RNA samples extracted from cells with the same treatment by using different primers for PC7, furin, PACE4, HIF-1α and GAPDH. PC7, furin and PACE4 downregulation was achieved by the corresponding SiRNA transfections as compared with GAPDH control. C. The expression levels of HIF-1α protein (from Fig. 1A) and PC7, furin, PACE4 and HIF-1α mRNAs (from Fig. 1B) were quantified, and normalized to their corresponding GAPDH loading controls, and depicted as relative numbers with respect to the results from cells without CoCl2 and siRNA treatments (value set at 1). The left and right panels are for A253 and D562 respectively.
Figure 2.
PC7 or furin can synergize with CoCl2 to regulate HIF-1α expression. A. The synergistic effect of PC SiRNA with CoCl2. The cells were first transfected with different SiRNAs for 1 day and then treated with CoCl2 for another day. The lysates were subjected to Western blot analysis for HIF-1α and GAPDH expression. B. The synergistic effect of PC SiRNA with the proteosome inhibitor MG-132. The cells were first transfected with different SiRNAs for 2 days and then treated with 2.5 μM of MG-132 for 30 min before harvest for Western blot analysis of HIF-1α and GAPDH.
PC7 or Furin Regulates HIF-1α Expression at the Translational level
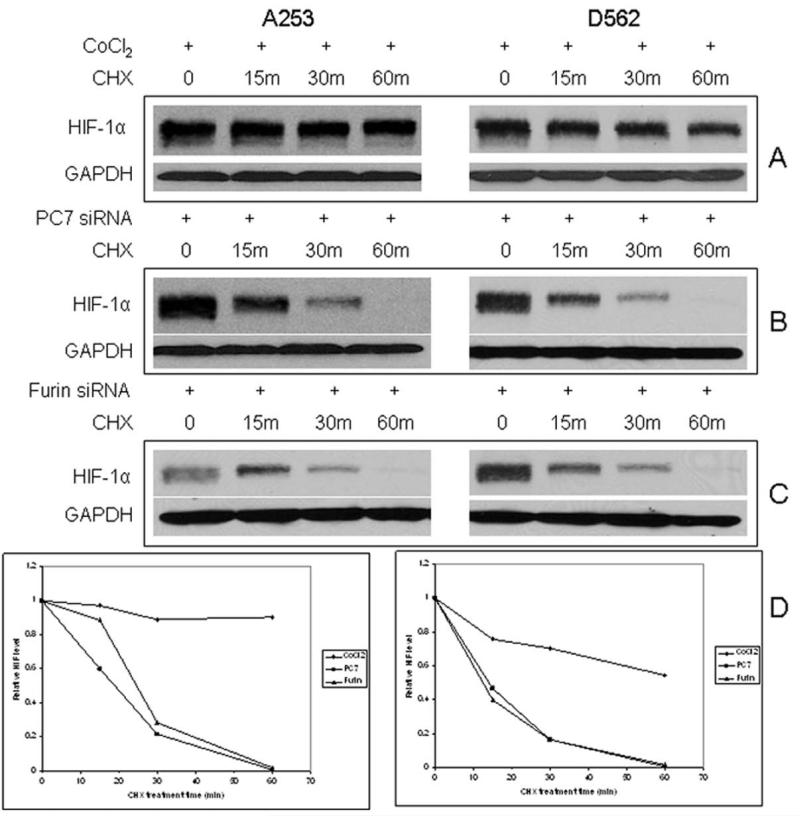
HIF-1α protein and activity can be regulated at multiple levels of gene expression. HIF-1α mRNA measured by RT-PCR indicates transcription activation was not responsible for the PC7/furin-mediated effect on HIF-1α in our cell system. We further investigated the potential post-transcriptional activation mechanism for PC7/furin by performing a cycloheximide (CHX) chase experiment. After HIF-1α was induced either by CoCl2 or by SiRNA, A253 or D562 cells were maintained in the absence or presence of CHX, in the latter case, blocking protein translation. The HIF-1α protein remained high for 60 min without any treatment (Fig. 3). As expected, CoCl2 treatment maintained HIF-1α at relatively constant level after 60 min, although a decrease was more obvious in D562 cells (Fig. 3A, 3D), consistent with the protein stabilization effect of CoCl2. Nevertheless, the augmented HIF-1α by PC7/furin SiRNA started to decrease rapidly at 15 min after CHX treatment, and barely could be detected after 60 min in Western blot analysis (Fig. 3B-D), indicating that PC7/furin SiRNA efficiently induced HIF-1α translation but did not prevent HIF-1α protein from degradation.
