Abstract
Purpose
To investigate the effect of Carr-Purcell (CP) pulse trains on transverse relaxation times, T2, of tissue water and metabolites (both non-coupled and J-coupled spins) in the rat brain at 9.4 T using LASER, CP-LASER and T2ρ-LASER sequences.
Methods
Proton NMR spectra were measured in rat brain in vivo at 9.4 T. Spectra were acquired at multiple echo times ranging from 18 to 402 ms. All spectra were analyzed using LCModel with simulated basis sets. Signals of metabolites as a function of echo time were fitted using a mono-exponential function to determine their T2 relaxation times.
Results
Measured T2s for tissue water and all metabolites were significantly longer with CP-LASER and T2ρ-LASER compared to LASER. The T2 increased by a factor of ~1.3 for non-coupled and weakly coupled spins (e.g., N-acetylaspartate and total creatine) and by a factor of ~2 (e.g., glutamine and taurine) to ~4 (e.g., glutamate and myo-inositol) for strongly coupled spins.
Conclusion
Application of a CP pulse train results in a larger increase in T2 relaxation times for strongly coupled spins than for non-coupled (singlet) and weakly coupled spins. This needs to be taken into account when correcting for T2 relaxation in CP-like sequences such as LASER.
Keywords: CP pulse train, Brain, LASER, LCModel, T2
INTRODUCTION
Localization by adiabatic selective refocusing (LASER) (1) and semi-LASER (2) sequences are widely used for in vivo single-voxel 1H NMR spectroscopy (MRS) studies in both humans and animals (3-9). These sequences offer excellent localization performance and greatly reduced chemical-shift displacement error, as well as diminished sensitivity to B1 inhomogeneity (i.e., adiabaticity).
The succession of multiple adiabatic full-passage (AFP) pulses in LASER and semi-LASER constitutes a Carr-Purcell (CP) train (10) which is known to minimize J-evolution for J-coupled metabolites (11). The time evolution due to J-modulation becomes negligible when where τcp is an interpulse delay (in s), J is the coupling constant (in Hz), and δ is the chemical shift difference (in Hz) between the coupled spins. The preservation of the spectral pattern for several J-coupled metabolites e.g., glutamate, glutamine and myo-inositol (mIns) has been demonstrated successfully at relatively long echo times (TE) in the human brain using CP-like PRESS sequences at 1.5 T (12) and 2 T (13). CPRESS (TE = 45 ms) was also shown to improve detection of mIns in patients with cognitive impairment compared to the standard PRESS sequence at 3 T (14).
In addition to minimizing J-modulation, CP pulse trains also refocus the effects of diffusion and chemical exchange (15), resulting in longer apparent transverse relaxation times (T2) than with standard sequences such as STEAM or PRESS. Accordingly, an increase in the apparent T2 of tissue water in the human brain was reported using CP-LASER at 4 T and 7 T compared to PRESS (5) and LASER (16). Similarly, an increase in apparent T2s was reported for non-coupled spins of N-acetylaspartate (NAA) and total creatine (tCr) with CP-LASER compared to PRESS (5). The term, apparent T2, refers to the observed decay constant of the transverse magnetization, irrespective of the physical processes that contribute to the signal decay. Henceforth, terms, apparent T2, T2, and transverse relaxation time, will be used interchangeably.
In spite of the increasing popularity of CP-like sequences such as CP-PRESS, LASER and semi-LASER, however, there have been very few studies of the effect of CP pulse trains on T2s of brain metabolites other than singlets. One study reported T2s of mIns and the multiplet of NAA in human brain with CP-PRESS at 1.5 T (12). In that study, the authors noted that T2 of mIns with CP-PRESS was somewhat longer than previously published with PRESS or STEAM. However, no precise quantitative comparison was made between PRESS and CP-PRESS, and T2s for J-coupled metabolites were limited to mIns and the multiplet of NAA. Therefore, the quantitative effect of CP trains on T2s of other coupled metabolites, such as glutamate, glutamine or taurine, remains to be determined.
The aim of the present study was to investigate the effect of CP pulse trains on the T2s of metabolites (both non-coupled and J-coupled spins) in the rat brain at 9.4 T using LASER, CP-LASER and T2ρ-LASER sequences.
METHODS
Male Sprague-Dawley rats (n = 6) were studied using a 9.4 T horizontal-bore magnet (Magnex Scientific, Oxford, UK) interfaced to an Agilent DirectDrive console (Agilent Technologies, CA, USA). The magnet was equipped with a gradient insert capable of reaching 400 mT/m in 300 μs (Resonance Research, Inc., MA, USA). The animals were anesthetized with 1.5% isoflurane in a mixture of 50% O2:50% N2O and placed in a cradle with the head fixed with bite-bar and ear rods. The body temperature was monitored throughout the study with a rectal thermal sensor and maintained at 37°C using a hot water circulation. All studies were approved by the Institutional Animal Care and Use Committee at the University of Minnesota.
Localized in vivo 1H NMR spectra from the brain were measured using LASER, CP-LASER and T2ρ-LASER sequences (Figure 1). A volume-of-interest (VOI) of 62.5 μL (5 × 2.5 × 5 mm3) was positioned on the midline 2 mm posterior to bregma and 3 mm ventral using transverse and sagittal RARE (17) images (repetition time, TR = 4 s, TE = 20 ms, echo train length = 8, field-of-view = 3 × 3 cm2, matrix size = 256 × 128, slice thickness = 1 mm, 10 slices) and included tissue from cortex, hippocampus, and striatum. For all NMR measurements, a home-built quadrature 400 MHz 1H surface RF coil (two loops, 14 mm diameter each) was used. B0 shimming in the defined VOI was performed using an adiabatic version of FAST(EST)MAP (18) which resulted in a water linewidth of 13 ± 1 Hz.
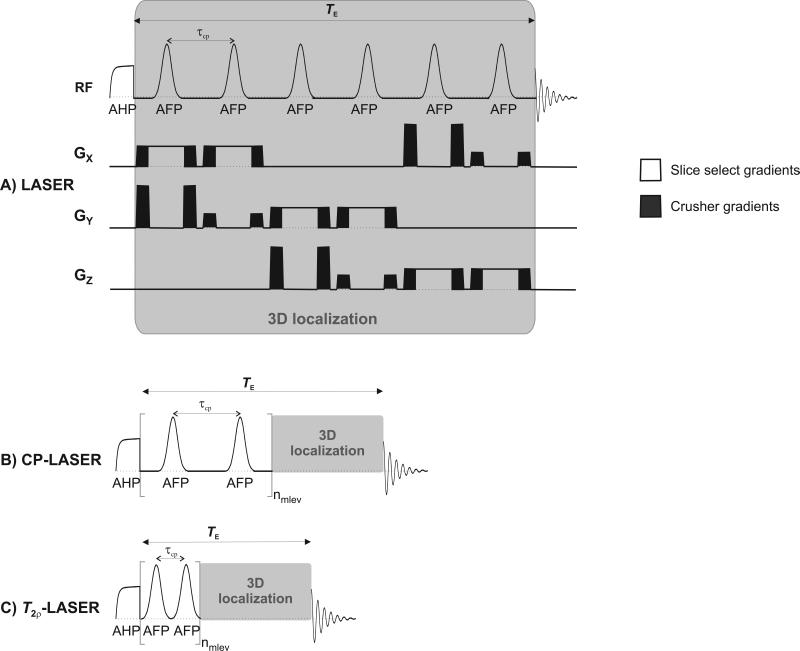
Figure 1.
Diagrams of A) LASER, B) CP-LASER and C) T2ρ-LASER sequences used for MRS acquisition. In LASER, the AFP pulses were applied in the presence of gradients for 3D localization (represented by the gray box). In CP- and T2ρ- LASER sequences, a CP train of AFP pulses (identical adiabatic pulses as utilized in LASER) was inserted prior to the LASER localization and was phase cycled according to the MLEV scheme. The τcp delay was 3 ms in CP-LASER and 1.5 ms in T2ρ-LASER (equivalent to the duration of one AFP pulse). No spoiler gradients were applied during the CP segment.
The LASER sequence consisted of a 4 ms nonselective adiabatic half-passage (AHP) pulse followed by three pairs of gradient selective AFP pulses of the HSn family (1.5 ms duration, n = 1, R = 25, (19)) for 3D-localization (Figure 1A). TE was defined as the total time from the end of the AHP pulse to the start of signal acquisition. The minimum TE was 18 ms (corresponding to τcp = 3 ms) due to additional time needed for slice selective and spoiling gradients. For T2 measurements, TE was increased by adding free precession delays evenly distributed around the six AFP pulses (1).
In CP-LASER (Figure 1B), TE was increased by adding AFP pulses rather than free precession delays. τcp was kept at 3 ms as in LASER. No gradient spoilers were used during the CP pulse train, and the CP pulses were phase cycled according to the MLEV scheme (20).
The T2ρ-LASER sequence (Figure 1C) was similar to CP-LASER except that there was no delay between the CP pulses such that τcp was 1.5 ms (equal to the duration of one AFP pulse).
For LASER, 1H spectra were acquired with eight TEs: 18, 25, 32, 46, 60, 100, 200 and 400 ms. For CP-LASER and T2ρ-LASER, CP train lengths (nmlev) of 8, 16, 32, 64 and 128 AFP pulses were used, corresponding to TEs of 42, 66, 114, 210 and 402 ms for CP-LASER and 30, 42, 66, 114 and 210 ms for T2ρ-LASER. These TEs correspond to the minimum TE used in LASER (i.e., 18 ms) plus the length of the CP pulse train (i.e., nmlev × τcp).
Water suppression was achieved using VAPOR (21). Spectra were acquired with TR of 4 s, a spectral width of 10 kHz and 10,000 data points. Each FID was individually saved for shot-to-shot B0 frequency correction. A non-suppressed water spectrum at each TE was also acquired for eddy current correction. For each pulse sequence, macromolecule spectra were acquired using the inversion-recovery technique (22) for all TEs except for TEs > 200 ms where macromolecule signal was negligible.
All in vivo 1H spectra were analyzed using LCModel version 6.1-4A (Stephen Provencher Inc., ON, Canada (23)). No baseline correction, zero-filling or apodization functions were applied to the in vivo data prior to the analysis. The spectra were fitted over the 0.5 to 4.2 ppm range. The model basis spectra for the various TEs used in this study were simulated using home-written programs based on density matrix formalism in Matlab (The MathWorks, Inc., Natick, MA, USA) (24) using measured and published chemical shifts and J-coupling values (25). The spin evolution occurring during the adiabatic RF pulses was also taken into account in the simulations. Separate basis spectra were generated for the singlet and multiplet signals of NAA, and the CH2 and CH3 groups of creatine and phosphocreatine (6). The signal from the methylene protons of tCr present in the macromolecule spectra (due to their short T1 relaxation time) was removed in post-processing using HSVD in Matlab.
The T2 values were determined by fitting the metabolite signals obtained from LCModel analysis as a function of TE using a two-parameter, mono-exponential decaying function in Matlab. Only metabolites with Cramér-Rao Lower bounds (CRLBs) < 50% at all TEs were considered (supplementary material), and T2 values are reported for metabolites for which fits with goodness of fit (R2) above 0.88 were obtained. The measured transverse relaxation times for each sequence are denoted by T2f (free precession), T2† and T2ρ for LASER, CP-LASER and T2p-LASER, respectively. The two-tailed, unpaired student's t-test was used to compare T2s between pulse sequences.