Figure 3.
PC7 and furin affect HIF-1α protein level by a different mechanism than CoCl2. Cells were treated with cycloheximide (CHX) for 15, 30 and 60 minutes after HIF-1α induction under 3 different conditions: A. CoCl2 treatment overnight, B. PC7 SiRNA, and C. furin SiRNA transfection for 2 days respectively. The cell lysates were collected and analyzed by Western blotting for HIF-1α and GAPDH. D. Quantitative analysis of the relative HIF-1α expression from the above experiments.
The Regulation of HIF-1α by PC7or Furin may Involve IGF-I Signaling
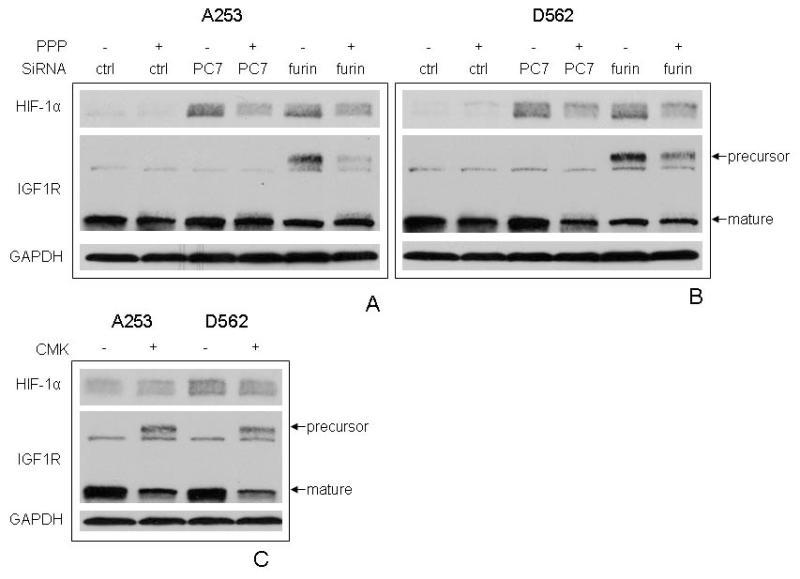
A number of oncoproteins, growth factors and cytokines regulate HIF-1α protein translation under normoxic conditions [37]. For instance, IGF-1 has been shown to upregulate HIF-1α expression in Kaposi sarcoma and colon cancer cells [34,38]. Interestingly, IGF-1 is also one of the major substrates processed by PCs [4]. To study the potential interaction between IGF signaling, PC, and HIF-1α, a specific IGF-1R tyrosine kinase inhibitor, picropodophyllin (PPP) [39], was employed to study the involvement of the PC substrate IGF-1R. Interestingly, PPP treatment attenuated the effect of both PC7 and furin SiRNA on HIF-1α enhancement in both A253 and D562 cells (Fig. 4A-B), suggesting IGF-1R signaling might be required. Since furin can process IGF-1R in these cell lines [21], we further looked at the IGF-1R expression by using an antibody which reacts with both proform and mature form of IGF-1R. As expected, furin SiRNA significantly increased the expression of the IGF-1R precursor due to compromised cleavage, and this effect was partially blocked by PPP. PC7 silencing did not affect IGF-1R maturation. CMK, an inhibitor of furin and other PCs [5], was found to inhibit IGF-1R processing, however, failed to induce HIF-1α protein (Fig. 4C).
Figure 4.
The possible involvement of IGF-I-IGF-IR pathway in the regulation of HIF-1α by PC7 and furin. A and B depict A253 and D562 cell lines, respectively. Cells were first transfected with control, PC7 or furin SiRNA for 1 day, and then treated in the presence or absence of picropodophyllin (PPP) for 1 more day. The lysates were analyzed for HIF-1α, IGF-IR and GAPDH expression. C. Cells were treated with CMK overnight and harvested for the analysis of HIF-1α, IGF-IR and GAPDH expression.