RESULTS
Spectral appearance
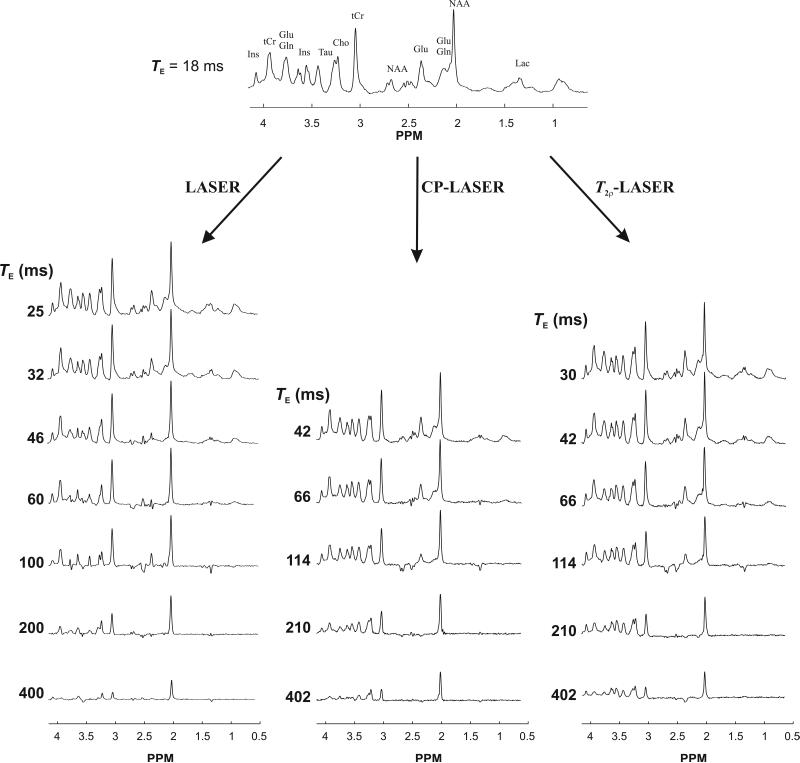
In vivo 1H NMR spectra acquired at various TEs with LASER, CP-LASER and T2ρ-LASER sequences in the rat brain are shown in Figure 2. In LASER, singlets showed signal reduction with increasing TE due to T2 relaxation while J-coupled metabolites underwent both J-evolution and T2 relaxation, which is apparent mainly in the 2.1 to 2.5 ppm (glutamate and glutamine) and 3.4 to 3.6 ppm (mIns) regions. In CP-LASER and T2ρ-LASER spectra, in contrast, the intensities of these multiplets were preserved at relatively long TEs: glutamate and glutamine resonances were still visible at 114 ms while mIns began to undergo noticeable J-modulation only around 210 ms.
Figure 2.
In vivo 1H NMR spectra measured with LASER, CP-LASER and T2ρ-LASER sequences from the same animal with different TEs (TR = 4 s, 128 averages were acquired for all spectra except for TE = 400 ms in LASER where 192 averages were acquired). The T2ρ-LASER spectrum at 402 ms was only acquired in this animal to show that even at such long TE several resonances in the 3 to 4.2 ppm range could be still observed compared to LASER and CP-LASER spectra.
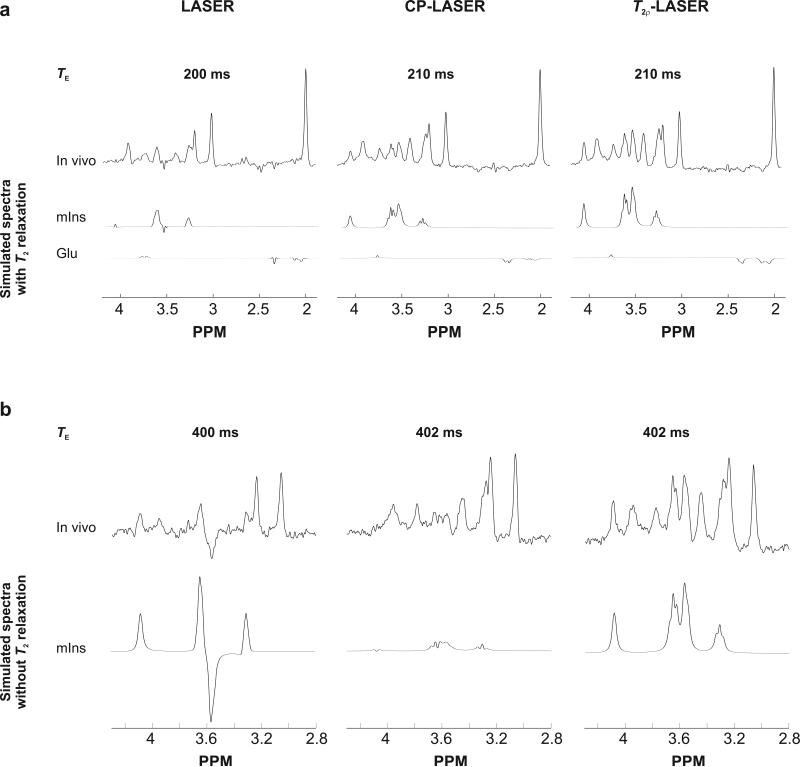
Spectra obtained with CP-LASER and T2ρ-LASER were remarkably similar at the same TE (Figure 2). However, small differences could be observed especially at long TEs (Figure 3). For example, the signal intensity of mIns at TE of 210 ms was lower with CP-LASER than with T2ρ-LASER despite having very similar spectral pattern (Figure 3a). In addition, glutamate exhibited different J-modulation with CP-LASER than T2ρ-LASER, and its intensity was higher with T2ρ-LASER. At TE of 402 ms, no mIns signal was observed with CP-LASER due to the J-modulation while under T2ρ-LASER mIns did not undergo any J-evolution (Figure 3b). These differences can be attributed to the different number of CP pulses and the different τcp delay between the CP pulses (3 ms versus 1.5 ms) in both sequences. The J-modulation is slowed down further in T2ρ-LASER compared to CP-LASER, especially for strongly coupled spin systems. Simulated spectra were in excellent agreement with the spectra measured in vivo (Figure 3).
Figure 3.
Comparison of LASER, CP-LASER and T2ρ-LASER spectra (128 averages, a 1.5 Hz Gaussian line broadening was applied prior to FT) measured at (a) TE = 200 ms and 210 ms and (b) TE = 400 ms and 402 ms from the same animal. In (a), the simulated spectra are scaled by T2 relaxation (amplitude matched to in vivo spectra). The observed differences in intensities between CP-LASER and T2ρ-LASER sequences are consistent with the density-matrix simulated NMR spectra. In (b), the simulated mIns spectra are shown with the same scaling adjusted so the intensity of mIns in T2ρ-LASER simulated spectrum matches the intensity of mIns in the T2ρ-LASER in vivo spectrum. Therefore the simulated spectra represent the relative signals intensity of mIns that would be expected if T2 was the same with LASER, CP-LASER and T2ρ-LASER. With the same T2 relaxation, the peak at 4.05 ppm would have almost identical amplitude with LASER and T2ρ-LASER. However, it is apparent that in vivo this peak is much smaller in LASER spectrum compared to T2ρ-LASER spectrum providing direct visual evidence that the T2 of mIns is longer with T2-LASER compared with LASER.
The simulated spectra are shown line-broadened to match in vivo spectra (8 Hz exponential line-broadening). Glu = glutamate, mIns = myo-inositol.
Macromolecule resonances were also affected by the CP pulse train. For instance, the signal intensities for the 0.91 ppm and 1.7 ppm resonances were higher with CP-LASER and T2ρ-LASER compared to LASER at similar echo times (Figure 2). However, no macromolecule signal was observed for any sequences at an echo time of 200 ms and longer.
Effect of CP pulse train on J-modulation
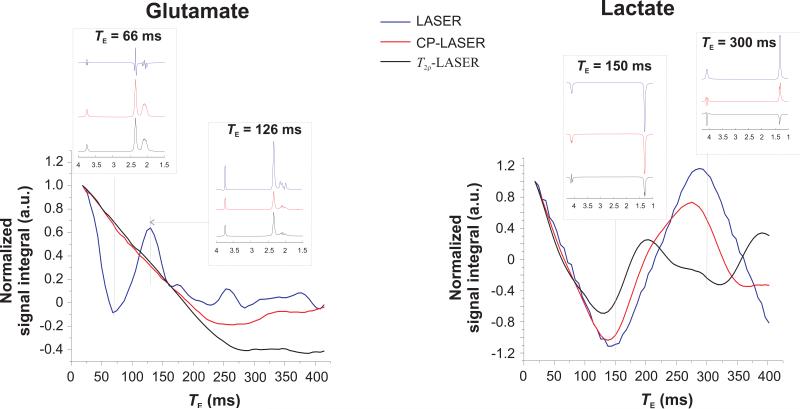
Under CP conditions, signal loss due to J-modulation was reduced much more significantly in strongly coupled spin systems than in weakly coupled spin systems as shown previously with simulations for alanine and taurine (26). This is illustrated in Figure 4 which shows simulations of total signal integral (without taking into account T2 relaxation) as a function of TE for glutamate (a strongly coupled spin system) and lactate (a weakly coupled spin system) for all three pulse sequences.
Figure 4.
Effect of CP pulse trains on the J-evolution of glutamate (strongly coupled system) and lactate (weakly coupled system). Signal integral (without taking into account T2 relaxation) which corresponds to the amplitude of the first point of the simulated FID normalized to 1 at TE of 18 ms is shown as a function of TE for LASER (blue line), CP-LASER (black line) and T2ρ-LASER (red line) sequences at 9.4 T. Inserts show the spectral pattern of glutamate at TE = 66 and 126 ms, and lactate at TE = 150 and 300 ms for the three sequences. Although the signal integral of glutamate with LASER is almost zero at TE = 66 ms, there is sufficient signal intensity for precise LCModel fitting. At TE = 126 ms, the signal intensity (without T2 relaxation) of glutamate with LASER is 2.5 times higher than that with T2ρ-LASER. It is possible that for certain TEs and certain metabolites, LASER sequence might have more signal than CP-LASER or T2ρ-LASER.
The simulated spectra are shown line-broadened (10 Hz exponential line-broadening).
For glutamate, simulations show that J-modulation is substantially reduced with CP-LASER and T2ρ-LASER compared to LASER, which is particularly apparent for TE < 150 ms. For lactate, in contrast, J-modulation is not significantly slowed by the CP train for TE < 100 ms, although it differs for each sequence at longer TE. The signal integral of lactate with LASER follows a sinusoidal modulation as TE increases, with the first minimum occurring at ~144 ms (i.e., 1/J) and the next maximum at ~288 Hz (i.e., 2/J) as expected for a weakly coupled ½ spin system. With the CP pulse train, the lactate integral is not refocused at multiples of 1/J.
This could also be observed in vivo (Figure 2): resonances of weakly coupled spins such as the methyl protons of lactate at 1.32 ppm and the NAA multiplet in the 2.49–2.67 ppm region showed noticeable J-modulation at much shorter TE than resonances of strongly coupled spins such as glutamate, taurine and mIns, with CP-LASER and T2ρ-LASER sequences.
Finally, simulations in Figure 4 also confirm that, while CP-LASER and T2ρ-LASER behave very similarly at shorter TE, significant differences in J-modulation become apparent at longer TE, as can also be observed in experimental data (Figure 3).
Transverse relaxation times
The T2 relaxation times of metabolites measured using all three pulse sequences are given in Table 1. T2s of all measured metabolites were significantly longer with CP-LASER and T2ρ-LASER compared to LASER. However, no significant difference was observed in measured T2s between CP-LASER and T2ρ-LASER sequences for any metabolite.