The Silencing of PC7 or Furin Upregulates VEGF Expression
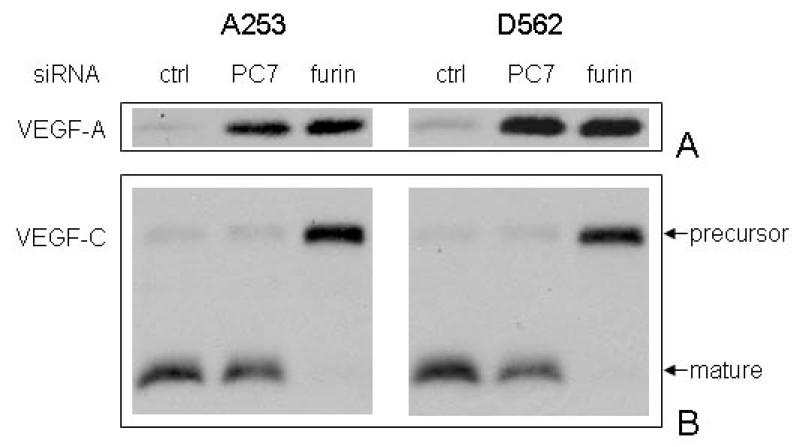
As a transcription factor, HIF-1 transactivates target genes associated with multiple aspects of tumor growth and progression [30]. VEGF-A is a well-characterized growth factor modulated by HIF-1α in tumor cells [26]. Thus, we examined the effect on VEGF-A of the increment in HIF-1α protein by knocking-down of PC7/furin. Conditional media was collected from SiRNA transfectants, concentrated and subjected to Western blotting analysis with VEGF-A specific antibody. As shown in Figure 5A, VEGF-A was strongly secreted into conditional media after PC7/furin SiRNA transfection as compared to control SiRNA, demonstrating that increased HIF-1α protein is functionally active. Furin is also responsible for the maturation of another member of VEGF family, VEGF-C [40], by proteolytic cleavage and the same conditional media was therefore analyzed. VEGF-C precursor was observed only in furin SiRNA treatment due to the downregulation of furin. PC7 again had no effect on VEGF-C maturation in these SCC cells (Fig. 5B).
Figure 5.
PC7 and furin SiRNA transfections led to alteration of VEGF-A and VEGF-C expression. Cells were transected with control, PC7 and PACE4 SiRNAs and the conditioned media were subsequently collected, concentrated and analyzed for VEGF-A and VEGF-C expression using Western blot analysis.
DISCUSSION
PCs participate in the process of tumorigenesis by supporting tumor cell proliferation, invasion and metastasis by proteolytic cleavage of multiple cancer-related substrates [41]. The functions of HIF-1α during cancer progression are achieved by its transcriptional activation of the promoters of many cancer-related target genes, some of which are also PC substrates [29]. The intercross between these two molecules was revealed by the finding that the furin gene promoter harbors a consensus HIF-1α binding sequence and under hypoxic condition, furin mRNA expression was rapidly increased due to transactivaton by HIF-1α [31,32]. Hypoxia can also trigger the relocation of furin from the trans-Golgi network to the endosomal compartment and to the plasma cell membrane of cancer cells, allowing for more efficient proprotein processing [42]. Conversely, normoxic conditions reverse this process, suggesting that furin may modulate the tumor cell behavior and its microenvironment at different stages of tumorigenesis depending on the tissue oxygen tension levels [42].
In this study, we provided a different perspective on the interplay between furin and HIF-1α in the context of tumor cell microenvironment. To our knowledge, this is the first report showing that furin SiRNA readily induced HIF-1α protein to a level comparable to that obtained by the hypoxia mimicker cobalt chloride, an inhibitor of HIF-1α degradation. This implies a reverse induction than the one described by McMahon et al (31), i.e., HIF-1α not only induces furin expression as previously published (31-32), but as shown indirectly in this report, furin should be able to suppress HIF-1α levels in what could be a regulatory feed-back loop.