Table 1.
T2 relaxation times (mean ± SD) measured with LASER, CP-LASER and T2ρ-LASER sequences and T2 ratios for CP-LASER and LASER and T2ρ-LASER and LASER obtained in the rat brain (n = 6) in vivo at 9.4 T. T2s of all measured metabolites were significantly longer (P < 0.05) with CP pulse sequences compared to LASER. T2 of water was also significantly longer with CP-LASER (P < 0.01) and T2ρ-LASER (P < 0.001) compared to LASER. Between CP-LASER and T2ρ-LASER sequences, the T2s of metabolites were not significantly different (P > 0.05) except in the case of water (P = 0.04). The high SDs observed for tCho and mIns were due to their T2 being longer than the maximum TE used in this study. The goodness of fit, R2 was > 0.9 for all metabolites except for the multiplet of NAA which was 0.88 with LASER.
| Spin systems | Compound | Group | LASER T2f (ms) | CP-LASER T2† (ms) | T2ρ-LASER T2ρ (ms) | T2†/T2f | T2ρ/T2f |
|---|---|---|---|---|---|---|---|
| Non-coupled and weakly coupled | Water | H2O | 44 ± 2 | 48 ± 2 | 50 ± 2 | 1.09 ± 0.07 | 1.16 ± 0.07 |
| NAA | 2CH3 | 321 ± 30 | 456 ± 26 | 449 ± 32 | 1.42 ± 0.16 | 1.40 ± 0.17 | |
| NAA | 3CH2 | 157 ± 41 | 187 ± 16 | 167 ± 25 | 1.19 ± 0.33 | 1.06 ± 0.32 | |
| tCr | N(CH3) | 170 ± 12 | 224 ± 7 | 231 ± 16 | 1.32 ± 0.10 | 1.36 ± 0.13 | |
| tCr | 2CH2 | 146 ± 16 | 175 ± 9 | 183 ± 20 | 1.20 ± 0.15 | 1.25 ± 0.20 | |
| tCho | entire molecule | 445 ± 67 | 523 ± 68 | ND | 1.17 ± 0.23 | - | |
| Strongly coupled | Glu | entire molecule | 70 ± 14 | 296 ± 44 | 270 ± 21 | 4.20 ± 1.02 | 3.84 ± 0.80 |
| Gln | entire molecule | 56 ± 12 | 116 ± 7 | 126 ± 19 | 2.08 ± 0.45 | 2.26 ± 0.58 | |
| mIns | entire molecule | 161 ± 26 | 584 ± 94 | 600 ± 90 | 3.62 ± 0.82 | 3.72 ± 0.82 | |
| taurine | entire molecule | 162 ± 28 | 355 ± 17 | 343 ± 27 | 2.19 ± 0.39 | 2.11 ± 0.40 |
Singlet resonances showed a significant increase in T2s with CP-LASER compared to LASER, ranging from 17% for tCho to 42% for NAA (Table 1). Similarly, the multiplet of NAA in the 2.47 to 2.67 ppm region showed a 6-19% increase in T2 with CP-LASER and T2ρ-LASER compared to LASER (Table 1). In comparison, the T2 of water increased by 9% with CP-LASER and 16% with T2ρ-LASER compared to LASER.
For strongly coupled systems, the increase in measured T2s with CP-LASER and T2ρ-LASER compared to LASER was much larger. For example, T2 of glutamate increased from 70 ms with LASER to 296 ms with CP-LASER. The increase in T2 with CP-LASER compared to LASER was about 2-fold for glutamine and taurine and about 4-fold for mIns and glutamate (Table 1). mIns exhibited the longest T2 under CP condition (600 ms). This lengthening of T2 is readily apparent when comparing the in vivo and simulated spectra of mIns and its resonance at 4.05 ppm (Figure 3B). Similarly, the prolonged T2 was confirmed directly for taurine using phantom data acquired with the three sequences (supplementary material).
The T2 of tCho could not be determined under T2ρ-LASER due to the strong cross-correlation between choline compounds and phosphorylethanolamine in the LCModel fit. In addition, the T2 of lactate could not be determined reliably with CP-LASER and T2ρ-LASER due to the lower signal integral of lactate with CP trains compared to LASER at most TEs (Figure 4).
DISCUSSION
In this study, we report for the first time quantitative measurement of T2 relaxation times for multiple J-coupled metabolites in the brain under CP conditions. While all metabolites had longer T2 with CP-LASER and T2ρ-LASER compared to LASER, the increase in T2 was much more pronounced for strongly coupled spins (up to 4-fold longer) than for non-coupled (singlet) and weakly coupled spins. These findings provide new insights into the relaxation pathways in coupled spin systems and have significant implications for accurate quantification of MR spectra acquired at high field.
Comparison of T2 measured with LASER, CP-LASER and T2ρ-LASER
T2s measured for non-coupled (singlet) spins including water were longer when measured with CP-LASER and T2ρ–LASER sequences compared to LASER. However, the increase in T2 was smaller for water than for other singlets such as the CH3 group of NAA or the CH3 and CH2 groups of tCr. Similar observations have been previously reported for water, NAA and tCr in the human brain (5). For water which has a larger apparent diffusion coefficient (ADC) than metabolites in addition to rapidly exchanging protons, the increase in T2 was smaller. This suggests that the diffusion component might not be efficiently refocused by the CP pulse trains (5). In contrast, NAA and tCr singlets have non-exchangeable protons and lower ADC than water, so that the CP pulse train suppresses the diffusion component efficiently, resulting in higher increase in T2 under CP train.
Although the molecular structures of glutamate and glutamine are similar, these metabolites seem to experience different relaxation pathways under the CP pulse train. This is evidenced by the much higher increase in T2s for glutamate (4-fold) compared to glutamine (2-fold) with CP-LASER and T2ρ-LASER compared to LASER. This different gain may be explained by the higher diffusion coefficient for glutamine compared to glutamate as previously reported in the rat brain (27). With a high diffusion coefficient, CP pulse trains may not refocus the diffusion component of T2 relaxation as efficiently (5).