The mechanism by which furin SiRNA upregulates HIF-1α protein under normoxic conditions is different to the one obtained by cobalt chloride treatment. CHX chase experiments indicate that its regulation is the result of increased HIF-1α translation. The oxygen-independent pathway of HIF-1α regulation has been identified [37] and can be utilized by certain growth factors such as IGF-1, EGF, TGFβ1 [34-36,43]. PPP, a specific IGF-1R tyrosine kinase inhibitor, neutralized the induction of HIF-1α by furin as well as by PC7 SiRNA (Fig. 4A), suggesting that the IGF-1R signaling pathway might be involved in the mode of action of PC SiRNA. Nevertheless, this mechanism appears to be unrelated to IGF-1R processing by PC based on three observations: firstly, PC7 did not process IGF-1R but still regulated HIF-1α (Fig. 4A-B); secondly, furin SiRNA reduced mature IGF-1R but induced HIF-1α; lastly, CMK, a pan-PC inhibitor, reduced mature IGF-1R but did not induce HIF-1α (Fig. 4C). These differences could be explained by efficiency differences in the siRNAs utilized for the two PCs and by the fact that CMK is a competitive inhibitor at the enzyme/protein level and obviously siRNAs act at a different level. It is plausible that PC7/furin SiRNAs induce HIF-1α by changing other aspects of IGF-1R signaling pathway such as altering, directly or indirectly, the IGF-1 availability in the tumor cell microenvironment. In addition to IGF-1R, it is possible that other downstream PC substrates may act as HIF-1α expression suppressors. Once PCs are knocked-down by siRNA, the inhibiting action of these substrates decrease or disappear, thus upregulating HIF-1α. Furthermore, our SiRNA data indicate that furin restrains the HIF-1α translation machinery and subsequently suppresses the activation of cancer-related growth factors such as VEGF-A in SCC cells, and simultaneously, activates by enzymatic cleavage other substrates including VEGF-C (Fig. 5).
Since PC7 knockout mice fail to show any obvious abnormal phenotype, PC7 processing of substrates maybe redundant because of overlapping funcions with other PCs [44,45]. In a human colon carcinoma cell model, PC7, along with furin or PC5, efficiently processed VEGF-C, VEGF-D and PDGF-B, while other studies showed that furin is the major IGF-1R convertase [7,9,46,47]. The processing of EGF and activation of EGFR signaling pathway by PC7 in human embryonic kidney 293 cell and a mouse neural crest-derived cell line appears to be a unique feature of PC7. In these cells, furin, PACE4, PC1/3, or PC5 were unable to process EGFR [48]. Our result showing that PC7 was incapable of activating IGF-1R is consistent with the above findings. Nevertheless, we found that PC7 did not participate in VEGF-C processing in SCC cells, which, as in many other examples reported in the literature, may be a cell-type specific effect. Taken together, the present report shows that PC7 regulates the expression of one of the major players in tumor biology, HIF-1α, and its transcriptional activity as manifested by VEGF-A induction. The mechanism of PC7-mediated HIF-1α regulation is similar to that of furin and involves inhibiting oxygen-independent HIF-1α protein translation perhaps related to IGF-1R signaling or changes in the balance of IGF, EGF and other growth factors.
Acknowledgements
This work was supported by grants from the National Institutes of Health, R01CA133001 and P30CA06927, and by an appropriation from the Commonwealth of Pennsylvania. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
List of Abbreviations
- HIF-1α
Hypoxia-inducible factor 1 alpha
- HNSCC
head and neck squamous cell carcinoma
- PC
proprotein convertases
- SCC
squamous cell carcinoma
- WT
Wild type
REFERENCES
- 1.Seidah NG. Prat A. The biology and therapeutic targeting of the proprotein convertases. Nat Rev Drug Discov. 2012;11:367–383. doi: 10.1038/nrd3699. [DOI] [PubMed] [Google Scholar]
- 2.Seidah NG. The proprotein convertases, 20 years later. Methods Mol Biol. 2011;768:23–57. doi: 10.1007/978-1-61779-204-5_3. [DOI] [PubMed] [Google Scholar]
- 3.Khatib AM, Siegfried G, Chretien M, Metrakos P, Seidah NG. Proprotein convertases in tumor progression and malignancy: novel targets in cancer therapy. The American journal of pathology. 2002;160:1921–1935. doi: 10.1016/S0002-9440(10)61140-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bassi DE, Fu J, Lopez de Cicco R, Klein-Szanto AJ. Proprotein convertases: “master switches” in the regulation of tumor growth and progression. Mol Carcinog. 2005;44:151–161. doi: 10.1002/mc.20134. [DOI] [PubMed] [Google Scholar]
- 5.de Cicco RL, Bassi DE, Benavides F, Conti CJ, Klein-Szanto AJ. Inhibition of proprotein convertases: approaches to block squamous carcinoma development and progression. Molecular carcinogenesis. 2007;46:654–659. doi: 10.1002/mc.20331. [DOI] [PubMed] [Google Scholar]
- 6.Khatib AM, Siegfried G, Prat A, et al. Inhibition of proprotein convertases is associated with loss of growth and tumorigenicity of HT-29 human colon carcinoma cells: importance of insulin-like growth factor-1 (IGF-1) receptor processing in IGF-1-mediated functions. J Biol Chem. 2001;276:30686–30693. doi: 10.1074/jbc.M101725200. [DOI] [PubMed] [Google Scholar]
- 7.Scamuffa N, Siegfried G, Bontemps Y, et al. Selective inhibition of proprotein convertases represses the metastatic potential of human colorectal tumor cells. J Clin Invest. 2008;118:352–363. doi: 10.1172/JCI32040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Siegfried G, Khatib AM, Benjannet S, Chretien M, Seidah NG. The proteolytic processing of pro-platelet-derived growth factor-A at RRKR(86) by members of the proprotein convertase family is functionally correlated to platelet-derived growth factor-A-induced functions and tumorigenicity. Cancer Res. 2003;63:1458–1463. [PubMed] [Google Scholar]
- 9.Siegfried G, Basak A, Cromlish JA, et al. The secretory proprotein convertases furin, PC5, and PC7 activate VEGF-C to induce tumorigenesis. The Journal of clinical investigation. 2003;111:1723–1732. doi: 10.1172/JCI17220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McMahon S, Laprise MH, Dubois CM. Alternative pathway for the role of furin in tumor cell invasion process. Enhanced MMP-2 levels through bioactive TGFbeta. Exp Cell Res. 2003;291:326–339. doi: 10.1016/s0014-4827(03)00407-5. [DOI] [PubMed] [Google Scholar]
- 11.Lalou C, Scamuffa N, Mourah S, et al. Inhibition of the proprotein convertases represses the invasiveness of human primary melanoma cells with altered p53, CDKN2A and N-Ras genes. PLoS One. 2010;5:e9992. doi: 10.1371/journal.pone.0009992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sun X, Essalmani R, Day R, Khatib AM, Seidah NG. Prat A. Proprotein convertase subtilisin/kexin type 9 deficiency reduces melanoma metastasis in liver. Neoplasia. 2012;14:1122–1131. doi: 10.1593/neo.121252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.D’Anjou F, Routhier S, Perreault JP, et al. Molecular Validation of PACE4 as a Target in Prostate Cancer. Transl Oncol. 2011;4:157–172. doi: 10.1593/tlo.10295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Couture F, D’Anjou F, Desjardins R, Boudreau F, Day R. Role of proprotein convertases in prostate cancer progression. Neoplasia. 2012;14:1032–1042. doi: 10.1593/neo.121368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kumar V, Behera R, Lohite K, Karnik S, Kundu GC. p38 kinase is crucial for osteopontin-induced furin expression that supports cervical cancer progression. Cancer research. 2010;70:10381–10391. doi: 10.1158/0008-5472.CAN-10-1470. [DOI] [PubMed] [Google Scholar]
- 16.Hubbard FC, Goodrow TL, Liu SC, et al. Expression of PACE4 in chemically induced carcinomas is associated with spindle cell tumor conversion and increased invasive ability. Cancer Res. 1997;57:5226–5231. [PubMed] [Google Scholar]
- 17.Mahloogi H, Bassi DE, Klein-Szanto AJ. Malignant conversion of non-tumorigenic murine skin keratinocytes overexpressing PACE4. Carcinogenesis. 2002;23:565–572. doi: 10.1093/carcin/23.4.565. [DOI] [PubMed] [Google Scholar]