Strongly coupled spins such as glutamate, taurine, and mIns exhibit a higher gain in T2s under the CP pulse train compared to non-coupled spins although their diffusion coefficients are closer to that of singlet resonances. This suggests that the prolonged T2 values cannot be solely attributed to suppression of the diffusion term since both non-coupled and J-coupled spins experience this relaxation mechanism. However, in strongly J-coupled systems, the additional increase in T2 during a CP sequence can be attributed to refocusing of cross-correlation or interference effects (28) between different dipole-dipole interactions (29).
Finally, T2s measured with CP-LASER and T2ρ-LASER were very similar, although differences were observed in the J-modulation pattern at longer TE. The similarity in T2 values can be attributed to the relatively short τcp of 3 ms used in CP-LASER, resulting in minimal dephasing of magnetization during the interpulse interval (30). Longer τcp delays (> 3 ms) in CP-LASER would result in shorter T2† compared to T2ρ. At very long τcp, T2† would approach that of T2f as previously reported (16,30,31).
The transverse relaxation times are dependent on magnetic field strength and the properties of the pulse sequence utilized (i.e., RF pulse patterns and timings) such that T2 values and T2 gains observed in this study at 9.4 T might not hold true for other sequences and other timings. For example, the T2s of singlets (e.g., NAA, tCr and tCho) measured with LASER in the present study were consistent with a previous rat study using a similar pulse sequence (7) but were longer (between 30% to 250% longer) than those reported with PRESS or SPECIAL sequences (32,33) at 9.4 T. Additionally, T2 values measured with LASER but not with CP-LASER and T2ρ-LASER can be affected by the chemical shift displacement errors. In the present study, the largest chemical shift displacement error of 7% would be observed for lactate due to the large bandwidth of the AFP pulses. However, for the strongly coupled metabolites such as glutamate, glutamine, taurine and mIns the chemical shift displacement error would be smaller (4%, 4%, 0.4%, and 2%, respectively).
Comparison of effect of CP train in human brain and rat brain
The increase in T2 under CP conditions (i.e., T2†/T2f) is dependent on B0, as reported previously (5), because the dynamic dephasing (i.e., diffusion and exchange) component of transverse relaxation increases with B0. The T2s measured for non-coupled spins (NAA, tCr and tCho) with CP-PRESS (12) in human brain at 1.5 T were only slightly longer compared to values obtained with the PRESS sequence (34). At higher fields, however, the increase in T2 with CP-LASER compared to PRESS was larger. The T2 of NAA singlet in human brain was 1.7 times longer at 4 T and 2.2 longer at 7 T with CP-LASER compared to PRESS (5), and 1.5 times longer at 4 T with CP-LASER (5) compared to LASER (6).
In the present study in rat brain, the increase in the T2 of NAA singlet at 9.4 T with CP-LASER compared to LASER was only a factor 1.4, which was lower than the increase observed in human brain at 4 T. Therefore the increase in T2 with CP-LASER compared to LASER appears smaller in rat brain than in human brain at a given B0. This is likely due to smaller microsusceptibility effects in rat brain, resulting in smaller B0 dephasing effects (35,36). A similar dependence of T2 on microsusceptibility and magnetic field can be expected for J-coupled spins.
Effect of RF pulse modulation on T2 relaxation times
The increase in T2 during CP pulse trains depends both on the amplitude and frequency-modulation functions of the AFP pulses used (37). It was experimentally demonstrated on phantom using 1H MRS that water exhibits longer T2ρ relaxation times under HS4 AFP pulses compared to HS1 AFP pulses with similar pulse duration and B1 field (31,38,39). Similar findings were recently reported for the CH3 group of NAA and tCr in the human brain in vivo at 4 T (39). This dependence of T2 on modulation functions is the result of spins experiencing different dipole-dipole and/or chemical exchange interactions during the different type of pulses (37) and forms the basis of a new MRI contrast (31). In the present study, HS1 pulses were used for both CP train as well as in LASER. Even larger increases in T2 can be expected if HS4 pulses are used.
Absolute quantification
Sequences such as LASER and semi-LASER are advantageous at high field because AFP pulses can be lengthened while retaining a large refocusing bandwidth, which minimizes chemical-shift displacement artifacts. Even though LASER and semi-LASER have longer TE than STEAM, the effect of this longer TE is mitigated by reduced J-modulation and increased T2. In fact, it has been shown that LASER and semi-LASER spectra can be very similar in appearance to short echo-time STEAM spectra while providing 2-fold higher signal-to-noise.
At high magnetic fields, the minimum achievable TE generally becomes longer due to increased RF power requirements, which leads to longer RF pulses. At the same time, T2s relaxation times become shorter at high field (36). Therefore, while often neglected at lower field, signal loss due to T2 relaxation may not be negligible at high field, especially in human brain.
Correction for T2 relaxation requires measurement of the T2s of metabolites. Our results show that the common practice of measuring T2 by inserting free precession delays does not provide accurate T2 values for CP-like sequences. For this type of sequences such as LASER and semi-LASER, T2s must be measured while keeping τcp constant to take into account the effect of successive 180° pulses. The potential effect of successive 180° pulses on T2 is important to keep in mind when performing absolute quantification.
CONCLUSION
This study reports for the first time quantitative measurements of T2s for multiple J-coupled metabolites. It is shown that the use of a CP pulse train results in a much larger increase in T2 for strongly coupled spins (up to 4-fold) than for non-coupled and weakly coupled spins. Therefore, pulse sequences with a succession of 180° pulses such as LASER are particularly advantageous for retaining signals from strongly coupled spins at relatively short TE. If correcting for T2 relaxation is needed in LASER and semi-LASER, care must be taken to use T2 values measured under CP-like conditions.