- 18.Mercapide J, Lopez De Cicco R, Bassi DE, Castresana JS, Thomas G, Klein-Szanto AJ. Inhibition of furin-mediated processing results in suppression of astrocytoma cell growth and invasiveness. Clin Cancer Res. 2002;8:1740–1746. [PubMed] [Google Scholar]
- 19.Bassi DE, Lopez De Cicco R, Mahloogi H, Zucker S, Thomas G, Klein-Szanto AJ. Furin inhibition results in absent or decreased invasiveness and tumorigenicity of human cancer cells. Proc Natl Acad Sci U S A. 2001;98:10326–10331. doi: 10.1073/pnas.191199198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bassi DE, Mahloogi H, Al-Saleem L, Lopez De Cicco R, Ridge JA, Klein-Szanto AJ. Elevated furin expression in aggressive human head and neck tumors and tumor cell lines. Molecular carcinogenesis. 2001;31:224–232. doi: 10.1002/mc.1057. [DOI] [PubMed] [Google Scholar]
- 21.Lopez de Cicco R, Bassi DE, Zucker S, Seidah NG, Klein-Szanto AJ. Human carcinoma cell growth and invasiveness is impaired by the propeptide of the ubiquitous proprotein convertase furin. Cancer research. 2005;65:4162–4171. doi: 10.1158/0008-5472.CAN-04-2820. [DOI] [PubMed] [Google Scholar]
- 22.Fu J, Bassi DE, Zhang J, et al. Enhanced UV-induced skin carcinogenesis in transgenic mice overexpressing proprotein convertases. Neoplasia. 2013;15:169–179. doi: 10.1593/neo.121846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fu J, Bassi DE, Zhang J, Li T, Nicolas E, Klein-Szanto AJ. Transgenic overexpression of the proprotein convertase furin enhances skin tumor growth. Neoplasia. 2012;14:271–282. doi: 10.1593/neo.12166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bassi DE, Lopez De Cicco R, Cenna J, Litwin S, Cukierman E, Klein-Szanto AJ. PACE4 expression in mouse basal keratinocytes results in basement membrane disruption and acceleration of tumor progression. Cancer research. 2005;65:7310–7319. doi: 10.1158/0008-5472.CAN-05-1213. [DOI] [PubMed] [Google Scholar]
- 25.Rundhaug JE, Hawkins KA, Pavone A, et al. SAGE profiling of UV-induced mouse skin squamous cell carcinomas, comparison with acute UV irradiation effects. Molecular carcinogenesis. 2005;42:40–52. doi: 10.1002/mc.20064. [DOI] [PubMed] [Google Scholar]
- 26.Majmundar AJ, Wong WJ, Simon MC. Hypoxia-inducible factors and the response to hypoxic stress. Mol Cell. 2010;40:294–309. doi: 10.1016/j.molcel.2010.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Semenza GL. Hypoxia-inducible factors in physiology and medicine. Cell. 2012;148:399–408. doi: 10.1016/j.cell.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Keith B, Johnson RS, Simon MC. HIF1alpha and HIF2alpha: sibling rivalry in hypoxic tumour growth and progression. Nat Rev Cancer. 2012;12:9–22. doi: 10.1038/nrc3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Semenza GL. Defining the role of hypoxia-inducible factor 1 in cancer biology and therapeutics. Oncogene. 2010;29:625–634. doi: 10.1038/onc.2009.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Semenza GL. Hypoxia-inducible factors: mediators of cancer progression and targets for cancer therapy. Trends Pharmacol Sci. 2012;33:207–214. doi: 10.1016/j.tips.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McMahon S, Grondin F, McDonald PP, Richard DE, Dubois CM. Hypoxia-enhanced expression of the proprotein convertase furin is mediated by hypoxia-inducible factor-1: impact on the bioactivation of proproteins. J Biol Chem. 2005;280:6561–6569. doi: 10.1074/jbc.M413248200. [DOI] [PubMed] [Google Scholar]
- 32.Silvestri L, Pagani A, Camaschella C. Furin-mediated release of soluble hemojuvelin: a new link between hypoxia and iron homeostasis. Blood. 2008;111:924–931. doi: 10.1182/blood-2007-07-100677. [DOI] [PubMed] [Google Scholar]
- 33.Fu J, Fong K, Bellacosa A, et al. VILIP-1 downregulation in non-small cell lung carcinomas: mechanisms and prediction of survival. PLoS One. 2008;3:e1698. doi: 10.1371/journal.pone.0001698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fukuda R, Hirota K, Fan F, Jung YD, Ellis LM, Semenza GL. Insulin-like growth factor 1 induces hypoxia-inducible factor 1-mediated vascular endothelial growth factor expression, which is dependent on MAP kinase and phosphatidylinositol 3-kinase signaling in colon cancer cells. J Biol Chem. 2002;277:38205–38211. doi: 10.1074/jbc.M203781200. [DOI] [PubMed] [Google Scholar]
- 35.Treins C, Giorgetti-Peraldi S, Murdaca J, Monthouel-Kartmann MN, Van Obberghen E. Regulation of hypoxia-inducible factor (HIF)-1 activity and expression of HIF hydroxylases in response to insulin-like growth factor I. Mol Endocrinol. 2005;19:1304–1317. doi: 10.1210/me.2004-0239. [DOI] [PubMed] [Google Scholar]
- 36.McMahon S, Charbonneau M, Grandmont S, Richard DE, Dubois CM. Transforming growth factor beta1 induces hypoxia-inducible factor-1 stabilization through selective inhibition of PHD2 expression. J Biol Chem. 2006;281:24171–24181. doi: 10.1074/jbc.M604507200. [DOI] [PubMed] [Google Scholar]
- 37.Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3:721–732. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- 38.Catrina SB, Botusan IR, Rantanen A, et al. Hypoxia-inducible factor-1alpha and hypoxia-inducible factor-2alpha are expressed in kaposi sarcoma and modulated by insulin-like growth factor-I. Clin Cancer Res. 2006;12:4506–4514. doi: 10.1158/1078-0432.CCR-05-2473. [DOI] [PubMed] [Google Scholar]
- 39.Girnita A, Girnita L, del Prete F, Bartolazzi A, Larsson O, Axelson M. Cyclolignans as inhibitors of the insulin-like growth factor-1 receptor and malignant cell growth. Cancer research. 2004;64:236–242. doi: 10.1158/0008-5472.can-03-2522. [DOI] [PubMed] [Google Scholar]
- 40.Lopez de Cicco R, Watson JC, Bassi DE, Litwin S, Klein-Szanto AJ. Simultaneous expression of furin and vascular endothelial growth factor in human oral tongue squamous cell carcinoma progression. Clin Cancer Res. 2004;10:4480–4488. doi: 10.1158/1078-0432.CCR-03-0670. [DOI] [PubMed] [Google Scholar]
- 41.Taylor NA, Van De Ven WJ, Creemers JW. Curbing activation: proprotein convertases in homeostasis and pathology. FASEB J. 2003;17:1215–1227. doi: 10.1096/fj.02-0831rev. [DOI] [PubMed] [Google Scholar]
- 42.Arsenault D, Lucien F, Dubois CM. Hypoxia enhances cancer cell invasion through relocalization of the proprotein convertase furin from the trans-Golgi network to the cell surface. J Cell Physiol. 2012;227:789–800. doi: 10.1002/jcp.22792. [DOI] [PubMed] [Google Scholar]
- 43.Zhong H, Chiles K, Feldser D, et al. Modulation of hypoxia-inducible factor 1 alpha expression by the epidermal growth factor/phosphatidylinositol 3-kinase/PTEN/AKT/FRAP pathway in human prostate cancer cells: implications for tumor angiogenesis and therapeutics. Cancer research. 2000;60:1541–1545. [PubMed] [Google Scholar]
- 44.Creemers JW, Khatib AM. Knock-out mouse models of proprotein convertases: unique functions or redundancy? Front Biosci. 2008;13:4960–4971. doi: 10.2741/3055. [DOI] [PubMed] [Google Scholar]
- 45.Seidah NG, Sadr MS, Chretien M, Mbikay M. The multifaceted Proprotein Convertases: their unique, redundant, complementary and opposite functions. J Biol Chem. 2013 doi: 10.1074/jbc.R113.481549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Siegfried G, Basak A, Prichett-Pejic W, et al. Regulation of the stepwise proteolytic cleavage and secretion of PDGF-B by the proprotein convertases. Oncogene. 2005;24:6925–6935. doi: 10.1038/sj.onc.1208838. [DOI] [PubMed] [Google Scholar]
- 47.McColl BK, Paavonen K, Karnezis T, et al. Proprotein convertases promote processing of VEGF-D, a critical step for binding the angiogenic receptor VEGFR-2. FASEB J. 2007;21:1088–1098. doi: 10.1096/fj.06-7060com. [DOI] [PubMed] [Google Scholar]
- 48.Rousselet E, Benjannet S, Marcinkiewicz E, Asselin MC, Lazure C, Seidah NG. Proprotein convertase PC7 enhances the activation of the EGF receptor pathway through processing of the EGF precursor. J Biol Chem. 2011;286:9185–9195. doi: 10.1074/jbc.M110.189936. [DOI] [PMC free article] [PubMed] [Google Scholar]