Supplementary Material
ACKNOWLEDGEMENT
The authors thank Chris Nelson, Manda Vollmers and Emily Colonna for expert technical assistance, Dr. Shalom Michaeli for helpful discussions and Dr. Jamie Walls for reading the manuscript. This work was supported by funding from NIH grants P41 EB015894 and P30 NS076408 and the W.M. KECK Foundation.
REFERENCES
- 1.Garwood M, DelaBarre L. The return of the frequency sweep: designing adiabatic pulses for contemporary NMR. J Magn Reson. 2001;153(2):155–177. doi: 10.1006/jmre.2001.2340. [DOI] [PubMed] [Google Scholar]
- 2.Scheenen TWJ, Klomp DWJ, Wijnen JP, Heerschap A. Short echo time 1H-MRSI of the human brain at 3T with minimal chemical shift displacement errors using adiabatic refocusing pulses. Magn Reson Med. 2008;59(1):1–6. doi: 10.1002/mrm.21302. [DOI] [PubMed] [Google Scholar]
- 3.Marjanska Mg, Auerbach EJ, Valabregue R. Van de Moortele P-F, Adriany G, Garwood M. Localized 1H NMR spectroscopy in different regions of human brain in vivo at 7T: T2 relaxation times and concentrations of cerebral metabolites. NMR Biomed. 2012;25(2):332–339. doi: 10.1002/nbm.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oz G, Tkac I. Short-echo, single-shot, full-intensity proton magnetic resonance spectroscopy for neurochemical profiling at 4 T: Validation in the cerebellum and brainstem. Magn Reson Med. 2011;65(4):901–910. doi: 10.1002/mrm.22708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Michaeli S, Garwood M, Zhu XH, DelaBarre L, Andersen P, Adriany G, Merkle H, Ugurbil K, Chen W. Proton T2 relaxation study of water, N-acetylaspartate, and creatine in human brain using Hahn and Carr-Purcell spin echoes at 4T and 7T. Magn Reson Med. 2002;47(4):629–633. doi: 10.1002/mrm.10135. [DOI] [PubMed] [Google Scholar]
- 6.Deelchand DK, Henry P-G, Ugurbil K, Marjanska M. Measurement of transverse relaxation times of J-coupled metabolites in the human visual cortex at 4 T. Magn Reson Med. 2012;67(4):891–897. doi: 10.1002/mrm.23080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Graaf R, Brown P, McIntyre S, Nixon T, Behar K, Rothman D. High magnetic field water and metabolite proton T1 and T2 relaxation in rat brain in vivo. Magn Reson Med. 2006;56(2):386–394. doi: 10.1002/mrm.20946. [DOI] [PubMed] [Google Scholar]
- 8.Marjanska M, Curran GL, Wengenack TM, Henry PG, Bliss RL, Poduslo JF, Jack CR, Jr., Ugurbil K, Garwood M. Monitoring disease progression in transgenic mouse models of Alzheimer's disease with proton magnetic resonance spectroscopy. Proc Natl Acad Sci U S A. 2005;102(33):11906–11910. doi: 10.1073/pnas.0505513102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mangia S, Liimatainen T, Garwood M, Tkac I, Henry P-G, Deelchand D, Michaeli S. Frequency offset dependence of adiabatic rotating frame relaxation rate constants: relevance to MRS investigations of metabolite dynamics in vivo. NMR Biomed. 2011;24(7):807–814. doi: 10.1002/nbm.1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carr HY, Purcell EM. Effects of Diffusion on Free Precession in Nuclear Magnetic Resonance Experiments. Phys Rev. 1954;94(3):630–638. [Google Scholar]
- 11.Allerhand A. Analysis of Carr---Purcell Spin-Echo NMR Experiments on Multiple-Spin Systems. I. The Effect of Homonuclear Coupling. J Chem Phys. 1966;44(1):1–9. [Google Scholar]
- 12.Soher BJ, Pattany PM, Matson GB, Maudsley AA. Observation of coupled 1H metabolite resonances at long TE. Magn Reson Med. 2005;53(6):1283–1287. doi: 10.1002/mrm.20491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hennig J, Thiel T, Speck O. Improved sensitivity to overlapping multiplet signals in in vivo proton spectroscopy using a multiecho volume selective (CPRESS) experiment. Magn Reson Med. 1997;37(6):816–820. doi: 10.1002/mrm.1910370603. [DOI] [PubMed] [Google Scholar]
- 14.Hancu I, Gillen R, Cowan J, Zimmerman EA. Improved myo-inositol detection through Carr-Purcell PRESS: A tool for more sensitive mild cognitive impairment diagnosis. Magn Reson Med. 2011;65(6):1515–1521. doi: 10.1002/mrm.22749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Allerhand A, Thiele E. Analysis of Carr---Purcell Spin-Echo NMR Experiments on Multiple-Spin Systems. II. The Effect of Chemical Exchange. J Chem Phys. 1966;45(3):902–916. [Google Scholar]
- 16.Bartha R, Michaeli S, Merkle H, Adriany G, Andersen P, Chen W, Ugurbil K, Garwood M. In vivo 1H2O T +2 measurement in the human occipital lobe at 4T and 7T by Carr- Purcell MRI: detection of microscopic susceptibility contrast. Magn Reson Med. 2002;47(4):742–750. doi: 10.1002/mrm.10112. [DOI] [PubMed] [Google Scholar]
- 17.Hennig J, Nauerth A, Friedburg H. RARE imaging: A fast imaging method for clinical MR. Magn Reson Med. 1986;3(6):823–833. doi: 10.1002/mrm.1910030602. [DOI] [PubMed] [Google Scholar]
- 18.Gruetter R, Tkac I. Field mapping without reference scan using asymmetric echo-planar techniques. Magn Reson Med. 2000;43(2):319–323. doi: 10.1002/(sici)1522-2594(200002)43:2<319::aid-mrm22>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 19.Silver MS, Joseph RI, Hoult DI. Highly selective π/2 and π pulse generation. J Magn Reson. 1984;59(2):347–351. [Google Scholar]
- 20.Levitt MH, Freeman R, Frenkiel T. Supercycles for broadband heteronuclear decoupling. J Magn Reson. 1982;50(1):157–160. [Google Scholar]
- 21.Tkac I, Starcuk Z, Choi IY, Gruetter R. In vivo 1H NMR spectroscopy of rat brain at 1 ms echo time. Magn Reson Med. 1999;41(4):649–656. doi: 10.1002/(sici)1522-2594(199904)41:4<649::aid-mrm2>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 22.Pfeuffer J, Tkac I, Provencher SW, Gruetter R. Toward an in vivo neurochemical profile: quantification of 18 metabolites in short-echo-time 1H NMR spectra of the rat brain. J Magn Reson. 1999;141(1):104–120. doi: 10.1006/jmre.1999.1895. [DOI] [PubMed] [Google Scholar]
- 23.Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med. 1993;30(6):672–679. doi: 10.1002/mrm.1910300604. [DOI] [PubMed] [Google Scholar]
- 24.Henry PG, Marjanska M, Walls JD, Valette J, Gruetter R, Ugurbil K. Proton-observed carbon-edited NMR spectroscopy in strongly coupled second-order spin systems. Magn Reson Med. 2006;55(2):250–257. doi: 10.1002/mrm.20764. [DOI] [PubMed] [Google Scholar]
- 25.Govindaraju V, Young K, Maudsley AA. Proton NMR chemical shifts and coupling constants for brain metabolites. NMR Biomed. 2000;13(3):129–153. doi: 10.1002/1099-1492(200005)13:3<129::aid-nbm619>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 26.Mayer D, Dreher W, Leibfritz D, Spielman DM. RF refocused echoes of J-coupled spin systems: Effects on RARE-based spectroscopic imaging. Magn Reson Med. 2007;57(5):967–971. doi: 10.1002/mrm.21206. [DOI] [PubMed] [Google Scholar]
- 27.Pfeuffer J, Tkac I, Gruetter R. Extracellular-intracellular distribution of glucose and lactate in the rat brain assessed noninvasively by diffusion-weighted 1H nuclear magnetic resonance spectroscopy in vivo. J Cereb Blood Flow Metab. 2000;20(4):736–746. doi: 10.1097/00004647-200004000-00011. [DOI] [PubMed] [Google Scholar]
- 28.Blicharski JS, Kruk D. NMR relaxation spectroscopy: Interference effects. Appl Magn Reson. 1999;17(2-3):367–374. [Google Scholar]
- 29.Pervushin K, Riek R, Wider G, Wuthrich K. Attenuated T2 relaxation by mutual cancellation of dipole-dipole coupling and chemical shift anisotropy indicates an avenue to NMR structures of very large biological macromolecules in solution. Proc Natl Acad Sci U S A. 1997;94(23):12366–12371. doi: 10.1073/pnas.94.23.12366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Santyr GE, Henkelman RM, Bronskill MJ. Variation in measured transverse relaxation in tissue resulting from spin locking with the CPMG sequence. J Magn Reson. 1988;79:28–44. [Google Scholar]
- 31.Michaeli S, Gröhn H, Gröhn O, Sorce DJ, Kauppinen R, Jr., Ugurbil K, Garwood M. Exchange-influenced T2rho contrast in human brain images measured with adiabatic radio frequency pulses. Magn Reson Med. 2005;53(4):823–829. doi: 10.1002/mrm.20428. CSS. [DOI] [PubMed] [Google Scholar]
- 32.Xin L, Gambarota G, Mlynarik V, Gruetter R. Proton T2 relaxation time of J-coupled cerebral metabolites in rat brain at 9.4 T. NMR Biomed. 2008;21(4):396–401. doi: 10.1002/nbm.1205. [DOI] [PubMed] [Google Scholar]
- 33.Lei H, Zhang Y, Zhu XH, Chen W. Changes in the proton T2 relaxation times of cerebral water and metabolites during forebrain ischemia in rat at 9.4 T. Magn Reson Med. 2003;49(6):979–984. doi: 10.1002/mrm.10490. [DOI] [PubMed] [Google Scholar]
- 34.Kreis R. Quantitative localized 1H MR spectroscopy for clinical use. Prog Nucl Magn Reson Spectrosc. 1997;31(2-3):155–195. [Google Scholar]
- 35.Zhang K, Sejnowski TJ. A universal scaling law between gray matter and white matter of cerebral cortex. Proc Natl Acad Sci U S A. 2000;97(10):5621–5626. doi: 10.1073/pnas.090504197. DO -5610.1073/pnas.090504197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Deelchand DK, Moortele P-FVd, Adriany G, Iltis I, Andersen P, Strupp JP, Thomas Vaughan J, Ugurbil K, Henry P-G. In vivo 1H NMR spectroscopy of the human brain at 9.4 T: Initial results. J Magn Reson. 2010;206(1):74–80. doi: 10.1016/j.jmr.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Michaeli S, Sorce DJ, Idiyatullin D, Ugurbil K, Garwood M. Transverse relaxation in the rotating frame induced by chemical exchange. J Magn Reson. 2004;169(2):293–299. doi: 10.1016/j.jmr.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 38.Mangia S, Liimatainen T, Garwood M, Michaeli S. Rotating frame relaxation during adiabatic pulses vs. conventional spin lock: simulations and experimental results at 4 T. Magn Reson Imaging. 2009;27(8):1074–1087. doi: 10.1016/j.mri.2009.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mangia S, Garwood M, Tkac I, Ugurbil K, Michaeli S. Probing dynamics of human brain metabolites with 1H MRS by T1rho and T2rho adiabatic relaxations. Proc Intl Soc Mag Reson Med. 2008;(16):693. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.